The Role of TNF-α in Neuropathic Pain: An Immunotherapeutic Perspective
Abstract
1. Introduction
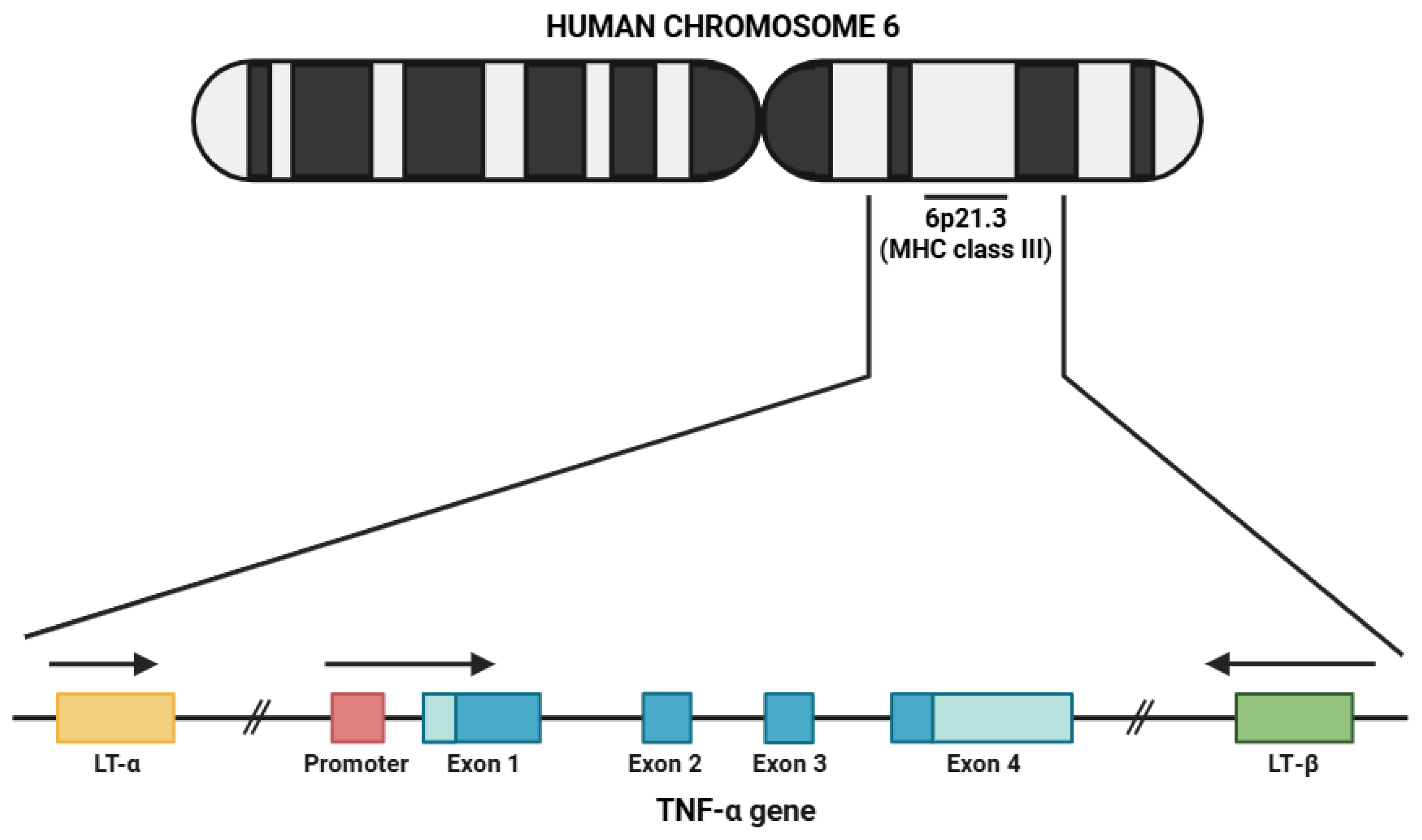
2. Biology of TNF-α
2.1. Characteristics of TNF-α
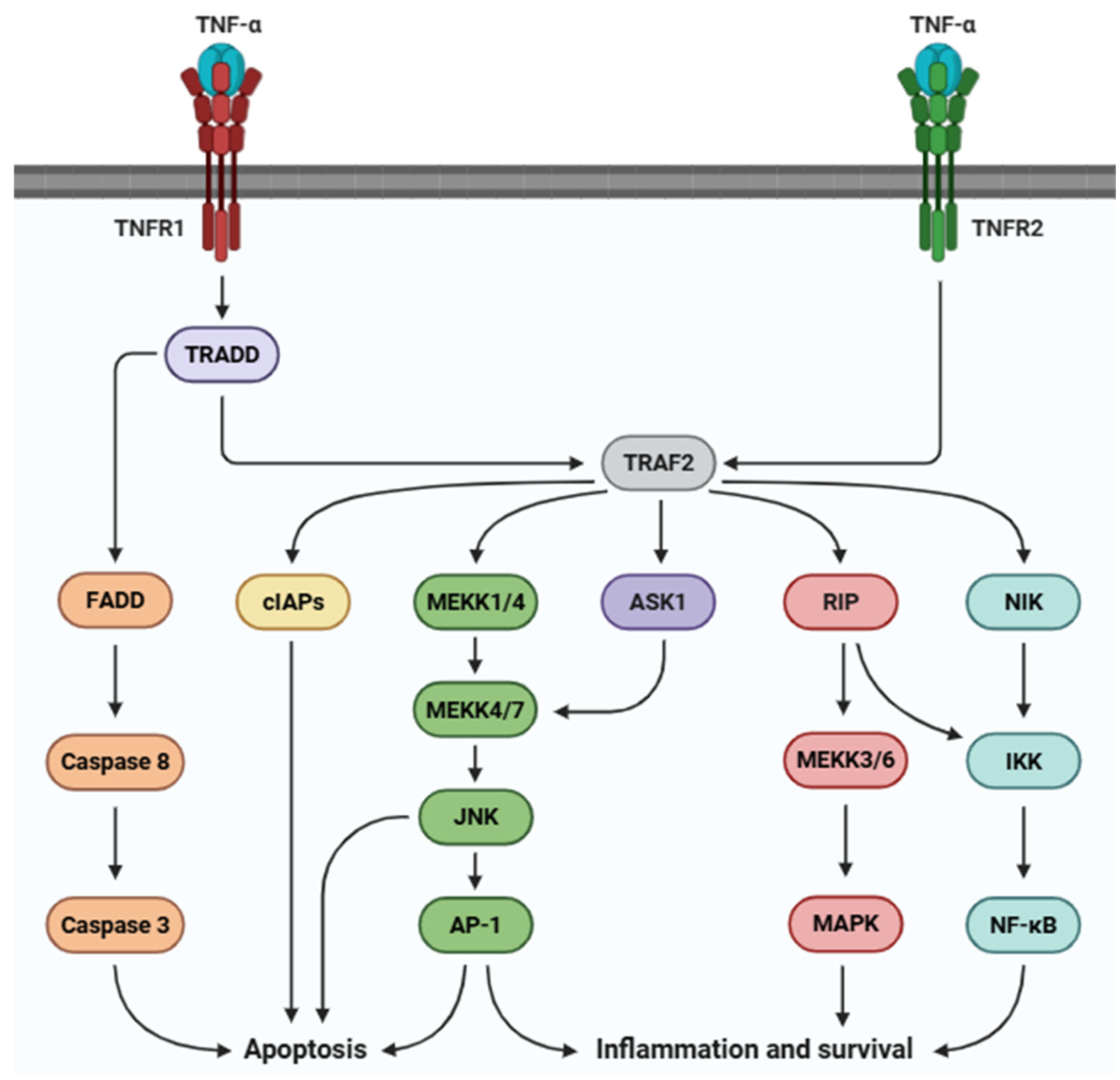
2.2. TNF-α Receptor: Structure and Localization
2.3. Biological Roles of TNF-α
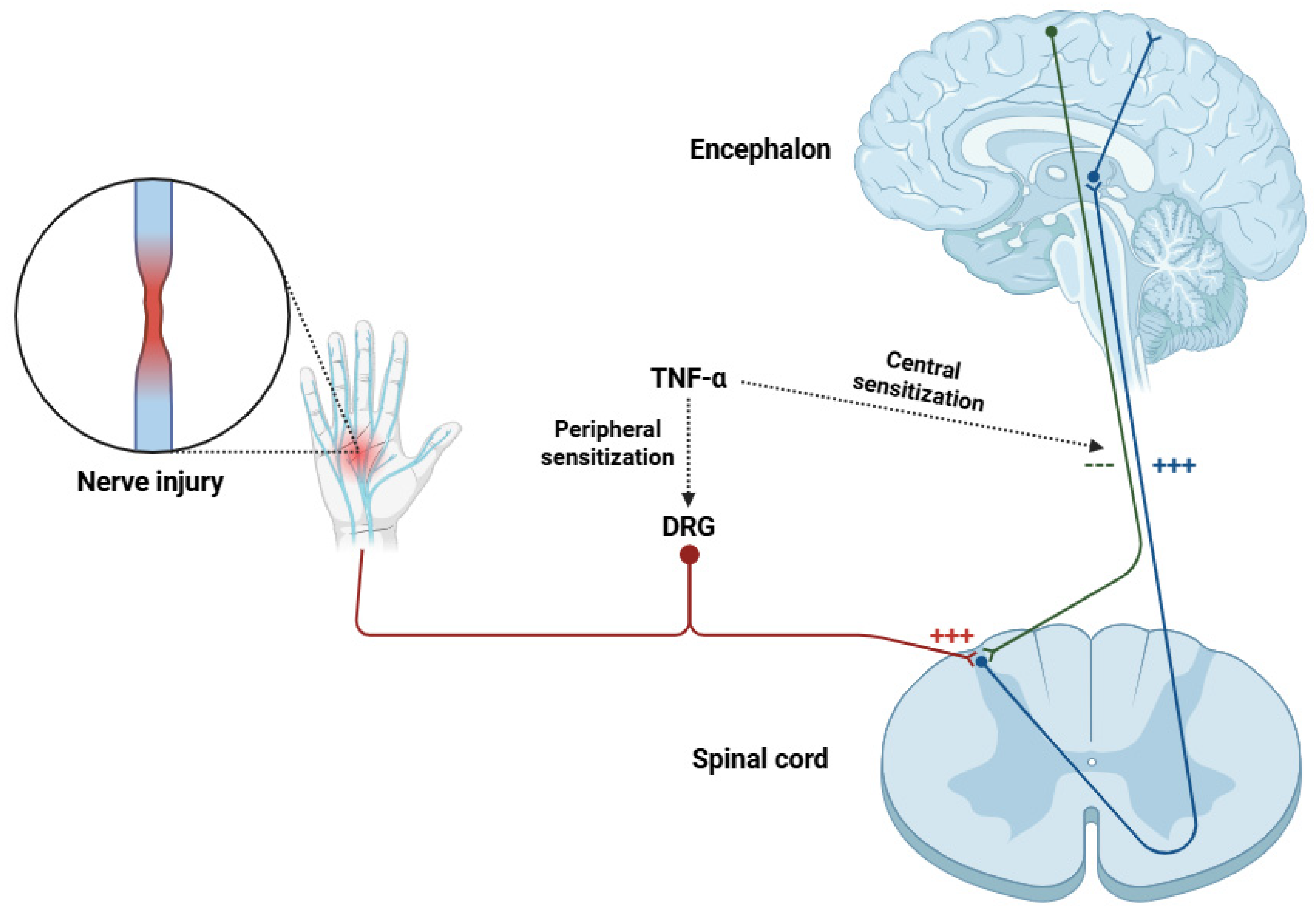
3. Role of TNF-α in Neuropathic Pain
4. Therapeutic Targeting of TNF-α in Neuropathic Pain
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADAM | A disintegrin and metalloprotease |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| AP-1 | Activator protein 1 |
| ASK1 | Apoptosis signal-regulating kinase 1 |
| BTZ | Bortezomib |
| CaV3.2 | Voltage-gated calcium channel 3.2 |
| CCI | Chronic constriction injury |
| CCL2 | C-C Motif chemokine ligand 2 |
| CGRP | Calcitonin gene-related peptide |
| cIAP | Cellular inhibitor of apoptosis protein |
| CIPN | Chemotherapy-induced peripheral neuropathy |
| CNS | Central nervous system |
| COX-2 | Cyclooxygenase 2 |
| CRPS | Complex regional pain syndrome |
| CXCL1 | C-X-C Motif Chemokine Ligand 1 |
| CXCL10 | C-X-C Motif Chemokine Ligand 10 |
| DPN | Diabetic polyneuropathy |
| DRG | Dorsal root ganglia |
| Elk-1 | ETS like-1 protein |
| EPSC | Excitatory postsynaptic current |
| EQ VAS | EuroQol visual analog scale |
| ERK | Extracellular signal-regulated kinase |
| EuroQol | European quality of life |
| FADD | Fas-associated death domain |
| GABA | Gamma-aminobutyric acid |
| GluA1 | Glutamate receptor subunit 1 (AMPA receptor) |
| IASP | International Association for the Study of Pain |
| ICAM-1 | Intercellular adhesion molecule 1 |
| IENFD | Intraepidermal nerve fiber density |
| IKK | IκB kinase |
| IL-1β | Interleukin 1 beta |
| IL-6 | Interleukin 6 |
| IQR 1–2 | Interquartile range of 1 to 2 |
| IRS | Insulin receptor substrate |
| ISS | Injury severity score |
| ITT | Intent-to-treat |
| JAK1 | Janus kinase 1 |
| JAK2 | Janus kinase 2 |
| JNK | c-Jun N-terminal kinase |
| LT-α | Lymphotoxin alpha |
| LT-β | Lymphotoxin beta |
| MAPK | Mitogen-activated protein kinase |
| MDSC | Myeloid-derived suppressor cell |
| MEKK1/4 | Mitogen-activated protein kinase/ERK kinases 1 and 4 |
| MEKK3/6 | Mitogen-activated protein kinase/ERK kinases 3 and 6 |
| MEKK4/7 | Mitogen-activated protein kinase/ERK kinases 4 and 7 |
| MHC | Major histocompatibility complex |
| MNCV | Motor nerve conduction velocity |
| NaV1.3 | Voltage-gated sodium channel 1.3 |
| NaV1.7 | Voltage-gated sodium channel 1.7 |
| NaV1.8 | Voltage-gated sodium channel 1.8 |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NF-κB p65 | Nuclear Factor kappa-light-chain-enhancer of activated B cells, p65 subunit |
| NIK | NF-κB-inducing kinase |
| NK | Natural killer cell |
| NMDA | N-methyl-D-aspartate |
| NO | Nitric oxide |
| NR1 | NMDA receptor subunit 1 |
| NR2 | NMDA receptor subunit 2 |
| p38 MAPK | p38 mitogen-activated protein kinase |
| p50 | NF-κB p50 subunit |
| p65 | NF-κB p65 (RelA) subunit |
| PKA | Protein kinase A |
| PKC | Protein kinase C |
| PNS | Peripheral nervous system |
| PP | Per protocol |
| pro-TNF-α | Pro-tumor necrosis factor alpha |
| PST | Partial sciatic nerve transection |
| RIP | Receptor-interacting protein |
| RIPK1 | Receptor-interacting serine/threonine-protein kinase 1 |
| ROS | Reactive oxygen species |
| SCI | Sciatic nerve injury |
| SNCV | Sensory nerve conduction velocity |
| SSFN | Sarcoidosis-associated small fiber neuropathy |
| STAT | Signal transducer and activator of transcription |
| STAT3 | Signal transducer and activator of transcription 3 |
| STAT5 | Signal transducer and activator of transcription 5 |
| SUCRA | Surface under the cumulative ranking curve |
| TACE | TNF-α converting enzyme |
| TG | Trigeminal ganglia |
| Th | T helper cell |
| Th1 | T helper cell type 1 |
| Th17 | T helper cell type 17 |
| TNF | Tumor necrosis factor |
| TNFR1 | Tumor necrosis factor receptor 1 |
| TNFR2 | Tumor necrosis factor receptor 2 |
| TNF-α | Tumor necrosis factor alpha |
| TRADD | TNF receptor-associated death domain |
| TRAF1 | TNF receptor-associated factor 1 |
| TRAF2 | TNF receptor-associated factor 2 |
| Treg | Regulatory T cell |
| VCAM-1 | Vascular cell adhesion molecule 1 |
| VGCC | Voltage-gated calcium channel |
| VGSC | Voltage-gated sodium channel |
References
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Vader, K.; Bostick, G.P.; Carlesso, L.C.; Hunter, J.; Mesaroli, G.; Perreault, K.; Tousignant-Laflamme, Y.; Tupper, S.; Walton, D.M.; Wideman, T.H.; et al. The Revised IASP Definition of Pain and Accompanying Notes: Considerations for the Physiotherapy Profession. Physiother. Can. 2021, 73, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Walters, E.T.; Williams, A.C.C. Evolution of mechanisms and behaviour important for pain. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2019, 374, 20190275. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.I.; Neumeister, M.W. Pain: Pathways and Physiology. Clin. Plast. Surg. 2020, 47, 173–180. [Google Scholar] [CrossRef]
- Nikolenko, V.N.; Shelomentseva, E.M.; Tsvetkova, M.M.; Abdeeva, E.I.; Giller, D.B.; Babayeva, J.V.; Achkasov, E.E.; Gavryushova, L.V.; Sinelnikov, M.Y. Nociceptors: Their Role in Body’s Defenses, Tissue Specific Variations and Anatomical Update. J. Pain Res. 2022, 15, 867–877. [Google Scholar] [CrossRef]
- Karcz, M.; Abd-Elsayed, A.; Chakravarthy, K.; Aman, M.M.; Strand, N.; Malinowski, M.N.; Latif, U.; Dickerson, D.; Suvar, T.; Lubenow, T.; et al. Pathophysiology of Pain and Mechanisms of Neuromodulation: A Narrative Review (A Neuron Project). J. Pain Res. 2024, 17, 3757–3790. [Google Scholar] [CrossRef]
- Todd, A.J. An Historical Perspective: The Second Order Neuron in the Pain Pathway. Front. Pain Res. 2022, 3, 845211. [Google Scholar] [CrossRef]
- Cao, B.; Xu, Q.; Shi, Y.; Zhao, R.; Li, H.; Zheng, J.; Liu, F.; Wan, Y.; Wei, B. Pathology of pain and its implications for therapeutic interventions. Signal. Transduct. Target. Ther. 2024, 9, 155. [Google Scholar]
- Kaplan, C.M.; Kelleher, E.; Irani, A.; Schrepf, A.; Clauw, D.J.; Harte, S.E. Deciphering nociplastic pain: Clinical features, risk factors and potential mechanisms. Nat. Rev. Neurol. 2024, 20, 347–363. [Google Scholar] [CrossRef]
- Petroianu, G.A.; Aloum, L.; Adem, A. Neuropathic pain: Mechanisms and therapeutic strategies. Front. Cell. Dev. Biol. 2023, 11, 1072629. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic Pain: From Mechanisms to Treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Song, X.J. Diabetic Neuropathic Pain: Directions for Exploring Treatments. Biomedicines 2024, 12, 589. [Google Scholar] [CrossRef] [PubMed]
- Mallick-Searle, T.; Snodgrass, B.; Brant, J.M. Postherpetic neuralgia: Epidemiology, pathophysiology, and pain management pharmacology. J. Multidiscip. Healthc. 2016, 9, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Swami, T.; Khanna, M.; Gupta, A.; Prakash, N.B. Neuropathic Pain in Guillain-Barre Syndrome: Association with Rehabilitation Outcomes and Quality of Life. Ann. Indian Acad. Neurol. 2021, 24, 708–714. [Google Scholar] [CrossRef]
- McBenedict, B.; Goh, K.S.; Yau, R.C.C.; Elamin, S.; Yusuf, W.H.; Verly, G.; Thomas, A.; Alphonse, B.; Ouabicha, K.; Valentim, G.; et al. Neuropathic Pain Secondary to Multiple Sclerosis: A Narrative Review. Cureus 2024, 16, e61587. [Google Scholar] [CrossRef]
- Zajączkowska, R.; Kocot-Kępska, M.; Leppert, W.; Wrzosek, A.; Mika, J.; Wordliczek, J. Mechanisms of Chemotherapy-Induced Peripheral Neuropathy. Int. J. Mol. Sci. 2019, 20, 1451. [Google Scholar] [CrossRef]
- Zorina-Lichtenwalter, K.; Parisien, M.; Diatchenko, L. Genetic studies of human neuropathic pain conditions: A review. Pain 2018, 159, 583–594. [Google Scholar] [CrossRef]
- Zimmer, Z.; Fraser, K.; Grol-Prokopczyk, H.; Zajacova, A. A global study of pain prevalence across 52 countries: Examining the role of country-level contextual factors. Pain 2022, 163, 1740–1750. [Google Scholar] [CrossRef]
- Viderman, D.; Tapinova, K.; Aubakirova, M.; Abdildin, Y.G. The Prevalence of Pain in Chronic Diseases: An Umbrella Review of Systematic Reviews. J. Clin. Med. 2023, 12, 7302. [Google Scholar] [CrossRef]
- Baskozos, G.; Hébert, H.L.; Pascal, M.M.; Themistocleous, A.C.; Macfarlane, G.J.; Wynick, D.; Bennett, D.L.; Smith, B.H. Epidemiology of neuropathic pain: An analysis of prevalence and associated factors in UK Biobank. Pain Rep. 2023, 8, e1066. [Google Scholar] [CrossRef]
- Liedgens, H.; Obradovic, M.; De Courcy, J.; Holbrook, T.; Jakubanis, R. A burden of illness study for neuropathic pain in Europe. Clinicoecon. Outcomes Res. 2016, 8, 113–126. [Google Scholar] [PubMed]
- DiBonaventura, M.D.; Sadosky, A.; Concialdi, K.; Hopps, M.; Kudel, I.; Parsons, B.; Cappelleri, J.C.; Hlavacek, P.; Alexander, A.H.; Stacey, B.R.; et al. The prevalence of probable neuropathic pain in the US: Results from a multimodal general-population health survey. J. Pain Res. 2017, 10, 2525–2538. [Google Scholar] [CrossRef] [PubMed]
- VanDenKerkhof, E.G.; Mann, E.G.; Torrance, N.; Smith, B.H.; Johnson, A.; Gilron, I. An Epidemiological Study of Neuropathic Pain Symptoms in Canadian Adults. Pain Res. Manag. 2016, 2016, 9815750. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Taguchi, T.; Yamashita, T.; Nakamura, M.; Ushida, T. The prevalence and impact of chronic neuropathic pain on daily and social life: A nationwide study in a Japanese population. Eur. J. Pain 2017, 21, 727–737. [Google Scholar] [CrossRef]
- Xiao, H.; Ma, K.; Huang, D.; Liu, X.G.; Liu, T.H.; Liu, Q.; Liu, G.Z.; Song, T.; Tao, W.; Wu, D.S.; et al. Expert consensus of the Chinese Association for the Study of Pain on ion channel drugs for neuropathic pain. World J. Clin. Cases 2021, 9, 2100–2109. [Google Scholar] [CrossRef]
- Mills, S.E.E.; Nicolson, K.P.; Smith, B.H. Chronic pain: A review of its epidemiology and associated factors in population-based studies. Br. J. Anaesth. 2019, 123, e273–e283. [Google Scholar] [CrossRef]
- Karcioglu, O.; Topacoglu, H.; Dikme, O.; Dikme, O. A systematic review of the pain scales in adults: Which to use? Am. J. Emerg. Med. 2018, 36, 707–714. [Google Scholar] [CrossRef]
- Kim, H.; Dionne, R.A. Individualized pain medicine. Drug Discov. Today Ther. Strateg. 2009, 6, 83–87. [Google Scholar] [CrossRef][Green Version]
- Staudt, M.D. The Multidisciplinary Team in Pain Management. Neurosurg. Clin. N. Am. 2022, 33, 241–249. [Google Scholar] [CrossRef]
- Lees, J.G.; Duffy, S.S.; Moalem-Taylor, G. Immunotherapy targeting cytokines in neuropathic pain. Front. Pharmacol. 2013, 4, 142. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chu, D.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From Clinical Significance to Quantification. Adv. Sci. 2021, 8, e2004433. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Huang, T.; Li, S.; Ma, A.; Pérez, J.L.; Sander, C.; Keskin, D.B.; Wu, C.J.; Fraenkel, E.; Hacohen, N. Dictionary of immune responses to cytokines at single-cell resolution. Nature 2024, 625, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Vanderwall, A.G.; Milligan, E.D. Cytokines in Pain: Harnessing Endogenous Anti-Inflammatory Signaling for Improved Pain Management. Front. Immunol. 2019, 10, 3009. [Google Scholar] [CrossRef]
- Hung, A.L.; Lim, M.; Doshi, T.L. Targeting cytokines for treatment of neuropathic pain. Scand. J. Pain 2017, 17, 287–293. [Google Scholar] [CrossRef]
- Duan, Y.W.; Chen, S.X.; Li, Q.Y.; Zang, Y. Neuroimmune Mechanisms Underlying Neuropathic Pain: The Potential Role of TNF-α-Necroptosis Pathway. Int. J. Mol. Sci. 2022, 23, 7191. [Google Scholar] [CrossRef]
- Clark, A.K.; Old, E.A.; Malcangio, M. Neuropathic pain and cytokines: Current perspectives. J. Pain Res. 2013, 6, 803–814. [Google Scholar]
- Kalliolias, G.D.; Ivashkiv, L.B. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 2016, 12, 49–62. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Bjorling, D.E. Tumor necrosis factor-α induces expression and release of interleukin-6 by human urothelial cells. Inflamm. Res. 2011, 60, 525–532. [Google Scholar] [CrossRef]
- Mark, K.S.; Trickler, W.J.; Miller, D.W. Tumor necrosis factor-alpha induces cyclooxygenase-2 expression and prostaglandin release in brain microvessel endothelial cells. J. Pharmacol. Exp. Ther. 2001, 297, 1051–1058. [Google Scholar] [CrossRef]
- Jana, B.; Andronowska, A.; Całka, J.; Mówińska, A. Biosynthetic pathway for leukotrienes is stimulated by lipopolysaccharide and cytokines in pig endometrial stromal cells. Sci. Rep. 2025, 15, 2806. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.M.; Lai, T.H.; Li, C.H.; Wu, W.B. TNF-α induces CXCL1 chemokine expression and release in human vascular endothelial cells in vitro via two distinct signaling pathways. Acta Pharmacol. Sin. 2014, 35, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, S.G.; Romão, P.R.; Figueiredo, F.; Morais, R.H.; Lima, H.C.; Ferreira, S.H.; Cunha, F.Q. TNF-alpha mediates the induction of nitric oxide synthase in macrophages but not in neutrophils in experimental cutaneous leishmaniasis. Eur. J. Immunol. 2003, 33, 297–306. [Google Scholar] [CrossRef]
- Durham, Z.L.; Hawkins, J.L.; Durham, P.L. Tumor necrosis factor-Alpha stimulates cytokine expression and transient sensitization of trigeminal nociceptive neurons. Arch. Oral Biol. 2017, 75, 100–106. [Google Scholar] [CrossRef]
- Kaye, A.D.; Perilloux, D.M.; Hawkins, A.M.; Wester, G.C.; Ragaland, A.R.; Hebert, S.V.; Kim, J.; Heisler, M.; Kelkar, R.A.; Chami, A.A.; et al. Tumor Necrosis Factor and Interleukin Modulators for Pathologic Pain States: A Narrative Review. Pain Ther. 2024, 13, 481–493. [Google Scholar] [CrossRef]
- Biesmans, S.; Bouwknecht, J.A.; Ver Donck, L.; Langlois, X.; Acton, P.D.; De Haes, P.; Davoodi, N.; Meert, T.F.; Hellings, N.; Nuydens, R. Peripheral Administration of Tumor Necrosis Factor-Alpha Induces Neuroinflammation and Sickness but Not Depressive-Like Behavior in Mice. Biomed. Res. Int. 2015, 2015, 716920. [Google Scholar] [CrossRef]
- Daëron, M. The immune system as a system of relations. Front. Immunol. 2022, 13, 984678. [Google Scholar] [CrossRef]
- Tuttle, J.; Drescher, E.; Simón-Campos, J.A.; Emery, P.; Greenwald, M.; Kivitz, A.; Rha, H.; Yachi, P.; Kiley, C.; Nirula, A. A Phase 2 Trial of Peresolimab for Adults with Rheumatoid Arthritis. N. Engl. J. Med. 2023, 388, 1853–1862. [Google Scholar] [CrossRef]
- Chen, S.X.; Liao, G.J.; Yao, P.W.; Wang, S.K.; Li, Y.Y.; Zeng, W.A.; Liu, X.G.; Zang, Y. Calpain-2 Regulates TNF-α Expression Associated with Neuropathic Pain Following Motor Nerve Injury. Neuroscience 2018, 376, 142–151. [Google Scholar] [CrossRef]
- LaBranche, T.P.; Bendele, A.M.; Omura, B.C.; Gropp, K.E.; Hurst, S.I.; Bagi, C.M.; Cummings, T.R.; Grantham, L.E., 2nd; Shelton, D.L.; Zorbas, M.A. Nerve growth factor inhibition with tanezumab influences weight-bearing and subsequent cartilage damage in the rat medial meniscal tear model. Ann. Rheum. Dis. 2017, 76, 295–302. [Google Scholar] [CrossRef]
- Gjefsen, E.; Bråten, L.C.H.; Goll, G.L.; Wigemyr, M.; Bolstad, N.; Valberg, M.; Schistad, E.I.; Marchand, G.H.; Granviken, F.; Selmer, K.K.; et al. The effect of infliximab in patients with chronic low back pain and Modic changes (the BackToBasic study): Study protocol of a randomized, double blind, placebo-controlled, multicenter trial. BMC Musculoskelet. Disord. 2020, 21, 698. [Google Scholar] [CrossRef] [PubMed]
- Bramson, C.; Herrmann, D.N.; Carey, W.; Keller, D.; Brown, M.T.; West, C.R.; Verburg, K.M.; Dyck, P.J. Exploring the role of tanezumab as a novel treatment for the relief of neuropathic pain. Pain Med. 2015, 16, 1163–1176. [Google Scholar] [CrossRef] [PubMed]
- Carswell, E.A.; Old, L.J.; Kassel, R.L.; Green, S.; Fiore, N.; Williamson, B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc. Natl. Acad. Sci. USA 1975, 72, 3666–3670. [Google Scholar] [CrossRef]
- Old, L.J. Tumor necrosis factor (TNF). Science 1985, 230, 630–632. [Google Scholar] [CrossRef]
- Kwon, J.; Chung, I.Y.; Benveniste, E.N. Cloning and sequence analysis of the rat tumor necrosis factor-encoding genes. Gene 1993, 132, 227–236. [Google Scholar] [CrossRef]
- Nedospasov, S.A.; Hirt, B.; Shakhov, A.N.; Dobrynin, V.N.; Kawashima, E.; Accolla, R.S.; Jongeneel, C.V. The genes for tumor necrosis factor (TNF-alpha) and lymphotoxin (TNF-beta) are tandemly arranged on chromosome 17 of the mouse. Nucleic Acids Res. 1986, 14, 7713–7725. [Google Scholar] [CrossRef]
- Wiens, G.D.; Glenney, G.W. Origin and evolution of TNF and TNF receptor superfamilies. Dev. Comp. Immunol. 2011, 35, 1324–1335. [Google Scholar] [CrossRef]
- Sethi, J.K.; Hotamisligil, G.S. Metabolic Messengers: Tumour necrosis factor. Nat. Metab. 2021, 3, 1302–1312. [Google Scholar] [CrossRef]
- Gearing, A.J.H.; Beckett, P.; Christodoulou, M.; Churchill, M.; Clements, J.M.; Crimmin, M.; Davidson, A.H.; Drummond, A.H.; Gallowav, W.A.; Gilbert, R.; et al. Matrix metalloproteinases and processing of pro-TNF-α. J. Leukoc. Biol. 1995, 57, 774–777. [Google Scholar] [CrossRef]
- DasGupta, S.; Murumkar, P.R.; Giridhar, R.; Yadav, M.R. Current perspective of TACE inhibitors: A review. Bioorg. Med. Chem. 2009, 17, 444–459. [Google Scholar] [CrossRef]
- Sedger, L.M.; McDermott, M.F. TNF and TNF-receptors: From mediators of cell death and inflammation to therapeutic giants—Past, present and future. Cytokine Growth Factor Rev. 2014, 25, 453–472. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Hung, M.-C.; Klostergaard, J. Human pro-tumor necrosis factor is a homotrimer. Biochemistry 1996, 35, 8216–8225. [Google Scholar] [CrossRef] [PubMed]
- Eck, M.J.; Sprang, S.R. The structure of tumor necrosis factor-alpha at 2.6 A resolution. Implications for receptor binding. J. Biol. Chem. 1989, 264, 17595–17605. [Google Scholar] [CrossRef] [PubMed]
- Daub, H.; Traxler, L.; Ismajli, F.; Groitl, B.; Itzen, A.; Rant, U. The trimer to monomer transition of Tumor Necrosis Factor-Alpha is a dynamic process that is significantly altered by therapeutic antibodies. Sci. Rep. 2020, 10, 9265. [Google Scholar] [CrossRef]
- Jones, E.Y.; Stuart, D.I.; Walker, N.P. The structure of tumour necrosis factor-implications for biological function. J. Cell. Sci. Suppl. 1990, 13, 11–18. [Google Scholar] [CrossRef]
- Liu, H.; Dai, L.; Hao, Z.; Huang, W.; Yang, Q. Hydrophobic cavity in C-terminus is essential for hTNF-α trimer conformation. Biochimie 2012, 94, 1001–1008. [Google Scholar] [CrossRef]
- Idriss, H.T.; Naismith, J.H. TNF alpha and the TNF receptor superfamily: Structure-function relationship(s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef]
- Li, Y.; Ye, R.; Dai, H.; Lin, J.; Cheng, Y.; Zhou, Y.; Lu, Y. Exploring TNFR1: From discovery to targeted therapy development. J. Transl. Med. 2025, 23, 71. [Google Scholar] [CrossRef]
- Beldi, G.; Khosravi, M.; Abdelgawad, M.E.; Salomon, B.L.; Uzan, G.; Haouas, H.; Naserian, S. TNFα/TNFR2 signaling pathway: An active immune checkpoint for mesenchymal stem cell immunoregulatory function. Stem. Cell. Res. Ther. 2020, 11, 281. [Google Scholar] [CrossRef]
- Papazian, I.; Tsoukala, E.; Boutou, A.; Karamita, M.; Kambas, K.; Iliopoulou, L.; Fischer, R.; Kontermann, R.E.; Denis, M.C.; Kollias, G.; et al. Fundamentally different roles of neuronal TNF receptors in CNS pathology: TNFR1 and IKKβ promote microglial responses and tissue injury in demyelination while TNFR2 protects against excitotoxicity in mice. J. Neuroinflamm. 2021, 18, 222. [Google Scholar] [CrossRef]
- Naserian, S.; Abdelgawad, M.E.; Afshar Bakshloo, M.; Ha, G.; Arouche, N.; Cohen, J.L.; Salomon, B.L.; Uzan, G. The TNF/TNFR2 signaling pathway is a key regulatory factor in endothelial progenitor cell immunosuppressive effect. Cell. Commun. Signal. 2020, 18, 94. [Google Scholar] [CrossRef] [PubMed]
- Sainz, J.; Salas-Alvarado, I.; López-Fernández, E.; Olmedo, C.; Comino, A.; García, F.; Blanco, A.; Gómez-Lopera, S.; Oyonarte, S.; Bueno, P.; et al. TNFR1 mRNA expression level and TNFR1 gene polymorphisms are predictive markers for susceptibility to develop invasive pulmonary aspergillosis. Int. J. Immunopathol. Pharmacol. 2010, 23, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Deguine, J.; Wei, J.; Barbalat, R.; Gronert, K.; Barton, G.M. Local TNFR1 Signaling Licenses Murine Neutrophils for Increased TLR-Dependent Cytokine and Eicosanoid Production. J. Immunol. 2017, 198, 2865–2875. [Google Scholar] [CrossRef] [PubMed]
- Pobezinskaya, Y.L.; Liu, Z. The role of TRADD in death receptor signaling. Cell Cycle 2012, 11, 871–876. [Google Scholar] [CrossRef]
- Priem, D.; Dondelinger, Y.; Bertrand, M.J.M. Monitoring RIPK1 Phosphorylation in the TNFR1 Signaling Complex. Methods Mol. Biol. 2018, 1857, 171–179. [Google Scholar]
- Hsu, H.; Shu, H.B.; Pan, M.G.; Goeddel, D.V. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell 1996, 84, 299–308. [Google Scholar] [CrossRef]
- Guo, D.; Dunbar, J.D.; Yang, C.H.; Pfeffer, L.M.; Donner, D.B. Induction of Jak/STAT signaling by activation of the type 1 TNF receptor. J. Immunol. 1998, 160, 2742–2750. [Google Scholar] [CrossRef]
- Jiang, Y.; Yu, M.; Hu, X.; Han, L.; Yang, K.; Ba, H.; Zhang, Z.; Yin, B.; Yang, X.P.; Li, Z.; et al. STAT1 mediates transmembrane TNF-alpha-induced formation of death-inducing signaling complex and apoptotic signaling via TNFR1. Cell Death Differ. 2017, 24, 660–671. [Google Scholar] [CrossRef]
- Marques-Fernandez, F.; Planells-Ferrer, L.; Gozzelino, R.; Galenkamp, K.M.; Reix, S.; Llecha-Cano, N.; Lopez-Soriano, J.; Yuste, V.J.; Moubarak, R.S.; Comella, J.X. TNFα induces survival through the FLIP-L-dependent activation of the MAPK/ERK pathway. Cell Death Dis. 2013, 4, e493. [Google Scholar] [CrossRef]
- Wang, L.W.; Chang, Y.C.; Chen, S.J.; Tseng, C.H.; Tu, Y.F.; Liao, N.S.; Huang, C.C.; Ho, C.J. TNFR1-JNK signaling is the shared pathway of neuroinflammation and neurovascular damage after LPS-sensitized hypoxic-ischemic injury in the immature brain. J. Neuroinflamm. 2014, 11, 215. [Google Scholar] [CrossRef]
- Swanson, K.A.; Nguyen, K.L.; Gupta, S.; Ricard, J.; Bethea, J.R. TNFR1/p38αMAPK signaling in Nex + supraspinal neurons regulates estrogen-dependent chronic neuropathic pain. Brain Behav. Immun. 2024, 119, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Kyriakis, J.M. Activation of the AP-1 transcription factor by inflammatory cytokines of the TNF family. Gene Expr. 1999, 7, 217–231. [Google Scholar] [PubMed]
- Al-Sadi, R.; Guo, S.; Ye, D.; Ma, T.Y. TNF-α modulation of intestinal epithelial tight junction barrier is regulated by ERK1/2 activation of Elk-1. Am. J. Pathol. 2013, 183, 1871–1884. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Regulation of NF-κB by TNF family cytokines. Semin. Immunol. 2014, 26, 253–266. [Google Scholar] [CrossRef]
- Ting, A.T.; Bertrand, M.J.M. More to Life than NF-κB in TNFR1 Signaling. Trends Immunol. 2016, 37, 535–545. [Google Scholar] [CrossRef]
- Choi, M.E.; Price, D.R.; Ryter, S.W.; Choi, A.M.K. Necroptosis: A crucial pathogenic mediator of human disease. JCI Insight. 2019, 4, e128834. [Google Scholar] [CrossRef]
- Ivagnès, A.; Messaoudene, M.; Stoll, G.; Routy, B.; Fluckiger, A.; Yamazaki, T.; Iribarren, K.; Duong, C.P.M.; Fend, L.; Caignard, A.; et al. TNFR2/BIRC3-TRAF1 signaling pathway as a novel NK cell immune checkpoint in cancer. Oncoimmunology 2017, 7, e1386826. [Google Scholar] [CrossRef] [PubMed]
- Siegmund, D.; Wagner, J.; Wajant, H. TNF Receptor Associated Factor 2 (TRAF2) Signaling in Cancer. Cancers 2022, 14, 4055. [Google Scholar] [CrossRef]
- Moatti, A.; Cohen, J.L. The TNF-α/TNFR2 Pathway: Targeting a Brake to Release the Anti-tumor Immune Response. Front. Cell Dev. Biol. 2021, 9, 725473. [Google Scholar] [CrossRef]
- Siegmund, D.; Ehrenschwender, M.; Wajant, H. TNFR2 unlocks a RIPK1 kinase activity-dependent mode of proinflammatory TNFR1 signaling. Cell Death Dis. 2018, 9, 921. [Google Scholar] [CrossRef]
- Zhao, X.; Lu, Y.; Cui, L. Neutrophil-sourced TNF in cancer: Deciphering an intricate orchestrator of immunosuppressive communication in the tumor microenvironment. Signal. Transduct. Target Ther. 2023, 8, 272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.C.; Kainz, V.; Burstein, R.; Levy, D. Tumor necrosis factor-α induces sensitization of meningeal nociceptors mediated via local COX and p38 MAP kinase actions. Pain 2011, 152, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Spanò, F.; Contatore, M.; Guastalla, A.; Penza, E.; Magnani, O.; Puppo, F. Infection risk associated with anti-TNF-α agents: A review. Expert. Opin. Drug Saf. 2015, 14, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zou, Y.; Li, G.; Zhao, S.; Zhang, W. Comparisons of infection events associated with tumor necrosis factor inhibitors in patients with inflammatory arthritis: A systematic review and network meta-analysis. Front. Pharmacol. 2024, 15, 1376262. [Google Scholar] [CrossRef]
- Sheng, W.S.; Hu, S.; Ni, H.T.; Rowen, T.N.; Lokensgard, J.R.; Peterson, P.K. TNF-alpha-induced chemokine production and apoptosis in human neural precursor cells. J. Leukoc. Biol. 2005, 78, 1233–1241. [Google Scholar] [CrossRef]
- Osborn, L.; Hession, C.; Tizard, R.; Vassallo, C.; Luhowskyj, S.; Chi-Rosso, G.; Lobb, R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell 1989, 59, 1203–1211. [Google Scholar] [CrossRef]
- Jang, D.I.; Lee, A.H.; Shin, H.Y.; Song, H.R.; Park, J.H.; Kang, T.B.; Lee, S.R.; Yang, S.H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Wu, X.; Xu, W.; Feng, X.; He, Y.; Liu, X.; Gao, Y.; Yang, S.; Shao, Z.; Yang, C.; Ye, Z. TNF-a mediated inflammatory macrophage polarization contributes to the pathogenesis of steroid-induced osteonecrosis in mice. Int. J. Immunopathol. Pharmacol. 2015, 28, 351–361. [Google Scholar] [CrossRef]
- Mehta, A.K.; Gracias, D.T.; Croft, M. TNF activity and T cells. Cytokine 2018, 101, 14–18. [Google Scholar] [CrossRef]
- Pesce, B.; Ribeiro, C.H.; Larrondo, M.; Ramos, V.; Soto, L.; Catalán, D.; Aguillón, J.C. TNF-α Affects Signature Cytokines of Th1 and Th17 T Cell Subsets through Differential Actions on TNFR1 and TNFR2. Int. J. Mol. Sci. 2022, 23, 9306. [Google Scholar] [CrossRef]
- Farrugia, M.; Baron, B. The role of TNF-α in rheumatoid arthritis: A focus on regulatory T cells. J. Clin. Transl. Res. 2016, 2, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.F.; Caetano, M.A.F.; Magalhães, H.I.R.; Castelucci, P. Study of tumor necrosis factor receptor in the inflammatory bowel disease. World J. Gastroenterol. 2023, 29, 2733–2746. [Google Scholar] [CrossRef] [PubMed]
- Mylonas, A.; Conrad, C. Psoriasis: Classical vs. Paradoxical. The Yin-Yang of TNF and Type I Interferon. Front. Immunol. 2018, 9, 2746. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Peng, L.; Till, B.; Liao, Y.; Yuan, S.; Yan, X.; Chen, L.; Fu, Q.; Qin, Z. Role of fibrosarcoma-induced CD11b+ myeloid cells and tumor necrosis factor-α in B cell responses. Oncogene 2022, 41, 1434–1444. [Google Scholar] [CrossRef]
- Alvarez, S.; Blanco, A.; Fresno, M.; Muñoz-Fernández, M.Á. TNF-α contributes to caspase-3 independent apoptosis in neuroblastoma cells: Role of NFAT. PLoS ONE 2011, 6, e16100. [Google Scholar] [CrossRef]
- An, L.; Shen, S.; Wang, L.; Li, Y.; Fahim, S.; Niu, Y.; Pan, S. TNF-alpha increases angiogenic potential in a co-culture system of dental pulp cells and endothelial cells. Braz. Oral Res. 2019, 33, e059. [Google Scholar] [CrossRef]
- Li, C.W.; Xia, W.; Huo, L.; Lim, S.O.; Wu, Y.; Hsu, J.L.; Chao, C.H.; Yamaguchi, H.; Yang, N.K.; Ding, Q.; et al. Epithelial-mesenchymal transition induced by TNF-α requires NF-κB-mediated transcriptional upregulation of Twist1. Cancer Res. 2012, 72, 1290–1300. [Google Scholar] [CrossRef]
- Alim, L.F.; Keane, C.; Souza-Fonseca-Guimaraes, F. Molecular mechanisms of tumour necrosis factor signalling via TNF receptor 1 and TNF receptor 2 in the tumour microenvironment. Curr. Opin. Immunol. 2024, 86, 102409. [Google Scholar] [CrossRef]
- Chen, X.; Oppenheim, J.J. TNF-alpha: An activator of CD4+FoxP3+TNFR2+ regulatory T cells. Curr. Dir. Autoimmun. 2010, 11, 119–134. [Google Scholar]
- Zhao, X.; Rong, L.; Zhao, X.; Li, X.; Liu, X.; Deng, J.; Wu, H.; Xu, X.; Erben, U.; Wu, P.; et al. TNF signaling drives myeloid-derived suppressor cell accumulation. J. Clin. Investig. 2012, 122, 4094–4104. [Google Scholar] [CrossRef]
- Rui, L.; Aguirre, V.; Kim, J.K.; Shulman, G.I.; Lee, A.; Corbould, A.; Dunaif, A.; White, M.F. Insulin/IGF-1 and TNF-alpha stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J. Clin. Investig. 2001, 107, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Barrett, E.J.; Barrett, M.O.; Cao, W.; Liu, Z. Tumor necrosis factor-alpha induces insulin resistance in endothelial cells via a p38 mitogen-activated protein kinase-dependent pathway. Endocrinology 2007, 148, 3356–3363. [Google Scholar] [CrossRef]
- Ahmad, R.; Bahman, F.; Kochumon, S.P.; Jacob, T.K.; Alrashed, F.; Wilson, A.; Arefanian, H.; Thomas, R.S.; Akhter, N.; Al-Mansour, N.; et al. 162-OR: Sustained TNF-α transcription triggered in obesity through H3K9/K18 acetylation is associated with metabolic impairments. Diabetes 2024, 73, 162. [Google Scholar] [CrossRef]
- Plantone, D.; Pardini, M.; Righi, D.; Manco, C.; Colombo, B.M.; De Stefano, N. The Role of TNF-α in Alzheimer’s Disease: A Narrative Review. Cells 2023, 13, 54. [Google Scholar] [CrossRef]
- Amin, R.; Quispe, C.; Docea, A.O.; Ydyrys, A.; Kulbayeva, M.; Durna Daştan, S.; Calina, D.; Sharifi-Rad, J. The role of Tumour Necrosis Factor in neuroinflammation associated with Parkinson’s disease and targeted therapies. Neurochem. Int. 2022, 158, 105376. [Google Scholar] [CrossRef]
- Zahid, M.; Busmail, A.; Penumetcha, S.S.; Ahluwalia, S.; Irfan, R.; Khan, S.A.; Rohit Reddy, S.; Vasquez Lopez, M.E.; Mohammed, L. Tumor Necrosis Factor Alpha Blockade and Multiple Sclerosis: Exploring New Avenues. Cureus 2021, 13, e18847. [Google Scholar] [CrossRef]
- Olmos, G.; Lladó, J. Tumor necrosis factor alpha: A link between neuroinflammation and excitotoxicity. Mediat. Inflamm. 2014, 2014, 861231. [Google Scholar] [CrossRef]
- Whiten, D.R.; Brownjohn, P.W.; Moore, S.; De, S.; Strano, A.; Zuo, Y.; Haneklaus, M.; Klenerman, D.; Livesey, F.J. Tumour necrosis factor induces increased production of extracellular amyloid-β- and α-synuclein-containing aggregates by human Alzheimer’s disease neurons. Brain Commun. 2020, 2, fcaa146. [Google Scholar] [CrossRef]
- Borrajo, A.; Rodriguez-Perez, A.I.; Diaz-Ruiz, C.; Guerra, M.J.; Labandeira-Garcia, J.L. Microglial TNF-α mediates enhancement of dopaminergic degeneration by brain angiotensin. Glia 2014, 62, 145–157. [Google Scholar] [CrossRef]
- Ashcroft, G.S.; Jeong, M.J.; Ashworth, J.J.; Hardman, M.; Jin, W.; Moutsopoulos, N.; Wild, T.; McCartney-Francis, N.; Sim, D.; McGrady, G.; et al. Tumor necrosis factor-alpha (TNF-α) is a therapeutic target for impaired cutaneous wound healing. Wound Repair Regen. 2012, 20, 38–49. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, C.; Graves, D.T. Abnormal cell responses and role of TNF-α in impaired diabetic wound healing. Biomed. Res. Int. 2013, 2013, 754802. [Google Scholar] [CrossRef] [PubMed]
- Malaviya, R.; Laskin, J.D.; Businaro, R.; Laskin, D.L. Targeting Tumor Necrosis Factor Alpha to Mitigate Lung Injury Induced by Mustard Vesicants and Radiation. Disaster Med. Public Health Prep. 2023, 17, e553. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Higerd-Rusli, G.P.; Ghovanloo, M.R.; Dib-Hajj, F.; Zhao, P.; Liu, S.; Kim, D.H.; Shim, J.S.; Park, K.S.; Waxman, S.G.; et al. Compartment-specific regulation of NaV1.7 in sensory neurons after acute exposure to TNF-α. Cell Rep. 2024, 43, 113685. [Google Scholar] [CrossRef] [PubMed]
- Leung, L.; Cahill, C.M. TNF-alpha and neuropathic pain—A review. J. Neuroinflamm. 2010, 7, 27. [Google Scholar] [CrossRef]
- Leo, M.; Argalski, S.; Schäfers, M.; Hagenacker, T. Modulation of Voltage-Gated Sodium Channels by Activation of Tumor Necrosis Factor Receptor-1 and Receptor-2 in Small DRG Neurons of Rats. Mediat. Inflamm. 2015, 2015, 124942. [Google Scholar] [CrossRef]
- He, X.H.; Zang, Y.; Chen, X.; Pang, R.P.; Xu, J.T.; Zhou, X.; Wei, X.H.; Li, Y.Y.; Xin, W.J.; Qin, Z.H.; et al. TNF-α contributes to up-regulation of Nav1.3 and Nav1.8 in DRG neurons following motor fiber injury. Pain 2010, 151, 266–279. [Google Scholar] [CrossRef]
- De Macedo, F.H.P.; Aires, R.D.; Fonseca, E.G.; Ferreira, R.C.M.; Machado, D.P.D.; Chen, L.; Zhang, F.X.; Souza, I.A.; Lemos, V.S.; Romero, T.R.L.; et al. TNF-α mediated upregulation of NaV1.7 currents in rat dorsal root ganglion neurons is independent of CRMP2 SUMOylation. Mol. Brain 2019, 12, 117. [Google Scholar] [CrossRef]
- Romanova, D.Y.; Balaban, P.M.; Nikitin, E.S. Sodium channels involved in the initiation of action potentials in invertebrate and mammalian neurons. Biophysica 2022, 2, 184–193. [Google Scholar] [CrossRef]
- Goodwin, G.; McMahon, S.B. The physiological function of different voltage-gated sodium channels in pain. Nat. Rev. Neurosci. 2021, 22, 263–274. [Google Scholar] [CrossRef]
- Wei, X.H.; Zang, Y.; Wu, C.Y.; Xu, J.T.; Xin, W.J.; Liu, X.G. Peri-sciatic administration of recombinant rat TNF-alpha induces mechanical allodynia via upregulation of TNF-alpha in dorsal root ganglia and in spinal dorsal horn: The role of NF-kappa B pathway. Exp. Neurol. 2007, 205, 471–484. [Google Scholar] [CrossRef]
- Czeschik, J.C.; Hagenacker, T.; Schäfers, M.; Büsselberg, D. TNF-alpha differentially modulates ion channels of nociceptive neurons. Neurosci. Lett. 2008, 434, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Dou, B.; Zhang, Y.; Chen, Z.; Li, Y.; Fan, Z.; Ma, Y.; Du, S.; Wang, J.; Xu, Z.; et al. Inflammation-the role of TRPA1 channel. Front. Physiol. 2023, 14, 1093925. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Feng, C.; He, H.; He, J.; Wang, J.; Li, X.; Wang, S.; Li, W.; Hou, J.; Liu, T.; et al. Sensitization of TRPV1 receptors by TNF-α orchestrates the development of vincristine-induced pain. Oncol. Lett. 2018, 15, 5013–5019. [Google Scholar] [CrossRef]
- Hoppanova, L.; Lacinova, L. Voltage-dependent CaV3.2 and CaV2.2 channels in nociceptive pathways. Pflugers Arch. 2022, 474, 421–434. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, S.; Gruber, S.; Mata, M.; Fink, D.J. Full-length membrane-bound tumor necrosis factor-α acts through tumor necrosis factor receptor 2 to modify phenotype of sensory neurons. Pain 2013, 154, 1778–1782. [Google Scholar] [CrossRef]
- Zhang, L.; Berta, T.; Xu, Z.Z.; Liu, T.; Park, J.Y.; Ji, R.R. TNF-α contributes to spinal cord synaptic plasticity and inflammatory pain: Distinct role of TNF receptor subtypes 1 and 2. Pain 2011, 152, 419–427. [Google Scholar] [CrossRef]
- Tolosa, L.; Caraballo-Miralles, V.; Olmos, G.; Lladó, J. TNF-α potentiates glutamate-induced spinal cord motoneuron death via NF-κB. Mol. Cell. Neurosci. 2011, 46, 176–186. [Google Scholar] [CrossRef]
- Guo, A.; Lau, C.G. TNF-α Orchestrates Experience-Dependent Plasticity of Excitatory and Inhibitory Synapses in the Anterior Piriform Cortex. Front. Neurosci. 2022, 16, 824454. [Google Scholar] [CrossRef]
- Gonçalves Dos Santos, G.; Delay, L.; Yaksh, T.L.; Corr, M. Neuraxial Cytokines in Pain States. Front. Immunol. 2020, 10, 3061. [Google Scholar] [CrossRef]
- Kemp, G.M.; Altimimi, H.F.; Nho, Y.; Heir, R.; Klyczek, A.; Stellwagen, D. Sustained TNF signaling is required for the synaptic and anxiety-like behavioral response to acute stress. Mol. Psychiatry 2022, 27, 4474–4484. [Google Scholar] [CrossRef]
- Heir, R.; Stellwagen, D. TNF-Mediated Homeostatic Synaptic Plasticity: From in vitro to in vivo Models. Front. Cell Neurosci. 2020, 14, 565841. [Google Scholar] [CrossRef] [PubMed]
- Jara, J.H.; Singh, B.B.; Floden, A.M.; Combs, C.K. Tumor necrosis factor alpha stimulates NMDA receptor activity in mouse cortical neurons resulting in ERK-dependent death. J. Neurochem. 2007, 100, 1407–1420. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Li, T.; Wang, R.; Deng, W.; Pu, H.; Deng, M. Roles of Phosphorylation of N-Methyl-D-Aspartate Receptor in Chronic Pain. Cell. Mol. Neurobiol. 2023, 43, 155–175. [Google Scholar] [CrossRef]
- Wigerblad, G.; Huie, J.R.; Yin, H.Z.; Leinders, M.; Pritchard, R.A.; Koehrn, F.J.; Xiao, W.H.; Bennett, G.J.; Huganir, R.L.; Ferguson, A.R.; et al. Inflammation-induced GluA1 trafficking and membrane insertion of Ca2+ permeable AMPA receptors in dorsal horn neurons is dependent on spinal tumor necrosis factor, PI3 kinase and protein kinase A. Exp. Neurol. 2017, 293, 144–158. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Wu, Z.; Lin, Q.; Yue, Y.; Fang, L. Regulation of AMPA receptors in spinal nociception. Mol. Pain. 2010, 6, 5. [Google Scholar] [CrossRef]
- Ai, H.; Yang, W.; Ye, M.; Lu, W.; Yao, L.; Luo, J.H. Differential regulation of AMPA receptor GluA1 phosphorylation at serine 831 and 845 associated with activation of NMDA receptor subpopulations. Neurosci. Lett. 2011, 497, 94–98. [Google Scholar] [CrossRef]
- Orellana, J.A.; Froger, N.; Ezan, P.; Jiang, J.X.; Bennett, M.V.; Naus, C.C.; Giaume, C.; Sáez, J.C. ATP and glutamate released via astroglial connexin 43 hemichannels mediate neuronal death through activation of pannexin 1 hemichannels. J. Neurochem. 2011, 118, 826–840. [Google Scholar] [CrossRef]
- Stück, E.D.; Christensen, R.N.; Huie, J.R.; Tovar, C.A.; Miller, B.A.; Nout, Y.S.; Bresnahan, J.C.; Beattie, M.S.; Ferguson, A.R. Tumor necrosis factor alpha mediates GABA(A) receptor trafficking to the plasma membrane of spinal cord neurons in vivo. Neural Plast. 2012, 2012, 261345. [Google Scholar] [CrossRef]
- Andrade, P.; Visser-Vandewalle, V.; Hoffmann, C.; Steinbusch, H.W.; Daemen, M.A.; Hoogland, G. Role of TNF-alpha during central sensitization in preclinical studies. Neurol. Sci. 2011, 32, 757–771. [Google Scholar] [CrossRef]
- Ji, R.R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef]
- Zhao, H.; Alam, A.; Chen, Q.; A Eusman, M.; Pal, A.; Eguchi, S.; Wu, L.; Ma, D. The role of microglia in the pathobiology of neuropathic pain development: What do we know? Br. J. Anaesth. 2017, 118, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Brás, J.P.; Bravo, J.; Freitas, J.; Barbosa, M.A.; Santos, S.G.; Summavielle, T.; Almeida, M.I. TNF-alpha-induced microglia activation requires miR-342: Impact on NF-kB signaling and neurotoxicity. Cell Death Dis. 2020, 11, 415. [Google Scholar] [CrossRef] [PubMed]
- Karavis, M.Y.; Siafaka, I.; Vadalouca, A.; Georgoudis, G. Role of Microglia in Neuropathic Pain. Cureus 2023, 15, e43555. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; You, B.; Jo, E.K.; Han, S.K.; Simon, M.I.; Lee, S.J. NADPH oxidase 2-derived reactive oxygen species in spinal cord microglia contribute to peripheral nerve injury-induced neuropathic pain. Proc. Natl. Acad. Sci. USA 2010, 107, 14851–14856. [Google Scholar] [CrossRef]
- Kuboyama, K.; Tsuda, M.; Tsutsui, M.; Toyohara, Y.; Tozaki-Saitoh, H.; Shimokawa, H.; Yanagihara, N.; Inoue, K. Reduced spinal microglial activation and neuropathic pain after nerve injury in mice lacking all three nitric oxide synthases. Mol. Pain 2011, 7, 50. [Google Scholar] [CrossRef]
- Pati, D.; Kash, T.L. Tumor necrosis factor-α modulates GABAergic and dopaminergic neurons in the ventrolateral periaqueductal gray of female mice. J. Neurophysiol. 2021, 126, 2119–2129. [Google Scholar] [CrossRef]
- Ignatowski, T.A.; Covey, W.C.; Knight, P.R.; Severin, C.M.; Nickola, T.J.; Spengler, R.N. Brain-derived TNFalpha mediates neuropathic pain. Brain Res. 1999, 841, 70–77. [Google Scholar] [CrossRef]
- Covey, W.C.; Ignatowski, T.A.; Renauld, A.E.; Knight, P.R.; Nader, N.D.; Spengler, R.N. Expression of neuron-associated tumor necrosis factor alpha in the brain is increased during persistent pain. Reg. Anesth. Pain Med. 2002, 27, 357–366. [Google Scholar]
- Motta, S.C.; Carobrez, A.P.; Canteras, N.S. The periaqueductal gray and primal emotional processing critical to influence complex defensive responses, fear learning and reward seeking. Neurosci. Biobehav. Rev. 2017, 76, 39–47. [Google Scholar] [CrossRef]
- Immordino-Yang, M.H.; Singh, V. Hippocampal contributions to the processing of social emotions. Hum. Brain Mapp. 2013, 34, 945–955. [Google Scholar] [CrossRef]
- Liu, K.Y.; Betts, M.J.; Hämmerer, D.; Düzel, E.; Mather, M.; Roiser, J.P.; Schneider, A.; Spottke, A.; Rostamzadeh, A.; Schott, B.H.; et al. Locus coeruleus signal intensity and emotion regulation in agitation in Alzheimer’s disease. Brain Commun. 2024, 7, fcae457. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wei, Y.; Zhou, J.; Zou, H.; Ma, L.; Liu, C.; Xiao, Z.; Liu, X.; Tan, X.; Yu, T.; et al. Activation of locus coeruleus-spinal cord noradrenergic neurons alleviates neuropathic pain in mice via reducing neuroinflammation from astrocytes and microglia in spinal dorsal horn. J. Neuroinflamm. 2022, 19, 123. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sateesh, S.; Jones, O.D.; Abraham, W.C. Pathway-specific TNF-mediated metaplasticity in hippocampal area CA1. Sci. Rep. 2022, 12, 1746. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, L.J.; Wang, J.; Li, D.; Ren, W.J.; Peng, J.; Wei, X.; Xu, T.; Xin, W.J.; Pang, R.P.; et al. TNF-α Differentially Regulates Synaptic Plasticity in the Hippocampus and Spinal Cord by Microglia-Dependent Mechanisms after Peripheral Nerve Injury. J. Neurosci. 2017, 37, 871–881. [Google Scholar] [CrossRef]
- Wang, R.; Man, Y.; Zhou, M.; Zhu, Y.; Wang, L.; Yang, J. Neuropathic pain-induced cognitive dysfunction and down-regulation of neuronal pentraxin 2 in the cortex and hippocampus. Neuroreport 2021, 32, 274–283. [Google Scholar] [CrossRef]
- Dellarole, A.; Morton, P.; Brambilla, R.; Walters, W.; Summers, S.; Bernardes, D.; Grilli, M.; Bethea, J.R. Neuropathic pain-induced depressive-like behavior and hippocampal neurogenesis and plasticity are dependent on TNFR1 signaling. Brain Behav. Immun. 2014, 41, 65–81. [Google Scholar] [CrossRef]
- Kim, J.M.; Kang, H.J.; Kim, J.W.; Kim, H.Y.; Jhon, M.; Lee, J.Y.; Kim, S.W.; Shin, I.S. Exploring the combined effects of serum tumor necrosis factor-alpha and serotonin on antidepressant efficacy in depression: A 12-week prospective analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2025, 138, 111328. [Google Scholar] [CrossRef]
- Mancini, M.; Natoli, S.; Gardoni, F.; Di Luca, M.; Pisani, A. Dopamine Transmission Imbalance in Neuroinflammation: Perspectives on Long-Term COVID-19. Int. J. Mol. Sci. 2023, 24, 5618. [Google Scholar] [CrossRef]
- Okamoto, N.; Hoshikawa, T.; Honma, Y.; Chibaatar, E.; Ikenouchi, A.; Harada, M.; Yoshimura, R. Effect modification of tumor necrosis factor-α on the kynurenine and serotonin pathways in major depressive disorder on type 2 diabetes mellitus. Eur. Arch. Psychiatry Clin. Neurosci. 2024, 274, 1697–1707. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Rakshit, S.; Molina, J.R. Immunotherapy in patients with autoimmune disease. J. Thorac. Dis. 2020, 12, 7032–7038. [Google Scholar] [CrossRef] [PubMed]
- Léger, J.M.; Guimarães-Costa, R.; Muntean, C. Immunotherapy in Peripheral Neuropathies. Neurotherapeutics 2016, 13, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Devlin, E.J.; Denson, L.A.; Whitford, H.S. Cancer Treatment Side Effects: A Meta-analysis of the Relationship Between Response Expectancies and Experience. J. Pain Symptom Manag. 2017, 54, 245–258.e2. [Google Scholar] [CrossRef]
- Tavares, L.C.P.; Caetano, L.V.N.; Ianhez, M. Side effects of chronic systemic glucocorticoid therapy: What dermatologists should know. An. Bras. Dermatol. 2024, 99, 259–268. [Google Scholar] [CrossRef]
- Kichloo, A.; Albosta, M.; Dahiya, D.; Guidi, J.C.; Aljadah, M.; Singh, J.; Shaka, H.; Wani, F.; Kumar, A.; Lekkala, M. Systemic adverse effects and toxicities associated with immunotherapy: A review. World J. Clin. Oncol. 2021, 12, 150–163. [Google Scholar] [CrossRef]
- Yamakawa, I.; Kojima, H.; Terashima, T.; Katagi, M.; Oi, J.; Urabe, H.; Sanada, M.; Kawai, H.; Chan, L.; Yasuda, H.; et al. Inactivation of TNF-α ameliorates diabetic neuropathy in mice. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E844–E852. [Google Scholar] [CrossRef]
- Schäfers, M.; Brinkhoff, J.; Neukirchen, S.; Marziniak, M.; Sommer, C. Combined epineurial therapy with neutralizing antibodies to tumor necrosis factor-alpha and interleukin-1 receptor has an additive effect in reducing neuropathic pain in mice. Neurosci. Lett. 2001, 310, 113–116. [Google Scholar] [CrossRef]
- Sommer, C.; Lindenlaub, T.; Teuteberg, P.; Schäfers, M.; Hartung, T.; Toyka, K.V. Anti-TNF-neutralizing antibodies reduce pain-related behavior in two different mouse models of painful mononeuropathy. Brain Res. 2001, 913, 86–89. [Google Scholar] [CrossRef]
- Sommer, C.; Schäfers, M.; Marziniak, M.; Toyka, K.V. Etanercept reduces hyperalgesia in experimental painful neuropathy. J. Peripher Nerv. Syst. 2001, 6, 67–72. [Google Scholar] [CrossRef]
- Vince, V.; Brelen, M.E.; Delbeke, J.; Colin, I.M. Anti-TNF-alpha reduces the inflammatory reaction associated with cuff electrode implantation around the sciatic nerve. J. Neuroimmunol. 2005, 165, 121–128. [Google Scholar] [CrossRef]
- Andrade, P.; Hoogland, G.; Del Rosario, J.S.; Steinbusch, H.W.; Visser-Vandewalle, V.; Daemen, M.A. Tumor necrosis factor-α inhibitors alleviation of experimentally induced neuropathic pain is associated with modulation of TNF receptor expression. J. Neurosci. Res. 2014, 92, 1490–1498. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Tagashira, H.; Nemoto, T.; Kita, S.; Kita, T.; Shinoda, Y.; Akiyoshi, K.; Yamaura, K.; Iwamoto, T. Perineural treatment with anti-TNF-α antibody ameliorates persistent allodynia and edema in novel mouse models with complex regional pain syndrome. J. Pharmacol. Sci. 2023, 153, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Li, Q.; Zhou, Y.; Chen, S.; Li, Y.; Zang, Y. Activation of the TNF-α-Necroptosis Pathway in Parvalbumin-Expressing Interneurons of the Anterior Cingulate Cortex Contributes to Neuropathic Pain. Int. J. Mol. Sci. 2023, 24, 15454. [Google Scholar] [CrossRef] [PubMed]
- Alé, A.; Bruna, J.; Morell, M.; van de Velde, H.; Monbaliu, J.; Navarro, X.; Udina, E. Treatment with anti-TNF alpha protects against the neuropathy induced by the proteasome inhibitor bortezomib in a mouse model. Exp. Neurol. 2014, 253, 165–173. [Google Scholar] [CrossRef]
- Jhan, M.K.; HuangFu, W.C.; Chen, Y.F.; Kao, J.C.; Tsai, T.T.; Ho, M.R.; Shen, T.J.; Tseng, P.C.; Wang, Y.T.; Lin, C.F. Anti-TNF-α restricts dengue virus-induced neuropathy. J. Leukoc. Biol. 2018, 104, 961–968. [Google Scholar] [CrossRef]
- Javed, S.; Kamili, Q.U.; Mendoza, N.; Tyring, S.K. Possible association of lower rate of postherpetic neuralgia in patients on anti-tumor necrosis factor-α. J. Med. Virol. 2011, 83, 2051–2055. [Google Scholar] [CrossRef]
- Karppinen, J.; Korhonen, T.; Malmivaara, A.; Paimela, L.; Kyllönen, E.; Lindgren, K.A.; Rantanen, P.; Tervonen, O.; Niinimäki, J.; Seitsalo, S.; et al. Tumor necrosis factor-alpha monoclonal antibody, infliximab, used to manage severe sciatica. Spine (Phila Pa 1976) 2003, 28, 750–753. [Google Scholar] [CrossRef]
- Korhonen, T.; Karppinen, J.; Malmivaara, A.; Autio, R.; Niinimäki, J.; Paimela, L.; Kyllönen, E.; Lindgren, K.A.; Tervonen, O.; Seitsalo, S.; et al. Efficacy of infliximab for disc herniation-induced sciatica: One-year follow-up. Spine (Phila Pa 1976) 2004, 29, 2115–2119. [Google Scholar] [CrossRef]
- Genevay, S.; Viatte, S.; Finckh, A.; Zufferey, P.; Balagué, F.; Gabay, C. Adalimumab in severe and acute sciatica: A multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010, 62, 2339–2346. [Google Scholar] [CrossRef]
- Guo, J.R.; Jin, X.J.; Shen, H.C.; Wang, H.; Zhou, X.; Liu, X.Q.; Zhu, N.N. A Comparison of the Efficacy and Tolerability of the Treatments for Sciatica: A Network Meta-Analysis. Ann. Pharmacother. 2017, 51, 1041–1052. [Google Scholar] [CrossRef]
- Huygen, F.J.; Niehof, S.; Zijlstra, F.J.; van Hagen, P.M.; van Daele, P.L. Successful treatment of CRPS 1 with anti-TNF. J. Pain Symptom Manag. 2004, 27, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Dirckx, M.; Groeneweg, G.; Wesseldijk, F.; Stronks, D.L.; Huygen, F.J. Report of a preliminary discontinued double-blind, randomized, placebo-controlled trial of the anti-TNF-α chimeric monoclonal antibody infliximab in complex regional pain syndrome. Pain Pract. 2013, 13, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, E.; Sandler, I.; Treister, R.; Suzan, E.; Haddad, M. Anti tumor necrosis factor—Alpha adalimumab for complex regional pain syndrome type 1 (CRPS-I): A case series. Pain Pract. 2013, 13, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, C.; Dirckx, M.; Huygen, F.J.P.M.; Tiemensma, J. Effectiveness of Infliximab in Patients with Complex Regional Pain Syndrome: A Case Series. J. Pain Res. 2023, 16, 1915–1926. [Google Scholar] [CrossRef]
- Tavee, J.O.; Karwa, K.; Ahmed, Z.; Thompson, N.; Parambil, J.; Culver, D.A. Sarcoidosis-associated small fiber neuropathy in a large cohort: Clinical aspects and response to IVIG and anti-TNF alpha treatment. Respir. Med. 2017, 126, 135–138. [Google Scholar] [CrossRef]

| Type of Neuropathy | Species Tested | Effects | References |
|---|---|---|---|
| Diabetic polyneuropathy (DPN) | Mice | TNF-α+/+ diabetic mice exhibited significant impairments in motor and sensory nerve conduction velocities (MNCV and SNCV), tail flick responses, and intraepidermal fiber density (IENFD), along with elevated expression of NF-κB p65 and cleaved caspase-3 in their DRGs | [176] |
| Chronic constriction injury (CCI) | Mice | Suppressed thermal hyperalgesia and mechanical allodynia | [177] |
| Mice | Suppressed thermal hyperalgesia and mechanical allodynia | [178] | |
| Mice | Suppressed thermal hyperalgesia and mechanical allodynia | [179] | |
| Rats | Perioperative anti-TNF-α treatment modulated the inflammation and fibrosis associated with CCI. Morphometric and immunohistochemical analyses demonstrated that a single systemic administration of anti-TNF-α attenuated early inflammatory responses | [180] | |
| Rats | Suppressed thermal hyperalgesia and mechanical allodynia | [181] | |
| Partial sciatic nerve transection (PST) | Mice | Suppressed thermal hyperalgesia and mechanical allodynia | [178] |
| Sciatic nerve injury (SCI) | Mice | Suppressed mechanical allodynia | [182] |
| Mice | Suppressed mechanical allodynia | [183] | |
| Chemotherapy-induced neuropathy (CIPN) | Mice | Treatment with anti-TNF-α significantly preserved the sensory nerve action potential amplitude and prevented the loss of myelinated and unmyelinated fibers in bortezomib (BTZ)-induced neurotoxicity | [184] |
| Dengue virus-induced neuropathy | Mice | Mitigated encephalitis | [185] |
| Type of Neuropathy | Drug Employed | Effects | References |
|---|---|---|---|
| Postherpetic neuralgia | Infliximab Adalimumab | A retrospective review across 12 dermatology clinics evaluated herpes zoster patients treated with TNF-α inhibitors, including infliximab and adalimumab. The analysis revealed a reduced incidence of postherpetic neuralgia in this cohort. | [186] |
| Sciatica | Infliximab | Within one hour of infusion, leg pain was reduced by 50%. After two weeks, 60% of patients receiving infliximab were pain-free, compared to 16% in the control group. This therapeutic effect persisted at three months, with 90% of infliximab-treated patients remaining pain-free vs. 46% in the control group. | [187] |
| Infliximab | A 3 mg/kg dose of infliximab provided sustained improvement in leg pain and disability over one year. Neurologic abnormalities improved in the infliximab group, although disk herniation volume reduction was very similar between groups. | [188] | |
| Adalimumab | Leg pain improved more significantly over time in the adalimumab group compared to the placebo group, although the effect size was modest. A significantly higher proportion of patients in the adalimumab group met the criteria for “responders” and “low residual disease impact”. Additionally, fewer surgical discectomies were required in the adalimumab group. | [189] | |
| Infliximab Adalimumab | Intravenous anti-TNF-α ranked highest for leg pain relief and subcutaneous anti-TNF-α ranked highest for lumbar pain relief, based on SUCRA analysis. All treatments had medium to high safety rankings in terms of withdrawal rates. | [190] | |
| Complex Regional Pain Syndrome (CRPS) | Infliximab | A significant decrease in local concentrations of TNF-α and IL-6 in blister fluid was observed. There was a slight improvement in clinical signs, with reductions in pain, temperature, and edema, as well as improved motor function. The patients also reported an overall improvement in well-being. By the end of the treatment clinical symptoms showed improvement. | [191] |
| Infliximab | There was no significant difference in the total ISS score between the treated and control groups. However, a trend was observed, suggesting a greater reduction in TNF-α in the infliximab group compared to the placebo group. Additionally, a subscale of the EuroQol (EQ VAS) showed a significant decrease in health status in the intervention group compared to the placebo group. | [192] | |
| Adalimumab | Three patient subgroups were identified, each consisting of three patients: “nonresponders”, “partial responders”, and “robust responders”, with the latter group showing improvement in nearly all parameters. Both the ITT and PP analyses revealed only a trend toward improvement in mechanical pain thresholds following treatment. | [193] | |
| Adalimumab | The infliximab dosage is 5 mg/kg, administered every four to six weeks. A total of 7 patients (of 15) completed a global perceived effect survey, all reporting improvement (IQR 1–2). | [194] | |
| Sarcoidosis-associated small fiber neuropathy (SSFN) | Infliximab | SSFN was diagnosed in 143 individuals, with 28 cases having other neuropathy causes. Pain and paresthesias were the most common symptoms, with 54% being non-length-dependent. Dysautonomia was present in 61 patients, mainly with cardiac symptoms. Symptomatic improvement was seen in 8 of 12 with infliximab. A total of 4 of 27 untreated patients showed improvement. | [195] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Domínguez, M. The Role of TNF-α in Neuropathic Pain: An Immunotherapeutic Perspective. Life 2025, 15, 785. https://doi.org/10.3390/life15050785
García-Domínguez M. The Role of TNF-α in Neuropathic Pain: An Immunotherapeutic Perspective. Life. 2025; 15(5):785. https://doi.org/10.3390/life15050785
Chicago/Turabian StyleGarcía-Domínguez, Mario. 2025. "The Role of TNF-α in Neuropathic Pain: An Immunotherapeutic Perspective" Life 15, no. 5: 785. https://doi.org/10.3390/life15050785
APA StyleGarcía-Domínguez, M. (2025). The Role of TNF-α in Neuropathic Pain: An Immunotherapeutic Perspective. Life, 15(5), 785. https://doi.org/10.3390/life15050785




