Diversity and Key Organisms in the Biocrust of a Tropical Granite-Gneiss Rocky Outcrop
Abstract
1. Introduction
2. Materials and Methods
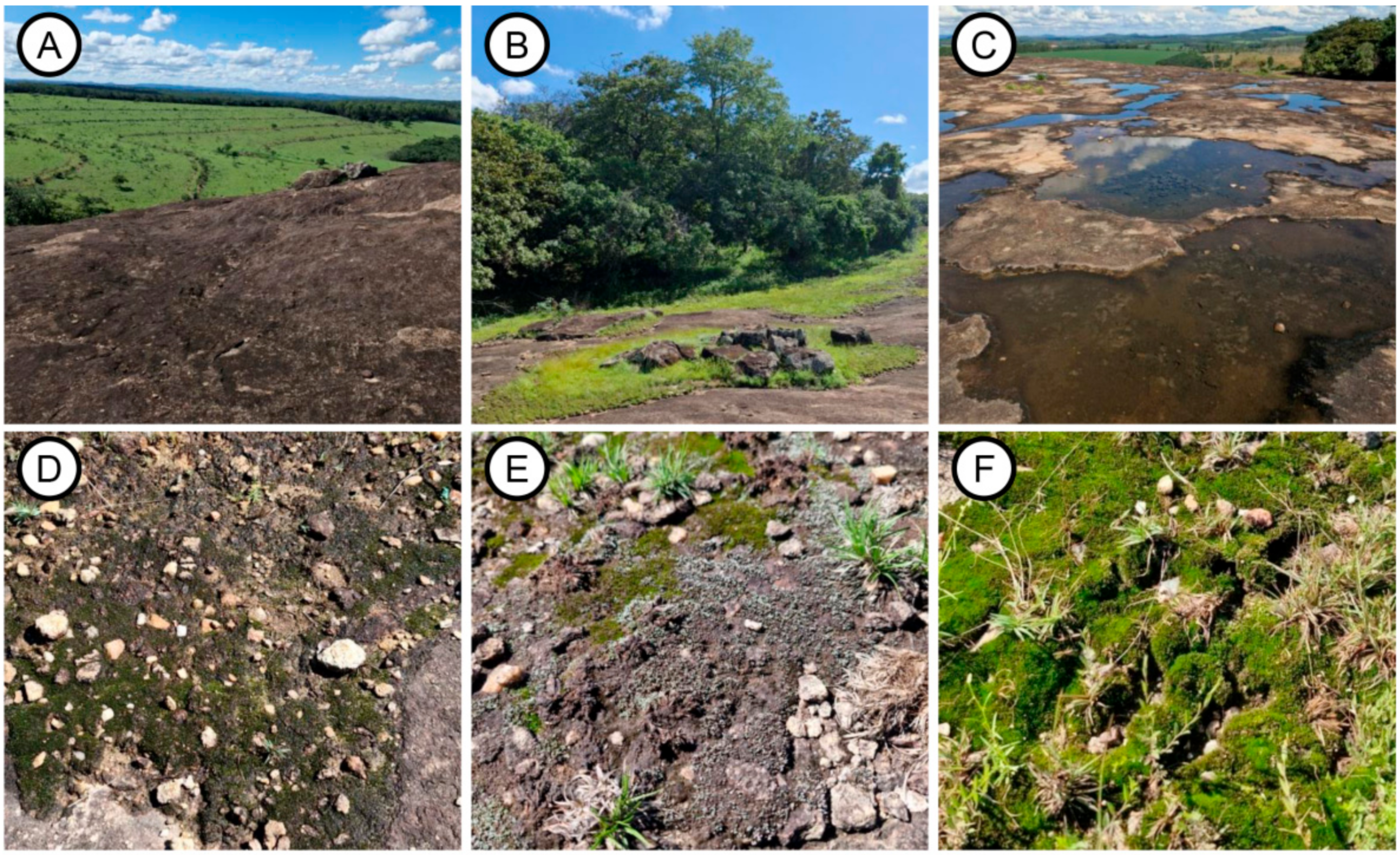
2.1. Study Area
2.2. Biocrust Survey
2.3. Statistical Analysis
3. Results
3.1. Biocrust Diversity in Granite-Gneiss Microhabitats
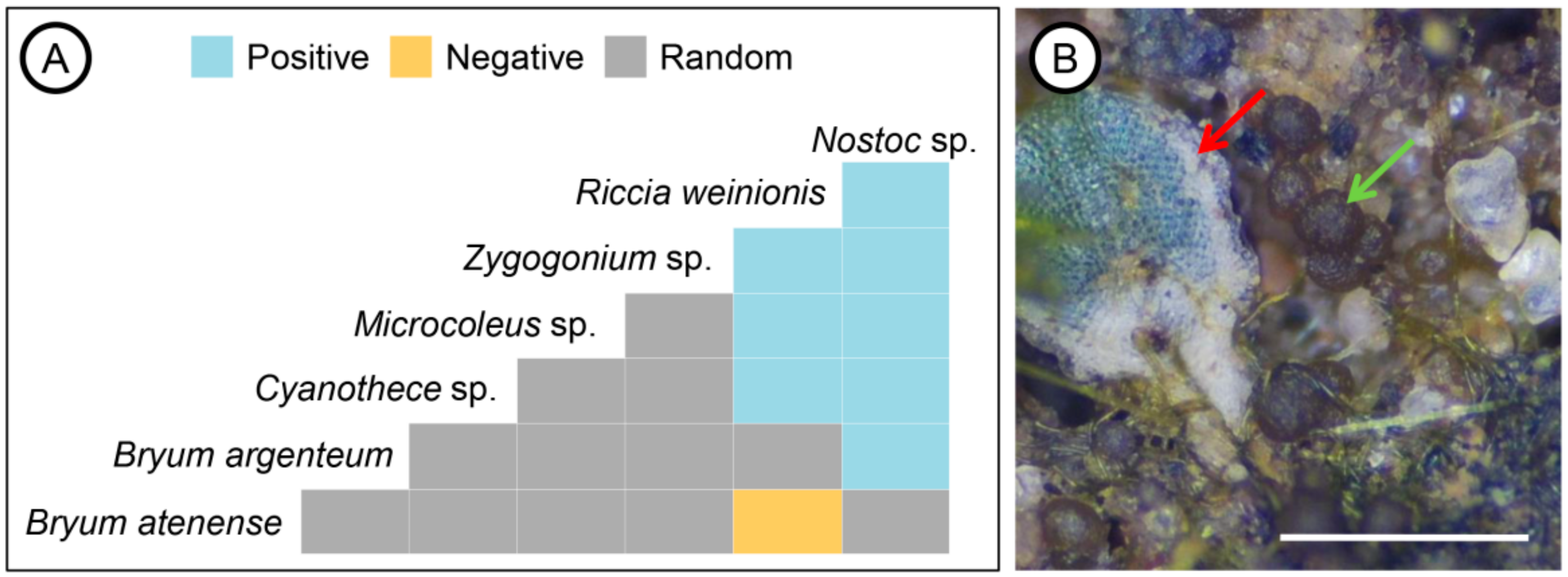
3.2. Co-Occurrence Interactions
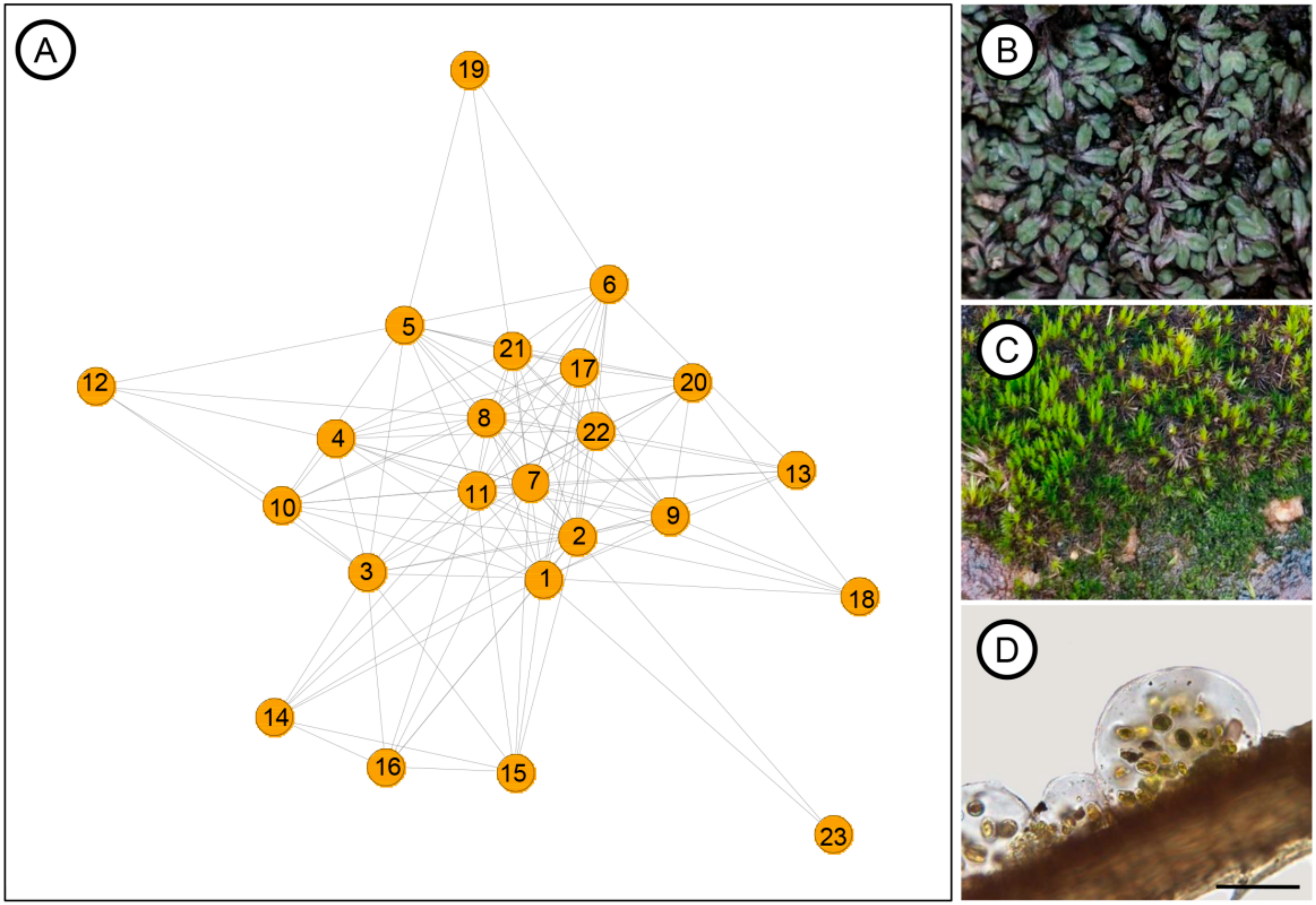
3.3. Community Network
4. Discussion
4.1. Biocrusts of Brazilian Rocky Outcrops: A Comparative Perspective
4.2. Insights into Biocrust Components’ Co-Occurrence
4.3. Lessons from Community Network Dynamics
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fitzsimons, J.A.; Michael, D.R. Rocky outcrops: A hard road in the conservation of critical habitats. Biol. Conserv. 2017, 211, 36–44. [Google Scholar] [CrossRef]
- Twidale, C.R. Granite Landforms; Elsevier: Amsterdam, The Netherlands, 2012; 372p. [Google Scholar]
- Larson, D.W.; Matthes, U.; Kelly, P.E. Cliff Ecology: Pattern and Process in Cliff Ecosystems; Cambridge University Press: Cambridge, UK, 2005; 360p. [Google Scholar]
- Twidale, C.R.; Romani, J.R.V. Landforms and Geology of Granite Terrains; CRC Press: London, UK, 2005; 362p. [Google Scholar]
- Migon, P. Granite Landscapes of the World; Oxford University Press: New York, NY, USA, 2006; 412p. [Google Scholar]
- Scarano, F.R. Rock outcrop vegetation in Brazil: A brief overview. Rev. Bras. Bot. 2007, 30, 561–568. [Google Scholar] [CrossRef]
- Oliveira, M.F.; Figueredo, C.C.; Konell, A.H.; Maciel-Silva, A.S. A first evaluation of biological soil crusts diversity in three distinctive rocky outcrops in Brazil. Flora 2024, 320, 152613. [Google Scholar] [CrossRef]
- Barthlott, W.; Porembski, S. Vascular Plants on Inselbergs: Systematic Overview. In Inselbergs: Biotic Diversity of Isolated Rock Outcrops in Tropical and Temperate Regions; Porembski, S., Barthlott, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 103–116. [Google Scholar]
- Jacobi, C.M.; Carmo, F.F.; Vincent, R.C.; Stehmann, J.R. Plant communities on ironstone outcrops: A diverse and endangered Brazilian ecosystem. Biodivers. Conserv. 2007, 16, 2185–2200. [Google Scholar] [CrossRef]
- Ferreira, V.L.; Stehmann, J.R. Saxicolous vascular flora of karst outcrops: An overlooked component of Brazilian biodiversity. Flora 2023, 305, 152314. [Google Scholar] [CrossRef]
- Medina, B.M.O.; Ribeiro, K.T.; Scarano, F.R. Plant-plant and plant-topography interactions on a rock outcrop at high altitude in southeastern Brazil. Biotropica 2006, 38, 27–34. [Google Scholar] [CrossRef]
- Jacobi, C.M.; Carmo, F.F. The contribution of ironstone outcrops to plant diversity in the Iron Quadrangle, a threatened Brazilian landscape. AMBIO 2008, 37, 324–326. [Google Scholar] [CrossRef]
- Porembski, S.; Becker, U.; Seine, R. Islands on Islands: Habitats on Inselbergs. In Inselbergs: Biotic Diversity of Isolated Rock Outcrops in Tropical and Temperate Regions; Porembski, S., Barthlott, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 49–67. [Google Scholar]
- Baskin, J.M.; Baskin, C.C. Endemism in rock outcrop plant communities of unglaciated eastern United States: An evaluation of the roles of the edaphic, genetic and light factors. J. Biogeogr. 1988, 15, 829–840. [Google Scholar] [CrossRef]
- Porembski, S.; Barthlott, W. Granitic and gneissic outcrops (inselbergs) as centers of diversity for desiccation-tolerant vascular plants. Plant Ecol. 2000, 151, 19–28. [Google Scholar] [CrossRef]
- Oliveira, R.S.; Abrahão, A.; Pereira, C.; Teodoro, G.S.; Brum, M.; Alcantara, S.; Lambers, H. Ecophysiology of Campos Rupestres Plants. In Ecology and Conservation of Mountaintop Grasslands in Brazil; Fernandes, G., Ed.; Springer: Cham, Switzerland, 2016; pp. 227–272. [Google Scholar]
- Carmo, F.F.; Jacobi, C.M. Diversity and plant trait-soil relationships among rock outcrops in the Brazilian Atlantic rainforest. Plant Soil 2016, 403, 7–20. [Google Scholar] [CrossRef]
- Colesie, C.; Gommeaux, M.; Green, T.G.A.; Büdel, B. Biological soil crusts in continental Antarctica: Garwood Valley, southern Victoria Land, and Diamond Hill, Darwin Mountains region. Antarct. Sci. 2014, 26, 115–123. [Google Scholar] [CrossRef]
- Chen, Y.; Lian, B.; Yin, Z.; Tang, Y. Weathering of carbonate rocks by biological soil crusts in karst areas. J. Earth Sci. 2014, 25, 662–667. [Google Scholar] [CrossRef]
- Hu, P.; Zhanga, W.; Xiao, L.; Yang, R.; Xiao, D.; Zhao, J.; Wang, W.; Chen, H.; Wang, K. Moss-dominated biological soil crusts modulate soil nitrogen following vegetation restoration in a subtropical karst region. Geoderma 2019, 352, 70–79. [Google Scholar] [CrossRef]
- Belnap, J.; Weber, B.; Büdel, B. Biological soil crusts as an organizing principle in drylands. In Biological Soil Crusts: An Organizing Principle in Drylands; Weber, B., Büdel, B., Belnap, J., Eds.; Springer: Cham, Switzerland, 2016; pp. 3–13. [Google Scholar]
- Weber, B.; Belnap, J.; Büdel, B.; Antoninka, A.J.; Barger, N.N.; Chaudhary, V.B.; Darrouzet-Nardi, A.; Eldridge, D.J.; Faist, A.M.; Ferrenberg, S.; et al. What is a biocrust? A refined, contemporary definition for a broadening research community. Biol. Rev. 2022, 97, 1768–1785. [Google Scholar] [CrossRef]
- Darby, B.J.; Neher, D.A. Microfauna within biological soil crusts. In Biological Soil Crusts: An Organizing Principle in Drylands; Weber, B., Büdel, B., Belnap, J., Eds.; Springer: Cham, Switzerland, 2016; pp. 139–157. [Google Scholar]
- Green, T.G.A.; Proctor, M.C.F. Physiology of photosynthetic organisms within biological soil crusts: Their adaptation, flexibility, and plasticity. In Biological Soil Crusts: An Organizing Principle in Drylands; Weber, B., Büdel, B., Belnap, J., Eds.; Springer: Cham, Switzerland, 2016; pp. 347–381. [Google Scholar]
- Dillon, J.G.; Castenholz, R.W. Scytonemin, a cyanobacterial sheath pigment, protects against UVC radiation: Implications for early photosynthetic life. J. Psychol. 1999, 35, 673–681. [Google Scholar] [CrossRef]
- Štepigová, J.; Vráblíková, H.; Lang, J.; Večeřová, K.; Barták, M. Glutathione and zeaxanthin formation during high light stress in foliose lichens. Plant Soil Environ. 2007, 53, 340–344. [Google Scholar] [CrossRef]
- Proctor, M.C.F. Structure and eco-physiological adaptation in bryophytes. In Bryophyte Systematics; Clarke, G.C.S., Duckett, J.G., Eds.; Academic Press: London, UK, 1979; pp. 479–509. [Google Scholar]
- Watson, W. Xerophytic adaptations of bryophytes in relation to habitat. New Phytol. 1914, 13, 149–169. [Google Scholar] [CrossRef]
- Glime, J.M. Water Relations: Plant Strategies. In Bryophyte Ecology; Glime, J.M., Ed.; Michigan Technological University and the International Association of Bryologists: Houghton, MI, USA, 2017; Volume 3, pp. 1–67. [Google Scholar]
- Vitt, D.H.; Crandall-Stotler, B.; Wood, A. Bryophytes: Survival in a dry world through tolerance and avoidance. In Plant Ecology and Evolution in Harsh Environments; Rajakaruna, N., Boyd, R.S., Harris, T.B., Eds.; Nova Science Pub Inc.: Hauppauge, NY, USA, 2014; pp. 267–295. [Google Scholar]
- Potts, M. Mechanisms of desiccation tolerance in cyanobacteria. Eur. J. Phycol. 1999, 34, 319–328. [Google Scholar] [CrossRef]
- Proctor, M.C.F.; Oliver, M.J.; Wood, A.J.; Alpert, P.; Stark, L.R.; Cleavitt, N.L.; Mishler, B.D. Desiccation-tolerance in bryophytes: A review. Bryologist 2007, 110, 595–621. [Google Scholar] [CrossRef]
- Kranner, I.; Beckett, R.; Hochman, A.; Nash, T.H., III. Desiccation-tolerance in lichens: A review. Bryologist 2008, 111, 576–593. [Google Scholar] [CrossRef]
- Sancho, L.G.; Belnap, J.; Colesie, C.; Raggio, J.; Weber, B. Carbon budgets of biological soil crusts at micro-, meso-, and global scales. In Biological Soil Crusts: An Organizing Principle in Drylands; Weber, B., Büdel, B., Belnap, J., Eds.; Springer: Cham, Switzerland, 2016; pp. 287–304. [Google Scholar]
- Barger, N.N.; Weber, B.; Garcia-Pichel, F.; Zaady, E.; Belnap, J. Patterns and controls on nitrogen cycling of biological soil crusts. In Biological Soil Crusts: An Organizing Principle in Drylands; Weber, B., Büdel, B., Belnap, J., Eds.; Springer: Cham, Switzerland, 2016; pp. 257–285. [Google Scholar]
- Abrantes, G.H.; Gücker, B.; Chaves, R.C.; Boëchat, I.G.; Figueredo, C.C. Epilithic biofilms provide large amounts of nitrogen to tropical mountain landscapes. Environ. Microbiol. 2023, 25, 3592–3603. [Google Scholar] [CrossRef]
- Belnap, B.; Büdel, B. Biological soil crusts as soil stabilizers. In Biological Soil Crusts: An Organizing Principle in Drylands; Weber, B., Büdel, B., Belnap, J., Eds.; Springer: Cham, Switzerland, 2016; pp. 305–320. [Google Scholar]
- Chamizo, S.; Belnap, J.; Eldridge, D.J.; Cantón, Y.; Issa, O.M. The role of biocrusts in arid land hydrology. In Biological Soil Crusts: An Organizing Principle in Drylands; Weber, B., Büdel, B., Belnap, J., Eds.; Springer: Cham, Switzerland, 2016; pp. 321–346. [Google Scholar]
- Oliveira, M.F.; Maciel-Silva, A.S. Biological soil crusts and how they might colonize other worlds: Insights from these Brazilian ecosystem engineers. J. Exp. Bot. 2022, 73, 4362–4379. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, M.B.; Cordeiro, J.; Silva, S.L.L.; Salim, I.H.; Reis, A.; Lacerda, T.J.; Seabra, E.A.L.; Oliveira, M.F.; Moura, S.P.; Santos, I.N.R.; et al. Rehabilitation of eroded trails and gullies on quartzite rock outcrops with native species in a high-altitude grassland. J. Environ. Manag. 2023, 326, 116569. [Google Scholar] [CrossRef] [PubMed]
- Paula, L.F.; Mota, N.F.; Viana, P.L.; Stehmann, J.R. Floristic and ecological characterization of habitat types on an inselberg in Minas Gerais, southeastern Brazil. Acta Bot. Bras. 2017, 31, 199–211. [Google Scholar] [CrossRef]
- Porembski, S. Biodiversity of Terrestrial Habitat Islands—The Inselberg Evidence. In Inselbergs: Biotic Diversity of Isolated Rock Outcrops in Tropical and Temperate Regions; Porembski, S., Barthlott, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 507–514. [Google Scholar]
- Martinelli, G. Mountain biodiversity in Brazil. Braz. J. Bot. 2007, 30, 587–597. [Google Scholar] [CrossRef]
- Porembski, S.; Silveira, F.A.; Fiedler, P.L.; Watve, A.; Rabarimanarivo, M.; Kouame, F.; Hopper, S.D. Worldwide destruction of inselbergs and related rock outcrops threatens a unique ecosystem. Biodivers. Conserv. 2016, 25, 2827–2830. [Google Scholar] [CrossRef]
- Safford, H.D.; Martinelli, G. Southeast Brazil. In Inselbergs: Biotic Diversity of Isolated Rock Outcrops in Tropical and Temperate Regions; Porembski, S., Barthlott, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 339–389. [Google Scholar]
- Gomes, P.; Alves, M. Floristic and vegetational aspects of an inselberg in the semi-arid region of Northeast Brazil. Edinb. J. Bot. 2009, 66, 329–346. [Google Scholar] [CrossRef]
- Couto, D.R.; Francisco, T.M.; Manhães, V.C.; Machado, H.D.; Pereira, M.C.A. Floristic composition of a Neotropical inselberg from Espírito Santo state, Brazil: An important area for conservation. Check List 2017, 13, 2043. [Google Scholar] [CrossRef]
- Paula, L.F.; Azevedo, L.O.; Mauad, L.P.; Cardoso, L.J.T.; Braga, J.M.A.; Kollmann, L.J.; Fraga, C.N.; Neto, L.M.; Labiak, P.H.; Mello-Silva, R.; et al. Sugarloaf Land in south-eastern Brazil: A tropical hotspot of lowland inselberg plant diversity. Biodivers. Data J. 2020, 8, e53135. [Google Scholar] [CrossRef]
- Filgueiras, T.S.; Nogueira, P.E.; Brochado, A.L.; Guala, G.F. Caminhamento: Um método expedito para levantamentos florísticos qualitativos. Cad. Geo. 1994, 12, 39–43. [Google Scholar]
- Frahm, J.P. Dicranaceae: Campylopodioideae, Paraleucobryoideae. In Flora Neotropica; New York Botanical Garden Press: New York, NY, USA, 1991. [Google Scholar]
- Gradstein, S.R.; Churchill, S.P.; Allen, N.S. Guide to the Bryophytes of Tropical America; Memoirs of the New York Botanical Garden: New York, NY, USA, 2001. [Google Scholar]
- Gradstein, S.R.; Costa, D.P. The Hepaticae and Anthocerotae of Brazil; New York Botanical Garden Press: New York, NY, USA, 2003. [Google Scholar]
- Pursell, R.A. Fissidentaceae. In Flora Neotropica; New York Botanical Garden Press: New York, NY, USA, 2007. [Google Scholar]
- Canestraro, B.K.; Peralta, D.F. Synopsis of Anomobryum and Bryum (Bryaceae, Bryophyta) in Brazil. Acta Bot. Bras. 2022, 36, e2021abb0283. [Google Scholar] [CrossRef]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota 1. Teil: Chroococcales. In Süsswasserflora von Mitteleuropa 19/1; Ettl, H., Gärtner, G., Heynig, G., Mollenhauer, D., Eds.; Spektrum Akademischer Verlag: Heidelberg, Germany, 2008. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota 2. Teil: Oscillatoriales. In Süsswasserflora von Mitteleuropa 19/2; Büdel, B., Gärtner, G., Krienitz, L., Schlager, M., Eds.; Spektrum Akademischer Verlag: Heidelberg, Germany, 2008. [Google Scholar]
- Guiry, M.D. Taxonomy and nomenclature of the Conjugatophyceae (=Zygnematophyceae). Algae 2013, 28, 1–29. [Google Scholar] [CrossRef]
- Komárek, J. Cyanoprokaryota 3. Teil: Heterocytous genera. In Süsswasserflora von Mitteleuropa 19/3; Büdel, B., Gärtner, G., Krienitz, L., Schlager, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Dal-Ferro, L.S.; Schenider, A.; Missiaggia, D.G.; Silva, L.J.; Maciel-Silva, A.S.; Figueredo, C.C. Organizing a global list of cyanobacteria and algae from soil biocrusts evidenced great geographic and taxonomic gaps. FEMS Microbiol. Ecol. 2024, 100, fiae086. [Google Scholar] [CrossRef]
- Veech, J.A. A probabilistic model for analysing species co-occurrence. Glob. Ecol. Biogeogr. 2013, 22, 252–260. [Google Scholar] [CrossRef]
- Griffith, D.M.; Veech, J.A.; Marsh, C.J. Cooccur: Probabilistic species co-occurrence analysis in R. J. Stat. Softw. 2016, 69, 1–17. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Yuan, M.M.; Guo, X.; Wu, L.; Zhang, Y.; Xiao, N.; Ning, D.; Shi, Z.; Zhou, X.; Wu, L.; Yang, Y.; et al. Climate warming enhances microbial network complexity and stability. Nat. Clim. Change 2021, 11, 343–348. [Google Scholar] [CrossRef]
- Pržulj, N.; Malod-Dognin, N. Network analytics in the age of big data. Science 2016, 353, 123–124. [Google Scholar] [CrossRef]
- Pombubpa, N.; Pietrasiak, N.; De Ley, P.; Stajich, J.E. Insights into dryland biocrust microbiome: Geography, soil depth and crust type affect biocrust microbial communities and networks in Mojave Desert, USA. FEMS Microbiol. Ecol. 2020, 96, fiaa125. [Google Scholar] [CrossRef]
- Guang, S.; Ying, Z.; Haotian, Y.; Xinrong, L. Biocrust mediates the complexity and stability of bacterial networks in both biocrust and subsoil layers in the Tengger Desert. Plant Soil 2023, 490, 217–237. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, B.; Wang, W.; Revillini, D.; Delgado-Baquerizo, M. Biocrust adaptations to microhabitat alter bacterial communities in a semiarid ecosystem. Plant Soil 2023, 492, 413–427. [Google Scholar] [CrossRef]
- Mugnai, G.; Pinchuk, I.; Borruso, L.; Tiziani, R.; Sannino, C.; Canini, F.; Turchetti, B.; Mimmo, T.; Zucconi, L.; Buzzini, P. The hidden network of biocrust successional stages in the High Arctic: Revealing abiotic and biotic factors shaping microbial and metazoan communities. Sci. Total Environ. 2024, 926, 171786. [Google Scholar] [CrossRef] [PubMed]
- Csardi, G.; Nepusz, T. The igraph software package for complex network research. Int. J. Complex Syst. 2006, 1695, 1–9. [Google Scholar]
- Seppelt, R.D.; Downing, A.J.; Deane-Coe, K.K.; Zhang, Y.; Zhang, J. Bryophytes within biological soil crusts. In Biological Soil Crusts: An Organizing Principle in Drylands; Weber, B., Büdel, B., Belnap, J., Eds.; Springer: Cham, Switzerland, 2016; pp. 101–120. [Google Scholar]
- Machado-de-Lima, N.M.; Fernandes, V.M.C.; Roush, D.; Ayuso, S.V.; Rigonato, J.; Garcia-Pichel, F.; Branco, L.H.Z. The compositionally distinct cyanobacterial biocrusts from Brazilian savanna and their environmental drivers of community diversity. Front. Microbiol. 2019, 10, 2798. [Google Scholar] [CrossRef] [PubMed]
- Machado-de-Lima, N.M.; Branco, L.H.Z. Biological soil crusts: New genera and species of Cyanobacteria from Brazilian semi-arid regions. Phytotaxa 2020, 470, 263–281. [Google Scholar] [CrossRef]
- Machado-de-Lima, N.M.; Munoz-Rojas, M.; Vazquez-Campos, X.; Branco, L.H.Z. Biocrust cyanobacterial composition, diversity, and environmental drivers in two contrasting climatic regions in Brazil. Geoderma 2021, 386, 114914. [Google Scholar] [CrossRef]
- Ferreira, V.C.R.; Sá Lima, L.G.; Branco, L.H.Z.; Santoro, K.R.; Corrêa, M.M.; Molica, R.J.R. Distinct responses of Scytonema hyalinum and Leptolyngbya sp. to water availability and biocrust formation. Braz. J. Microbiol. 2025. [Google Scholar] [CrossRef]
- Moreira-Grez, B.; Tam, K.; Cross, A.T.; Yong, J.W.; Kumaresan, D.; Nevill, P.; Farrell, M.; Whiteley, A.S. The bacterial microbiome associated with arid biocrusts and the biogeochemical influence of biocrusts upon the underlying soil. Front. Microbiol. 2019, 10, 2143. [Google Scholar] [CrossRef]
- Steven, B.; Gallegos-Graves, L.V.; Yeager, C.M.; Belnap, J.; Evans, R.D.; Kuske, C.R. Dryland biological soil crust cyanobacteria show unexpected decreases in abundance under long-term elevated CO2. Environ. Microbiol. 2012, 14, 3247–3258. [Google Scholar] [CrossRef]
- Szyja, M.; Menezes, A.G.S.; Oliveira, F.D.A.; Leal, I.; Tabarelli, M.; Büdel, B.; Wirth, R. Neglected but potent dry forest players: Ecological role and ecosystem service provision of biological soil crusts in the human-modified Caatinga. Front. Ecol. Evol. 2019, 7, 1–18. [Google Scholar] [CrossRef]
- Webber, C.L.; Bremer, U.F.; Taghizadeh-Mehrjardi, R.; Weber, B.; Rosa, A.; Scholten, T.; Seitz, S. Biological soil crusts as a major ecosystem component in sandization areas of the Brazilian Pampa. Geoderma Reg. 2023, 34, e00682. [Google Scholar] [CrossRef]
- Oliveira, M.F.; Santos, P.O.; Oliveira, G.F.; Trajano, G.O.; Maciel-Silva, A.S. A promising application of biodegradable glue to improve the efficiency of biocrust inoculation on mining tailings in Brazil. Restor. Ecol. 2024, 33, e14365. [Google Scholar] [CrossRef]
- Bowker, M.A.; Belnap, J.; Büdel, B.; Sannier, C.; Pietrasiak, N.; Eldridge, D.J.; Rivera-Aguilar, V. Controls on distribution patterns of biological soil crusts at micro- to global scales. In Biological Soil Crusts: An Organizing Principle in Drylands; Weber, B., Büdel, B., Belnap, J., Eds.; Springer: Cham, Switzerland, 2016; pp. 173–197. [Google Scholar]
- Benites, V.M.; Schaefer, C.E.G.; Simas, F.N.; Santos, H.G. Soils associated with rock outcrops in the Brazilian mountain ranges Mantiqueira and Espinhaço. Braz. J. Bot. 2007, 30, 569–577. [Google Scholar] [CrossRef]
- Downing, A.J. Distribution of bryophytes on limestones in eastern Australia. Bryologist 1992, 95, 5–14. [Google Scholar] [CrossRef]
- Zander, R.H. Genera of the Pottiaceae: Mosses of Harsh Environments; The Buffalo Society of Natural Sciences: Buffalo, NY, USA, 1993. [Google Scholar]
- Rogers, R.W. Zonation of the liverwort Riccia in a temporary watercourse in subtropical, semi-arid Australia. Aust. J. Bot. 1994, 42, 657–662. [Google Scholar] [CrossRef]
- Eldridge, D.J.; Delgado-Baquerizo, M. The influence of climatic legacies on the distribution of dryland biocrust communities. Glob. Change Biol. 2018, 25, 327–336. [Google Scholar] [CrossRef]
- Belnap, J.; Gardner, J.S. Soil microstructure in soils of the Colorado Plateau: The role of the cyanobacterium Microcoleus vaginatus. Great Basin Nat. 1993, 53, 40–47. [Google Scholar]
- Garcia-Pichel, F.; Wojciechowski, M.F. The evolution of a capacity to build supra-cellular ropes enabled filamentous cyanobacteria to colonize highly erodible substrates. PLoS ONE 2009, 4, e78011–e78016. [Google Scholar] [CrossRef]
- Xiao, J.; Lan, S.; Farías, M.E.; Qian, L.; Xia, L.; Song, S.; Wu, L. The living forms of Microcoleus vaginatus and their contributions to the aggregate structure of biocrusts. FEMS Microbiol. Ecol. 2023, 99, fiad040. [Google Scholar] [CrossRef]
- Büdel, B.; Dulić, T.; Darienko, T.; Rybalka, N.; Friedl, T. Cyanobacteria and Algae of Biological Soil Crusts. In Biological Soil Crusts: An Organizing Principle in Drylands; Weber, B., Büdel, B., Belnap, J., Eds.; Springer: Cham, Switzerland, 2016; pp. 55–80. [Google Scholar]
- Lan, S.; Thomas, A.D.; Rakes, J.B.; Garcia-Pichel, F.; Wu, L.; Hu, C. Cyanobacterial community composition and their functional shifts associated with biocrust succession in the Gurbantunggut Desert. Environ. Microbiol. Rep. 2021, 13, 884–898. [Google Scholar] [CrossRef]
- Peñaloza-Bojacá, G.F.; Oliveira, B.A.; Araújo, C.A.T.; Fantecelle, L.B.; Santos, N.D.; Maciel-Silva, A.S. Bryophytes on Brazilian ironstone outcrops: Diversity, environmental filtering, and conservation implications. Flora 2018, 238, 162–174. [Google Scholar] [CrossRef]
- Oliveira, B.A.; Oliveira, M.F.; Maciel-Silva, A.S. What can bryophyte diversity on Cangas (ironstone outcrops) teach us? J. Veg. Sci. 2021, 32, e13029. [Google Scholar] [CrossRef]
- Frahm, J.P. Diversity, life strategies, origins and distribution of tropical inselbergs bryophytes. An. Inst. Biol. Univ. Nac. Autón. México 1996, 67, 73–86. [Google Scholar]
- Adams, D.G.; Duggan, P.S. Cyanobacteria–bryophyte symbioses. J. Exp. Bot. 2008, 59, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.G. Cyanobacteria in symbiosis with hornworts and liverworts. In Cyanobacteria in Symbiosis; Rai, A.N., Bergman, B., Rasmussen, U., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 117–135. [Google Scholar]
- Solheim, B.; Zielke, M. Associations Between Cyanobacteria and Mosses. In Cyanobacteria in Symbiosis; Rai, A.N., Bergman, B., Rasmussen, U., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 137–152. [Google Scholar]
- Rousk, K. Biotic and abiotic controls of nitrogen fixation in cyanobacteria–moss associations. New Phytol. 2022, 235, 1330–1335. [Google Scholar] [CrossRef]
- Liu, X.; Rousk, K. The moss traits that rule cyanobacterial colonization. Ann. Bot. 2021, 129, 147–160. [Google Scholar] [CrossRef]
- Reddy, G.B.; Giddens, J. Nitrogen fixation by moss-algal association in grassland. Soil Biol. Biochem. 1981, 13, 537–538. [Google Scholar] [CrossRef]
- Rossi, F.; Philippis, R. Role of cyanobacterial exopolysaccharides in phototrophic biofilms and in complex microbial mats. Life 2015, 5, 1218–1238. [Google Scholar] [CrossRef]
- Zi, R.; Zhao, L.; Fang, Q.; Fang, F.; Yin, X.; Qian, X.; Fan, C.; Han, Z. Effect of Nostoc commune cover on shallow soil moisture, runoff and erosion in the subtropics. Geoderma 2024, 447, 116931. [Google Scholar] [CrossRef]
- Román, J.R.; Roncero-Ramos, B.; Chamizo, S.; Rodríguez-Caballero, E.; Cantón, Y. Restoring soil functions by means of cyanobacteria inoculation: Importance of soil conditions and species selection. Land Degrad. Dev. 2018, 29, 3184–3193. [Google Scholar] [CrossRef]
- Reddy, K.J.; Haskell, J.B.; Sherman, D.M.; Sherman, L.A. Unicellular, aerobic nitrogen-fixing cyanobacteria of the genus Cyanothece. J. Bacteriol. 1993, 175, 1284–1292. [Google Scholar] [CrossRef]
- Langhans, T.M.; Storm, C.; Schwabe, A. Community assembly of biological soil crusts of different successional stages in a temperate sand ecosystem, as assessed by direct determination and enrichment techniques. Microb. Ecol. 2009, 58, 394–407. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Sun, Y.; Zhu, R.L. The oil bodies of liverworts: Unique and important organelles in land plants. Crit. Rev. Plant Sci. 2013, 32, 293–302. [Google Scholar] [CrossRef]
- Romani, F.; Flores, J.R.; Tolopka, J.I.; Suárez, G.; He, X.; Moreno, J.E. Liverwort oil bodies: Diversity, biochemistry, and molecular cell biology of the earliest secretory structure of land plants. J. Exp. Bot. 2022, 73, 4427–4439. [Google Scholar] [CrossRef]
- Whitehead, J.; Wittemann, M.; Cronberg, N. Allelopathy in bryophytes—A review. Lindbergia 2018, 41, 1–7. [Google Scholar] [CrossRef]
- Hierro, J.L.; Callaway, R.M. The ecological importance of allelopathy. Annu. Rev. Ecol. Evol. Syst. 2021, 52, 25–45. [Google Scholar] [CrossRef]
- Gomes, M.P.; Garcia, Q.S.; Barreto, L.C.; Pimenta, L.P.S.; Matheus, M.T.; Figueredo, C.C. Allelopathy: An overview from micro-to macroscopic organisms, from cells to environments, and the perspectives in a climate-changing world. Biologia 2017, 72, 113–129. [Google Scholar] [CrossRef]
- Wilson, J.B.; Steel, J.B.; Newman, J.E.; Tangney, R.S. Are bryophyte communities different? J. Bryol. 1995, 18, 689–705. [Google Scholar] [CrossRef]
- Slack, N.G. Bryophytes and ecological niche theory. Bot. J. Linn. Soc. 1990, 104, 187–213. [Google Scholar] [CrossRef]
- Colesie, C.; Felde, V.J.M.N.; Büdel, B. Composition and Macrostructure of Biological Soil Crusts. In Biological Soil Crusts: An Organizing Principle in Drylands; Weber, B., Büdel, B., Belnap, J., Eds.; Springer: Cham, Switzerland, 2016; pp. 159–172. [Google Scholar]
- Delmas, E.; Besson, M.; Brice, M.H.; Burkle, L.A.; Dalla Riva, G.V.; Fortin, M.J.; Gravel, D.; Guimarães, P.R., Jr.; Hembry, D.H.; Newman, E.A.; et al. Analysing ecological networks of species interactions. Biol. Rev. 2019, 94, 16–36. [Google Scholar] [CrossRef]
- Schultz, N.L.; Sluiter, I.R.; Allen, G.G.; Machado-de-Lima, N.M.; Muñoz-Rojas, M. Biocrust amendments to topsoils facilitate biocrust restoration in a post-mining arid environment. Front. Microbiol. 2022, 13, 882673. [Google Scholar] [CrossRef]
- Rezasoltani, S.; Champagne, P.; Mann, V. Improvement in mine tailings biophysicochemical properties by means of cyanobacterial inoculation. Waste Biomass Valor. 2024, 15, 1689–1699. [Google Scholar] [CrossRef]
- Liao, K.; Tao, Y.; Tu, J.; Zeng, Y.; Li, Y.; Wang, P.; Li, X.; He, F.; Chen, L. Induced and natural moss soil crusts accelerate the C, N, and P cycles of PbZn tailings. Sci. Total Environ. 2024, 909, 168657. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Maestre, F.T.; Eldridge, D.J.; Bowker, M.A.; Ochoa, V.; Gozalo, B.; Berdugo, M.; Val, J.; Singh, B.K. Biocrust-forming mosses mitigate the negative impacts of increasing aridity on ecosystem multifunctionality in drylands. New Phytol. 2016, 209, 1540–1552. [Google Scholar] [CrossRef] [PubMed]
- Bowker, M.A. Biological soil crust rehabilitation in theory and practice: An underexploited opportunity. Restor. Ecol. 2007, 15, 13–23. [Google Scholar] [CrossRef]
- Antoninka, A.; Faist, A.; Rodriguez-Caballero, E.; Young, K.E.; Chaudhary, V.B.; Condon, L.A.; Pyke, D.A. Biological soil crusts in ecological restoration: Emerging research and perspectives. Restor. Ecol. 2020, 28, S3–S8. [Google Scholar] [CrossRef]
- Malešević, T.P.; Meriluoto, J.; Mihalj, I.; Važić, T.; Marković, R.; Jurca, T.; Codd, G.A.; Svirčev, Z. Restoration of damaged drylands through acceleration of biocrust development. Catena 2024, 244, 108265. [Google Scholar] [CrossRef]


| Phylum | Family | Taxa | Freq. |
|---|---|---|---|
| Cyanobacteria | Cyanothecaceae | Cyanothece Komárek | 05 |
| Microcystaceae | Gloeothece Nägeli | 03 | |
| Microcoleaceae | Microcoleus Desmazières ex Gomont | 11 | |
| Nostocaceae | Nostoc Vaucher ex Bornet &Flahault | 11 | |
| Scytonemataceae | Scytonema C.Agardh ex Bornet &Flahault | 34 | |
| Stigonemataceae | Stigonema C.Agardh ex Bornet &Flahault | 06 | |
| Total | 70 | ||
| Chlorophyta | Chlorococcaceae | Chlorococcum Meneghini | 07 |
| Radiococcaceae | Gloeocystis Nägeli | 04 | |
| Total | 11 | ||
| Charophyta | Desmidiaceae | Actinotaenium (Nägeli) Teiling | 04 |
| Euastrum Ehrenberg ex Ralfs | 01 | ||
| Zygnemataceae | Zygogonium Kützing | 06 | |
| Total | 11 | ||
| Bacillariophyta | Pinnulariaceae | Pinnularia borealis Ehrenberg | 04 |
| Total | 04 | ||
| Bryophyta | Bryaceae | Anomobryum conicum (Hornsch.) Broth. | 14 |
| Bryum arachnoideum Müll. Hal. | 03 | ||
| Bryum argenteum Hedw. | 17 | ||
| Bryum atenense Williams | 15 | ||
| Bryum orthodontioides Müll.Hal. | 01 | ||
| Dicranaceae | Campylopus lamellatus Mont. | 07 | |
| Dicranella lindigiana (Hampe) Mit. | 02 | ||
| Fabroniaceae | Fabronia ciliaris (Brid.) Brid. | 01 | |
| Fissidentaceae | Fissidens weirii Mitt. | 01 | |
| Total | 61 | ||
| Marchantiophyta | Cephaloziellaceae | Cylindrocolea rhizantha (Mont.) R.M.Schust. | 01 |
| Ricciaceae | Riccia weinionis Steph. | 08 | |
| Total | 09 |
| Taxa Pair | p_lt | p_gt |
|---|---|---|
| Riccia weinionis and Cyanothece | 0.999 | 0.004 |
| Riccia weinionis and Microcoleus | 0.997 | 0.031 |
| Riccia weinionis and Nostoc | 1.000 | <0.001 |
| Riccia weinionis and Zygogonium | 0.999 | 0.012 |
| Riccia weinionis and Bryum atenense | 0.007 | 1.000 |
| Bryum argenteum and Nostoc | 0.995 | 0.031 |
| Nostoc and Zygogonium | 0.995 | 0.047 |
| Nostoc and Cyanothece | 0.999 | 0.019 |
| Nostoc and Microcoleus | 0.995 | 0.036 |
| Phylum | Taxa | Degree | Betweenness Centrality |
|---|---|---|---|
| Cyanobacteria | Cyanothece | 10 | 2.508 |
| Gloeothece | 7 | 5.799 | |
| Microcoleus | 13 | 1.537 | |
| Nostoc | 16 | 11.290 | |
| Scytonema | 22 | 12.181 | |
| Stigonema | 10 | 7.012 | |
| Chlorophyta | Chlorococcum | 12 | 10.260 |
| Gloeocystis | 10 | 15.954 | |
| Charophyta | Actinotaenium | 10 | 14.892 |
| Euastrum | 5 | 6.082 | |
| Zygogonium | 12 | 5.484 | |
| Bacillariophyta | Pinnularia borealis | 13 | 15.843 |
| Anomobryum conicum | 18 | 20.988 | |
| Bryophyta | Bryum arachnoideum | 7 | 5.839 |
| Bryum argenteum | 18 | 6.684 | |
| Bryum atenense | 17 | 36.327 | |
| Bryum orthodontioides | 3 | 3.386 | |
| Campylopus lamellatus | 13 | 16.104 | |
| Dicranella lindigiana | 5 | 3.512 | |
| Fabronia ciliaris | 2 | 0.698 | |
| Fissidens weirii | 7 | 5.799 | |
| Marchantiophyta | Cylindrocolea rhizantha | 7 | 5.799 |
| Riccia weinionis | 11 | 1.737 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, M.F.; Figueredo, C.C.; Maciel-Silva, A.S. Diversity and Key Organisms in the Biocrust of a Tropical Granite-Gneiss Rocky Outcrop. Life 2025, 15, 759. https://doi.org/10.3390/life15050759
Oliveira MF, Figueredo CC, Maciel-Silva AS. Diversity and Key Organisms in the Biocrust of a Tropical Granite-Gneiss Rocky Outcrop. Life. 2025; 15(5):759. https://doi.org/10.3390/life15050759
Chicago/Turabian StyleOliveira, Mateus Fernandes, Cleber Cunha Figueredo, and Adaíses Simone Maciel-Silva. 2025. "Diversity and Key Organisms in the Biocrust of a Tropical Granite-Gneiss Rocky Outcrop" Life 15, no. 5: 759. https://doi.org/10.3390/life15050759
APA StyleOliveira, M. F., Figueredo, C. C., & Maciel-Silva, A. S. (2025). Diversity and Key Organisms in the Biocrust of a Tropical Granite-Gneiss Rocky Outcrop. Life, 15(5), 759. https://doi.org/10.3390/life15050759






