Three-Dimensional Bioprinting Techniques in Skin Regeneration: Current Insights and Future Perspectives
Abstract
1. Introduction
1.1. Skin Anatomy and Physiology
1.2. Skin Injuries
1.3. Skin Treatments
1.4. New Approaches for Skin Regeneration
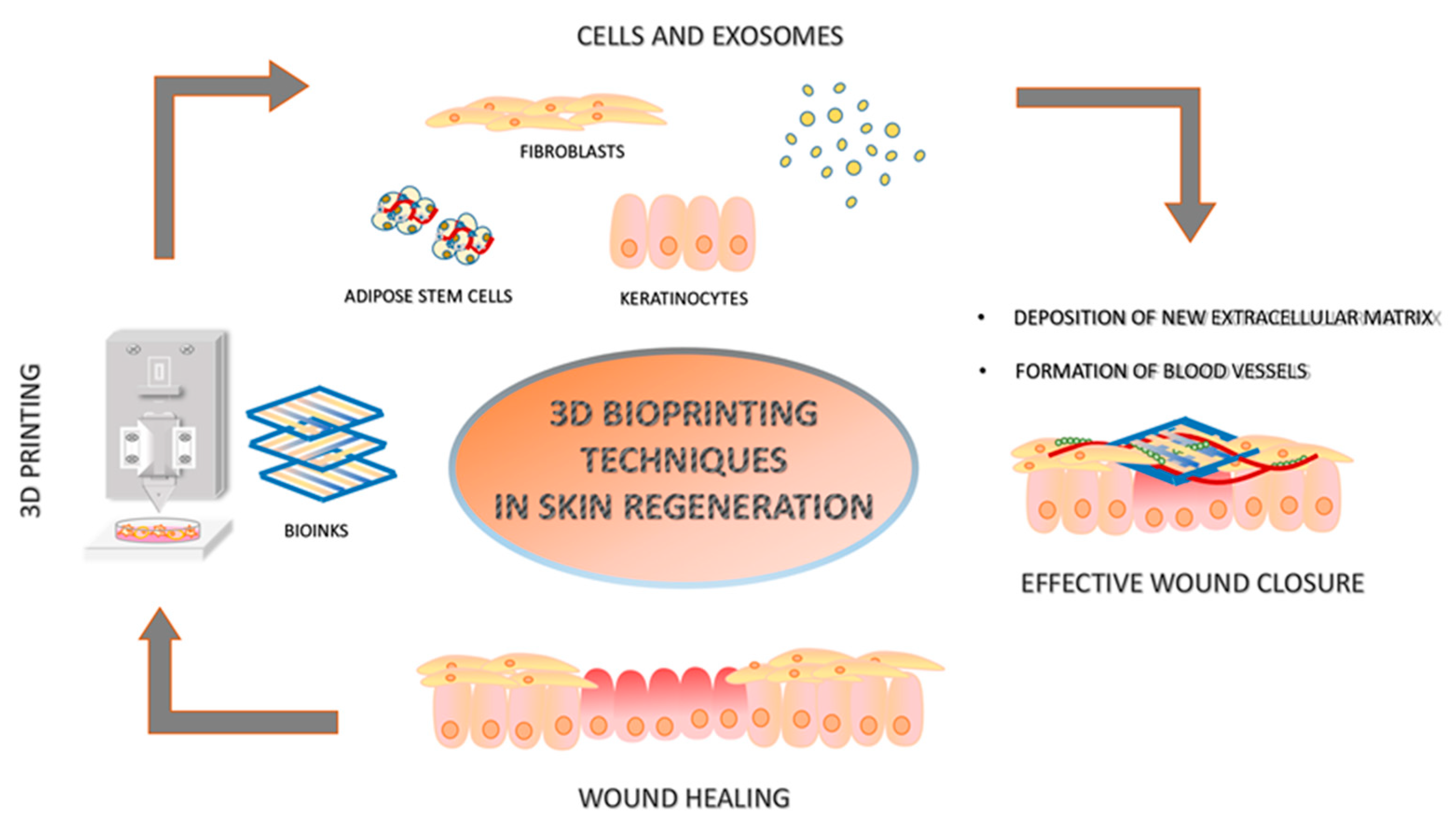
2. Three-Dimensional Printing in Skin Regeneration
3. Bioinks
3.1. Bioinks Without Cells
3.2. Bioinks with Cells
3.2.1. Fibroblasts
3.2.2. Fibroblasts and Keratinocytes Combined with Spheroids of Dermal Papilla Cells Spheroid
3.2.3. Fibroblasts, Keratinocytes and Endothelial Cells
3.2.4. Adipose- and Bone-Marrow-Derived Stem Cells
4. EVs in Skin Regeneration
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Halem, M.; Karimkhani, C. Dermatology of the head and neck: Skin cancer and benign skin lesions. Dent. Clin. N. Am. 2012, 56, 771–790. [Google Scholar] [CrossRef] [PubMed]
- Yousef, H.; Alhajj, M.; Fakoya, A.O.; Sharma, S. Anatomy, Skin (Integument), Epidermis; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Lopez-Ojeda, W.; Pandey, A.; Alhajj, M.; Oakley, A.M. Anatomy, Skin (Integument); StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Berkey, C.A.; Elsafty, O.; Riggs, M.M.; Dauskardt, R.H. Characterization and modeling of partial-thickness cutaneous injury from debris-simulating kinetic projectiles. Commun. Eng. 2022, 1, 33. [Google Scholar] [CrossRef]
- Takeo, M.; Lee, W.; Ito, M. Wound healing and skin regeneration. Cold Spring Harb. Perspect. Med. 2015, 5, a023267. [Google Scholar] [CrossRef] [PubMed]
- Chuong, C.M.; Randall, V.A.; Widelitz, R.B.; Wu, P.; Jiang, T.X. Physiological regeneration of skin appendages and implications for regenerative medicine. Physiology 2012, 27, 61–72. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, X.; Wang, C.; Li, L.; Ma, L.; Gao, C. Bioengineering skin constructs. In Stem Cell Biology and Tissue Engineering in Dental Sciences; Elsevier: Amsterdam, The Netherlands, 2015; pp. 703–719. [Google Scholar]
- Ji, S.; Zhu, Z.; Sun, X.; Fu, X. Functional hair follicle regeneration: An updated review. Signal Transduct. Target. Ther. 2021, 6, 66. [Google Scholar] [CrossRef]
- Plotczyk, M.; Jimenez, F. Hair follicles in wound healing and skin remodelling. In Hair Follicle Regeneration; Springer: Berlin/Heidelberg, Germany, 2022; pp. 291–304. [Google Scholar]
- Nuutila, K. Hair Follicle Transplantation for Wound Repair. Adv. Wound Care 2021, 10, 153–163. [Google Scholar] [CrossRef]
- Weng, T.; Wu, P.; Zhang, W.; Zheng, Y.; Li, Q.; Jin, R.; Chen, H.; You, C.; Guo, S.; Han, C.; et al. Regeneration of skin appendages and nerves: Current status and further challenges. J. Transl. Med. 2020, 18, 53. [Google Scholar] [CrossRef]
- Xie, J.; Yao, B.; Han, Y.; Huang, S.; Fu, X. Skin appendage-derived stem cells: Cell biology and potential for wound repair. Burns Trauma 2016, 4, 38. [Google Scholar] [CrossRef]
- Abuhamad, A.Y.; Masri, S.; Fadilah, N.I.M.; Alamassi, M.N.; Maarof, M.; Fauzi, M.B. Application of 3D-Printed Bioinks in Chronic Wound Healing: A Scoping Review. Polymers 2024, 16, 2456. [Google Scholar] [CrossRef]
- Fitridge, R.; Thompson, M. (Eds.) Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists; University of Adelaide Press: Adelaide, AU, USA, 2011. [Google Scholar]
- Teot, L.; Mustoe, T.A.; Middelkoop, E.; Gauglitz, G.G. (Eds.) Textbook on Scar Management: State of the Art Management and Emerging Technologies; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Wilgus, T.A. Inflammation as an orchestrator of cutaneous scar formation: A review of the literature. Plast. Aesthet. Res. 2020, 7, 54. [Google Scholar] [CrossRef]
- Koller, J. Effects of radiation on the integrity and functionality of amnion and skin grafts. In Sterilisation of Tissues Using Ionising Radiations; Elsevier: Amsterdam, The Netherlands, 2005; pp. 197–220. [Google Scholar]
- Brown, T.M.; Krishnamurthy, K. Histology, Dermis; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Blume, P. Chapter 19: Skin Grafts. Lower Extremity Soft Tissue and Cutaneous Plastic Surgery, 2nd ed.; WB Saunders: Philadelphia, PA, USA, 2012; pp. 207–224. [Google Scholar]
- Crawford, M.E. Autografts, allografts and xenografts in cutaneous surgery. Low. Extrem. Soft Tissue Cutan. Plast. Surg. 2012, 225. [Google Scholar] [CrossRef]
- Ray, S.; Rao, K. Full thickness skin grafts. In Skin Grafts-Indications, Applications and Current Research; StatPearls: Treasure Island, FL, USA, 2011. [Google Scholar]
- Seyhan, T. Split-thickness skin grafts. In Skin Grafts-Indications, Applications and Current Research; InTech: Vienna, Austria, 2011; pp. 1–16. [Google Scholar]
- Shahrokhi, S.; Arno, A.; Jeschke, M.G. The use of dermal substitutes in burn surgery: Acute phase. Wound Repair Regen. 2014, 22, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Lomba, E.; Lozano-Masdemont, B.; Sanchez-Herrero, A.; Aviles-Izquierdo, J.A. Dermal substitutes: An alternative for the reconstruction of large full-thickness defects in the plantar surface. An. Bras. Dermatol. 2021, 96, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Pleguezuelos-Beltran, P.; Galvez-Martin, P.; Nieto-Garcia, D.; Marchal, J.A.; Lopez-Ruiz, E. Advances in spray products for skin regeneration. Bioact. Mater. 2022, 16, 187–203. [Google Scholar] [CrossRef]
- Dai, L.G.; Fu, K.Y.; Hsieh, P.S.; Hung, Y.M.; Wang, Y.W.; Hsia, L.C.; Chang, S.C.; Wang, C.H.; Teng, S.C.; Chen, S.G.; et al. Evaluation of Wound Healing Efficacy of an Antimicrobial Spray Dressing at Skin Donor Sites. Wounds 2015, 27, 224–228. [Google Scholar]
- Frankowski, J.; Kurzatkowska, M.; Sobczak, M.; Piotrowska, U. Utilization of 3D bioprinting technology in creating human tissue and organoid models for preclinical drug research—State-of-the-art. Int. J. Pharm. 2023, 644, 123313. [Google Scholar] [CrossRef]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef]
- Seol, Y.J.; Kang, H.W.; Lee, S.J.; Atala, A.; Yoo, J.J. Bioprinting technology and its applications. Eur. J. Cardiothorac Surg. 2014, 46, 342–348. [Google Scholar] [CrossRef]
- Shapira, A.; Dvir, T. 3D Tissue and Organ Printing-Hope and Reality. Adv. Sci. 2021, 8, 2003751. [Google Scholar] [CrossRef]
- Liu, N.; Ye, X.; Yao, B.; Zhao, M.; Wu, P.; Liu, G.; Zhuang, D.; Jiang, H.; Chen, X.; He, Y.; et al. Advances in 3D bioprinting technology for cardiac tissue engineering and regeneration. Bioact. Mater. 2021, 6, 1388–1401. [Google Scholar] [CrossRef]
- Kawai, Y.; Tohyama, S.; Arai, K.; Tamura, T.; Soma, Y.; Fukuda, K.; Shimizu, H.; Nakayama, K.; Kobayashi, E. Scaffold-Free Tubular Engineered Heart Tissue From Human Induced Pluripotent Stem Cells Using Bio-3D Printing Technology in vivo. Front. Cardiovasc. Med. 2021, 8, 806215. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Seol, Y.J.; Ko, I.K.; Kang, H.W.; Lee, Y.K.; Yoo, J.J.; Atala, A.; Lee, S.J. 3D Bioprinted Human Skeletal Muscle Constructs for Muscle Function Restoration. Sci. Rep. 2018, 8, 12307. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.G.; Park, S.A.; Lee, S.H.; Choi, J.S.; Cho, H.; Lee, S.J.; Kwon, Y.W.; Kwon, S.K. Transplantation of a 3D-printed tracheal graft combined with iPS cell-derived MSCs and chondrocytes. Sci. Rep. 2020, 10, 4326. [Google Scholar] [CrossRef]
- Jaksa, L.; Aryeetey, O.J.; Hatamikia, S.; Nagl, K.; Buschmann, M.; Dieter, H.P.; Kronreif, G.; Lorenz, A. 3D-Printed multi-material liver model with simultaneous mechanical and radiological tissue-mimicking features for improved realism. Int. J. Bioprint 2023, 9, 721. [Google Scholar] [CrossRef]
- Kaur, A.; Midha, S.; Giri, S.; Mohanty, S. Functional Skin Grafts: Where Biomaterials Meet Stem Cells. Stem Cells Int. 2019, 2019, 1286054. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef]
- Tricarico, C.; Clancy, J.; D’Souza-Schorey, C. Biology and biogenesis of shed microvesicles. Small GTPases 2017, 8, 220–232. [Google Scholar] [CrossRef]
- Zhou, X.; Xie, F.; Wang, L.; Zhang, L.; Zhang, S.; Fang, M.; Zhou, F. The function and clinical application of extracellular vesicles in innate immune regulation. Cell. Mol. Immunol. 2020, 17, 323–334. [Google Scholar] [CrossRef]
- Borges, F.T.; Reis, L.A.; Schor, N. Extracellular vesicles: Structure, function, and potential clinical uses in renal diseases. Braz. J. Med. Biol. Res. 2013, 46, 824–830. [Google Scholar] [CrossRef]
- Mirjana, S.; Nada, B.; Valerija, D. Variability of Satureja cuneifolia Ten. essential oils and their antimicrobial activity depending on the stage of development. Eur. Food Res. Technol. 2004, 218, 367–371. [Google Scholar] [CrossRef]
- Oke, F.; Aslim, B.; Ozturk, S.; Altundag, S. Essential oil composition, antimicrobial and antioxidant activities of Satureja cuneifolia Ten. Food Chem. 2009, 112, 874–879. [Google Scholar] [CrossRef]
- Ilhan, E.; Cesur, S.; Guler, E.; Topal, F.; Albayrak, D.; Guncu, M.M.; Cam, M.E.; Taskin, T.; Sasmazel, H.T.; Aksu, B.; et al. Development of Satureja cuneifolia-loaded sodium alginate/polyethylene glycol scaffolds produced by 3D-printing technology as a diabetic wound dressing material. Int. J. Biol. Macromol. 2020, 161, 1040–1054. [Google Scholar] [CrossRef] [PubMed]
- Gunes-Bayir, A.; Kocyigit, A.; Guler, E.M.; Bilgin, M.G.; Ergun, I.S.; Dadak, A. Effects of carvacrol on human fibroblast (WS-1) and gastric adenocarcinoma (AGS) cells in vitro and on Wistar rats in vivo. Mol. Cell. Biochem. 2018, 448, 237–249. [Google Scholar] [CrossRef]
- Wan, W.; Cai, F.; Huang, J.; Chen, S.; Liao, Q. A skin-inspired 3D bilayer scaffold enhances granulation tissue formation and anti-infection for diabetic wound healing. J. Mater. Chem. B 2019, 7, 2954–2961. [Google Scholar] [CrossRef]
- Yu, B.; He, C.; Wang, W.; Ren, Y.; Yang, J.; Guo, S.; Zheng, Y.; Shi, X. Asymmetric Wettable Composite Wound Dressing Prepared by Electrospinning with Bioinspired Micropatterning Enhances Diabetic Wound Healing. ACS Appl. Bio. Mater. 2020, 3, 5383–5394. [Google Scholar] [CrossRef]
- Dwijaksara, N.L.B.; Andromeda, S.; Gusti, A.W.R.; Siswanto, P.A.; Devi, N.L.P.M.L.; Arnita, N.P.S. Innovations in bioink materials and 3D bioprinting for precision tissue engineering. Metta J. Ilmu Multidisiplin 2024, 4, 101–118. [Google Scholar] [CrossRef]
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.H.; Yang, M.H.; Tyan, Y.C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Muscolino, E.; Di Stefano, A.B.; Trapani, M.; Sabatino, M.A.; Giacomazza, D.; Alessi, S.; Cammarata, E.; Moschella, F.; Cordova, A.; Toia, F.; et al. kappa-Carrageenan and PVA blends as bioinks to 3D print scaffolds for cartilage reconstruction. Int. J. Biol. Macromol. 2022, 222, 1861–1875. [Google Scholar] [CrossRef]
- Guan, G.; Qizhuang, L.; Liu, S.; Jiang, Z.; Zhou, C.; Liao, W. 3D-bioprinted peptide coupling patches for wound healing. Mater. Today Bio. 2022, 13, 100188. [Google Scholar] [CrossRef]
- Bashiri, Z.; Rajabi Fomeshi, M.; Ghasemi Hamidabadi, H.; Jafari, D.; Alizadeh, S.; Nazm Bojnordi, M.; Orive, G.; Dolatshahi-Pirouz, A.; Zahiri, M.; Reis, R.L.; et al. 3D-printed placental-derived bioinks for skin tissue regeneration with improved angiogenesis and wound healing properties. Mater. Today Bio. 2023, 20, 100666. [Google Scholar] [CrossRef]
- Virzì, N.F.; Diaz-Rodriguez, P.; Concheiro, A.; Pittalà, V.; Alvarez-Lorenzo, C. Xanthan gum/guar gum-based 3D-printed scaffolds for wound healing: Production, characterization, and biocompatibility screening. Carbohydr. Polym. Technol. Appl. 2024, 7, 100523. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Z.; Jorgensen, A.M.; Yang, Y.; Jin, Q.; Zhang, G.; Cao, G.; Fu, Y.; Zhao, W.; Ju, J.; et al. Bioprinting a skin patch with dual-crosslinked gelatin (GelMA) and silk fibroin (SilMA): An approach to accelerating cutaneous wound healing. Mater. Today Bio. 2023, 18, 100550. [Google Scholar] [CrossRef]
- Ullah, F.; Javed, F.; Mushtaq, I.; Rahman, L.U.; Ahmed, N.; Din, I.U.; Alotaibi, M.A.; Alharthi, A.I.; Ahmad, A.; Bakht, M.A.; et al. Development of highly-reproducible hydrogel based bioink for regeneration of skin-tissues via 3-D bioprinting technology. Int. J. Biol. Macromol. 2023, 230, 123131. [Google Scholar] [CrossRef]
- Li, Y.; Wu, J.; He, C.; He, H.; Xie, M.; Yao, K.; He, J.; Duan, Y.; Zhaung, L.; Wang, P.; et al. 3D Prestress Bioprinting of Directed Tissues. Adv. Healthc. Mater. 2023, 12, e2301487. [Google Scholar] [CrossRef]
- Seok, J.M.; Ahn, M.; Kim, D.; Lee, J.S.; Lee, D.; Choi, M.J.; Yeo, S.J.; Lee, J.H.; Lee, K.; Kim, B.S.; et al. Decellularized matrix bioink with gelatin methacrylate for simultaneous improvements in printability and biofunctionality. Int. J. Biol. Macromol. 2024, 262, 130194. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, R.; Wang, C.; Guo, Y.; Sun, W.; Ouyang, L. Recombinant Human Collagen-Based Bioinks for the 3D Bioprinting of Full-thickness Human Skin Equivalent. Int. J. Bioprint 2022, 8, 611. [Google Scholar] [CrossRef]
- Zhu, M.; Hu, T.; Song, W.; Cui, X.; Tian, Y.; Yao, B.; Wu, M.; Huang, S.; Niu, Z. Guanidinylated/PEGylated chitosan in the bioink promotes the formation of multi-layered keratinocytes in a human skin equivalent. Carbohydr. Polym. 2023, 314, 120964. [Google Scholar] [CrossRef]
- Kang, M.S.; Kwon, M.; Lee, S.H.; Kim, W.H.; Lee, G.W.; Jo, H.J.; Kim, B.; Yang, S.Y.; Kim, K.S.; Han, D.W. 3D Printing of Skin Equivalents with Hair Follicle Structures and Epidermal-Papillary-Dermal Layers Using Gelatin/Hyaluronic Acid Hydrogels. Chem. Asian J. 2022, 17, e202200620. [Google Scholar] [CrossRef]
- Cavallo, A.; Al Kayal, T.; Mero, A.; Mezzetta, A.; Pisani, A.; Foffa, I.; Vecoli, C.; Buscemi, M.; Guazzelli, L.; Soldani, G.; et al. Marine Collagen-Based Bioink for 3D Bioprinting of a Bilayered Skin Model. Pharmaceutics 2023, 15, 1331. [Google Scholar] [CrossRef]
- Lameirinhas, N.S.; Teixeira, M.C.; Carvalho, J.P.F.; Valente, B.F.A.; Pinto, R.J.B.; Oliveira, H.; Luis, J.L.; Pires, L.; Oliveira, J.M.; Vilela, C.; et al. Nanofibrillated cellulose/gellan gum hydrogel-based bioinks for 3D bioprinting of skin cells. Int. J. Biol. Macromol. 2023, 229, 849–860. [Google Scholar] [CrossRef]
- Ahn, M.; Cho, W.W.; Lee, H.; Park, W.; Lee, S.H.; Back, J.W.; Gao, Q.; Gao, G.; Cho, D.W.; Kim, B.S. Engineering of Uniform Epidermal Layers via Sacrificial Gelatin Bioink-Assisted 3D Extrusion Bioprinting of Skin. Adv. Healthc. Mater. 2023, 12, e2301015. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Qin, C.; Wu, J.; Zhang, H.; Zhuang, H.; Zhang, M.; Zhang, Z.; Ma, L.; Wang, X.; Ma, B.; et al. 3D Printing of Strontium Silicate Microcylinder-Containing Multicellular Biomaterial Inks for Vascularized Skin Regeneration. Adv. Healthc. Mater. 2021, 10, e2100523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lai, L.; Fu, H.; Fu, Q.; Chen, M. 3D-Bioprinted Biomimetic Multilayer Implants Comprising Microfragmented Adipose Extracellular Matrix and Cells Improve Wound Healing in a Murine Model of Full-Thickness Skin Defects. ACS Appl. Mater. Interfaces 2023, 15, 29713–29728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Fu, Q.; Fu, H.; Zeng, J.; Jia, L.; Chen, M. 3D-bioprinted human lipoaspirate-derived cell-laden skin constructs for healing of full-thickness skin defects. Int. J. Bioprint. 2023, 9, 718. [Google Scholar] [CrossRef]
- Fu, H.; Zhang, D.; Zeng, J.; Fu, Q.; Chen, Z.; Sun, X.; Yang, Y.; Li, S.; Chen, M. Application of 3D-printed tissue-engineered skin substitute using innovative biomaterial loaded with human adipose-derived stem cells in wound healing. Int. J. Bioprint 2023, 9, 674. [Google Scholar] [CrossRef]
- Kim, B.S.; Kwon, Y.W.; Kong, J.S.; Park, G.T.; Gao, G.; Han, W.; Kim, M.B.; Lee, H.; Kim, J.H.; Cho, D.W. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: A step towards advanced skin tissue engineering. Biomaterials 2018, 168, 38–53. [Google Scholar] [CrossRef]
- Chen, S.; Wang, H.; Su, Y.; John, J.V.; McCarthy, A.; Wong, S.L.; Xie, J. Mesenchymal stem cell-laden, personalized 3D scaffolds with controlled structure and fiber alignment promote diabetic wound healing. Acta Biomater. 2020, 108, 153–167. [Google Scholar] [CrossRef]
- Zhang, W.; Ling, Y.; Sun, Y.; Xiao, F.; Wang, L. Extracellular Vesicles Derived from Mesenchymal Stem Cells Promote Wound Healing and Skin Regeneration by Modulating Multiple Cellular Changes: A Brief Review. Genes 2023, 14, 1516. [Google Scholar] [CrossRef]
- Li, M.; Wang, T.; Tian, H.; Wei, G.; Zhao, L.; Shi, Y. Macrophage-derived exosomes accelerate wound healing through their anti-inflammation effects in a diabetic rat model. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3793–3803. [Google Scholar] [CrossRef]
- Cao, W.; Meng, X.; Cao, F.; Wang, J.; Yang, M. Exosomes derived from platelet-rich plasma promote diabetic wound healing via the JAK2/STAT3 pathway. iScience 2023, 26, 108236. [Google Scholar] [CrossRef]
- Nagelkerke, A.; Ojansivu, M.; van der Koog, L.; Whittaker, T.E.; Cunnane, E.M.; Silva, A.M.; Dekker, N.; Stevens, M.M. Extracellular vesicles for tissue repair and regeneration: Evidence, challenges and opportunities. Adv. Drug Deliv. Rev. 2021, 175, 113775. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, Y.; Zhang, S.; He, F.; Shi, T.; Li, J.; Wang, Z.; Jia, J. Exosome-based bioinks for 3D bioprinting applications in tissue engineering and regenerative medicine. Int. J. Bioprinting 2023, 9, 0114. [Google Scholar] [CrossRef]
- Bari, E.; Di Gravina, G.M.; Scocozza, F.; Perteghella, S.; Frongia, B.; Tengattini, S.; Segale, L.; Torre, M.L.; Conti, M. Silk Fibroin Bioink for 3D Printing in Tissue Regeneration: Controlled Release of MSC extracellular Vesicles. Pharmaceutics 2023, 15, 383. [Google Scholar] [CrossRef] [PubMed]
- Hodge, J.G.; Robinson, J.L.; Mellott, A.J. Mesenchymal Stem Cell Extracellular Vesicles from Tissue-Mimetic System Enhance Epidermal Regeneration via Formation of Migratory Cell Sheets. Tissue Eng. Regen. Med. 2023, 20, 993–1013. [Google Scholar] [CrossRef]
- Li, L.; Wang, Z.; Wang, K.; Fu, S.; Li, D.; Wang, M.; Cao, Y.; Zhu, H.; Li, Z.; Weng, L.; et al. Paintable Bioactive Extracellular Vesicle Ink for Wound Healing. ACS Appl. Mater. Interfaces 2023, 15, 25427–25436. [Google Scholar] [CrossRef]
- Ferroni, L.; Gardin, C.; D’Amora, U.; Calza, L.; Ronca, A.; Tremoli, E.; Ambrosio, L.; Zavan, B. Exosomes of mesenchymal stem cells delivered from methacrylated hyaluronic acid patch improve the regenerative properties of endothelial and dermal cells. Biomater. Adv. 2022, 139, 213000. [Google Scholar] [CrossRef]
- Borena, B.M.; Martens, A.; Broeckx, S.Y.; Meyer, E.; Chiers, K.; Duchateau, L.; Spaas, J.H. Regenerative Skin Wound Healing in Mammals: State-of-the-Art on Growth Factor and Stem Cell Based Treatments. Cell. Physiol. Biochem. 2015, 36, 1–23. [Google Scholar] [CrossRef]
- Yannas, I.V.; Lee, E.; Orgill, D.P.; Skrabut, E.M.; Murphy, G.F. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc. Natl. Acad. Sci. USA 1989, 86, 933–937. [Google Scholar] [CrossRef]
- Savoji, H.; Godau, B.; Hassani, M.S.; Akbari, M. Skin Tissue Substitutes and Biomaterial Risk Assessment and Testing. Front. Bioeng. Biotechnol. 2018, 6, 86. [Google Scholar] [CrossRef]
- Sen, C.K. Human Wound and Its Burden: Updated 2020 Compendium of Estimates. Adv. Wound Care 2021, 10, 281–292. [Google Scholar] [CrossRef]
- Morton, L.M.; Phillips, T.J. Wound healing and treating wounds: Differential diagnosis and evaluation of chronic wounds. J. Am. Acad. Dermatol. 2016, 74, 589–605; quiz 605-586. [Google Scholar] [CrossRef] [PubMed]
- Ricci, G.; Gibelli, F.; Sirignano, A. Three-Dimensional Bioprinting of Human Organs and Tissues: Bioethical and Medico-Legal Implications Examined through a Scoping Review. Bioengineering 2023, 10, 1052. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Shih, S.; Khachemoune, A. Skin substitutes for acute and chronic wound healing: An updated review. J. Dermatolog. Treat. 2020, 31, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Caron, C.; Mahmoud, I.; Derish, I.; Schwertani, A.; Cecere, R. Extracellular Vesicles as a Cell-free Therapy for Cardiac Repair: A Systematic Review and Meta-analysis of Randomized Controlled Preclinical Trials in Animal Myocardial Infarction Models. Stem Cell Rev. Rep. 2022, 18, 1143–1167. [Google Scholar] [CrossRef]
- Ho, J.K.; Shao, H.; You, C.; Pan, X.; Wang, X.; Chen, G.; Khan, A.; Han, C. Successful Application of Tissue Engineering Skin to Third Degree Burn Wound on Lateral Thorax-A Case Study. Biomed. J. Sci. Tech. Res. 2019, 16, 12122–12125. [Google Scholar]
- Demircan, M.; Cicek, T.; Yetis, M.I. Preliminary results in single-step wound closure procedure of full-thickness facial burns in children by using the collagen-elastin matrix and review of pediatric facial burns. Burns 2015, 41, 1268–1274. [Google Scholar] [CrossRef]
- Golchin, A.; Farahany, T.Z.; Khojasteh, A.; Soleimanifar, F.; Ardeshirylajimi, A. The Clinical Trials of Mesenchymal Stem Cell Therapy in Skin Diseases: An Update and Concise Review. Curr. Stem Cell Res. Ther. 2019, 14, 22–33. [Google Scholar] [CrossRef]
- Jafarzadeh, A.; Pour Mohammad, A.; Keramati, H.; Zeinali, R.; Khosravi, M.; Goodarzi, A. Regenerative medicine in the treatment of specific dermatologic disorders: A systematic review of randomized controlled clinical trials. Stem Cell Res. Ther. 2024, 15, 176. [Google Scholar] [CrossRef]
- Wei, Y.; Li-Tsang, C.W.P.; Liu, J.; Xie, L.; Yue, S. 3D-printed transparent facemasks in the treatment of facial hypertrophic scars of young children with burns. Burns 2017, 43, e19–e26. [Google Scholar] [CrossRef]
- Alhazmi, B.; Alshomer, F.; Alazzam, A.; Shehabeldin, A.; Almeshal, O.; Kalaskar, D.M. Digital workflow for fabrication of bespoke facemask in burn rehabilitation with smartphone 3D scanner and desktop 3D printing: Clinical case study. 3D Print. Med. 2022, 8, 12. [Google Scholar] [CrossRef]
- Cibelli, J.; Emborg, M.E.; Prockop, D.J.; Roberts, M.; Schatten, G.; Rao, M.; Harding, J.; Mirochnitchenko, O. Strategies for improving animal models for regenerative medicine. Cell Stem Cell 2013, 12, 271–274. [Google Scholar] [CrossRef]
- Derman, I.D.; Rivera, T.; Garriga Cerda, L.; Singh, Y.P.; Saini, S.; Abaci, H.E.; Ozbolat, I.T. Advancements in 3D skin bioprinting: Processes, bioinks, applications and sensor integration. Int. J. Extrem Manuf. 2025, 7, 012009. [Google Scholar] [CrossRef]
| Bioinks | Results | Reference |
|---|---|---|
| Gelatinmethacryloyl/hyaluronic acid methacryloyl (GelMA)/HAMA) added with glutamine-histidine-arginine-glutamic acid-aspartic acid-glycine-serine (QHREDGS) peptide | Full-thickness skin wound repair together with new vessels formation and ECM deposition in in vivo rat skin wound models. | [50] |
| 5% placenta-derived extracellular matrix—sodium alginate (ECM-Alg)/Gel | Neo-angiogenesis in ex vivo CAM assay on chick embryos. Almost 87% of wound closure of full-thickness wound mice models together with new vascularization and collagen synthesis. | [51] |
| 12% w/v xanthan gum/guar gum (XG)/GG) | Pro-angiogenic activity involves the formation of blood vessels and good tissue integration. | [52] |
| Bioinks | Loaded Cells | Results | Reference |
|---|---|---|---|
| Gelatin Methacryloyl/methacrylated silk fibroin (GelMA)/SilMA) | Mice-derived FBs (Fibroblasts) | Full-thickness and vascularized skin wound repair together with ECM deposition in in vivo mice models. | [53] |
| Polyethylene oxide-co-Chitosan-co-polymethylmethacrylic-acid (PEO-CS-PMMA) added with Nicotinamide | Human dermal fibroblasts (HDFs) | Good biocompatibility for cells and good structural and mechanical properties for 3D bioprinting. | [54] |
| Gelatin and microbial transglutaminase (mTG) | Human skin FBs (HFF-1), mouse FB cells (L929), human bronchial epithelioid cells and mouse embryo osteoblast precursor cells (MC3T3) | In vivo full-thickness skin immunodeficient mice treated with stretched aligned HFF-1 laden scaffolds (SCLS) decreased wound dimensions. Moreover, a thick granulation tissue was generated, and the FBs inside it expressed higher levels of PCNA, demonstrating their high proliferation activity, and high VEGF levels, thus promoting angiogenic phenomena. | [55] |
| Gelatin Methacryloyl (GelMA) and a skin-derived extracellular matrix (SdECM) | Fibroblasts (FBs) | The hybrid GelMA/SdECM bioink exhibited higher viscosity compared to GelMA alone, improving printing stability. It also promoted greater fibroblast viability (97.6%) and proliferation, with increased expression of skin-related markers such as type I collagen and FGF, indicating enhanced biological functionality for skin tissue engineering. | [56] |
| Gelatin Methacryloyl—recombinant type III collagen (GelMA-rhCol3-3.2) | Human dermal fibroblasts (HDFs) and then human epidermal keratinocytes (HaCaTs) cells on top | Almost complete wound closure in full-thickness excisional wound rat models. Moreover, new hair follicles appeared, and collagen deposition was demonstrated. | [57] |
| Guanidinylated/PEGylated chitosan (GPCS) | Human dermal fibroblasts (HDFs) and then human epidermal keratinocytes (HaCaTs) cells on top | Generation of a thick skin with proliferating KCs layered on different planes in in vitro experiments. | [58] |
| Gelatin methacryloyl/hyaluronic acid methacryloyl (GelMA)/HAMA) added with 2 cm × 2 cm × 0.2 cm lattice cuboid | Normal human dermal FBs (NHDFs) and hair follicle dermal papilla cells (HFDPCs) spheroids. HaCaTs were seeded on the top | HaCaTs differentiated and penetrated into the bioprinted structure. Then, they surrounded HFDPC spheroids to form a complex in whom spontaneously hair-follicle-like unit (HFU) generated. | [59] |
| Semi-cross-linked alginate (ALG) mixed with 20 mg/mL marine collagen from basa fish skin (COL-ALG-20) | Normal human dermal fibroblasts (NHDFs) and human epidermal keratinocytes (HaCaTs) | NHDFs and HaCaTs proliferated inside the bioink and secreted new ECM. | [60] |
| Nanofibrillated Cellulose and Gellan Gum (NFC/GG) | Human epidermal keratinocytes (HaCaTs) cells | Very good cell survival after 7 days and very good rheological and mechanical properties for 3D bioprinting. | [61] |
| Sacrificial gelatin bioink | Human neonatal epidermal keratinocytes (HEKs) | HEKs adhered in the bioink and differentiated from the lower to the upper layer of the skin by forming a full-thickness skin model made by stratum basale, spinosum, granulosum, lucidum and corneum. | [62] |
| Cells in 2% Strontium silicate mixed with GAM (Co-2SS-GAM) | Human umbilical vascular endothelial cells (HUVECs) and human dermal fibroblasts (HDFs) | Thick epidermis regeneration with a dermal structure rich in collagen fibers and neo-vessels with the presence of hair follicles and complete epithelialization. | [63] |
| Microfragmented adipose extracellular matrix- Gelatin Methacryloyl- hyaluronic acid methacryloyl (mFAECM-GelMA-HAMA) | human epidermal keratinocytes (HaCaTs), Fibroblasts, human umbilical vascular endothelial cells (HUVECs) | Dermis regeneration in full-thickness excisional skin defect mice model by promoting the contraction of the new tissue inside the wound, a new vasculrization and collagen III deposition. | [64] |
| Human adipose tissue decellularized ECM-Gelatin Methacryloyl- hyaluronic acid methacryloyl (adECM-GelMA-HAMA) | Adipose-derived stem cells (ADSCs) | Complete wound closure together with collagen III deposition and neo-vessels formation in in vivo full-thickness injury mice model. | [65] |
| Human adipose tissue decellularized ECM-Gelatin Methacryloyl- hyaluronic acid methacryloyl (adECM-GelMA-HAMA) | Human adipose-derived stem cells (hADSCs) | Recovery of the entire area of wounded skin in full-thickness skin wound mice models, almost without scar tissue formation. Rich amount of collagen together with a modulated inflammatory cells response. | [66] |
| Decellularized and solubilized porcine skin tissue (S-dECM) | Human neonatal epidermal KCs (HEKs), endothelial progenitor cells (EPCs) and ASCs | Wound healing for over 21 days with the re-epithelialization and neovascularization phenomena, together with the enhancement of the blood flow in in vivo wound mice models. | [67] |
| 3D PLC- enriched with 0.5% F-127 and immersed in a 0.5% gelatin solution | Bone-marrow-derived mesenchymal stem cells (BM-MSCs) | The developed material not only promotes granulation tissue formation, angiogenesis, and collagen deposition but also induces macrophage polarization toward the M2 phenotype, enhancing the expression of anti-inflammatory cytokines such as IL-4 and IL-10, which are essential for inflammation resolution and the healing process | [68] |
| Bioinks | Loaded Material | Results | Reference |
|---|---|---|---|
| Sodium alginate (SA) and silk fibroin (SF) hydrogels | Mesenchymal stem cell secretome (MSC-secretome) | Lyosecretome (freeze-dried MSC-secretome) was incorporated at 20 mg/mL into SA-SF hydrogels. The presence of SF significantly delayed lipid release, while EV release was modulated by both diffusion and matrix erosion. | [74] |
| X-Block hydrogel | Adipose stem cell secretome (ASC-secretome) | The ‘‘100 kDa’’ fraction of 3D ASC-secretome was able to positively modulate human KC (KCs) morphology towards the formation of a stratified cell layer, their differentiation, metabolism, migration and proliferation in in vitro assays. | [75] |
| EVM2-Gel ink | M2 macrophage-derived Evs (EVM2) | Improvement of wound healing in in vivo models, in a dose-dependent way, by inducing the switch of macrophage phenotype from M1 to M2 and by promoting HUVECs proliferation and migration. High secretion of collagen fibers. | [76] |
| MeHA | Exosomes derived from human mesenchymal stem cells (hMSC-EXOs) | Increased cell proliferation and migration after 24 and 48 h of stimulation with hMSC-EXOs released from the patch in in vitro assays. | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Stefano, A.B.; Urrata, V.; Schilders, K.; Franza, M.; Di Leo, S.; Moschella, F.; Cordova, A.; Toia, F. Three-Dimensional Bioprinting Techniques in Skin Regeneration: Current Insights and Future Perspectives. Life 2025, 15, 787. https://doi.org/10.3390/life15050787
Di Stefano AB, Urrata V, Schilders K, Franza M, Di Leo S, Moschella F, Cordova A, Toia F. Three-Dimensional Bioprinting Techniques in Skin Regeneration: Current Insights and Future Perspectives. Life. 2025; 15(5):787. https://doi.org/10.3390/life15050787
Chicago/Turabian StyleDi Stefano, Anna Barbara, Valentina Urrata, Kim Schilders, Mara Franza, Simona Di Leo, Francesco Moschella, Adriana Cordova, and Francesca Toia. 2025. "Three-Dimensional Bioprinting Techniques in Skin Regeneration: Current Insights and Future Perspectives" Life 15, no. 5: 787. https://doi.org/10.3390/life15050787
APA StyleDi Stefano, A. B., Urrata, V., Schilders, K., Franza, M., Di Leo, S., Moschella, F., Cordova, A., & Toia, F. (2025). Three-Dimensional Bioprinting Techniques in Skin Regeneration: Current Insights and Future Perspectives. Life, 15(5), 787. https://doi.org/10.3390/life15050787











