Stress-Induced Depression and Its Effects on Tooth Wear in Rats: A 3D Dental Scan Imaging Perspective
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Body Weight, Food, and Water Measurements
2.3. Stress-Induced Depression
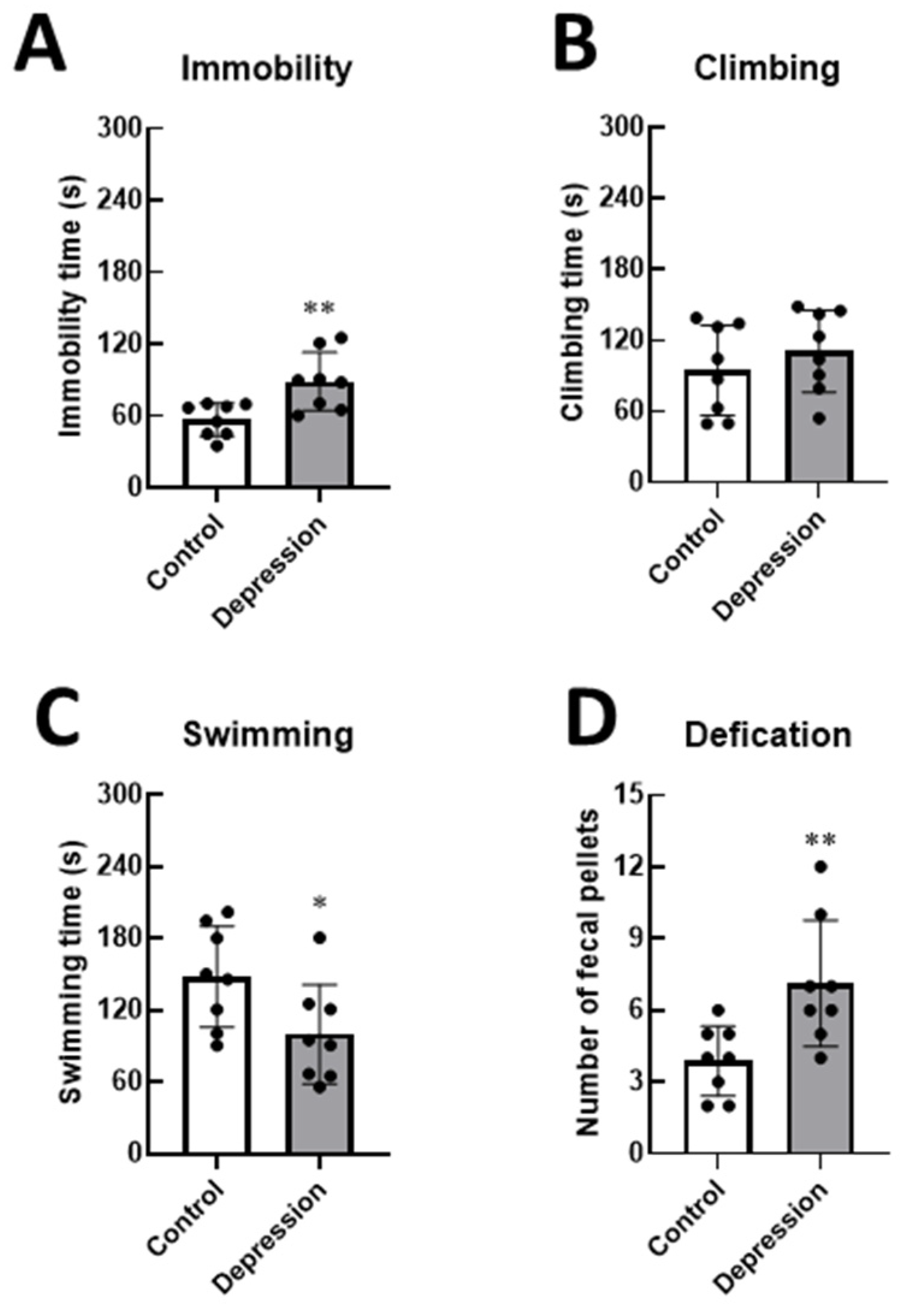
2.4. Forced Swim Test (FST)
2.5. Urinary Corticosterone Assay
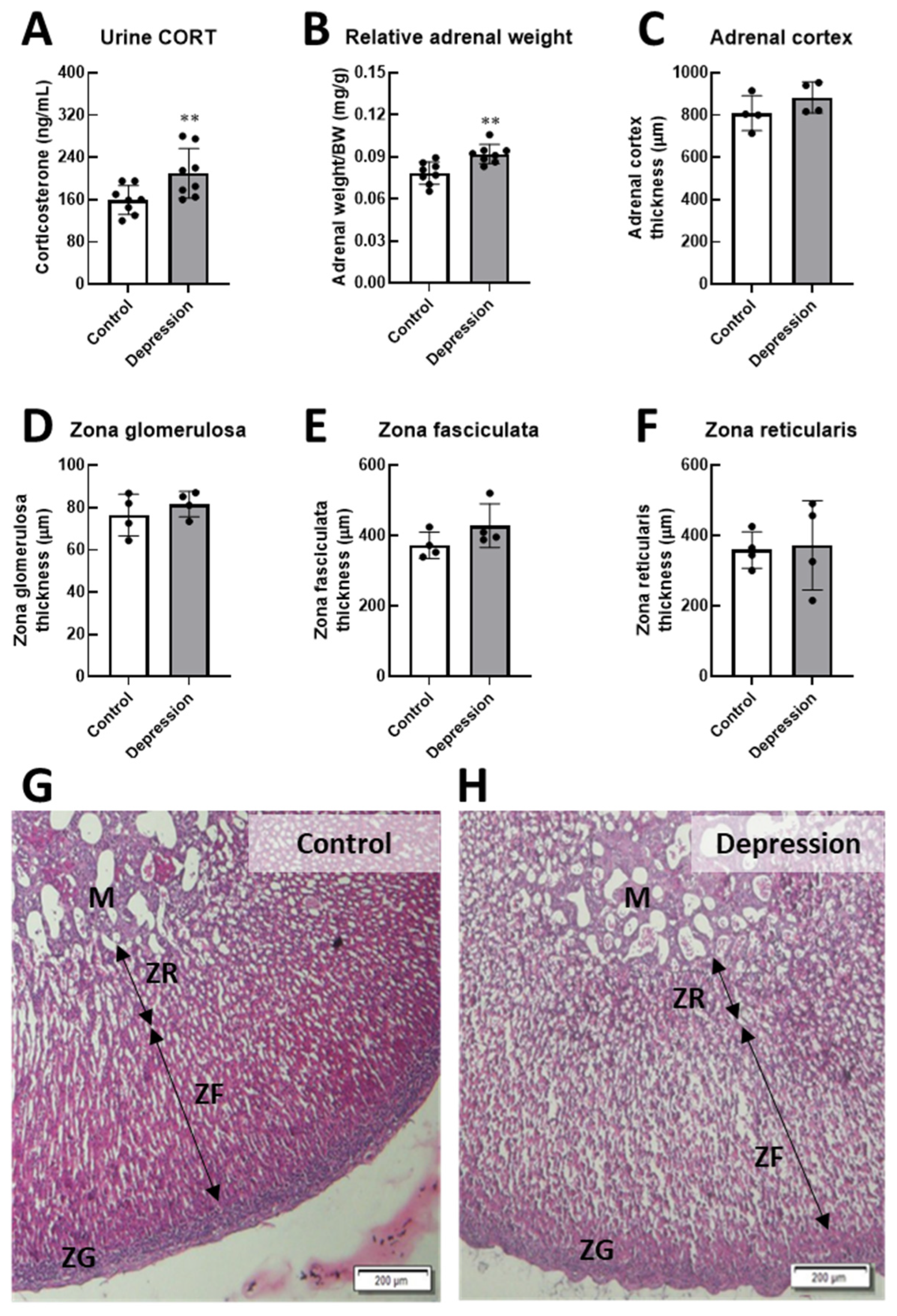
2.6. Adrenal Preparation and Histological Analysis
2.7. 3D Model Acquisition
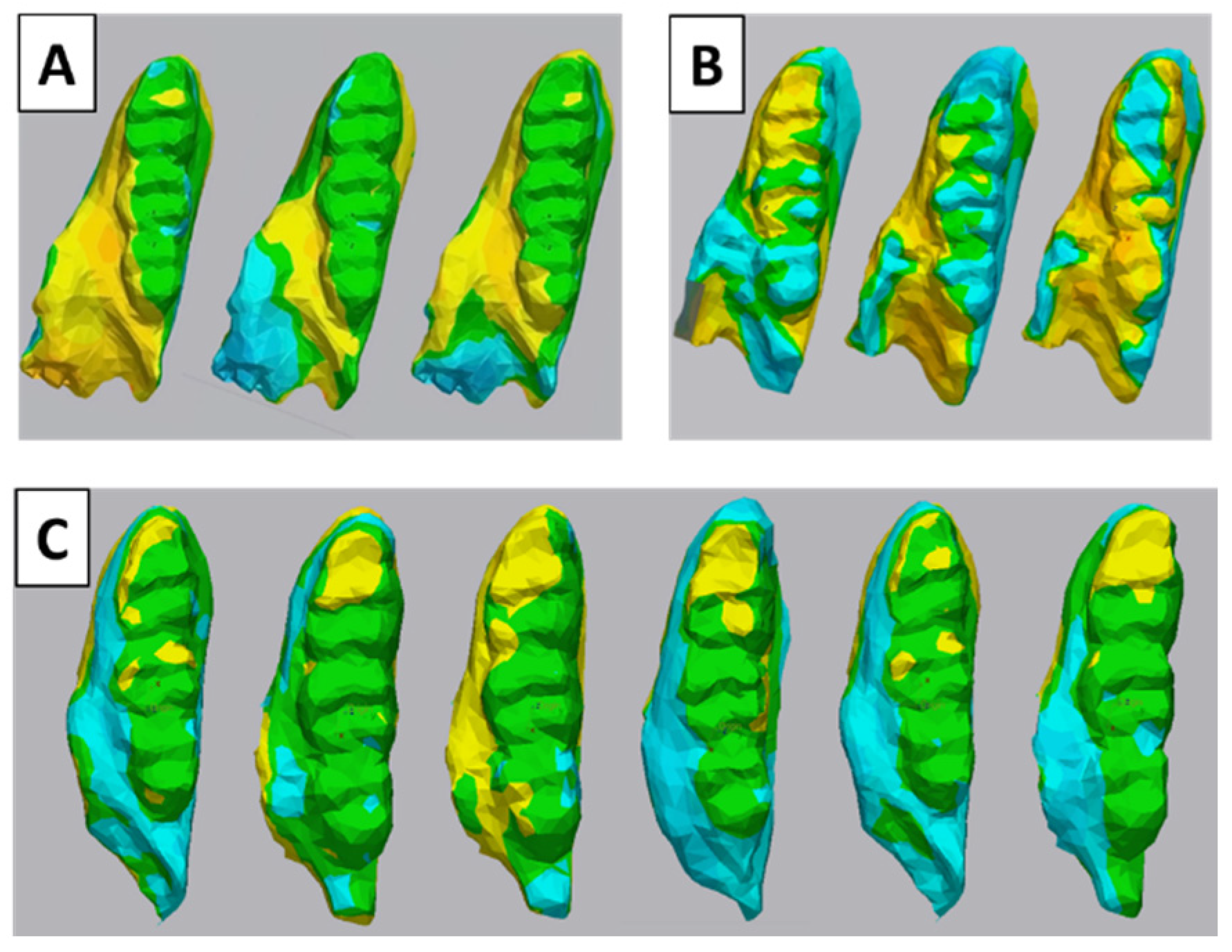
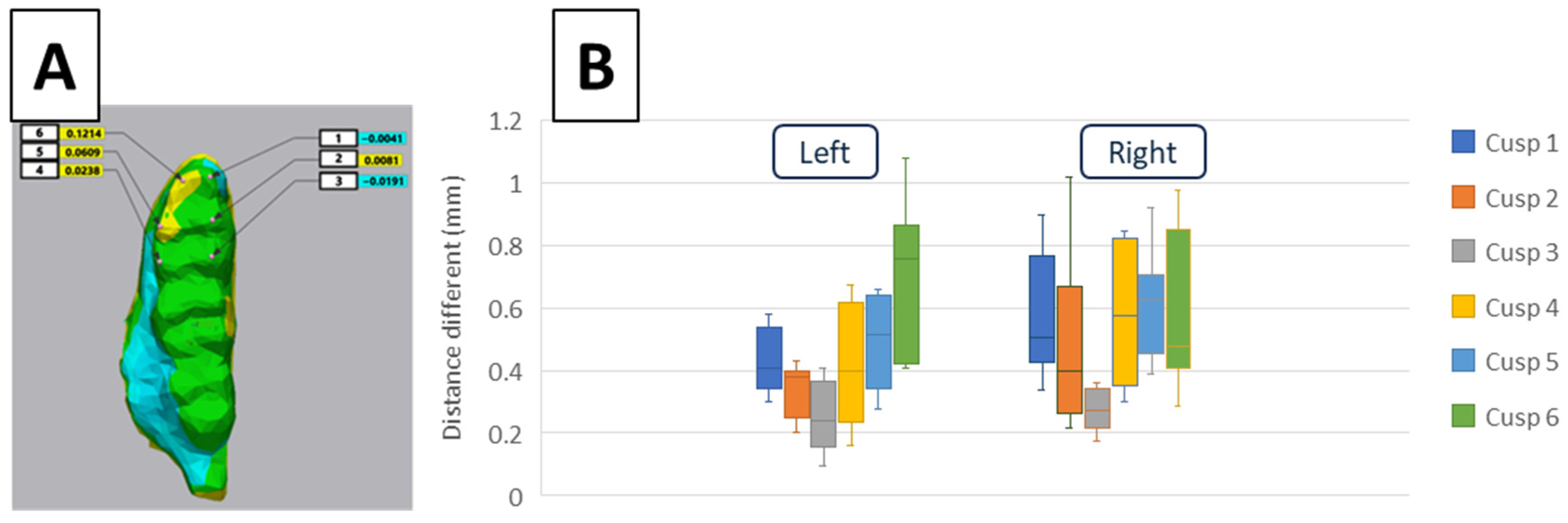
2.8. The 3D Cusps’ Morphologic Analysis
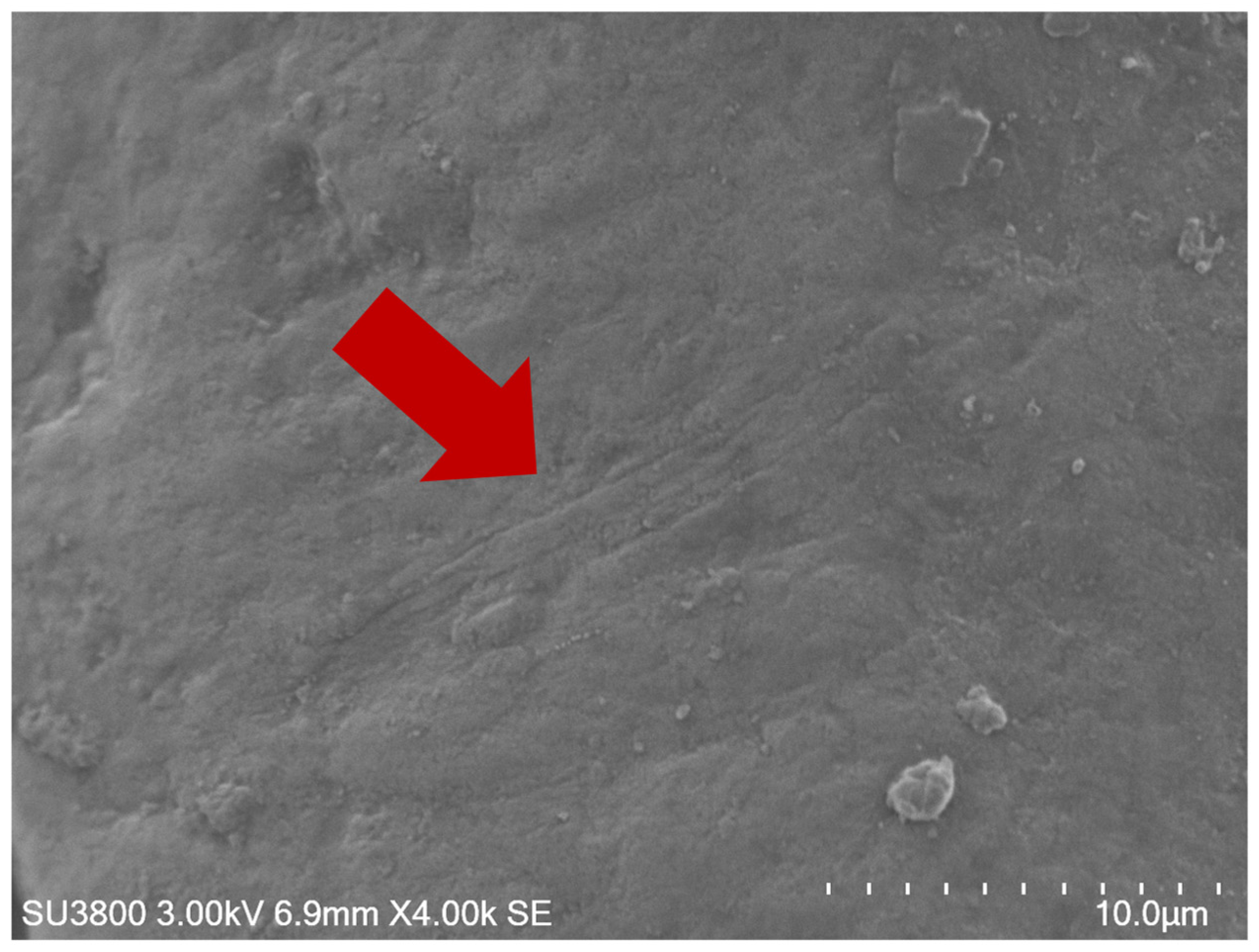
2.9. Scanning Electron Microscopy (SEM)
2.10. Statistical Analysis
3. Results
3.1. Physical and Behavioral Responses in Stress-Induced Depression
3.2. 3D Tooth Wear Analysis
3.3. Microscopic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Major Depression—National Institute of Mental Health (NIMH). Available online: https://www.nimh.nih.gov/health/statistics/major-depression (accessed on 5 March 2025).
- Institute for Health Metrics and Evaluation. Global Health Data Exchange (GHDx). Available online: https://vizhub.healthdata.org/gbd-results (accessed on 5 March 2025).
- Evans-Lacko, S.; Aguilar-Gaxiola, S.; Al-Hamzawi, A.; Alonso, J.; Benjet, C.; Bruffaerts, R.; Chiu, W.T.; Florescu, S.; de Girolamo, G.; Gureje, O.; et al. Socio-Economic Variations in the Mental Health Treatment Gap for People with Anxiety, Mood, and Substance Use Disorders: Results from the WHO World Mental Health (WMH) Surveys. Psychol. Med. 2018, 48, 1560–1571. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, G.; Ginszt, M.; Zawadka, M.; Rutkowska, K.; Podstawka, Z.; Szkutnik, J.; Majcher, P.; Gawda, P. The Relationship between Stress and Masticatory Muscle Activity in Female Students. J. Clin. Med. 2021, 10, 3459. [Google Scholar] [CrossRef]
- Knezevic, E.; Nenic, K.; Milanovic, V.; Knezevic, N.N. The Role of Cortisol in Chronic Stress, Neurodegenerative Diseases, and Psychological Disorders. Cells 2023, 12, 2726. [Google Scholar] [CrossRef]
- Lapmanee, S.; Supkamonseni, N.; Bhubhanil, S.; Treesaksrisakul, N.; Sirithanakorn, C.; Khongkow, M.; Namdee, K.; Surinlert, P.; Tipbunjong, C.; Wongchitrat, P. Stress-Induced Changes in Cognitive Function and Intestinal Barrier Integrity Can Be Ameliorated by Venlafaxine and Synbiotic Supplementations. PeerJ 2024, 12, e17033. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, T.; Kelly, A.; Randall, C.L.; Tranby, E.; Franstve-Hawley, J. Association Between Mental Health and Oral Health Status and Care Utilization. Front. Oral Health 2021, 2, 732882. [Google Scholar] [CrossRef]
- Bafageeh, F.; Loux, T. Depression Symptoms Linked to Multiple Oral Health Outcomes in US Adults. JDR Clin. Trans. Res. 2025, 10, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Cademartori, M.G.; Gastal, M.T.; Nascimento, G.G.; Demarco, F.F.; Corrêa, M.B. Is Depression Associated with Oral Health Outcomes in Adults and Elders? A Systematic Review and Meta-Analysis. Clin. Oral Investig. 2018, 22, 2685–2702. [Google Scholar] [CrossRef]
- Kisely, S.; Sawyer, E.; Siskind, D.; Lalloo, R. The Oral Health of People with Anxiety and Depressive Disorders—A Systematic Review and Meta-Analysis. J. Affect. Disord. 2016, 200, 119–132. [Google Scholar] [CrossRef]
- Songphaeng, T.; Lapmanee, S.; Bhubhanil, S.; Momdee, K.; Rojviriya, C.; Kitsahawong, K.; Chailertvanitkul, P.; Welbat, J.U.; Morkmued, S. Atomoxetine and Escitalopram Migrate the Derangement of the Temporomandibular Joint Morphologic and Histologic Changes in Rats Exposed to Stress-Induced Depression. J. Oral Sci. 2023, 65, 219–225. [Google Scholar] [CrossRef]
- Zieliński, G.; Pająk-Zielińska, B.; Pająk, A.; Wójcicki, M.; Litko-Rola, M.; Ginszt, M. Global Co-Occurrence of Bruxism and Temporomandibular Disorders: A Meta-Regression Analysis. Dent. Med. Probl. 2025; online ahead of print. [Google Scholar] [CrossRef]
- Storari, M.; Serri, M.; Aprile, M.; Denotti, G.; Viscuso, D. Bruxism in Children: What Do We Know? Narrative Review of the Current Evidence. Eur. J. Paediatr. Dent. 2023, 24, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Romero, E.A.; Martínez-Pozas, O.; García-González, M.; de-Pedro, M.; González-Álvarez, M.E.; Esteban-González, P.; Cid-Verdejo, R.; Villafañe, J.H. Association between Sleep Disorders and Sleep Quality in Patients with Temporomandibular Joint Osteoarthritis: A Systematic Review. Biomedicines 2022, 10, 2143. [Google Scholar] [CrossRef]
- Onbasi, Y.; Abu-Hossin, S.; Paulig, M.; Berger, L.; Wichmann, M.; Matta, R.-E. Trueness of Full-Arch Dental Models Obtained by Digital and Conventional Impression Techniques: An in Vivo Study. Sci. Rep. 2022, 12, 22509. [Google Scholar] [CrossRef] [PubMed]
- Gkantidis, N.; Dritsas, K.; Ren, Y.; Halazonetis, D.; Katsaros, C. An Accurate and Efficient Method for Occlusal Tooth Wear Assessment Using 3D Digital Dental Models. Sci. Rep. 2020, 10, 10103. [Google Scholar] [CrossRef]
- Al-Seelawi, Z.; Hermann, N.V.; Peutzfeldt, A.; Baram, S.; Bakke, M.; Sonnesen, L.; Tsakanikou, A.; Rahiotis, C.; Benetti, A.R. Clinical and Digital Assessment of Tooth Wear. Sci. Rep. 2024, 14, 592. [Google Scholar] [CrossRef] [PubMed]
- Lapmanee, S.; Bhubhanil, S.; Sriwong, S.; Khongkow, M.; Namdee, K.; Wongchitrat, P.; Pongkorpsakol, P. Venlafaxine and Synbiotic Attenuated Learned Fearlike Behavior and Recognition Memory Impairment in Immobilized-Stressed Rats. Physiol. Pharmacol. 2023, 27, 171–181. [Google Scholar] [CrossRef]
- Gencturk, S.; Unal, G. Rodent Tests of Depression and Anxiety: Construct Validity and Translational Relevance. Cogn. Affect. Behav. Neurosci. 2024, 24, 191–224. [Google Scholar] [CrossRef]
- Chen, C.P.; Zhang, J.; Zhang, B.; Hassan, M.G.; Hane, K.; Chen, C.C.; Navarro Palacios, A.A.; Kapila, S.; Jheon, A.H.; Goodwin, A.F. Unilateral Loss of Maxillary Molars in Young Mice Leads to Bilateral Condylar Adaptation and Degenerative Disease. JBMR Plus 2022, 6, e10638. [Google Scholar] [CrossRef]
- Pan, S.-Y.; Gu, Y.-R.; Zhao, G.; Wang, Y.; Qin, Z.-H.; Tang, Q.-Y.; Qin, Y.-Y.; Luo, L. NADPH Mimics the Antidepressant Effects of Exercise in a Chronic Unpredictable Stress Rat Model. Biochem. Biophys. Res. Commun. 2024, 731, 150360. [Google Scholar] [CrossRef]
- Becker, L.; Mallien, A.S.; Pfeiffer, N.; Brandwein, C.; Talbot, S.R.; Bleich, A.; Palme, R.; Potschka, H.; Gass, P. Evidence-Based Severity Assessment of the Forced Swim Test in the Rat. PLoS ONE 2023, 18, e0292816. [Google Scholar] [CrossRef]
- Lapointe, J.-M.; Snyder, P.A.; Reagan, W.J. Evaluation of Urinary Corticosterone as a Biomarker of Stress in Rats Using Fenitrothion as a Chemical Stressor. J. Immunotoxicol. 2016, 13, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Lapmanee, S.; Charoenphandhu, J.; Teerapornpuntakit, J.; Krishnamra, N.; Charoenphandhu, N. Agomelatine, Venlafaxine, and Running Exercise Effectively Prevent Anxiety- and Depression-like Behaviors and Memory Impairment in Restraint Stressed Rats. PLoS ONE 2017, 12, e0187671. [Google Scholar] [CrossRef] [PubMed]
- Shan, T.; Ma, J.; Ma, Q.; Guo, K.; Guo, J.; Li, X.; Li, W.; Liu, J.; Huang, C.; Wang, F.; et al. Β2-AR-HIF-1α: A Novel Regulatory Axis for Stress-Induced Pancreatic Tumor Growth and Angiogenesis. Curr. Mol. Med. 2013, 13, 1023–1034. [Google Scholar] [CrossRef]
- Tseilikman, V.; Komelkova, M.; Kondashevskaya, M.V.; Manukhina, E.; Downey, H.F.; Chereshnev, V.; Chereshneva, M.; Platkovskii, P.; Goryacheva, A.; Pashkov, A.; et al. A Rat Model of Post-Traumatic Stress Syndrome Causes Phenotype-Associated Morphological Changes and Hypofunction of the Adrenal Gland. Int. J. Mol. Sci. 2021, 22, 13235. [Google Scholar] [CrossRef] [PubMed]
- Soto-Goñi, X.A.; Alen, F.; Buiza-González, L.; Marcolino-Cruz, D.; Sánchez-Sánchez, T.; Ardizone-García, I.; Aneiros-López, F.; Jiménez-Ortega, L. Adaptive Stress Coping in Awake Bruxism. Front. Neurol. 2020, 11, 564431. [Google Scholar] [CrossRef]
- Chemelo, V.D.S.; Né, Y.G.d.S.; Frazão, D.R.; de Souza-Rodrigues, R.D.; Fagundes, N.C.F.; Magno, M.B.; da Silva, C.M.T.; Maia, L.C.; Lima, R.R. Is There Association Between Stress and Bruxism? A Systematic Review and Meta-Analysis. Front. Neurol. 2020, 11, 590779. [Google Scholar] [CrossRef]
- Xiao, F.; Hu, A.; Meng, B.; Zhang, Y.; Han, W.; Su, J. PVH-Peri5 Pathway for Stress-Coping Oromotor and Anxious Behaviors in Mice. J. Dent. Res. 2023, 102, 227–237. [Google Scholar] [CrossRef]
- Kubo, K.; Iinuma, M.; Chen, H. Mastication as a Stress-Coping Behavior. Biomed. Res. Int. 2015, 2015, 876409. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Xu, X.; Zhou, P.; Zhang, H.; Yang, H.; Zhang, M.; Zhang, J.; Lu, L.; Liu, J.; et al. Long-Term Effect of Bilateral Anterior Elevation of Occlusion on the Temporomandibular Joints. Oral Dis. 2022, 28, 1911–1920. [Google Scholar] [CrossRef]
- Bronkhorst, H.; Kalaykova, S.; Huysmans, M.-C.; Loomans, B.; Pereira-Cenci, T. Tooth Wear and Bruxism: A Scoping Review. J. Dent. 2024, 145, 104983. [Google Scholar] [CrossRef]
- Oudkerk, J.; Grenade, C.; Davarpanah, A.; Vanheusden, A.; Vandenput, S.; Mainjot, A.K. Risk Factors of Tooth Wear in Permanent Dentition: A Scoping Review. J. Oral Rehabil. 2023, 50, 1110–1165. [Google Scholar] [CrossRef] [PubMed]
- Beddis, H.P.; Davies, S.J. Relationships between Tooth Wear, Bruxism and Temporomandibular Disorders. Br. Dent. J. 2023, 234, 422–426. [Google Scholar] [CrossRef]
- Kato, T.; Higashiyama, M.; Katagiri, A.; Toyoda, H.; Yamada, M.; Minota, N.; Katsura-Fuchihata, S.; Zhu, Y. Understanding the Pathophysiology of Sleep Bruxism Based on Human and Animal Studies: A Narrative Review. J. Oral Biosci. 2023, 65, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Buchhardt, J.; Kiess, W.; Körner, A.; Biemann, R.; Hirsch, C.; Child Study Team, L. The Influence of Steroid Hormones on Tooth Wear in Children and in Adolescents. J. Clin. Med. 2022, 11, 3603. [Google Scholar] [CrossRef] [PubMed]
- Sato, C.; Sato, S.; Takashina, H.; Ishii, H.; Onozuka, M.; Sasaguri, K. Bruxism Affects Stress Responses in Stressed Rats. Clin. Oral Investig. 2010, 14, 153–160. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, M.; Chen, Y.-J.; Li, Q.; Wu, A.-Z. Psychological Stress Induces Temporary Masticatory Muscle Mechanical Sensitivity in Rats. J. Biomed. Biotechnol. 2011, 2011, 720603. [Google Scholar] [CrossRef]
- Aung, P.T.; Kato, C.; Fujita, A.; Abe, Y.; Ogawa, T.; Ishidori, H.; Okihara, H.; Kokai, S.; Ono, T. Effects of Low Occlusal Loading on the Neuromuscular Behavioral Development of Cortically-Elicited Jaw Movements in Growing Rats. Sci. Rep. 2021, 11, 7175. [Google Scholar] [CrossRef]
- Krivanek, J.; Soldatov, R.A.; Kastriti, M.E.; Chontorotzea, T.; Herdina, A.N.; Petersen, J.; Szarowska, B.; Landova, M.; Matejova, V.K.; Holla, L.I.; et al. Dental Cell Type Atlas Reveals Stem and Differentiated Cell Types in Mouse and Human Teeth. Nat. Commun. 2020, 11, 4816. [Google Scholar] [CrossRef]
- Güven, M.; Elalmiş, D.D.; Binokay, S.; Tan, U. Population-Level Right-Paw Preference in Rats Assessed by a New Computerized Food-Reaching Test. Int. J. Neurosci. 2003, 113, 1675–1689. [Google Scholar] [CrossRef]
- Nissan, J.; Gross, M.D.; Shifman, A.; Tzadok, L.; Assif, D. Chewing Side Preference as a Type of Hemispheric Laterality. J. Oral Rehabil. 2004, 31, 412–416. [Google Scholar] [CrossRef]
- Martins, F.L.P.d.S.P.; Inete, M.B.; Souza, Y.D.d.E.S.; Costa, R.L.V.; Gabbay, R.D.; Moraes, T.M.; Costa, V.V.L.; Paracampo, C.C.P.; Albuquerque, L.C.d.; Gomes, D.L. Association between Self-Perception of Chewing, Chewing Behavior, and the Presence of Gastrointestinal Symptoms in Candidates for Bariatric Surgery. Nutrients 2024, 16, 1096. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, J.; Huang, D.; Wang, Z.; Hu, M.; Liu, H.; Jiang, H. A Comparative Study of Condyle Position in Temporomandibular Disorder Patients with Chewing Side Preference Using Cone-Beam Computed Tomography. J. Oral Rehabil. 2022, 49, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Sritara, S.; Matsumoto, Y.; Lou, Y.; Qi, J.; Aida, J.; Ono, T. Association between the Temporomandibular Joint Morphology and Chewing Pattern. Diagnostics 2023, 13, 2177. [Google Scholar] [CrossRef]
- Tiwari, S.; Nambiar, S.; Unnikrishnan, B. Chewing Side Preference—Impact on Facial Symmetry, Dentition and Temporomandibular Joint and Its Correlation with Handedness. J. Orofac. Sci. 2017, 9, 22. [Google Scholar] [CrossRef]
- Lewis, R.; Dwyer-Joyce, R.S. Wear of Human Teeth: A Tribological Perspective. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2005, 219, 1–18. [Google Scholar] [CrossRef]
- Levrini, L.; Di Benedetto, G.; Raspanti, M. Dental Wear: A Scanning Electron Microscope Study. BioMed Res. Int. 2014, 2014, 340425. [Google Scholar] [CrossRef]
- Wetselaar, P.; Manfredini, D.; Ahlberg, J.; Johansson, A.; Aarab, G.; Papagianni, C.E.; Reyes Sevilla, M.; Koutris, M.; Lobbezoo, F. Associations between Tooth Wear and Dental Sleep Disorders: A Narrative Overview. J. Oral Rehabil. 2019, 46, 765–775. [Google Scholar] [CrossRef]
- Díaz-Flores García, V.; Freire, Y.; David Fernández, S.; Gómez Sánchez, M.; Tomás Murillo, B.; Suárez, A. Intraoral Scanning for Monitoring Dental Wear and Its Risk Factors: A Prospective Study. Healthcare 2024, 12, 1069. [Google Scholar] [CrossRef]
- Schlenz, M.A.; Schlenz, M.B.; Wöstmann, B.; Glatt, A.S.; Ganss, C. Intraoral Scanner-Based Monitoring of Tooth Wear in Young Adults: 24-Month Results. Clin. Oral Investig. 2023, 27, 2775–2785. [Google Scholar] [CrossRef]
- Schlenz, M.A.; Schlenz, M.B.; Wöstmann, B.; Glatt, A.S.; Ganss, C. Intraoral Scanner-Based Monitoring of Tooth Wear in Young Adults: 36-Month Results. Clin. Oral Investig. 2024, 28, 350. [Google Scholar] [CrossRef]
- van Nistelrooij, N.; Maier, E.; Bronkhorst, H.; Crins, L.; Xi, T.; Loomans, B.A.C.; Vinayahalingam, S. Automated Monitoring of Tooth Wear Progression Using AI on Intraoral Scans. J. Dent. 2024, 150, 105323. [Google Scholar] [CrossRef] [PubMed]
- Ungar, P.S. Mammalian Dental Function and Wear: A Review. Biosurf. Biotribol. 2015, 1, 25–41. [Google Scholar] [CrossRef]
- Yen, C.; Valentine, E.P.; Chiang, M.-C. The Use of Transcranial Magnetic Stimulation in Attention Optimization Research: A Review from Basic Theory to Findings in Attention-Deficit/Hyperactivity Disorder and Depression. Life 2024, 14, 329. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plongniras, P.; Lapmanee, S.; Thonapan, N.; Thangsombat, P.; Janthaphim, P.; Lertkarnvijai, C.; Chailertvanitkul, P.; Morkmued, S. Stress-Induced Depression and Its Effects on Tooth Wear in Rats: A 3D Dental Scan Imaging Perspective. Life 2025, 15, 712. https://doi.org/10.3390/life15050712
Plongniras P, Lapmanee S, Thonapan N, Thangsombat P, Janthaphim P, Lertkarnvijai C, Chailertvanitkul P, Morkmued S. Stress-Induced Depression and Its Effects on Tooth Wear in Rats: A 3D Dental Scan Imaging Perspective. Life. 2025; 15(5):712. https://doi.org/10.3390/life15050712
Chicago/Turabian StylePlongniras, Preeyarat, Sarawut Lapmanee, Natchayaporn Thonapan, Phuripong Thangsombat, Phongsakorn Janthaphim, Chanakarn Lertkarnvijai, Pattama Chailertvanitkul, and Supawich Morkmued. 2025. "Stress-Induced Depression and Its Effects on Tooth Wear in Rats: A 3D Dental Scan Imaging Perspective" Life 15, no. 5: 712. https://doi.org/10.3390/life15050712
APA StylePlongniras, P., Lapmanee, S., Thonapan, N., Thangsombat, P., Janthaphim, P., Lertkarnvijai, C., Chailertvanitkul, P., & Morkmued, S. (2025). Stress-Induced Depression and Its Effects on Tooth Wear in Rats: A 3D Dental Scan Imaging Perspective. Life, 15(5), 712. https://doi.org/10.3390/life15050712








