Prognostic Impact of COVID-19 Inflammation Score Response: A Sub-Group Analysis on Critically Ill Patients of the RuxCoFlam Trial
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
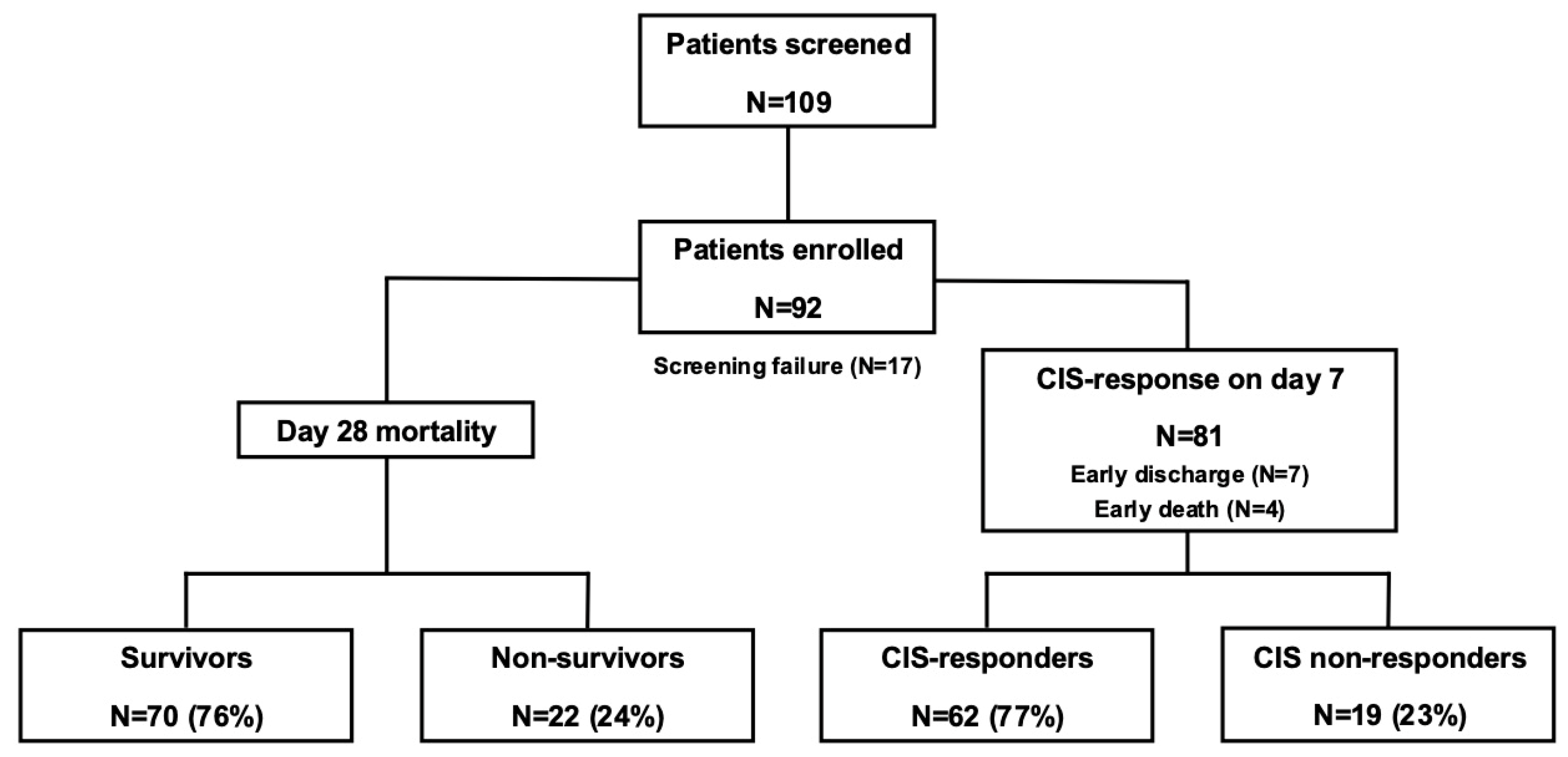
3.1. Patient Disposition and Rux Treatment
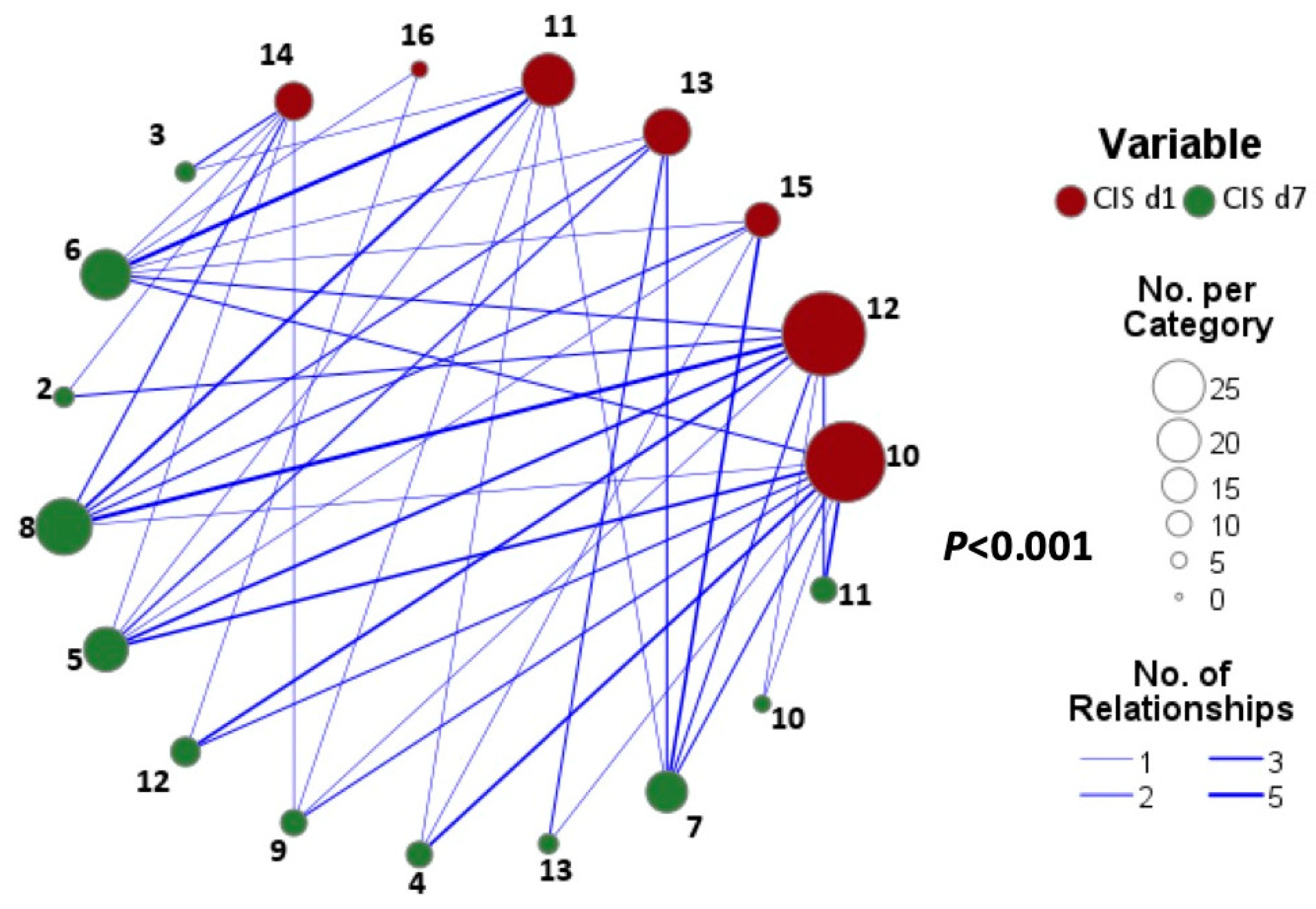
3.2. CIS Repsonose on Day 7
3.3. Survival Probabilities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aPTT | Activated partial thromboplastin time |
| BID | Twice daily |
| BMI | Body mass index |
| °C | Degree Celsius |
| CI | Confidence interval |
| CIS | COVID-19 inflammation score |
| CoV-2 | Coronavirus type 2 |
| COVID-19 | Coronavirus disease 2019 |
| CRP | C-reactive protein |
| Ct | Cycle threshold |
| CT | Computed tomography |
| ECMO | Extracorporeal membrane oxygenation |
| FDA | Food and Drug Administration |
| G/L | Giga/liter |
| HR | Hazard ratio |
| ICU | Intensive care unit |
| IFN-γ | Interferon-γ, |
| IL-6 | Interleukin-6 |
| JAK | Janus kinase |
| mg/L | Milligram/liter |
| MPN | Myeloproliferative neoplasm |
| OR | Odds ratio |
| Pts | Patients |
| RRT | Renal replacement therapy |
| Rux | Ruxolitinib |
| SAPS II | Simplified Acute Physiology Score II |
| SOFA | Sequential Organ Failure Assessment |
| SpO2 | Peripheral arterial oxygen saturation |
| ULN | Upper limit of normal |
| US | United states |
| WBC | White blood cell |
| WHO | World Health Organization |
References
- Goker Bagca, B.; Biray Avci, C. The potential of JAK/STAT pathway inhibition by ruxolitinib in the treatment of COVID-19. Cytokine Growth Factor. Rev. 2020, 54, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Han, M.K.; Antila, M.; Ficker, J.H.; Gordeev, I.; Guerreros, A.; Bernus, A.L.; Roquilly, A.; Sifuentes-Osornio, J.; Tabak, F.; Teijeiro, R.; et al. Ruxolitinib in addition to standard of care for the treatment of patients admitted to hospital with COVID-19 (RUXCOVID): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Rheumatol. 2022, 4, e351–e361. [Google Scholar] [CrossRef] [PubMed]
- Botta, C.; Indrieri, A.; Garofalo, E.; Biamonte, F.; Bruni, A.; Pasqua, P.; Cesario, F.; Costanzo, F.S.; Longhini, F.; Mendicino, F. COVID-19: High-JAKing of the Inflammatory “Flight” by Ruxolitinib to Avoid the Cytokine Storm. Front. Oncol. 2020, 10, 599502. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, A.; Wiesmann, T.; Vogelmeier, C.F.; Mack, E.; Skevaki, C.; Gaik, C.; Keller, C.; Figiel, J.; Sohlbach, K.; Rolfes, C.; et al. Ruxolitinib for the treatment of SARS-CoV-2 induced acute respiratory distress syndrome (ARDS). Leukemia 2020, 34, 2276–2278. [Google Scholar] [CrossRef]
- Neubauer, A.; Johow, J.; Mack, E.; Burchert, A.; Meyn, D.; Kadlubiec, A.; Torje, I.; Wulf, H.; Vogelmeier, C.F.; Hoyer, J.; et al. The janus-kinase inhibitor ruxolitinib in SARS-CoV-2 induced acute respiratory distress syndrome (ARDS). Leukemia 2021, 35, 2917–2923. [Google Scholar] [CrossRef]
- Mortara, A.; Mazzetti, S.; Margonato, D.; Delfino, P.; Bersano, C.; Catagnano, F.; Lauriola, M.; Grosso, P.; Perseghin, G.; Ippoliti, G. Compassionate use of ruxolitinib in patients with SARS-CoV-2 infection not on mechanical ventilation: Short-term effects on inflammation and ventilation. Clin. Transl. Sci. 2021, 14, 1062–1068. [Google Scholar] [CrossRef]
- Ovilla-Martinez, R.; Cota-Rangel, X.; De La Pena-Celaya, J.; Alvarado-Zepeda, M.A.; Jimenez Sastre, A.; Azuara Forcelledo, H.; Ordonez Rodriguez, B.; Pulido Broca, J.; Molina Jaimes, A.; Muniz-Carvajal, A.; et al. Ruxolitinib as a treatment strategy for SARS-CoV-2 pneumonia: Clinical experience in a real-world setting. J. Infect. Dev. Ctries. 2022, 16, 63–72. [Google Scholar] [CrossRef]
- Rein, L.; Calero, K.; Shah, R.; Ojielo, C.; Hudock, K.M.; Lodhi, S.; Sadaka, F.; Bellam, S.; Palma, C.; Hager, D.N.; et al. Randomized Phase 3 Trial of Ruxolitinib for COVID-19-Associated Acute Respiratory Distress Syndrome. Crit. Care Med. 2022, 50, 1701–1713. [Google Scholar] [CrossRef]
- Hammersen, J.; Birndt, S.; Dohner, K.; Reuken, P.; Stallmach, A.; Sauerbrey, P.; La Rosee, F.; Pfirrmann, M.; Fabisch, C.; Weiss, M.; et al. The JAK1/2 inhibitor ruxolitinib in patients with COVID-19 triggered hyperinflammation: The RuxCoFlam trial. Leukemia 2023, 37, 1879–1886. [Google Scholar] [CrossRef]
- La Rosee, F.; Bremer, H.C.; Gehrke, I.; Kehr, A.; Hochhaus, A.; Birndt, S.; Fellhauer, M.; Henkes, M.; Kumle, B.; Russo, S.G.; et al. The Janus kinase 1/2 inhibitor ruxolitinib in COVID-19 with severe systemic hyperinflammation. Leukemia 2020, 34, 1805–1815. [Google Scholar] [CrossRef]
- Feng, C.; Hua, Z.; He, L.; Yao, S.; Zou, H.; Zhu, Y.; Wang, Z.; Wang, Y. A convenient and practical index for predicting the induction response in adult patients with hemophagocytic lymphohistiocytosis: Ferritin/platelet ratio. Ann. Hematol. 2024, 103, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Monk, M.; Torres, J.; Vickery, K.; Jayaraman, G.; Sarva, S.T.; Kesavan, R. A Comparison of ICU Mortality Scoring Systems Applied to COVID-19. Cureus 2023, 15, e35423. [Google Scholar] [CrossRef] [PubMed]
- Allyn, J.; Ferdynus, C.; Bohrer, M.; Dalban, C.; Valance, D.; Allou, N. Simplified Acute Physiology Score II as Predictor of Mortality in Intensive Care Units: A Decision Curve Analysis. PLoS ONE 2016, 11, e0164828. [Google Scholar] [CrossRef]
- Le-Gall, J.R.; Lemeshow, S.; Saulnier, F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993, 270, 2957–2963. [Google Scholar] [CrossRef]
- Vincent, J.L.; de Mendonca, A.; Cantraine, F.; Moreno, R.; Takala, J.; Suter, P.M.; Sprung, C.L.; Colardyn, F.; Blecher, S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit. Care Med. 1998, 26, 1793–1800. [Google Scholar] [CrossRef]
- Rubio-Rivas, M.; Mora-Lujan, J.M.; Formiga, F.; Arevalo-Canas, C.; Lebron Ramos, J.M.; Villalba Garcia, M.V.; Fonseca Aizpuru, E.M.; Diez-Manglano, J.; Arnalich Fernandez, F.; Romero Cabrera, J.L.; et al. WHO Ordinal Scale and Inflammation Risk Categories in COVID-19. Comparative Study of the Severity Scales. J. Gen. Intern. Med. 2022, 37, 1980–1987. [Google Scholar] [CrossRef]
- Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; Elmahi, E.; et al. Recovery Collaborative Group. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Catanzaro, M.; Fagiani, F.; Racchi, M.; Corsini, E.; Govoni, S.; Lanni, C. Immune response in COVID-19: Addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal Transduct. Target. Ther. 2020, 5, 84. [Google Scholar] [CrossRef]
- Chandel, A.; Leazer, S.; Alcover, K.C.; Farley, J.; Berk, J.; Jayne, C.; McNutt, R.; Olsen, M.; Allard, R.; Yang, J.; et al. Intensive Care and Organ Support Related Mortality in Patients With COVID-19: A Systematic Review and Meta-Analysis. Crit. Care Explor. 2023, 5, e0876. [Google Scholar] [CrossRef]
- Bos, L.D.J.; Ware, L.B. Acute respiratory distress syndrome: Causes, pathophysiology, and phenotypes. Lancet 2022, 400, 1145–1156. [Google Scholar] [CrossRef]
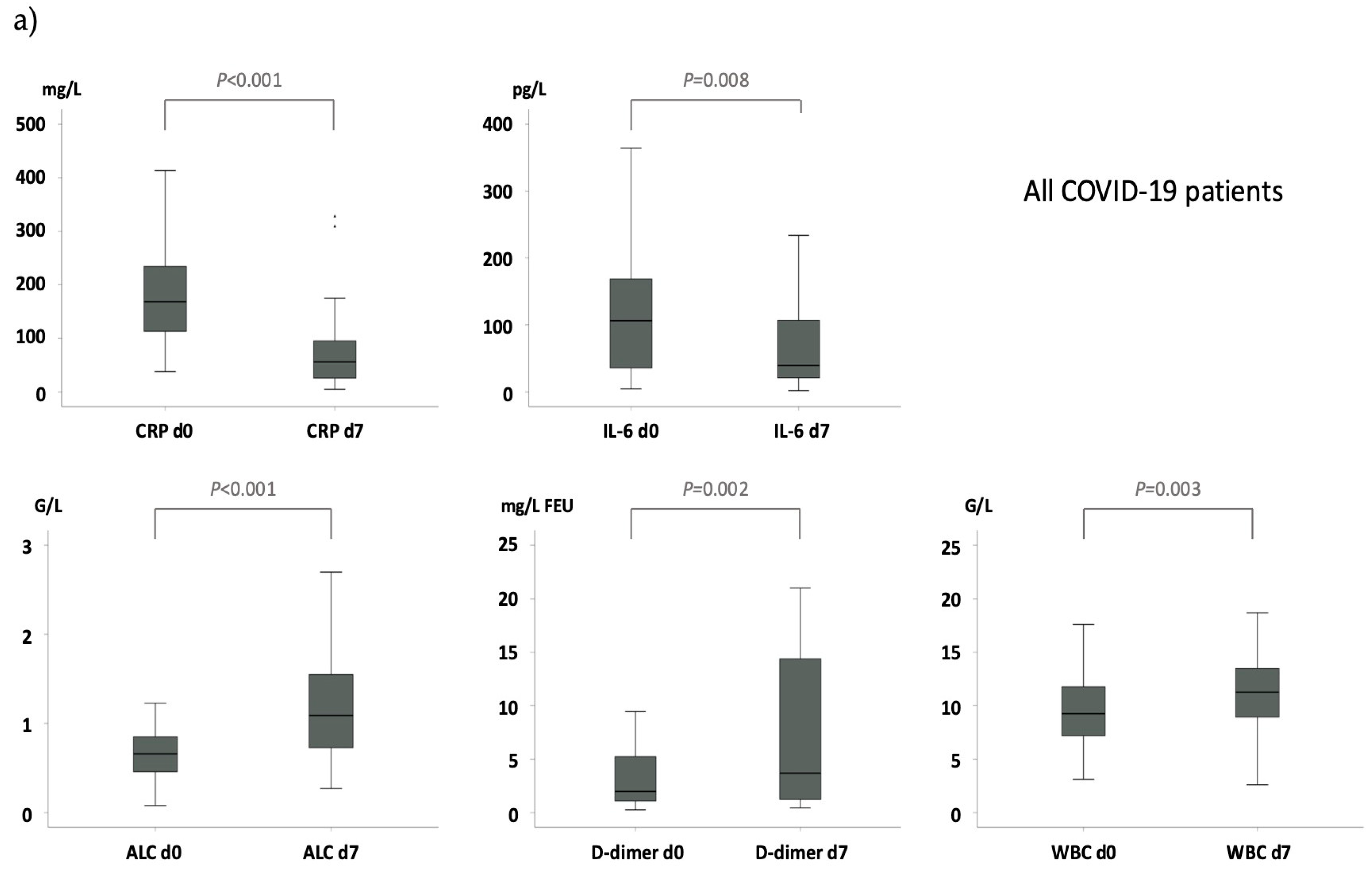
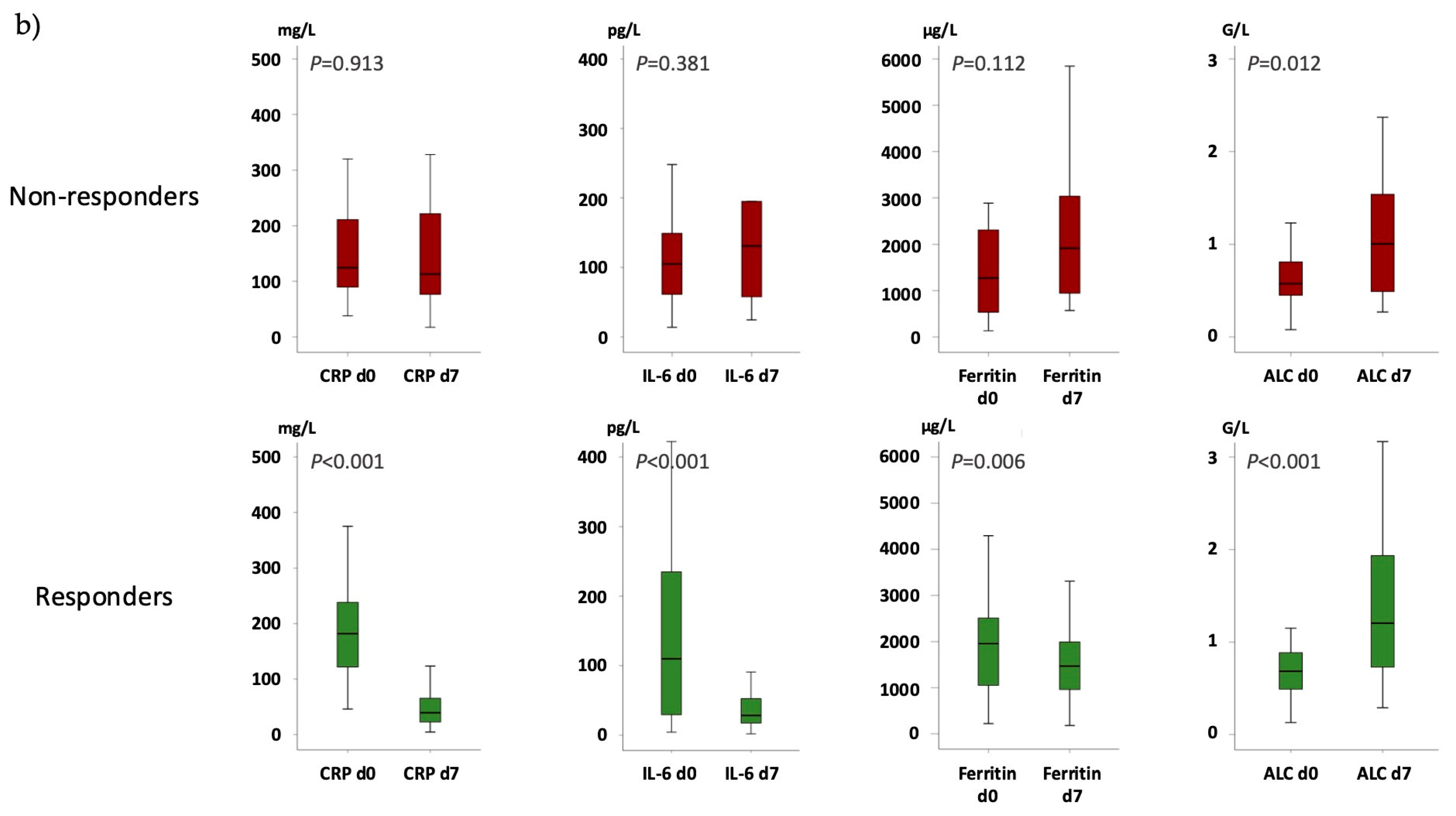
 indicates no difference in the delta (Δ) from day 7 to day 0.
indicates no difference in the delta (Δ) from day 7 to day 0.
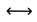 indicates no difference in the delta (Δ) from day 7 to day 0.
indicates no difference in the delta (Δ) from day 7 to day 0.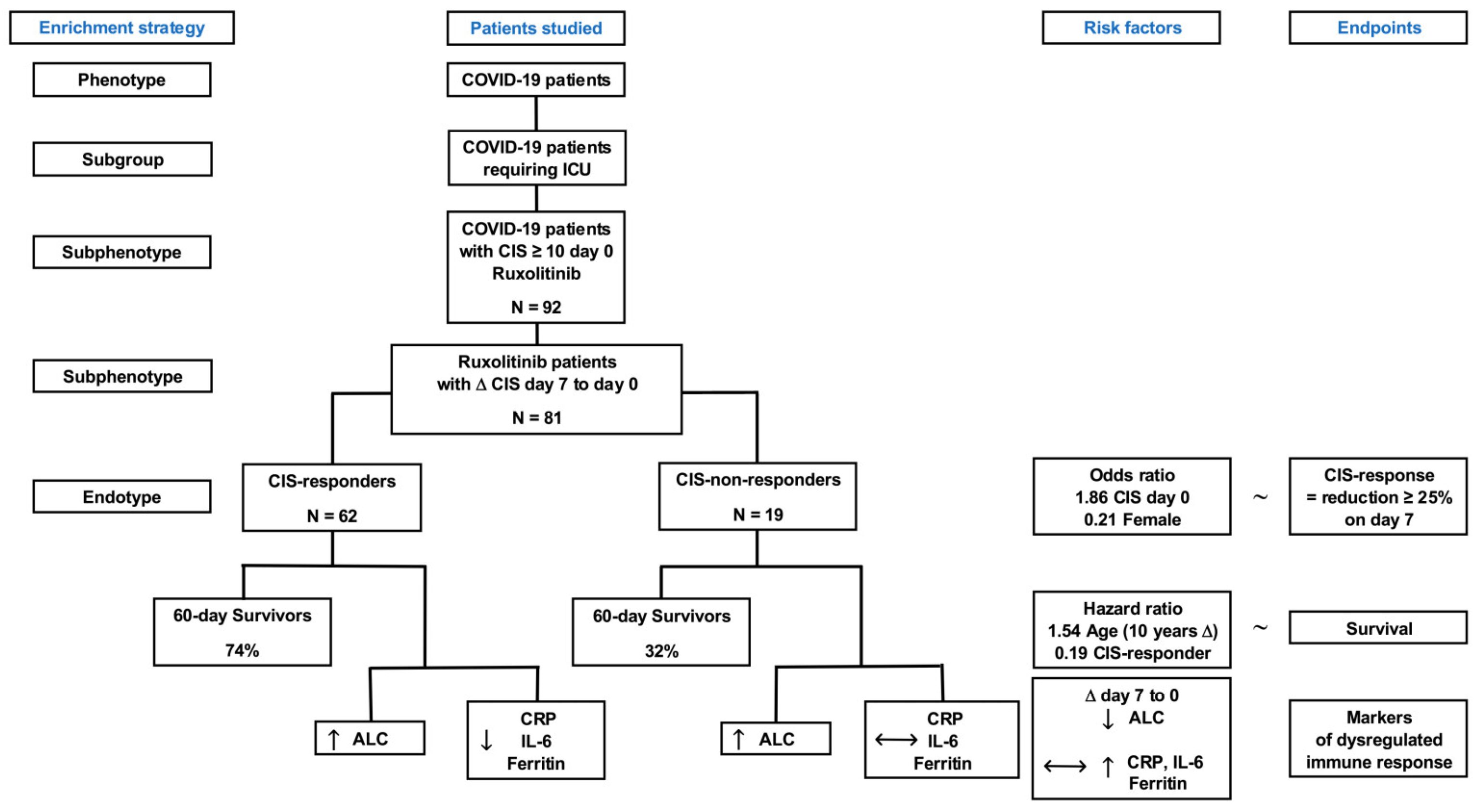
| Patient Characteristics | Cases n (%) | Mean (SD) | Median (Min–Max) |
|---|---|---|---|
| Total | 92 (100) | ||
| Age (years) | 92 | 58.3 (±12.2) | 58 (25–85) |
| Gender | 92 | ||
| Female | 22 (23.9) | ||
| Male | 70 (76.1) | ||
| BMI (kg/m2) | 89 | 33.2 (±14.0) | 29 (20–52) |
| Blood group | 92 | ||
| O | 25 (27.8) | ||
| B | 11 (12.2) | ||
| AB | 8 (8.9) | ||
| A | 46 (51.1) | ||
| n. a. | 2 (2.2) | ||
| SARS-CoV-2 | 92 | ||
| ct-value | 90 | 27.0 (±6.3) | 27 (15–40) |
| Days of symptoms | 85 | 9.9 (±5.2) | 10 (1–24) |
| CIS on day 0 | 92 | 12.0 (±1.7) | 12 (10–16) |
| Severity of disease | 92 | ||
| SAPSII | 92 | 34.4 (±9.0) | 32 (15–63) |
| SOFA | 91 | 5.4 (±2.2) | 5 (2–12) |
| WHO scale | 92 | 6.1 (±1.0) | 6 (5–7) |
| Comorbidities * | 92 | ||
| Arterial hypertension | 49 (53.3) | ||
| Cardiovascular disease | 32 (34.8) | ||
| Diabetes mellitus | 23 (25.0) | ||
| Hyperlipoproteinemia | 13 (14.1) | ||
| COPD/Asthma bronchiale | 18 (19.6) | ||
| Metabolic syndrome | 9 (9.8) | ||
| Arthritis | 4 (4.3) | ||
| Treatments * | 92 | ||
| Corticosteroids | 91 (98.9) | ||
| Remdesivir | 48 (52.2) | ||
| Corticosteroids and remdesivir | 47 (51.1) | ||
| Catecholamines | 62 (67.4) | ||
| Renal replacement | 8 (8.7) | ||
| ECMO | 23 (25.0) | ||
| Maximum ruxolitinib dose | 92 | ||
| 10 mg | 40 (43.5) | ||
| 15 mg | 24 (26.1) | ||
| 20 mg | 28 (30.4) | ||
| ICU length of stay (days) | 92 | 18.7 (±14.2) | 15 (2–73) |
| Complications * | 92 | ||
| Bacterial superinfection | 65 (70.7) | ||
| Bacteremia | 41 (44.6) | ||
| Viral superinfection/reactivation | 6 (6.5) | ||
| Fungal superinfection | 33 (35.9) | ||
| Fungemia | 3 (3.3) | ||
| Thrombosis/PAE | 13 (14.1) | ||
| Bleedings | 30 (32.6) |
| (A) CIS Response ~ | Variable | Reference Group | Odds Ratio | 95% CI | p-Value |
|---|---|---|---|---|---|
| Age (10 y difference) | continuous | 0.88 | [0.56–1.38] | 0.567 | |
| Blood group A | categorial | All other | 1.73 | [0.61–4.91] | 0.302 |
| BMI (kg/m2) | continuous | 0.99 | [0.96–1.02] | 0.415 | |
| CIS d0 | continuous | 1.86 | [1.21–2.84] | 0.005 | |
| ct-value | continuous | 1.04 | [0.96–1.14] | 0.346 | |
| Gender female | categorial | male | 0.21 | [0.07–0.66] | 0.007 |
| SAPSII | continuous | 0.99 | [0.94–1.06] | 0.814 | |
| SOFA | continuous | 0.91 | [0.72–1.15] | 0.435 | |
| Days symptom onset | continuous | 0.96 | [0.86–1.06] | 0.387 | |
| WHO scale 5 | categorial | 6 and 7 | 1.01 | [0.35–2.93] | 0.984 |
| (B) CIS Response ~ | Variable | Reference Group | Odds Ratio | 95% CI | p-Value |
| Age (10 y difference) | continuous | 0.66 | [0.38–1.14] | 0.134 | |
| Blood group A | categorial | All other | 2.65 | [0.70–10.02] | 0.151 |
| BMI (kg/m2) | continuous | 0.98 | [0.92–1.05] | 0.524 | |
| CIS d0 | continuous | 1.56 | [1.01–2.41] | 0.046 | |
| ct-value | continuous | 1.03 | [0.93–1.15] | 0.576 | |
| Gender female | categorial | male | 0.21 | [0.06–0.82] | 0.024 |
| SAPSII | continuous | 0.99 | [0.90–1.10] | 0.886 | |
| SOFA | continuous | 0.83 | [0.58–1.20] | 0.315 | |
| Days symptom onset | continuous | 1.00 | [0.86–1.16] | 0.975 | |
| WHO scale 5 | categorial | 6 and 7 | 0.56 | [0.12–2.67] | 0.467 |
| (C) Survival (d60) ~ | Variable | Reference Group | Hazard Ratio | 95% CI | p-Value |
| Age (10 y difference) | continuous | 1.76 | [1.29–2.40] | <0.001 | |
| Blood group A | categorial | All other | 0.83 | [0.42–1.64] | 0.593 |
| BMI (kg/m2) | continuous | 0.99 | [0.96–1.02] | 0.502 | |
| CIS d0 | continuous | 0.95 | [0.77–1.17] | 0.613 | |
| CIS response | categorial | 0.24 | [0.11–0.50] | <0.001 | |
| ct-value | continuous | 1.01 | [0.96–1.07] | 0.631 | |
| Gender female | categorial | male | 1.50 | [0.70–3.23] | 0.301 |
| SAPSII | continuous | 1.06 | [1.03–1.10] | <0.001 | |
| SOFA | continuous | 1.07 | [0.92–1.23] | 0.394 | |
| Days symptom onset | continuous | 1.02 | [0.95–1.09] | 0.561 | |
| WHO scale 5 | categorial | 6 and 7 | 0.81 | [0.41–1.64] | 0.562 |
| (D) Survival (d60) ~ | Variable | Reference Group | Hazard Ratio | 95% CI | p-Value |
| Age (10 y difference) | continuous | 1.54 | [1.10–2.17] | 0.012 | |
| Blood group A | categorial | All other | 0.80 | [0.34–1.88] | 0.605 |
| BMI (kg/m2) | continuous | 0.99 | [0.95–1.02] | 0.439 | |
| CIS d0 | continuous | 0.96 | [0.72–1.26] | 0.752 | |
| CIS response | categorial | 0.19 | [0.08–0.45] | <0.001 | |
| ct-value | continuous | 1.06 | [1.00–1.12] | 0.071 | |
| Gender female | categorial | male | 0.80 | [0.27–2.37] | 0.684 |
| SAPSII | continuous | 1.03 | [0.98–1.09] | 0.280 | |
| SOFA | continuous | 1.01 | [0.79–1.29] | 0.936 | |
| Days symptom onset | continuous | 1.03 | [0.94–1.12] | 0.577 | |
| WHO scale 5 | categorial | 6 and 7 | 0.68 | [0.29–1.58] | 0.370 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weiss, M.; Hammersen, J.; Rudolphi, S.; Formann, I.; Träger, K.; Rücker, F.G.; Grüner, B.; Allgöwer, A.; Birndt, S.; Fabisch, C.; et al. Prognostic Impact of COVID-19 Inflammation Score Response: A Sub-Group Analysis on Critically Ill Patients of the RuxCoFlam Trial. Life 2025, 15, 781. https://doi.org/10.3390/life15050781
Weiss M, Hammersen J, Rudolphi S, Formann I, Träger K, Rücker FG, Grüner B, Allgöwer A, Birndt S, Fabisch C, et al. Prognostic Impact of COVID-19 Inflammation Score Response: A Sub-Group Analysis on Critically Ill Patients of the RuxCoFlam Trial. Life. 2025; 15(5):781. https://doi.org/10.3390/life15050781
Chicago/Turabian StyleWeiss, Manfred, Jakob Hammersen, Sebastian Rudolphi, Isabell Formann, Karl Träger, Frank G. Rücker, Beate Grüner, Andreas Allgöwer, Sebastian Birndt, Christian Fabisch, and et al. 2025. "Prognostic Impact of COVID-19 Inflammation Score Response: A Sub-Group Analysis on Critically Ill Patients of the RuxCoFlam Trial" Life 15, no. 5: 781. https://doi.org/10.3390/life15050781
APA StyleWeiss, M., Hammersen, J., Rudolphi, S., Formann, I., Träger, K., Rücker, F. G., Grüner, B., Allgöwer, A., Birndt, S., Fabisch, C., Hochhaus, A., Döhner, K., Rosée, P. L., & Stegelmann, F. (2025). Prognostic Impact of COVID-19 Inflammation Score Response: A Sub-Group Analysis on Critically Ill Patients of the RuxCoFlam Trial. Life, 15(5), 781. https://doi.org/10.3390/life15050781







