Ultrafiltration and Fluid Excretion in Echinoids Involves the Axial Organ with Elimination via the Intestine
Abstract
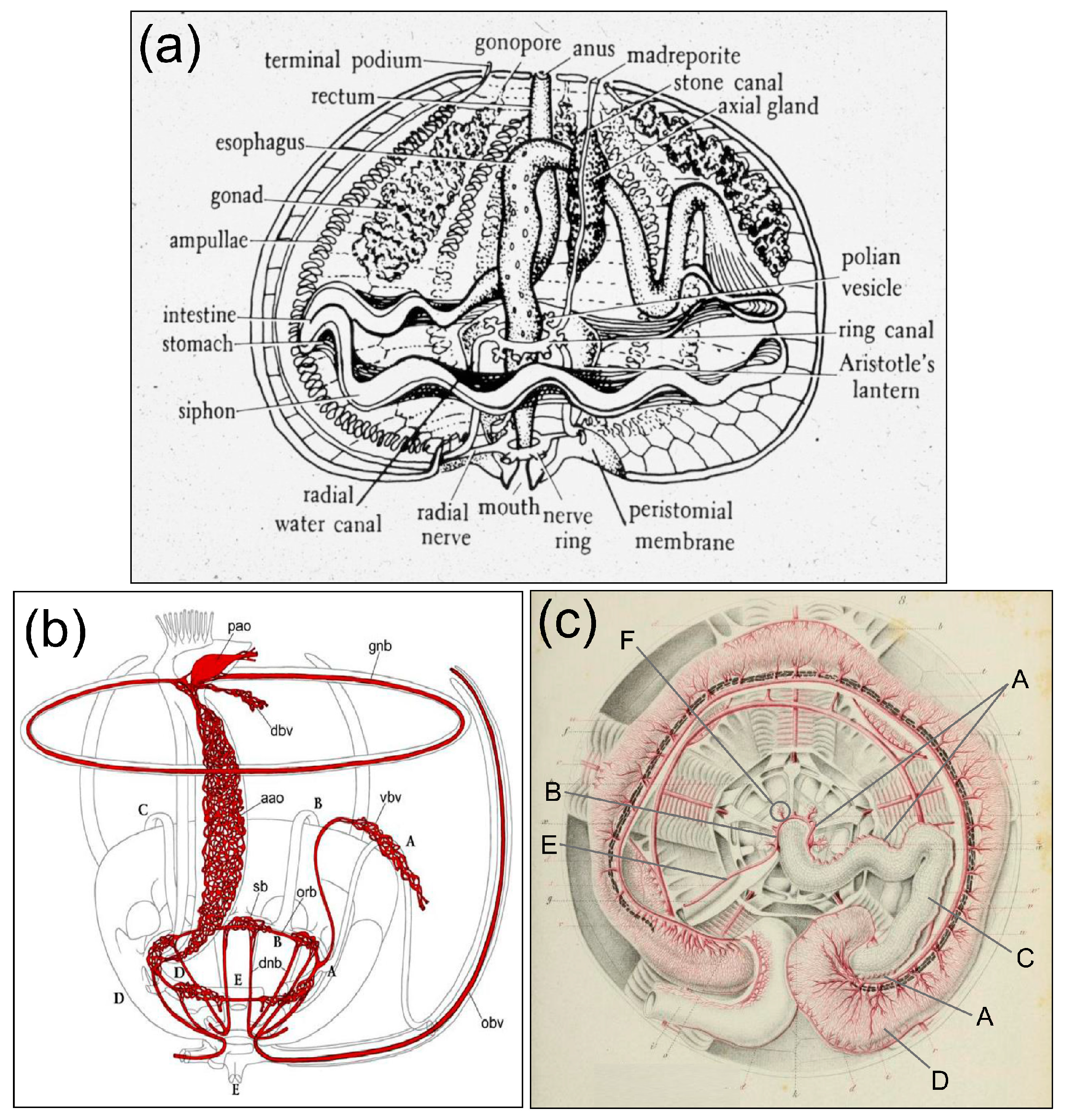
1. Introduction
2. Materials and Methods
2.1. Transcript Analyses
2.2. Sea Urchin Acquisition and Care
2.3. Fluorescein Injection
3. Results
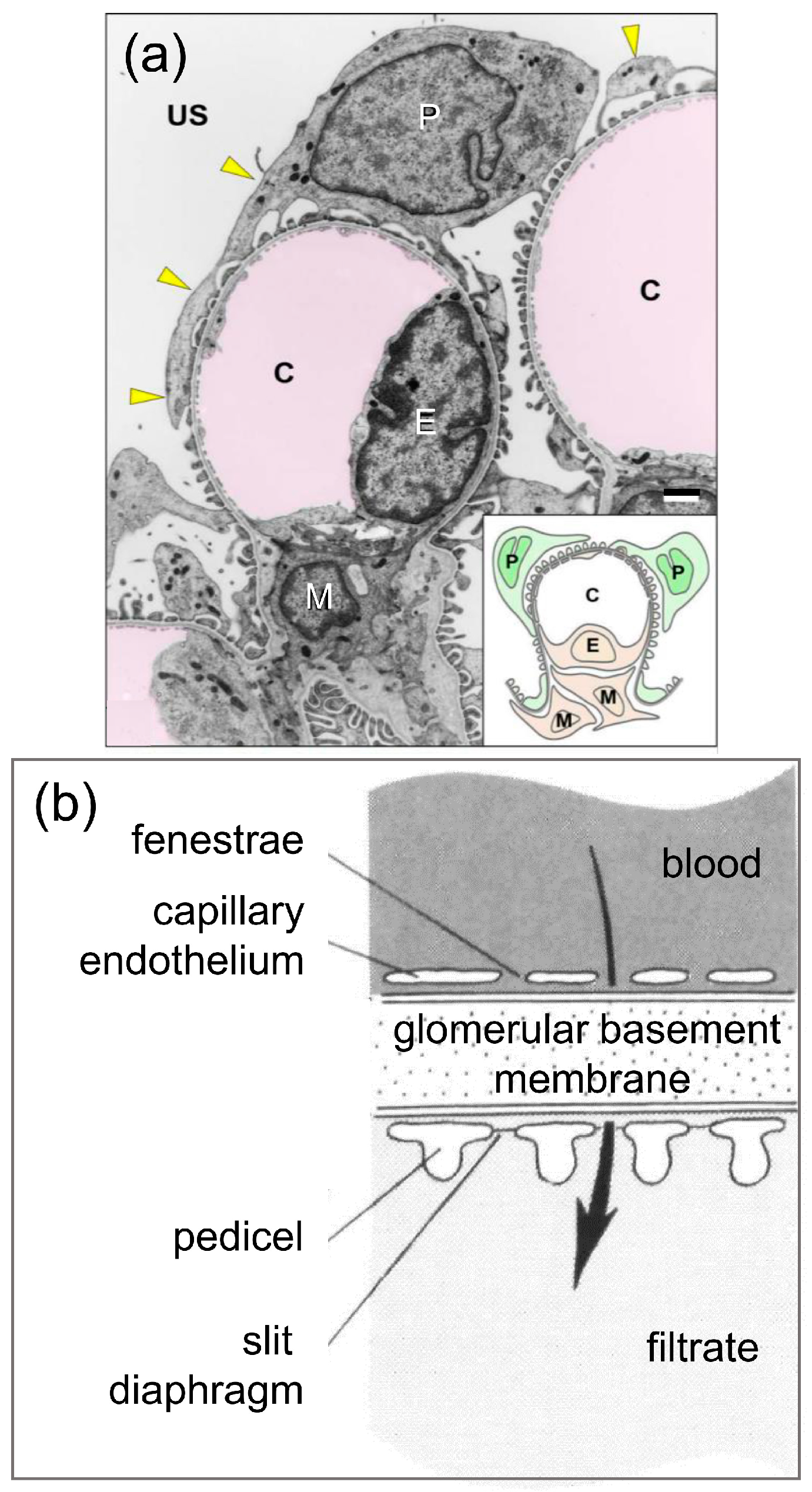
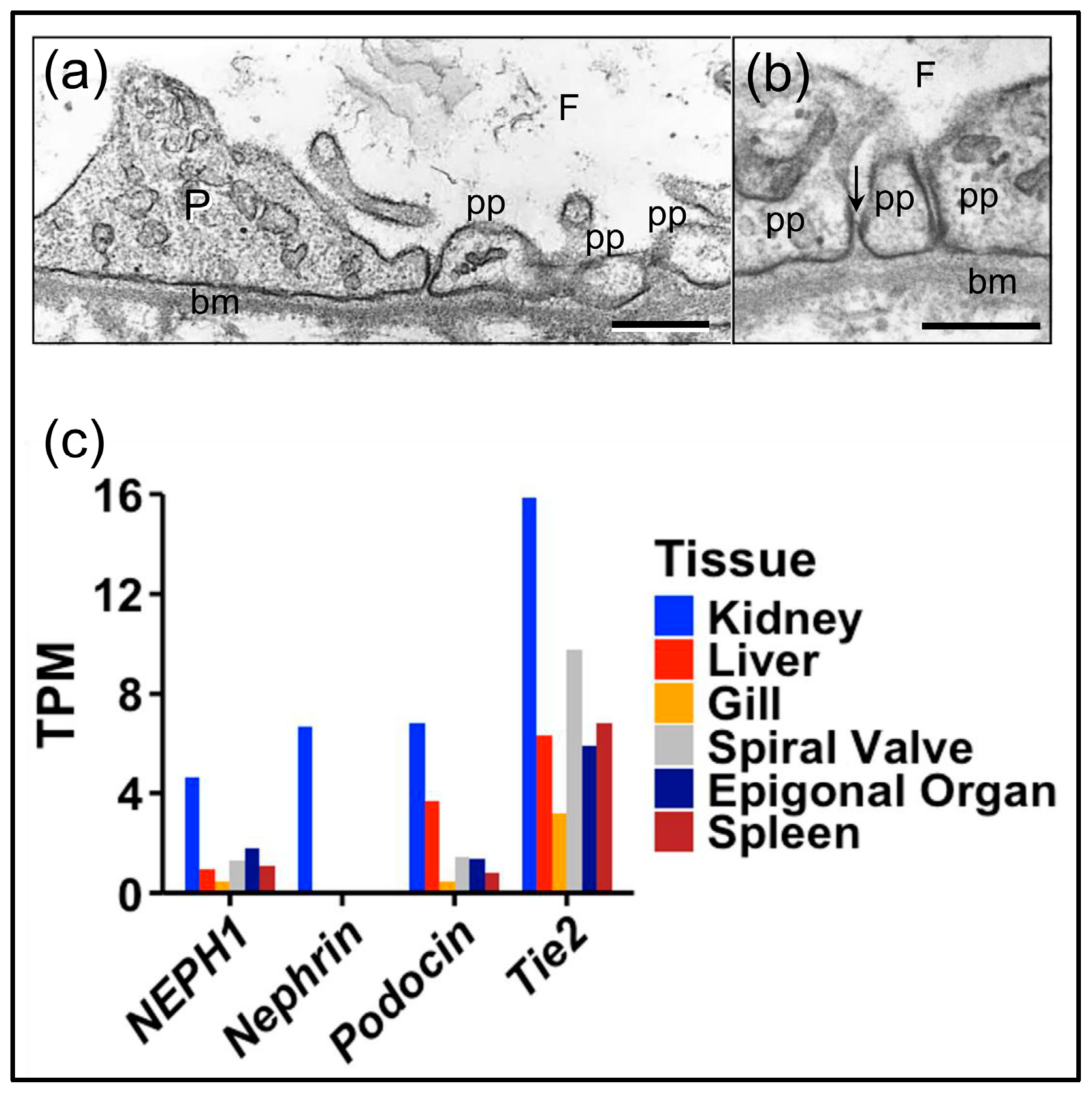
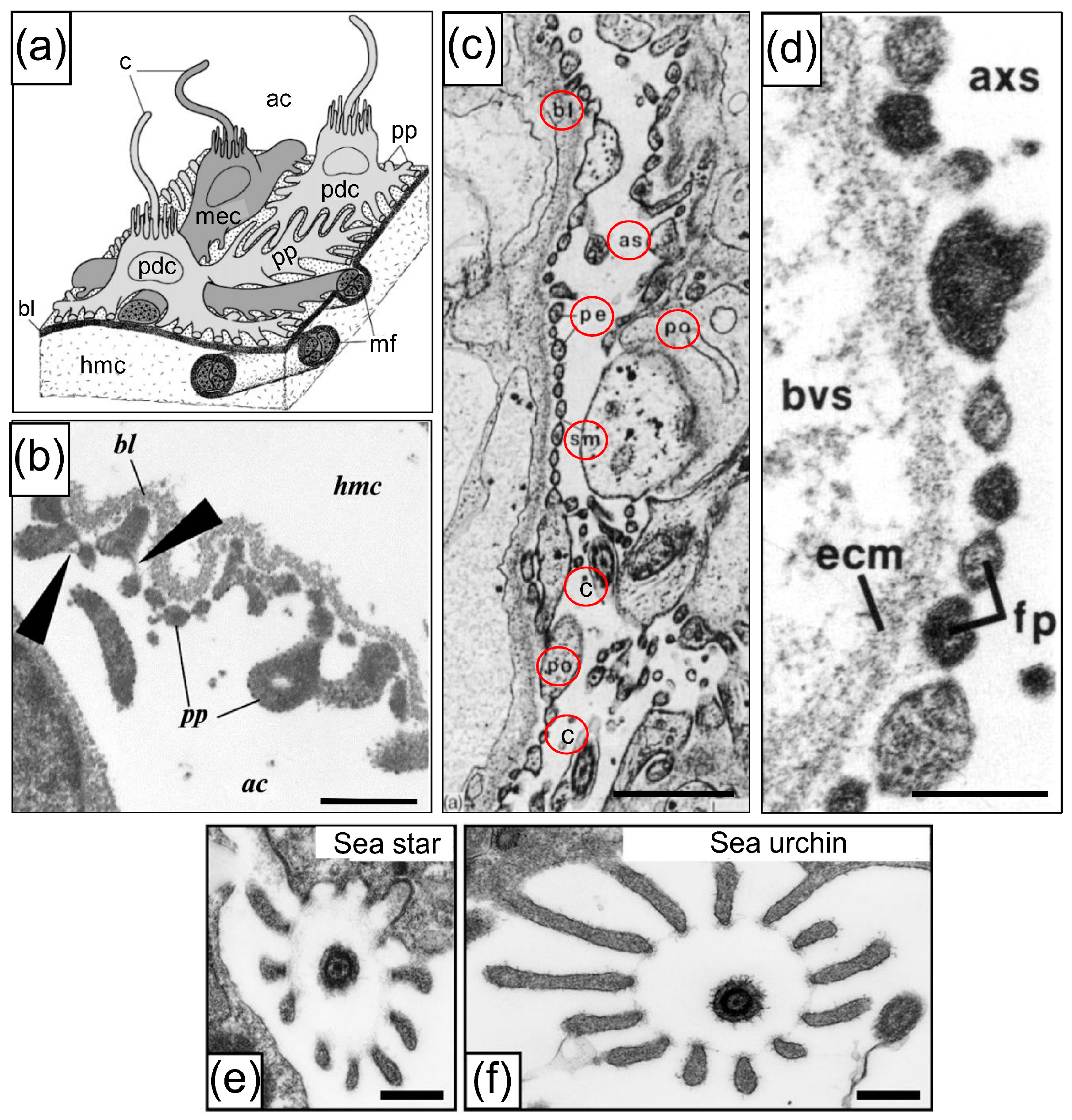
3.1. Podocyte-Specific Genes Are Expressed in the Axial Organ of Sea Urchins in Agreement with Expression in the Glomeruli of Sharks
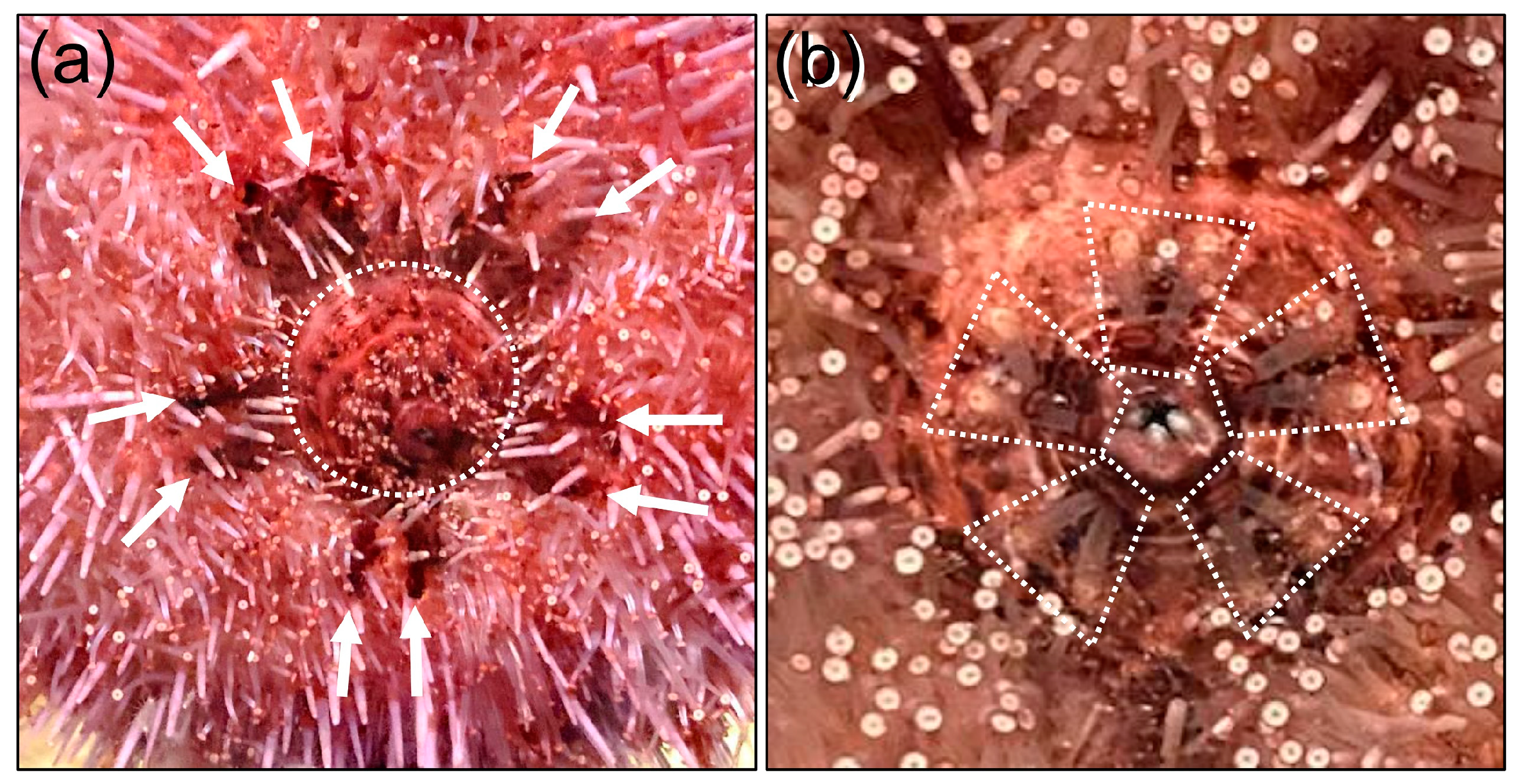
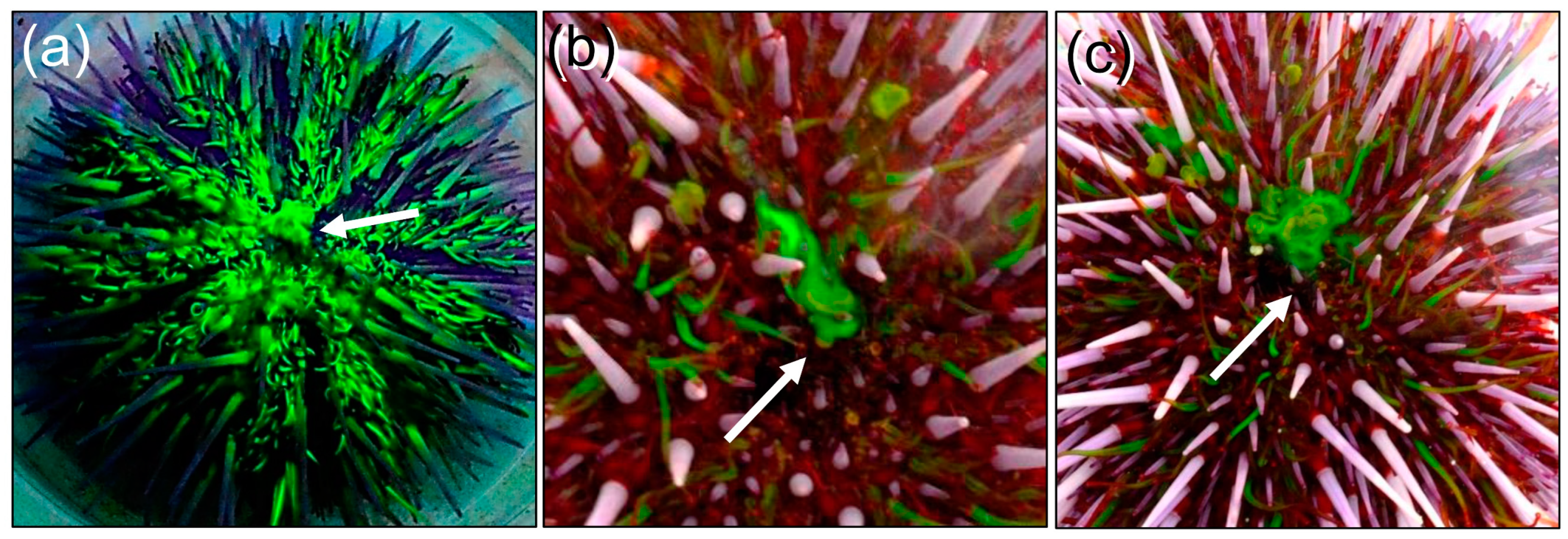
3.2. Fluorescein Excretion in S. purpuratus Occurs Through the Intestine and Anus Rather than the Madreporite or Gills
4. Discussion
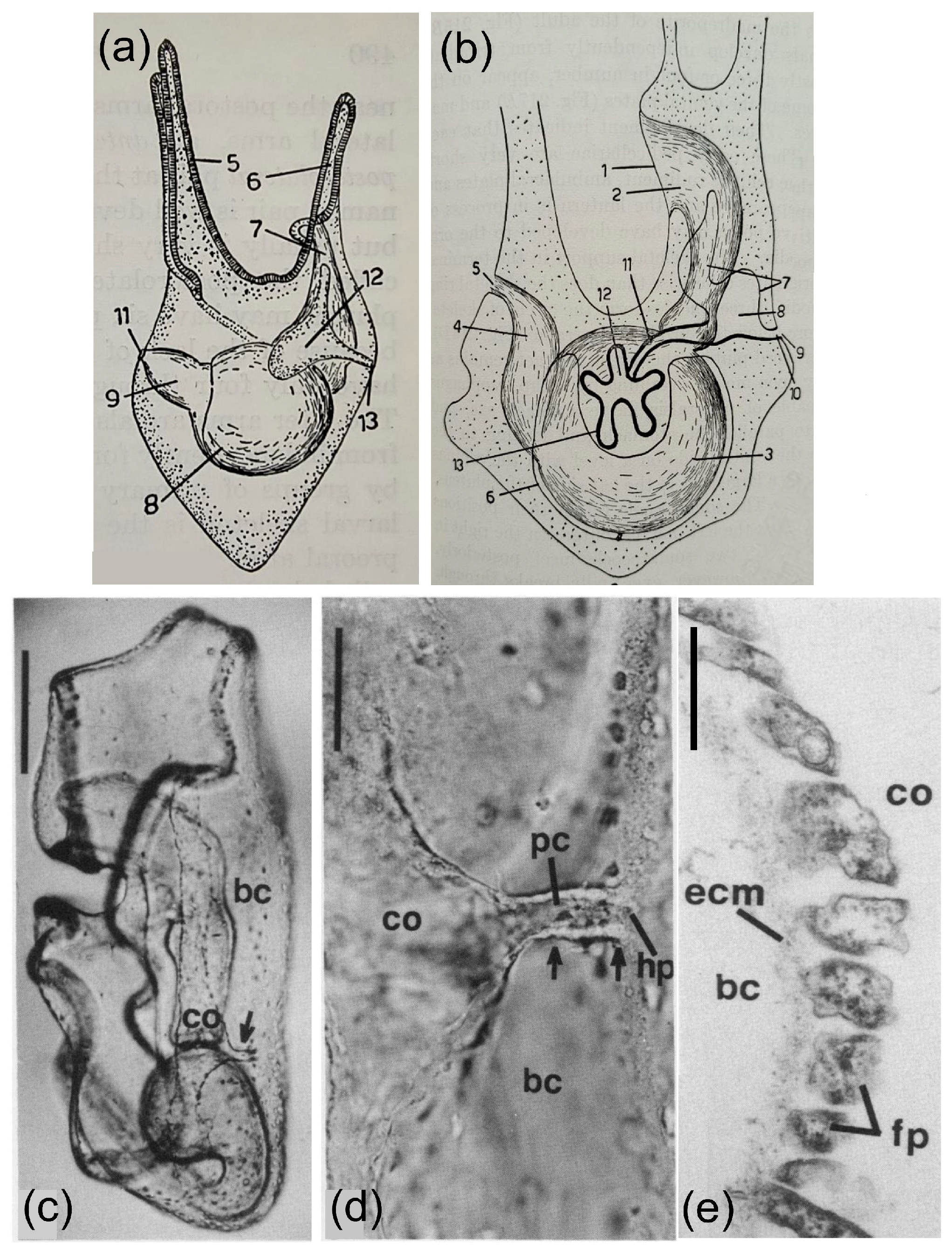
The Haemal System as the Connection Between the Coelomic Fluid, the Water Vascular System, the Axial Organ, and the Intestine
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GBM | Glomerular basement membrane |
| TPM | Transcript per million |
| mpi | Minutes post-injection |
| hpi | Hours post-injections |
| NEPH1 | Nephrin/podocin-interacting protein 1 |
| TEM | Transmission electron micrograph |
| UV | Ultraviolet |
References
- Scott, R.P.; Quaggin, S.E. The cell biology of renal filtration. J. Cell Biol. 2015, 2092, 199–210. [Google Scholar] [CrossRef]
- Garg, R. A review of podocyte biology. Am. J. Nephrol. 2018, 47 (Suppl. 1), 3–13. [Google Scholar] [CrossRef] [PubMed]
- Lacy, E.R.; Reale, E. Urinary System, Chapter 14. In Sharks, Skates, and Rays: The Biology of Elasmobranch Fishes; Hamlett, W.C., Ed.; The Johns Hopkins University Press: Baltimore, MD, USA, 1987; pp. 353–397. ISBN 978-0801860485. [Google Scholar]
- Ichimura, K.; Sakai, T. Evolutionary morphology of podocytes and primary urine-producing apparatus. Anat. Sci. Int. 2017, 92, 161–172. [Google Scholar] [CrossRef]
- Lacy, E.R.; Castellucci, M.; Reale, E. The elasmobranch renal corpuscle: Fine structure of Bowman’s capsule and the glomerular capillary wall. Anat. Rec. 1987, 218, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Holland, L.Z.; Holland, N.D. The invertebrate chordate amphioxus gives clues to vertebrate origins. Curr. Top. Dev. Biol. 2022, 147, 563–594. [Google Scholar] [CrossRef] [PubMed]
- Ruppert, E.E. Evolutionary origin of the vertebrate nephron. Am. Zool. 1994, 34, 542–553. [Google Scholar] [CrossRef]
- Cannon, J.T.; Vellutini, B.C.; Smith, J.; Ronquist, F.; Jondelius, U.; Hejnol, A. Xenacoelomorpha is the sister group to nephrozoa. Nature 2016, 530, 89–93. [Google Scholar] [CrossRef]
- Hejnol, A.; Obst, M.; Stamatakis, A.; Off, M.; Rouse, G.W.; Edgecombe, G.D.; Martinez, P.; Baguñà, J.; Bailly, X.; Jondelius, U.; et al. Assessing the root of bilaterian animals with scalable phylogenomic methods. Proc. R. Soc. B 2009, 276, 4261–4270. [Google Scholar] [CrossRef]
- Gąsiorowski, L.; Andrikou, C.; Janssen, R.; Bump, P.; Budd, G.E.; Lowe, C.J.; Hejnol, A. Molecular evidence for a single origin of ultrafiltration-based excretory organs. Curr. Biol. 2021, 31, 3629–3638. [Google Scholar] [CrossRef]
- Dow, J.A.T.; Simons, M.; Romero, M.F. Drosophila melanogaster: A simple genetic model of kidney structure, function and disease. Nat. Rev. Nephrol. 2022, 18, 417. [Google Scholar] [CrossRef]
- Andrikou, C.; Thiel, D.; Ruiz-Santiesteban, J.A.; Hejnol, A. Active mode of excretion across digestive tissues predates the origin of excretory organs. PLoS Biol. 2019, 17, e3000408. [Google Scholar] [CrossRef] [PubMed]
- Bartholomaeus, T.; Ax, P. Protonephridia and metanephridia—Their relation within the bilateria. J. Zool. Syst. Evol. Res. 1992, 30, 21–45. [Google Scholar] [CrossRef]
- Ruppert, E.E.; Smith, P.R. The functional organization of filtration nephridia. Biol. Rev. 1988, 63, 231–258. [Google Scholar] [CrossRef]
- Ezhova, O.V.; Egorova, E.A.; Malakhov, V.V. Ultrastructural evidence of the excretory function in the asteroid axial organ (Asteroidea, Echinodermata). Dokl. Biol. Sci. 2016, 468, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Ezhova, O.V.; Malakhov, V.V. The axial complex of echinoderms represents the kidney and is homologous to the hemichordate heart-kidney. Paleontol. J. 2021, 55, 1029–1038. [Google Scholar] [CrossRef]
- Grantham, J.J.; Wallace, D.P. Return of the secretory kidney. Am. J. Physiol.-Ren. Physiol. 2002, 282, F1–F9. [Google Scholar] [CrossRef]
- Bartolomaeus, T.; von Döhren, J. Comparative morphology and evolution of the nephridia in nemertea. J. Nat. Hist. 2010, 44, 37–40. [Google Scholar] [CrossRef]
- Bartolomaeus, T.; Maslakova, S.; von Döhren, J. Protonephridia in the larvae of the paleonemertean species Carinoma mutabilis (Carinomidae, Nemertea) and Cephalothrix (Procephalothrix) filiformis (Cephalothricidae, Nemertea). Zoomorphology 2014, 133, 43–57. [Google Scholar] [CrossRef]
- Baeumler, N.; Haszprunar, G.; Ruthensteiner, B. Development of the excretory system in a polyplacophoran mollusc: Stages in metanephridial system development. Front. Zool. 2012, 9, 23. [Google Scholar] [CrossRef]
- Kieneke, A.; Ahlrichs, W.H.; Martínez Arbizu, P.; Bartolomaeus, T. Ultrastructure of protonephridia in Xenotrichula carolinensis syltensis and Chaetonotus maximus (Gastrotricha: Chaetonotida): Comparative evaluation of the gastrotrich excretory organs. Zoomorphology 2008, 127, 1–20. [Google Scholar] [CrossRef]
- Hessler, R.R.; Elofsson, R. Segmental podocytic excretory glands in the thorax of Hutchinsoniella macracantha (Cephalocarida). J. Crustac. Biol. 1995, 15, 61–69. [Google Scholar] [CrossRef]
- Smith, P.R.; Ruppert, E.E.; Gardiner, S.L. A deuterostome-like nephridium in the mitraria larva of Owenia fusiformis (Polychaeta, Annelida). Biol. Bull. 1987, 172, 315–323. [Google Scholar] [CrossRef]
- Welsch, U.; Rehkämper, G. Podocytes in the axial organ of echinoderms. J. Zool. 1987, 213, 45–50. [Google Scholar] [CrossRef]
- Ruppert, E.E.; Balser, E.J. Nephridia in the larvae of hemichordates and echinoderms. Biol. Bull. 1986, 171, 188–196. [Google Scholar] [CrossRef]
- Stach, T.; Eisler, K. The ontogeny of the nephridial system of the larval amphioxus (Branchiostoma lanceolatum). Acta Zool. 1998, 79, 113–118. [Google Scholar] [CrossRef]
- Ruppert, E.E. Morphology of Hatschek’s nephridium in larval and juvenile stages of Branchiostoma virginiae (Cephalochordata). Isr. J. Zool. 1996, 42, S161–S182. [Google Scholar]
- Nakao, T. The excretory organ of amphioxus (Branchiostoma) belcheri. J. Ultrastruct. Res. 1965, 12, 1–12. [Google Scholar] [CrossRef]
- Lacalli, T.C. Tunicate tails, stolons, and the origin of the vertebrate trunk. Biol. Rev. 1999, 74, 177–198. [Google Scholar] [CrossRef]
- Abdul Jaffar Ali, H.; Tamilselvi, M.; Akram, A.S.; Kaleem Arshan, M.L.; Sivakumar, V. Comparative study on bioremediation of heavy metals by solitary ascidian, Phallusia nigra, between Thoothukudi and Vizhinjam ports of India. Ecotoxicol. Environ. Saf. 2015, 121, 93–99. [Google Scholar] [CrossRef]
- Markus, J.A.; Lambert, C.C. Urea and ammonia excretion by solitary ascidians. J. Exp. Mar. Biol. Ecol. 1983, 66, 1–10. [Google Scholar] [CrossRef]
- Goodbody, I. Nitrogen excretion in Ascidiacea: II. Storage excretion and the uricolytic enzyme system. J. Exp. Biol. 1965, 42, 299–305. [Google Scholar] [CrossRef]
- Das, S.M. The physiology of excretion in Molgula (Tunicata, Ascidiacea). Biol. Bull. 1948, 95, 307–319. [Google Scholar] [CrossRef]
- Heron, A.C. A new type of excretory mechanism in the tunicates. Mar. Biol. 1976, 36, 191–197. [Google Scholar] [CrossRef]
- Balser, E.J.; Ruppert, E.E.; Jaeckle, W.B. Ultrastructure of the coeloms of auricularia larvae (Holothuroidea: Echinodermata): Evidence for the presence of an axocoel. Biol. Bull. 1993, 185, 86–96. [Google Scholar] [CrossRef]
- Hara, Y.; Kuraishi, R.; Uemura, I.; Katow, H. Asymmetric formation and possible function of the primary pore canal in plutei of Temnopleurus hardwick. Dev. Growth Differ. 2003, 45, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.-J.; Su, Y.-H. Opposing nodal and BMP signals regulate left–right asymmetry in the sea urchin larva. PLoS Biol. 2012, 10, e1001402. [Google Scholar] [CrossRef] [PubMed]
- Bury, H. Studies on the embryology of the echinoderms. Q. J. Microsc. Sci. 1889, 29, 409–450. [Google Scholar] [CrossRef]
- MacBride, E.W. The development of Echinus esculentus, together with some points in the development of E. miliaris and E. acuntus. Philos. Trans. R. Soc. B 1903, 195, 285–327. [Google Scholar] [CrossRef][Green Version]
- Hyman, L.H. The Invertebrates: Echinodermata, The Coelomate Bilateria; McGraw-Hill Book Co., Inc.: New York, NY, USA, 1955; Volume IV, 763p, ISBN 978-0070316638. [Google Scholar]
- Valencia, J.E.; Feuda, R.; Mellott, D.O.; Burke, R.D.; Peter, I.S. Ciliary photoreceptors in sea urchin larvae indicate pan-deuterostome cell type conservation. MBC Biol. 2021, 19, 257. [Google Scholar] [CrossRef]
- Gemmill, J.F. The development and certain points in the adult structure of the starfish Asterias rubens, L. Philos. Trans. R. Soc. Lond. B 1914, 105, 213–294. [Google Scholar]
- Ferguson, J.C. Cell production in the Tiedemann bodies and haemal organs of the starfish, Asterias forbesi. Trans. Am. Microsc. Soc. 1966, 85, 2000–2209. [Google Scholar] [CrossRef]
- Bachmann, S.; Goldschmid, A. Fine structure of the axial complex of Sphaerechinus granularis (Lam.) (Echinodermata: Echinoidea). Cell Tissue Res. 1978, 193, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Boolootian, R.; Campbell, J. A primitive heart in the echinoid Strongylocentrotus purpuratus. Science 1964, 145, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Farmanfarmaian, A. The controversial echinoid heart and hemal system--function effectiveness in respiratory exchange. Comp. Biochem. Physiol. 1986, 24, 855–863. [Google Scholar] [CrossRef]
- Millot, N.; Vevers, H.G. The morphology and histochemistry of the echinoid axial organ. Philos. Trans. R. Soc. B 1968, 253, 201–230. [Google Scholar] [CrossRef]
- Millott, N. Injury and the axial organ of echinoids. Cell. Mol. Life Sci. 1969, 25, 756. [Google Scholar] [CrossRef]
- Golconda, P.; Buckley, K.M.; Reynolds, C.; Romanello, J.; Smith, L.C. The axial organ and the pharynx are sites of hematopoiesis in the sea urchin. Front. Immunol. 2019, 10, 870. [Google Scholar] [CrossRef]
- Bachmann, S.; Goldschmid, A. The echinoid axial complex and Tiedemann bodies The different pathways and accumulation sites of coelomocytes with regard to waste disposal in the organism. In Echinderms: Present and Past; Jangoux, M., Ed.; AA Balkema: Rotterdam, The Netherlands, 1980; pp. 254–257. ISBN 9061910773. [Google Scholar]
- Ziegler, A.; Faber, C.; Bartolomaeus, T. Comparative morphology of the axial complex and interdependence of internal organ systems in sea urchins (Echinodermata: Echinoidea). Front. Zool. 2009, 6, 10. [Google Scholar] [CrossRef]
- Ziegler, A.; Faber, C.; Mueller, S.; Bartolomaeus, T. Systematic comparison and reconstruction of sea urchin (Echinoidea) internal anatomy: A novel approach using magnetic resonance imaging. BMC Biol. 2008, 6, 33. [Google Scholar] [CrossRef]
- Ezhova, O.V.; Malakhov, V.V.; Egorova, E.A. Axial complex and associated structures of the sea urchin Strongylocentrotus pallidus (Sars, G.O. 1871) (Echinodermata: Echinoidea). J. Morphol. 2018, 279, 792–808. [Google Scholar] [CrossRef]
- Petrunkevitch, A. Morphology of Invertebrate Types; The Macmillan Company: Boston, MA, USA, 1916; ISBN 13: 978-1164899778. [Google Scholar]
- Perrier, M.E. Recherches sur l’appareil circulatoroir des oursins. Arch. Zool. Exp. Gén. 1875, 4, 605–643. [Google Scholar]
- Balser, E.J.; Ruppert, E.E. Ultrastructure of axial vascular and coelomic organs in comasterid featherstars (Echinoderma: Crinoidea). Acta Zool. 1993, 74, 87–101. [Google Scholar] [CrossRef]
- Bargmann, W.; von Hehn, G. On the axial organ (“mysterious gland”) of Asterias rubens L. Z. Zellforsch. Mikrosk. Anat. 1968, 88, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Webber, W.A.; Lee, J. Fine structure of mammalian renal cilia. Anat. Rec. 1975, 182, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.C. Seawater inflow through the madreporite and internal body regions of a starfish (Leptasterias hexactis) as demonstrated with fluorescent microbeads. J. Exp. Zool. 1990, 255, 262–271. [Google Scholar] [CrossRef]
- Tu, Q.; Cameron, R.A.; Worley, K.C.; Gibbs, R.A.; Davidson, E.H. Gene structure in the sea urchin Strongylocentrotus purpuratus based on transcriptome analysis. Genome Res. 2012, 22, 2079–2087. [Google Scholar] [CrossRef] [PubMed]
- Bakke, F.K.; Gundappa, M.K.; Matz, H.; Stead, D.A.; Macqueen, D.J.; Dooley, H. Exploration of the nurse shark (Ginglymostoma cirratum) plasma immunoproteome using high-resolution LC-MS/MS. Front. Immunol. 2022, 13, 873390. [Google Scholar] [CrossRef]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef]
- O’goshi, K.-I.; Serup, J. Review: Safety of sodium fluorescein for in vivo study of skin. Ski. Res. Technol. 2006, 12, 155–161. [Google Scholar] [CrossRef]
- Kuroda, D.; Kinouchi, H.; Kanemaru, K.; Nishiyama, Y.; Ogiwara, M.; Yoshioda, H.; Horikoshi, T. Intra-arterial injection fluorescein videoangiography in aneurysm surgery. Neurosurgery 2013, 72 (Suppl. 2), ons141–ons150. [Google Scholar] [CrossRef]
- Elliot, L.F.; Russell, M.P.; Hernandez, J.C. Estimating echinoid test volume from height and diameter measurements. In Echinoderms in a Changing World, Proceedings of the 13th International Echinoderm Conference, Hobart Tasmania, Australia, 5–9 January 2009; Johnson, C., Ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 105–112. ISBN 9781138000100. [Google Scholar]
- Terwilliger, D.P.; Buckley, K.M.; Brockton, V.; Ritter, N.J.; Smith, L.C. Distinctive expression patterns of 185/333 genes in the purple sea urchin, Strongylocentrotus purpuratus: An unexpectedly diverse family of transcripts in response to LPS, beta-1,3-glucan, and dsRNA. BMC Mol. Biol. 2007, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Sellin, L.; Huber, T.B.; Gerke, P.; Quack, I.; Pavenstädt, H.; Walz, G. NEPH1 defines a novel family of podocin-interacting proteins. FASEB J. 2003, 17, 115–117. [Google Scholar] [CrossRef]
- He, L.; Sun, Y.; Patrakka, J.; Mostad, P.; Norlin, J.; Xiao, Z.; Andrae, J.; Tryggvason, K.; Samuelsson, T.; Betsholtz, C.; et al. Glomerulus-specific mRNA transcripts and proteins identified through kidney expressed sequence tag database analysis. Kidney Int. 2007, 71, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Kocylowski, M.K.; Aypek, H.; Bildl, W.; Helmstädter, M.; Trachte, P.; Dumoulin, B.; Wittösch, S.; Kühne, L.; Aukschun, U.; Teetzen, C.; et al. A slit-diaphragm-associated protein network for dynamic control of renal filtration. Nat. Commun. 2022, 13, 6446. [Google Scholar] [CrossRef]
- Kawachi, H.; Fukusumi, Y. New insight into podocyte slit diaphragm, a therapeutic target of proteinuria. Clin. Exp. Nephrol. 2020, 24, 193–204. [Google Scholar] [CrossRef]
- Ruotsalainen, V.; Ljungberg, P.; Wartiovaara, J.; Lenkkeri, U.; Kestila, M.; Jalanko, H.; Holmberg, C.; Tryggvason, K. Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc. Natl. Acad. Sci. USA 1999, 96, 7962–7967. [Google Scholar] [CrossRef] [PubMed]
- Kestilä, M.; Lenkkeri, U.; Männikkö, M.; Lamerdin, J.; McCready, P.; Putaala, H.; Ruotsalainen, V.; Morita, T.; Nissinen, M.; Herva, R.; et al. Positionally cloned gene for a novel glomerular protein--nephrin--is mutated in congenital nephrotic syndrome. Mol. Cell 1998, 1, 575–582. [Google Scholar] [CrossRef]
- Holzman, L.B.; St John, P.L.; Kovari, I.A.; Verma, R.; Holthofer, H.; Abrahamson, D.R. Nephrin localizes to the slit pore of the glomerular epithelial cell. Kidney Int. 1999, 56, 1481–1491. [Google Scholar] [CrossRef]
- Roselli, S.; Boute, N.; Sich, M.; Benessy, F.; Attie, T.; Gubler, M.C.; Antignac, C. Podocin localizes in the kidney to the slit diphragm area. Am. J. Pathol. 2002, 160, 131–139. [Google Scholar] [CrossRef]
- Xu, P.X.; Zheng, W.; Huang, L.; Maire, P.; Laclef, C.; Silvius, D. Six1 is required for the early organogenesis of mammalian kidney. Development 2003, 130, 3085–3094. [Google Scholar] [CrossRef]
- Kobayashi, A.; Valerius, M.T.; Mugford, J.W.; Carroll, T.J.; Self, M.; Oliver, G.; McMahon, A.P. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 2008, 3, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Andrikou, C.; Gąsiorowski, L.; Hejnol, A. Cell types, morphology, and evolution of animal excretory organs. In Origin and Evolution of Metazoan Cell Types; Leys, S.P., Hejnol, A., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 128–164. [Google Scholar] [CrossRef]
- Kolatsi-Joannou, M.; Li, X.Z.; Suda, T.; Yuan, H.T.; Woolf, A.S. Expression and potential role of angiopoietins and Tie-2 in early development of the mouse metanephros. Dev. Dyn. 2001, 2222, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.T.; Suri, C.; Yancopoulos, G.D.; Woolf, A.S. Expression of angiopoietin-1, angiopoietin-2, and the Tie-2 receptor tyrosine kinase during mouse kidney. J. Am. Soc. Nephrol. 1999, 10, 1722–1736. [Google Scholar] [CrossRef]
- Smith, L.C.; Chang, L.; Britten, R.J.; Davidson, E.H. Sea urchin genes expressed in activated coelomocytes are identified by expressed sequence tags. Complement homologues and other putative immune response genes suggest immune system homology within the deuterostomes. J. Immunol. 1996, 156, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.E.; Dhillon, J.; Miller, C.A.; Messier-Solek, C.; Rast, J.P.; Majeske, A.J.; Zuelke, D.; Rast, J.P.; Smith, L.C. SpTie1/2 from the sea urchin Strongylocentrotus purpuratus, is an orthologue of vertebrate Tie1 and Tie2 and is expressed in coelomocytes, axial organ and embryos. Dev. Comp. Immunol. 2010, 34, 884–895. [Google Scholar] [CrossRef] [PubMed]
- Hibino, T.; Loza-Coll, M.; Messier, C.; Majeske, A.J.; Cohen, A.; Terwilliger, D.P.; Buckley, K.M.; Brockton, V.; Nair, S.V.; Berney, K.; et al. The immune gene repertoire encoded in the purple sea urchin genome. Dev. Biol. 2006, 300, 349–365. [Google Scholar] [CrossRef]
- Telmer, C.A.; Karimi, K.; Chess, M.M.; Agalakov, S.; Arshinoff, B.I.; Lotay, V.; Wang, D.Z.; Chu, S.; Pells, T.J.; Vize, P.D.; et al. Echinobase: A resource to support the echinoderm research community. Genetics 2024, 227, iyae002. [Google Scholar] [CrossRef]
- Robertson, J.D. Ionic regulation in some marine invertebrates. J. Exp. Biol. 1949, 26, 182–200. [Google Scholar] [CrossRef]
- Bradley, T.J. Chapter 5, Osmoconformers. In Animal Osmoregulation; Oxford Animal Biology Series; Bradley, T.J., Ed.; Oxford University Press: Oxford, UK, 2008. [Google Scholar] [CrossRef]
- Hyodo, S.; Kakumura, K.; Takagi, W.; Hasegawa, K.; Yamaguchi, Y. Morphological and functional characteristics of the kidney of cartilaginous fishes: With special reference to urea reabsorption. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R1381–R1395. [Google Scholar] [CrossRef]
- Ezhova, O.V.; Lavrova, E.A.; Malakhov, V.V. Microscopic anatomy of the axial complex in the starfish Asterias rubens (Echinodermata, Asteroidea). Biol. Bull. 2013, 40, 643–653. [Google Scholar] [CrossRef]
- Ezhova, O.V.; Malakhov, V.V. Axial complex of Crinoidea: A comparison with other Ambulacraria. J. Morphol. 2020, 281, 1456–1475. [Google Scholar] [CrossRef]
- Morris, V.B.; Selvakumaraswamy, P.; Whan, R.; Byrne, M. Development of the five primary podia from the coeloms of a sea star larva: Homology with the echinoid echinoderms and other deuterostomes. Proc. R. Soc. B 2009, 276, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, T.; Suzuki, K.; Watanabe, Y. Intra-arterial fluorescence angiography with injection of fluorescein sodium from the superficial temporal artery during aneurysm surgery: Technical notes. Neurol. Med. Chir. 2014, 54, 490–496. [Google Scholar] [CrossRef]
- Keerl, R.; Weber, R.K.; Draf, W.; Wienke, A.; Schaefer, S.D. Use of sodium fluorescein solution for detection of cerebrospinal fluid fistulas: An analysis of 420 administrations and reported complications in Europe and the United States. Laryngoscope 2004, 114, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Cobb, J.L.S.; Sneddon, E. An ultra-structural study of the gills of Echinus esculentus. Cell Tissue Res. 1977, 182, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Hillier, B.J.; Vacquier, V.D. Amassin, an olfactomedin protein, mediates the massive intercellular adhesion of sea urchin coelomocytes. J. Cell Biol. 2003, 160, 597–604. [Google Scholar] [CrossRef]
- D’Andrea-Winslow, L.; Radke, D.W.; Utecht, T.; Kaneko, T.; Akasaka, D. Sea urchin coelomocyte arylsulfatase: A modulator of the echinoderm clotting pathway. Integr. Zool. 2012, 7, 61–73. [Google Scholar] [CrossRef]
- Tamori, M.; Matsuno, A.; Takahashi, K. Structure and function of the pore canals of the sea urchin madreporite. Philos. Trans. R. Soc. Lond. B. 1996, 351, 659–676. [Google Scholar]
- Ferguson, J.C. Translocative functions of the enigmatic organs of starfish—The axial organ, hemal vessels, Tiedemann’s bodies, and rectal caeca: An autoradiographic study. Biol. Bull. 1984, 166, 140–155. [Google Scholar] [CrossRef]
- Ferguson, J.C. Rate of water admission through the madreporite of a starfish. J. Exp. Biol. 1989, 148, 147–156. [Google Scholar] [CrossRef]
- Juhasz-Dora, T.; James, P.; Evensen, T.; Lindberg, S.-K. Hidden in plain sight: Hyperspectral documentation of complex biofluorescence produced by the green sea urchin (Strongylocentrotus droebachiensis). Methods Appl. Fluoresc. 2024, 12, 025002. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, S.; Pohla, H.; Goldschmid, A. Phagocytes in the axial complex of the sea urchin, Sphaerenchinus granularis (Lam.). Cell Tissue Res. 1980, 213, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Holland, N.D.; Ghiselin, M.T. Siphons and siphonal grooves in the digestive systems of the echinoidea (Echinodermata). Zoomorphology 2009, 127, 259–264. [Google Scholar] [CrossRef]
- Burton, M.P.M. Haemal system of regular echinoids. Nature 1964, 204, 1218. [Google Scholar] [CrossRef]
- Ou, Z.; Duh, Y.-S.; Rommelfanger, N.J.; Keck, C.H.C.; Jiang, S.; Brinson, K.; Zhao, S.; Schmidt, E.L.; Wu, X.; Yang, F.; et al. Achieving optical transparency in live animals with absorbing molecules. Science 2024, 385, eadm6869. [Google Scholar] [CrossRef]
- Clarke, D.N.; Formery, L.; Lowe, C.J. See-Star: A versatile hydrogel-based protocol for clearing large, opaque and calcified marine invertebrates. Evodevo 2024, 15, 8. [Google Scholar] [CrossRef]

| Vertebrate Protein | Function | Human Accession Number (GenBank, NCBI.gov) | Sea Urchin Protein Match 1 | Sea Urchin Gene Match, e Value | Genome Location; LOC Number 2 RNAseq; WHL22 Number 3 | Tissue of Elevated Gene Expression Post-Metamorphosis 3 |
|---|---|---|---|---|---|---|
| Human nephrin | slit diaphragm structure | AAG17141.1 | Nephrin | 8 × 10−122 | LOC592278 WHL22.293015.0 | Axial organ |
| Nephrin-like | Gene name search | LOC581043 WHL22.22857 | Axial organ, coelomocytes | |||
| Mouse NEPH1 | nephrin/podocin-interacting protein | AAN73043.1 | Kin of IRRE-like protein 4 | 1 × 10−80 | LOC100890311 WHL22.526524.1 | Axial organ elevated relative to other adult tissues |
| Human Tie2 | receptor tyrosine kinase, angio-poietin receptor | KAI4006839.1 | Tie1/2 5 | 6 × 10−109 | LOC110977718 or LOCtek WHL22.41577 | Coelomocytes and axial organ |
| Human SIX1 | Homeobox transcription factor | CAA62974.1 | Six1/2 6 | 2 × 10−130 | LOC110974175 WHL22.121485 | Axial organ, coelomocytes, testes, and radial nerve |
| Human SIX2 | Homeobox transcription factor | NP_058628.3 | Six1/2 6 | 2 × 10−121 |
| Vertebrate Protein | Query Genbank Accession Number | e Value of Top tBlastn Hit 1 |
|---|---|---|
| Mouse NEPH1 | AAN73043.1 | 0 |
| Human Nephrin | AAG17141.1 | 0 |
| Danio podocin | NP_001018155.2 | 8 × 10−61 |
| Human Tie2 | KAI4006839.1 | 0 |
| Human six1 | CAA62974.1 | Not found 2 |
| Human six2 | NP_058628.3 | Not found 2 |
| Sea Urchin Gene | Genome Location Number 1 | Transcript Number 2 | Elevated Gene Expression Post-Metamorphosis |
|---|---|---|---|
| Ammonium transporter 1 | LOC576563 | WHL22.591474 | Intestine |
| V-type H+-ATPase, type A | LOC585744 | WHL22.470441 | Intestine, axial organ coelomocytes, nerve, juvenile |
| Aquaporin 12A-like | LOC115918744 | WHL22.456167 | Intestine |
| Aquaporin | LOC105444576 | WHL22.273709 | Axial organ |
| Aquaporin 3 | LOC105442651 | WHL22.528654 | Axial organ, intestine, testes, nerve, juvenile |
| Aquaporin 8 | LOC589852 LOC762564 | WHL22.362925 WHL22.318239 | Intestine, axial organ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, L.C.; Hill, T.M. Ultrafiltration and Fluid Excretion in Echinoids Involves the Axial Organ with Elimination via the Intestine. Life 2025, 15, 767. https://doi.org/10.3390/life15050767
Smith LC, Hill TM. Ultrafiltration and Fluid Excretion in Echinoids Involves the Axial Organ with Elimination via the Intestine. Life. 2025; 15(5):767. https://doi.org/10.3390/life15050767
Chicago/Turabian StyleSmith, L. Courtney, and Thomas M. Hill. 2025. "Ultrafiltration and Fluid Excretion in Echinoids Involves the Axial Organ with Elimination via the Intestine" Life 15, no. 5: 767. https://doi.org/10.3390/life15050767
APA StyleSmith, L. C., & Hill, T. M. (2025). Ultrafiltration and Fluid Excretion in Echinoids Involves the Axial Organ with Elimination via the Intestine. Life, 15(5), 767. https://doi.org/10.3390/life15050767






