Alkaloid Profile Characterisation and Bioactivity Evaluation of Bolivian Hippeastrum Species (Amaryllidaceae) as Cholinesterase Inhibitors
Abstract
1. Introduction
2. Materials and Methods
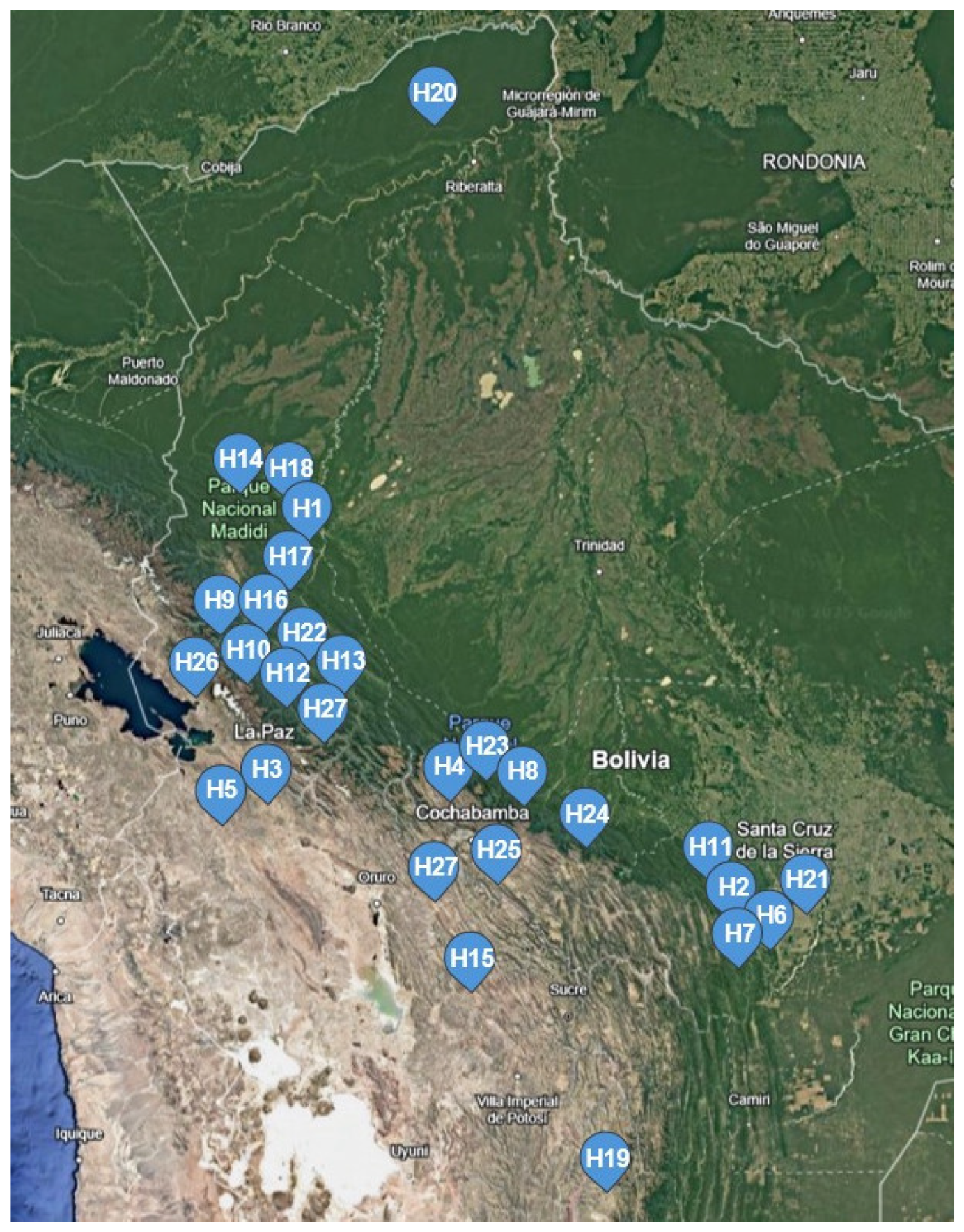
2.1. Plant Material
2.2. Alkaloid Extraction
2.3. GC-MS Analysis
2.4. Identification of Alkaloids
2.5. Quantification of Alkaloids
2.6. AChE and Butyrylcholinesterase (BuChE) Inhibition Assays
3. Results
3.1. Identification and Quantification of Bioactive Compounds
3.1.1. Lycorine Type Alkaloids
3.1.2. Homolycorine Type Alkaloids
3.1.3. Galanthindol Type Alkaloids
3.1.4. Haemanthamine/Crinine Type Alkaloids
3.1.5. Narciclasine Type Alkaloids
3.1.6. Pretazettine Type Alkaloids
3.1.7. Montanine Type Alkaloids
3.1.8. Galanthamine Type Alkaloids
3.1.9. Ismine Type Alkaloids
3.2. AChE and BuChE Inhibitory Activities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AChE | Acethylcholinesterase |
| BuChE | Butyrylcholinesterase |
| AA | Amaryllidaceae alkaloid |
References
- Cordell, G.A.; Quinn-Beattie, M.L.; Farnsworth, N.R. The potential of alkaloids in drug discovery. Phytother. Res. 2001, 15, 183–205. [Google Scholar] [CrossRef]
- Ka, S.; Koirala, M.; Mérindol, N.; Desgagné-Penix, I. Biosynthesis and Biological Activities of Newly Discovered Amaryllidaceae Alkaloids. Molecules 2020, 25, 4901. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, E.; Masi, M.; Evidente, M.; Superchi, S. Amaryllidaceae alkaloids: Absolute configuration and biological activity. Chirality 2017, 29, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Berkov, S.; Osorio, E.; Viladomat, F.; Bastida, J. Chemodiversity, chemotaxonomy and chemoecology of Amaryllidaceae alkaloids. In Alkaloids: Chemistry and Biology; Knölker, H.-J., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 83, pp. 113–185. [Google Scholar]
- Spies, P.; Grobler, J.P.; Spies, J.J. A review of phylogenetic relationships in the genus Clivia. Philos. Trans. Genet. 2011, 1, 168–207. [Google Scholar]
- Singh, A.; Desgagné-Penix, I. Biosynthesis of the Amaryllidaceae alkaloids. Plant Sci. Today 2014, 1, 114–120. [Google Scholar] [CrossRef]
- Kilgore, M.B.; Kutchan, T.M. The Amaryllidaceae alkaloids: Biosynthesis and methods for enzyme discovery. Phytochem. Rev. 2015, 15, 317–337. [Google Scholar] [CrossRef]
- Cahlíková, L.; Vrabec, R.; Pidaný, F.; Peřinová, R.; Maafi, N.; Mamun, A.A.; Ritomská, A.; Wijaya, V.; Blunden, G. Recent progress on biological activity of Amaryllidaceae and further isoquinoline alkaloids in connection with Alzheimer’s Disease. Molecules 2021, 26, 5240. [Google Scholar] [CrossRef]
- Maelicke, A.; Samochocki, M.; Jostock, R.; Fehrenbacher, A.; Ludwig, J.; Albuquerque, E.X.; Zerlin, M. Allosteric sensitization of nicotinic receptors by galantamine, a new treatment strategy for Alzheimer’s disease. Biol. Psychiatry 2001, 49, 279–288. [Google Scholar] [CrossRef]
- Heinrich, M.; Teoh, H.L. Galanthamine from snowdrop-the development of a modern drug against Alzheimer’s disease from local Caucasian knowledge. J. Ethnopharmacol. 2004, 92, 147–162. [Google Scholar] [CrossRef]
- Marco-Contelles, J.; do Carmo Carreiras, M.; Rodríguez, C.; Villarroya, M.; García, A.G. Synthesis and pharmacology of galantamine. Chem. Rev. 2006, 106, 116–133. [Google Scholar] [CrossRef]
- Bastida, J.; Berkov, S.; Torras Claveria, L.; Pigni, N.B.; de Andrade, J.P.; Martínez, V.; Codina, C.; Viladomat, F. Chemical and biological aspects of Amaryllidaceae alkaloids. In Recent Advances in Pharmaceutical Sciences; Muñoz-Torrero, D., Ed.; Transworld Research Network: Trivandrum, India, 2011; Volume 661, pp. 65–100. [Google Scholar]
- Samochocki, M.; Höffle, A.; Fehrenbacher, A.; Jostock, R.; Ludwig, J.; Christner, C.; Radina, M.; Zerlin, M.; Ullmer, C.; Pereira, E.F.R.; et al. Galantamine is an allosterically potentiating ligand of neuronal nicotinic but not of muscarinic acetylcholine receptors. J. Pharmacol. Exp. Ther. 2003, 305, 1024–1036. [Google Scholar] [CrossRef]
- Zarotsky, V.; Sramek, J.J.; Cutler, N.R. Galantamine hydrobromide: An agent for Alzheimer’s disease. Am. J. Health Syst. Pharm. 2003, 60, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Omoruyi, S.I.; Ibrakaw, A.S.; Ekpo, O.E.; Boatwright, J.S.; Cupido, C.N.; Hussein, A.A. Neuroprotective activities of Crossyne flava bulbs and Amaryllidaceae alkaloids: Implications for Parkinson’s disease. Molecules 2021, 26, 3990. [Google Scholar] [CrossRef] [PubMed]
- Griffin, C.; Karnik, A.; McNulty, J.; Pandey, S. Pancratistatin selectively targets cancer cell mitochondria and reduces growth of human colon tumor xenografts. Mol. Cancer Ther. 2011, 10, 57–68. [Google Scholar] [CrossRef]
- Griffin, C.; McNulty, J.; Pandey, S. Pancratistatin induces apoptosis and autophagy in metastatic prostate cancer cells. Int. J. Oncol. 2011, 38, 1549–1556. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hao, B.; Shen, S.F.; Zhao, Q.J. Cytotoxic and antimalarial Amaryllidaceae alkaloids from the bulbs of Lycoris radiata. Molecules 2013, 18, 2458–2468. [Google Scholar] [CrossRef]
- Cao, Z.; Yang, P.; Zhou, Q. Multiple biological functions and pharmacological effects of lycorine. Sci. China Chem. 2013, 56, 1382–1391. [Google Scholar] [CrossRef]
- Ang, S.; Liu, X.M.; Huang, X.J.; Zhang, D.M.; Zhang, W.; Wang, L.; Ye, W.C. Four new Amaryllidaceae alkaloids from Lycoris radiata and their cytotoxicity. Planta Med. 2015, 81, 1712–1718. [Google Scholar] [CrossRef]
- Cedrón, J.C.; Ravelo, Á.G.; León, L.G.; Padrón, J.M.; Estévez-Braun, A. Antiproliferative and structure activity relationships of Amaryllidaceae alkaloids. Molecules 2015, 20, 13854–13863. [Google Scholar] [CrossRef]
- Doskočil, I.; Hošáková, A.; Šafratová, M.; Benešová, N.; Havlík, J.; Havelek, R.; Kuneš, J.; Královec, K.; Chebek, J.; Cahlíková, L. Cytotoxic activities of Amaryllidaceae alkaloids against gastrointestinal cancer cells. Phytochem. Lett. 2015, 13, 394–398. [Google Scholar] [CrossRef]
- Koutova, D.; Maafi, N.; Muthna, D.; Kralovec, K.; Kroustkova, J.; Pidany, F.; Timbilla, A.A.; Cermakova, E.; Cahlikova, L.; Rezacova, M.; et al. Antiproliferative activity and apoptosis-inducing mechanism of Amaryllidaceae alkaloid montanine on A549 and MOLT-4 human cancer cells. Biomed. Pharmacother. 2023, 166, 115295. [Google Scholar] [CrossRef] [PubMed]
- Gasca, C.A.; Moreira, N.C.S.; de Almeida, F.C.; Gomes, J.V.D.; Castillo, W.O.; Fagg, C.W.; Magãlhaes, P.O.; Fonseca-Bazzo, Y.M.; Sakamoto-Hojo, E.; de Medeiros, Y.K.; et al. Acetylcholinesterase inhibitory activity, anti-inflammatory, and neuroprotective potential of Hippeastrum psittacinum (Ker Gawl.) herb (Amaryllidaceae). Food Chem. Toxicol. 2020, 145, 111703. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, M.F.; Fahim, J.R.; Allam, A.E.; Shoman, M.E.; Zawily, A.E.; Kamel, M.S.; Shimizu, K.; Attia, E.Z. Studies on the nonalkaloidal secondary metabolites of Hippeastrum vittatum (L’Her.) Herb. Bulbs. ACS Omega 2023, 23, 26749–26761. [Google Scholar] [CrossRef]
- Pipkin, J.L.; Larson, D.A. Characterization of the ‘very’ lysine-rich histones of active and quiescent anther tissues of Hippeastrum belladonna. Exp. Cell Res. 1972, 71, 249–260. [Google Scholar] [CrossRef]
- Tallini, L.R.; Osorio, E.H.; Dos Santos, V.D.; Borges, W.S.; Kaiser, M.; Viladomat, F.; Zuanazzi, J.A.S.; Bastida, J. Hippeastrum reticulatum (Amaryllidaceae): Alkaloid profiling, biological activities and molecular docking. Molecules 2017, 22, 2191. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, J.E.; Piñeiro, M.; Martinez-Peinado, N.; Barrera, P.; Sosa, M.; Bastida, J.; Alonso-Padilla, J.; Ferensin, G.E. Candimine from Hippeastrum escoipense (Amaryllidaceae): Anti-Trypanosoma cruzi activity and synergistic effect with benznidazole. Phytomedicine 2023, 114, 154788. [Google Scholar] [CrossRef]
- Piñeiro, M.; Ortiz, J.E.; Zapata, R.M.S.; Barrera, P.A.; Sosa, M.A.; Roitman, G.; Bastida, J.; Ferensin, G.E. Antiparasitic activity of Hippeastrum species and synergistic interaction between montanine and benznidazole against Trypanosoma cruzi. Microorganisms 2023, 11, 144. [Google Scholar] [CrossRef]
- Bessa, C.D.P.B.; Feu, A.E.; de Menezes, R.P.B.; Scotti, M.T.; Lima, J.M.G.; Lima, M.L.; Tempone, A.G.; de Andrade, J.P.; Bastida, J.; Borges, W.S. Multitarget anti-parasitic activities of isoquinoline alkaloids isolated from Hippeastrum aulicum (Amaryllidaceae). Phytomedicine 2024, 128, 155414. [Google Scholar] [CrossRef]
- Giordani, R.B.; Pagliosa, L.B.; Henriques, A.T.; Zuanazzi, J.A.S.; Dutilh, J.H.A. Investigação do potencial antioxidante e anticolinesterásico de Hippeastrum (Amaryllidaceae). Quim Nova 2008, 31, 2042–2046. [Google Scholar] [CrossRef]
- De Andrade, J.P.; Pigni, N.B.; Torras-Claveria, L.; Guo, Y.; Berkov, S.; Reyes-Chilpa, R.; El Amrani, A.; Zuanazzi, J.A.S.; Codina, C.; Viladomat, F.; et al. Alkaloids from the Hippeastrum genus: Chemistry and Biological Activity. Rev. Latinoamer. Quim. 2012, 40, 2. [Google Scholar]
- Segaran, G.; Sathiavelu, M. Antibacterial activity of an ornamental plant Hippeastrum fosteri. Bangladesh J. Pharmacol. 2021, 16, 49–51. [Google Scholar] [CrossRef]
- Shahzad, A.; Afzal, S.; Ahmad, I. Spectrophotometric quantification of total phenol, flavonoid and alkaloid content and in-vitro investigation of antioxidant and cytotoxic potential of Hippeastrum equestre bulbs. Pharm. Chem. J. 2022, 56, 1263–1271. [Google Scholar] [CrossRef]
- Tallini, L.R.; Giordani, R.B.; de Andrade, J.P.; Bastida, J.; Zuanazzi, J.A.S. Structural diversity and biological potential of alkaloids from the genus Hippeastrum, Amaryllidaceae: An update. Rev. Bras. Farmacogn. 2021, 31, 648–657. [Google Scholar] [CrossRef]
- Lara Rico, R.F.; Vásquez Chávez, R.; Atahuachi Burgos, M. The Genus Hippeastrum (Amaryllidaceae) in Bolivia; Pacific Bulb Society: Leonia, NJ, USA, 2021. [Google Scholar]
- Moya Huanca, A.L.; Villalba Vargas, D.; Zenteno-Ruiz, F.S. Una especie nueva de Hippeastrum (Amaryllidaceae) de los bosques secos yungueños de Bolivia. Novon J. Bot. Nomencl. 2022, 30, 43–48. [Google Scholar] [CrossRef]
- Meerow, A.W.; Snijman, D.A. Amaryllidaceae. In Flowering Plants: Monocotyledons; Kubitzki, K., Ed.; Springer: Berlin/Heidelberg, Germany, 1998; Volume 3, pp. 83–110. [Google Scholar]
- Lara Rico, R.F.; Vásquez Chávez, R. Notas acerca del género Hippeastrum (Amaryllidaceae) en Bolivia, II. Fontqueria 2015, 56, 403–438. [Google Scholar]
- Lara Rico, F.; Vásquez, R.; Atahuachi, B.M. Jarajorechis, hipeastras o amarilis nativas de Bolivia. In Guías de Biodiversidad Boliviana; Fundación Patiño: Santa Cruz de la Sierra, Bolivia, 2022. [Google Scholar]
- Rodríguez-Escobar, M.L.; Tallini, L.R.; Lisa-Molina, J.; Berkov, S.; Viladomat, F.; Meerow, A.; Bastida, J.; Torras-Claveria, L. Chemical and Biological Aspects of Different Species of the Genus Clinanthus Herb. (Amaryllidaceae) from South America. Molecules 2023, 28, 5408. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–90. [Google Scholar] [CrossRef]
- López, S.; Bastida, J.; Viladomat, F.; Codina, C. Acetylcholinesterase inhibitory activity of some Amaryllidaceae alkaloids and Narcissus extracts. Life Sci. 2002, 71, 2521–2529. [Google Scholar] [CrossRef]
- Roy, M.; Liang, L.; Xiao, X.; Feng, P.; Ye, M.; Liu, J. Lycorine: A prospective natural lead for anticancer drug discovery. Biomed. Pharmacother. 2018, 107, 615–624. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, T.; Cheng, Z.; Zhu, N.; Wang, H.; Lin, L.; Wang, Z.; Yi, H.; Hu, M. Lycorine inhibits glioblastoma multiforme growth through EGFR suppression. J. Exp. Clin. Cancer Res. 2018, 37, 157. [Google Scholar] [CrossRef]
- Hu, M.; Peng, S.; He, Y.; Qin, M.; Cong, X.; Xing, Y.; Liu, M.; Yi, Z. Lycorine is a novel inhibitor of the growth and metastasis of hormone-refractory prostate cancer. Oncotarget 2015, 20, 15348–15361. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Y.; Tang, Q.; Zhang, Q.; Hu, C.P.; Huang, J.B.; Sheng, F.F.; Liu, Y.L.; Zou, M.; Li, G.B.; Zhang, R. Lycorine induces mitochondria-dependent apoptosis in hepatoblastoma HepG2 cells through ROCK1 activation. Front. Pharmacol. 2019, 10, 651. [Google Scholar] [CrossRef]
- Giordani, R.B.; Weizenmann, M.; Rosemberg, D.B.; De Carli, G.A.; Bogo, M.R.; Zuanazzi, J.A.S.; Tasca, T. Trichomonas vaginalis nucleoside triphosphate diphosphohydrolase and ecto-5′-nucleotidase activities are inhibited by lycorine and candimine. Parasitol. Int. 2010, 59, 226–231. [Google Scholar] [CrossRef]
- Nair, J.J.; Van Staden, J. The plant family Amaryllidaceae as a source of cytotoxic homolycorine alkaloid principles. S. Afr. J. Bot. 2021, 136, 157–174. [Google Scholar] [CrossRef]
- Unver, N.; Kaya, G.I.; Werner, C.; Verpoorte, R.; Gözler, B. Galanthindole: A new indole alkaloid from Galanthus plicatus ssp. byzantinus. Planta Med. 2003, 69, 869–871. [Google Scholar] [CrossRef] [PubMed]
- McNulty, J.; Nair, J.J.; Codina, C.; Bastida, J.; Pandey, S.; Gerasimoff, J.; Griffin, C. Selective apoptosis-inducing activity of Crinum-type Amaryllidaceae alkaloids. Phytochemistry 2007, 68, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Kaya, G.I.; Sarıkaya, B.; Onur, M.A.; Somer, N.U.; Viladomat, F.; Codina, C.; Bastida, J.; Lauinger, I.L.; Kaiser, M.; Tasdemir, D. Antiprotozoal alkaloids from Galanthus trojanus. Phytochem. Lett. 2011, 4, 301–305. [Google Scholar] [CrossRef]
- Kornienko, A.; Evidente, A. Chemistry, biology and medicinal potential of narciclasine and its congeners. Chem. Rev. 2008, 108, 1982–2014. [Google Scholar] [CrossRef]
- Hu, Z.; Guo, J.; Ma, D.; Wang, Z.; Liu, Y.; Wang, Q. Discovery of crinasiadine, trisphaeridine, bicolorine, and their derivatives as anti-tobacco mosaic virus (TMV) agents. Int. J. Mol. Sci. 2025, 26, 1103. [Google Scholar] [CrossRef]
- Koutová, D.; Maafi, N.; Havelek, R.; Opletal, L.; Blunden, G.; Řezáčová, M.; Cahlíková, L. Chemical and biological aspects of Montanine-type alkaloids isolated from plants of the Amaryllidaceae family. Molecules 2020, 25, 2337. [Google Scholar] [CrossRef]
- Ortiz, J.E.; Pigni, N.B.; Andujar, S.A.; Roitman, G.; Suvire, F.D.; Enriz, R.D.; Tapia, A.; Bastida, J.; Feresin, G.E. Alkaloids from Hippeastrum argentinum and their cholinesterase-inhibitory activities: An in vitro and in silico study. J. Nat. Prod. 2016, 79, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Aljarba, N.H.; Ali, H.; Alkahtani, S. Synergistic dose permutation of isolated alkaloid and sterol for anticancer effect on young swiss albino mice. Drug Des. Dev. Ther. 2021, 15, 4043–4052. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, T.U.; Merindol, N.; Liyanage, N.S.; Gélinas, S.-E.; Lionel, B.; Seydou, K.; Seck, M.; Evidente, A.; Desgagné-Penix, I. Antiviral alkaloids from Crinum jagus: Extraction, synergistic effects, and activity against dengue virus and human coronavirus OC43. Heliyon 2025, 11, e42580. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.-P.; Liu, E.Y.L.; Chen, Z.-C.; Xu, M.L.; Yu, A.X.D.; Wu, Q.-Y.; Xia, Y.-J.; Duan, R.; Dong, T.T.X.; Tsim, K.W.K. Synergistic inhibition of acetylcholinesterase by alkaloids derived from Stephaniae Tetrandrae Radix, Coptidis Rhizoma and Phellodendri Chinensis Cortex. Molecules 2019, 24, 4567. [Google Scholar] [CrossRef]
- De Andrade, J.P.; Guo, Y.; Font-Bardia, M.; Calvet, T.; Dutilh, J.; Viladomat, F.; Codina, C.; Nair, J.J.; Zuanazzi, J.A.S.; Bastida, J. Crinine-type alkaloids from Hippeastrum aulicum and H. calyptratum. Phytochemistry 2014, 103, 188–195. [Google Scholar] [CrossRef]


| Code | Altitude (m.a.s.l.) | Average Annual Precipitation (mm) | Soil Type | Average Annual Temperature (°C) |
|---|---|---|---|---|
| H1, H14, H17, H18 | 300–500 | 1800–2000 | clayey | 20–25 |
| H2, H6, H21 | 200–600 | 1200–1400 | sandy | 22–28 |
| H3 | 3600 | 800 | rocky | 15–10 |
| H4, H8, H23 | 2500–2800 | 1300–1500 | alluvial | 18–22 |
| H5, H12, H16 | 1800–2920 | 1500–3000 | silty | 15–20 |
| H7 | 200 | 1200 | sandy | 23–30 |
| H9 | 2200 | 1200 | clayey | 14–18 |
| H10 | 2500 | 1500 | sandy | 17–22 |
| H11 | 500 | 1100 | rocky | 20–25 |
| H13 | 1800–2200 | 1500 | alluvial | 16–20 |
| H15 | 2800 | 800 | rocky | 15–10 |
| H19 | 2500 | 1200 | rocky | 15–20 |
| H20 | 200 | 2000 | clayey | 24–30 |
| H22 | 500 | 1600 | silty | 22–28 |
| H24 | 2200 | 1300 | alluvial | 18–22 |
| H25 | 2300 | 1100 | clayey | 19–23 |
| H26 | 2800 | 1200 | rocky | 16–10 |
| H27 | 2000 | 1000 | alluvial | 15–19 |
| Code | Species | Collection Code | Botanical Validation | Herbarium | Geographical Location |
|---|---|---|---|---|---|
| H1 | Hippeastrum cardenasii | ACE131 | [RL], [MA] | BOLV | La Paz (P.N. Madidi), Bolivia |
| H2 | Hippeastrum chionedyanthum | ACE124 | [RL], [MA] | BOLV | Santa Cruz (Bermejo), Bolivia |
| H3 | Hippeastrum cybister | MLRE01 | [AF] | LPB | La Paz (Sendero del Águila), Bolivia |
| H4 | Hippeastrum cybister | ACE121 | [RL], [MA] | BOLV | Cochabamba (Tiquipaya), Bolivia |
| H5 | Hippeastrum escobaruriae | ACE111 | [RL], [MA] | BOLV | La Paz (Coroico), Bolivia |
| H6 | Hippeastrum evansiarum | MM3849 | [MM] | USZ | Santa Cruz (P.N. Madidi), Bolivia |
| H7 | Hippeastrum evansiarum | ACE118 | [RL], [MA] | BOLV | Santa Cruz (La Angostura), Bolivia |
| H8 | Hippeastrum fragrantissimum | ACE113 | [RL], [MA] | BOLV | Cochabamba (Tablasmonte), Bolivia |
| H9 | Hippeastrum haywardii | MM5811 | [AF], [CM] | LPB | La Paz (Larecaja), Bolivia |
| H10 | Hippeastrum haywardii | ACE 140 | [RL], [MA] | BOLV | La Paz (Guanay), Bolivia |
| H11 | Hippeastrum incachacanum | ACE 125 | [RL], [MA] | BOLV | Santa Cruz (Piedramesa), Bolivia |
| H12 | Hippeastrum lapacense | ACE 128 | [RL], [MA] | BOLV | La Paz (Coroico), Bolivia |
| H13 | Hippeastrum lara-ricoi | ACE132 | [RL], [MA] | BOLV | La Paz (Sud Yungas), Bolivia |
| H14 | Hippeastrum leopoldii | ACE139 | [RL], [MA] | BOLV | La Paz (P.N. Madidi), Bolivia |
| H15 | Hippeastrum mollevillquense | ACE129 | [RL], [MA] | BOLV | Potosí (Mollevillque), Bolivia |
| H16 | Hippeastrum nelsonii | ACE109 | [RL], [MA] | BOLV | La Paz (Guanay), Bolivia |
| H17 | Hippeastrum paquichanum | ACE138 | [RL], [MA] | BOLV | La Paz (P.N. Madidi), Bolivia |
| H18 | Hippeastrum pardinum | ACE137 | [RL], [MA] | BOLV | La Paz (P.N. Madidi), Bolivia |
| H19 | Hippeastrum parodii | ACE116 | [RL], [MA] | BOLV | Chuquisaca (Inca Huasi), Bolivia |
| H20 | Hippeastrum psittacinum | ACE133 | [RL], [MA] | BOLV | Pando, Bolivia |
| H21 | Hippeastrum puniceum | AF25733 | [AF], [CM] | LPB | Santa Cruz (Andrés Ibáñez), Bolivia |
| H22 | Hippeastrum puniceum | MM | [MM] | USZ | Santa Cruz (Jardín Botánico), Bolivia |
| H23 | Hippeastrum sp. | ACE 105 | [RL], [MA] | BOLV | Cochabamba (Tablasmonte), Bolivia |
| H24 | Hippeastrum umabisanum | ACE152 | [RL], [MA] | BOLV | Chuquisaca, Bolivia |
| H25 | Hippeastrum vittatum var. tweedianum | ACE107 | [RL], [MA] | BOLV | Cochabamba (Naranjitos), Bolivia |
| H26 | Hippeastrum warszewiczianum | ACE153 | [RL], [MA] | BOLV | La Paz (Sorata), Bolivia |
| H27 | Hippeastrum yungacense | AF25732 | [AF], [CM] | LPB | La Paz (Tamanpaya), Bolivia |
| Code | Extraction Yield (mg/g DW) |
|---|---|
| H1 | 2.0 |
| H2 | 6.7 |
| H3 | 6.3 |
| H4 | 3.0 |
| H5 | 11.7 |
| H6 | 1.9 |
| H7 | 5.0 |
| H8 | 24.0 |
| H9 | 15.0 |
| H10 | 20.8 |
| H11 | 1.0 |
| H12 | 9.0 |
| H13 | 9.8 |
| H14 | 11.8 |
| H15 | 19.0 |
| H16 | 5.0 |
| H17 | 14.0 |
| H18 | 0.9 |
| H19 | 4.0 |
| H20 | 4.0 |
| H21 | 4.1 |
| H22 | 1.0 |
| H23 | 6.0 |
| H24 | 5.1 |
| H25 | 10.0 |
| H26 | 10.1 |
| H27 | 10.0 |
| Alkaloids | RI | RT (min) | [M+] |
|---|---|---|---|
| Lycorine type | |||
| Anhydrolycorine | 2818.0 | 24.6375 | 250 |
| Assoanine | 2909.0 | 25.7095 | 266 |
| Dihydrolycorine | 2745.0 | 23.7785 | 288 |
| Galanthine | 3022.4 | 27.0459 | 242 |
| Lycorine | 3138.3 | 28.4106 | 226 |
| Tortuosine | 3212.8 | 29.2889 | 296 |
| 11,12-Dehydroanhydrolycorine | 2948.8 | 26.1784 | 248 |
| Homolycorine type | |||
| Candimine | 3305.2 | 30.3770 | 125 |
| Cliviasine | 3181.0 | 28.9140 | 96 |
| Hippeastrine | 3269.9 | 29.9614 | 125 |
| Homolycorine | 2624.8 | 22.3619 | 109 |
| Nerinine | 3177.9 | 28.8780 | 109 |
| 8-O-Demethylhomolycorine | 2739.7 | 27.0306 | 109 |
| 2-Hydroxyhomolycorine | 3329.0 | 30.6576 | 109 |
| 2-Methoxy-8-O-demethylhomolycorine | 3021.1 | 27.0308 | 109 |
| Galanthindole type | |||
| Galanthindole | 2553.9 | 24.1963 | 281 |
| Crinine/Haemanthamine type | |||
| Maritidine | 2566.1 | 21.6712 | 287 |
| O-Methylmaritidine | 2761.6 | 23.9741 | 301 |
| Vittatine/crinine | 2769.7 | 24.0685 | 271 |
| 8-O-Demethylmaritidine | 2810.6 | 24.5511 | 273 |
| Crinane-3-one | 2852.4 | 27.5250 | 271/181 |
| Crinamine | 2945.0 | 26.1345 | 272 |
| Hippeastidine | 2947.4 | 26.1626 | 319 |
| Haemanthamine | 3008.2 | 26.8789 | 272 |
| Flexinine | 3042.0 | 27.2765 | 258 |
| Undulatine | 3123.8 | 28.2400 | 331 |
| Narciclasine type | |||
| Trisphaeridine | 2346.1 | 20.9836 | 223 |
| Pretazettine type | |||
| Isotazettino | 3077.1 | 27.6901 | 247 |
| 3-O-Demethyltazettine | 3049.9 | 27.3696 | 247 |
| 3-epi-Macronine | 3186.6 | 28.9797 | 245 |
| Tazettine | 2991.6 | 26.6831 | 247 |
| O-Methyltazettine | 2935.3 | 26.0200 | 261 |
| 5-Methoxyhomolycorine | 3346.8 | 30.8677 | 139 |
| Montanine type | |||
| Pancracine | 3027.5 | 27.1062 | 287 |
| Pancratinine C | 2893.0 | 25.5216 | 287 |
| 2-O-Methylpancracine | 2967.6 | 26.4007 | 301 |
| Galanthamine type | |||
| Sanguinine | 2666.4 | 22.8524 | 273 |
| Galanthamine | 2406.0 | 22.5833 | 286 |
| Narwedine | 2746.7 | 23.7978 | 284 |
| 3-O-Acetylgalanthamine | 2824.7 | 24.7172 | 270 |
| Lycoramine | 2699.5 | 23.2424 | 288 |
| Lycoraminone | 2731.4 | 23.6183 | 286 |
| Galanthamine-N-oxide | 2638.4 | 22.5226 | 286 |
| Epigalanthamine | 2670.0 | 22.8942 | 287 |
| Norlycoramine | 2736.6 | 23.6786 | 274 |
| Ismine type | |||
| Ismine | 2497.4 | 20.6170 | 238 |
| Other types of alkaloids | |||
| 5,6-Dihydrolycorine | 2384.9 | 21.6068 | 238 |
| Demethylismine | 2473.5 | 20.5799 | 224 |
| AChE | BuChE | ||||
|---|---|---|---|---|---|
| Code | Species | µg·mL−1 ± SD | Adjusted p Value | µg·mL−1 ± SD | Adjusted p Value |
| H1 | H. cardenasii | 49.67 ± 1.20 | <0.0001 | 149.13 ± 3.02 | <0.0001 |
| H2 | H. chionedyanthum | 6.03 ± 0.45 | 0.3775 | NA | - |
| H4 | H. cybister | 106.83 ± 4.15 | <0.0001 | NA | - |
| H5 | H. escobaruriae | 64.46 ± 4.50 | <0.0001 | NA | - |
| H8 | H. fragrantissimum | 11.85 ± 0.22 | 0.0032 | 103.62 ± 3.60 | <0.0001 |
| H9 | H. haywardii | 3.54 ± 0.09 | 0.9688 | 23.26 ± 0.24 | 0.0037 |
| H10 | H. haywardii | 9.08 ± 0.64 | 0.0450 | 69.49 ± 2.73 | <0.0001 |
| H12 | H. lapacense | 75.07 ± 1.35 | <0.0001 | NA | - |
| H13 | H. lara-ricoi | 2.32 ± 0.07 | 0.9998 | 53.15 ± 2.44 | <0.0001 |
| H14 | H. leopoldii | 77.85 ± 3.38 | <0.0001 | NA | - |
| H15 | H. mollevillquense | 36.52 ± 2.85 | <0.0001 | NA | - |
| H16 | H. nelsonii | 51.66 ± 3.89 | <0.0001 | 116.02 ± 5.00 | <0.0001 |
| H18 | H. pardinum | 102.90 ± 4.76 | <0.0001 | NA | - |
| H19 | H. parodii | 35.13 ± 1.75 | <0.0001 | 119.23 ± 1.95 | <0.0001 |
| H20 | H. psittacinum | 94.45 ± 6.22 | <0.0001 | NA | - |
| H21 | H. puniceum | 141.46 ± 8.01 | <0.0001 | NA | - |
| H23 | H. sp. | 33.58 ± 1.18 | <0.0001 | 111.70 ± 5.20 | <0.0001 |
| H24 | H. umabisanum | 162.10 ± 6.12 | <0.0001 | NA | - |
| H25 | H. vittatum | 36.68 ± 3.50 | <0.0001 | 169.65 ± 7.53 | <0.0001 |
| H26 | H. warszewiczianum | 47.43 ± 1.79 | <0.0001 | NA | - |
| H27 | H. yungacense | 6.42 ± 0.21 | 0.3058 | 35.97 ± 0.81 | <0.0001 |
| GAL | Galanthamine | 0.35 ± 0.07 | 3.58 ± 0.14 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Escobar, M.L.; Lara, R.F.; Atahuachi, M.; Fuentes, A.F.; Maldonado, C.; Bastida, J.; Tallini, L.R.; Torras-Claveria, L. Alkaloid Profile Characterisation and Bioactivity Evaluation of Bolivian Hippeastrum Species (Amaryllidaceae) as Cholinesterase Inhibitors. Life 2025, 15, 719. https://doi.org/10.3390/life15050719
Rodríguez-Escobar ML, Lara RF, Atahuachi M, Fuentes AF, Maldonado C, Bastida J, Tallini LR, Torras-Claveria L. Alkaloid Profile Characterisation and Bioactivity Evaluation of Bolivian Hippeastrum Species (Amaryllidaceae) as Cholinesterase Inhibitors. Life. 2025; 15(5):719. https://doi.org/10.3390/life15050719
Chicago/Turabian StyleRodríguez-Escobar, María Lenny, Raúl Fernando Lara, Margoth Atahuachi, Alfredo F. Fuentes, Carla Maldonado, Jaume Bastida, Luciana R. Tallini, and Laura Torras-Claveria. 2025. "Alkaloid Profile Characterisation and Bioactivity Evaluation of Bolivian Hippeastrum Species (Amaryllidaceae) as Cholinesterase Inhibitors" Life 15, no. 5: 719. https://doi.org/10.3390/life15050719
APA StyleRodríguez-Escobar, M. L., Lara, R. F., Atahuachi, M., Fuentes, A. F., Maldonado, C., Bastida, J., Tallini, L. R., & Torras-Claveria, L. (2025). Alkaloid Profile Characterisation and Bioactivity Evaluation of Bolivian Hippeastrum Species (Amaryllidaceae) as Cholinesterase Inhibitors. Life, 15(5), 719. https://doi.org/10.3390/life15050719










