Effects of Resistance Training on Sarcopenia Risk Among Healthy Older Adults: A Scoping Review of Physiological Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
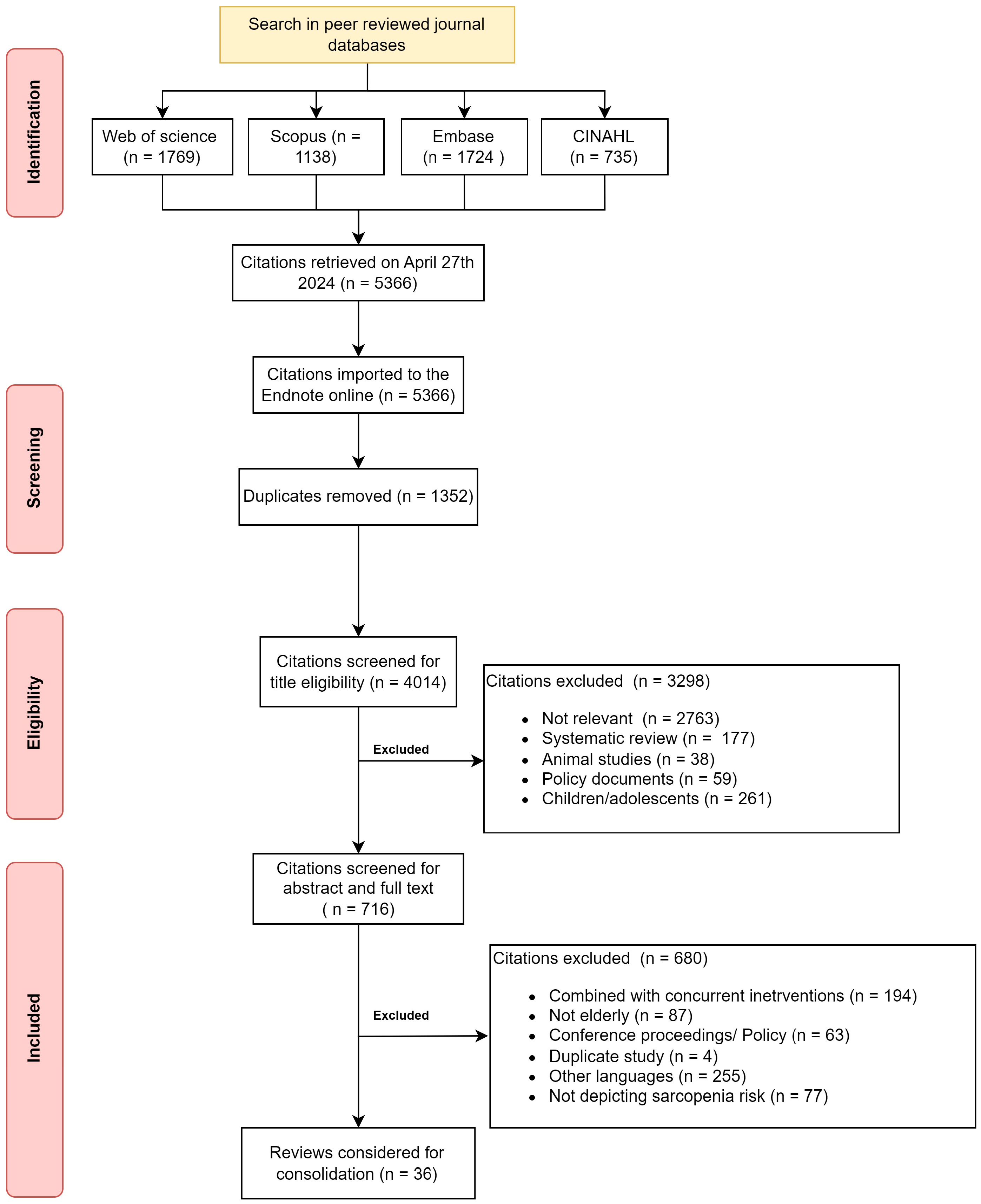
2.1. Information Sources and Search
2.2. Eligibility Criteria and Source Selection
2.3. Data Charting Process and Data Items
2.4. Synthesis of Results
3. Results
3.1. Characteristics of the Included Studies
3.2. Population
3.3. Intervention
3.4. Outcomes
3.5. Key Findings
3.5.1. Positive Findings
3.5.2. Null Findings
4. Discussion
4.1. Dose of Resistance Exercise Program
4.2. Caution with Resistance Exercise Training
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMC | bone mineral content |
| BMI | body mass index |
| BP | blood pressure |
| CG | control group |
| COP | center of pressure |
| CRP | C-reactive protein |
| DEXA | dual energy X-ray absorptiometry |
| EMG | electromyography |
| FEV | force expiratory volume |
| FFA | free fatty acid |
| HIIRT | high-intensity interval resistance training |
| HSP | heat shock proteins |
| IGF | insulin like growth factor |
| IL | interleukin |
| IPAQ | international physical activity questionnaire |
| LIPA | light-intensity physical activity |
| MEP | maximal expiratory pressure |
| MET | metabolic equivalent |
| MIP | maximal expiratory pressure |
| MVPA | moderate to vigorous physical activity |
| MVV | maximal voluntary ventilation |
| MWD | minute walk distance |
| PA | physical activity |
| PRT | progressive resistance training |
| RM | repetition maximum |
| ROS | reactive oxygen species |
| RPE | rate of perceived exertion |
| RT | resistance training |
| SBP | systolic blood pressure |
| SMI | skeletal muscle index |
| SPPB | short physical performance battery |
| STS | sit to stand |
| TG | triglycerides |
| TMT | trail making test |
| TNF | tissue necrosis factor |
| TUG | Time-Up-Go test |
| VAT | visceral adipose tissue |
| VO2 | oxygen consumed for the workload |
References
- Vezzoli, A.; Mrakic-Sposta, S.; Montorsi, M.; Porcelli, S.; Vago, P.; Cereda, F.; Longo, S.; Maggio, M.; Narici, M. Moderate Intensity Resistive Training Reduces Oxidative Stress and Improves Muscle Mass and Function in Older Individuals. Antioxidants 2019, 8, 431. [Google Scholar] [CrossRef] [PubMed]
- Yeung, S.S.Y.; Reijnierse, E.M.; Pham, V.K.; Trappenburg, M.C.; Lim, W.K.; Meskers, C.G.M.; Maier, A.B. Sarcopenia and its association with falls and fractures in older adults: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2019, 10, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Volpi, E.; Nazemi, R.; Fujita, S. Muscle tissue changes with aging. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Billot, M.; Calvani, R.; Urtamo, A.; Sánchez-Sánchez, J.L.; Ciccolari-Micaldi, C.; Chang, M.; Roller-Wirnsberger, R.; Wirnsberger, G.; Sinclair, A.; Vaquero-Pinto, N.; et al. Preserving Mobility in Older Adults with Physical Frailty and Sarcopenia: Opportunities, Challenges, and Recommendations for Physical Activity Interventions. Clin. Interv. Aging 2020, 15, 1675–1690. [Google Scholar] [CrossRef]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Heidari, D.; Shirvani, H.; Bazgir, B.; Shamsoddini, A. The Resistance Training Effects on Skeletal Muscle Stem Cells in Older Adult: A Systematic Review and Meta-Analysis. Cell J. 2023, 25, 513–523. [Google Scholar] [CrossRef]
- Lidegaard, M.; Jensen, R.B.; Andersen, C.H.; Zebis, M.K.; Colado, J.C.; Wang, Y.; Heilskov-Hansen, T.; Andersen, L.L. Effect of brief daily resistance training on occupational neck/shoulder muscle activity in office workers with chronic pain: Randomized controlled trial. BioMed Res. Int. 2013, 2013, 262386. [Google Scholar] [CrossRef]
- Ho, S.Y.; Chung, Y.C.; Wu, H.J.; Ho, C.C.; Chen, H.T. Effect of high intensity circuit training on muscle mass, muscular strength, and blood parameters in sedentary workers. PeerJ 2024, 12, e17140. [Google Scholar] [CrossRef]
- Perreault, K.; Courchesne-Loyer, A.; Fortier, M.; Maltais, M.; Barsalani, R.; Riesco, E.; Dionne, I.J. Sixteen weeks of resistance training decrease plasma heat shock protein 72 (eHSP72) and increase muscle mass without affecting high sensitivity inflammatory markers’ levels in sarcopenic men. Aging Clin. Exp. Res. 2016, 28, 207–214. [Google Scholar] [CrossRef]
- Heo, S.-J.; Jee, Y.-S. Intensity-effects of strengthening exercise on thigh muscle volume, pro- or anti-inflammatory cytokines, and immunocytes in the older adults: A randomized controlled trial. Arch. Gerontol. Geriatr. 2024, 116, 105136. [Google Scholar] [CrossRef]
- Kong, L.N.; Lyu, Q.; Liu, D.X.; Hu, P. Effects of exercise interventions on physical, psychological and social outcomes in frail older adults: An overview of systematic reviews. J. Clin. Nurs. 2024. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Gu, H.; Cai, X.; Zhang, Y.; Hou, X.; Yu, J.; Sun, T. The Effects of Exercise for Cognitive Function in Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2023, 20, 1088. [Google Scholar] [CrossRef]
- Khodadad Kashi, S.; Mirzazadeh, Z.S.; Saatchian, V. A Systematic Review and Meta-Analysis of Resistance Training on Quality of Life, Depression, Muscle Strength, and Functional Exercise Capacity in Older Adults Aged 60 Years or More. Biol. Res. Nurs. 2023, 25, 88–106. [Google Scholar] [CrossRef] [PubMed]
- Yuenyongchaiwat, K.; Akekawatchai, C.; Khattiya, J. Effects of a Pedometer-Based Walking Home Program Plus Resistance Training on Inflammatory Cytokines and Depression in Thai Older People with Sarcopenia: A Three-Arm Randomized Controlled Trial. Clin. Gerontol. 2023, 46, 717–728. [Google Scholar] [CrossRef]
- Vikberg, S.; Sörlén, N.; Brandén, L.; Johansson, J.; Nordström, A.; Hult, A.; Nordström, P. Effects of Resistance Training on Functional Strength and Muscle Mass in 70-Year-Old Individuals With Pre-sarcopenia: A Randomized Controlled Trial. J. Am. Med. Dir. Assoc. 2019, 20, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Van Roie, E.; Delecluse, C.; Coudyzer, W.; Boonen, S.; Bautmans, I. Strength training at high versus low external resistance in older adults: Effects on muscle volume, muscle strength, and force-velocity characteristics. Exp. Gerontol. 2013, 48, 1351–1361. [Google Scholar] [CrossRef]
- Abreu, E.L.; An-Lin, C.; Kelly, P.J.; Chertoff, K.; Brotto, L.; Griffith, E.; Kinder, G.; Uridge, T.; Zachow, R.; Brotto, M. Skeletal Muscle Troponin as a Novel Biomarker to Enhance Assessment of the Impact of Strength Training on Fall Prevention in the Older Adults. Nurs. Res. 2014, 63, 75–82. [Google Scholar] [CrossRef]
- Adnan, R.; Din, H.M.; Ashari, A.; Minhat, H.S. Effectiveness of a Community-Based Muscle Strengthening Exercise Program to Increase Muscle Strength Among Pre-frail Older Persons in Malaysia: A Pilot Study. Front. Public Health 2021, 9, 610184. [Google Scholar] [CrossRef]
- Akatsu, H.; Manabe, T.; Kawade, Y.; Masaki, Y.; Hoshino, S.; Jo, T.; Kobayashi, S.; Hayakawa, T.; Ohara, H. Effect of Ankle Weights as a Frailty Prevention Strategy in the Community-Dwelling Elderly: A Preliminary Report. Int. J. Environ. Res. Public Health 2022, 19, 7350. [Google Scholar] [CrossRef]
- Aragao-Santos, J.C.; De Resende-Neto, A.G.; Nogueira, A.C.; Feitosa-Neta, M.D.; Brandao, L.H.; Chaves, L.M.; Da Silva-Grigoletto, M.E. The effects of functional and traditional strength training on different strength parameters of elderly women: A randomized and controlled trial. J. Sports Med. Phys. Fit. 2019, 59, 380–386. [Google Scholar] [CrossRef]
- Baggen, R.J.; Van Roie, E.; van Dieën, J.H.; Verschueren, S.M.; Delecluse, C. Weight bearing exercise can elicit similar peak muscle activation as medium-high intensity resistance exercise in elderly women. Eur. J. Appl. Physiol. 2018, 118, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, A.; Krawczyk, S.N.; Potiaumpai, M.; Signorile, J.F. High-speed circuit training vs hypertrophy training to improve physical function in sarcopenic obese adults: A randomized controlled trial. Exp. Gerontol. 2014, 60, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Binder, E.F.; Yarasheski, K.E.; Steger-May, K.; Sinacore, D.R.; Brown, M.; Schechtman, K.B.; Holloszy, J.O. Effects of progressive resistance training on body composition in frail older adults: Results of a randomized, controlled trial. J. Gerontol.—Ser. A Biol. Sci. Med. Sci. 2005, 60, 1425–1431. [Google Scholar] [CrossRef]
- Candow, D.G.; Chilibeck, P.D.; Abeysekara, S.; Zello, G.A. Short-term heavy resistance training eliminates age-related deficits in muscle mass and strength in healthy older males. J. Strength Cond. Res. 2011, 25, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Cebrià I Iranzo, M.À.; Balasch-Bernat, M.; Tortosa-Chuliá, M.Á.; Balasch-Parisi, S. Effects of Resistance Training of Peripheral Muscles Versus Respiratory Muscles in Older Adults With Sarcopenia Who are Institutionalized: A Randomized Controlled Trial. J. Aging Phys. Act. 2018, 26, 637–646. [Google Scholar] [CrossRef]
- Chang, S.F.; Chiu, S.C. Effect of resistance training on quality of life in older people with sarcopenic obesity living in long-term care institutions: A quasi-experimental study. J. Clin. Nurs. 2020, 29, 2544–2556. [Google Scholar] [CrossRef]
- Chun-De, L.; Jau-Yih, T.; Li-Fong, L.; Shih-Wei, H.; Jan-Wen, K.; Lin-Chuan, C.; Tsan-Hon, L.; Liao, C.-D.; Tsauo, J.-Y.; Lin, L.-F.; et al. Effects of elastic resistance exercise on body composition and physical capacity in older women with sarcopenic obesity: A CONSORT-compliant prospective randomized controlled trial. Medicine 2017, 96, e7115. [Google Scholar] [CrossRef]
- Conlon, J.; Newton, R.; Tufano, J.; Peñailillo, L.; Banyard, H.; Hopper, A.; Ridge, A.; Haff, G.; Conlon, J.A.; Newton, R.U.; et al. The efficacy of periodised resistance training on neuromuscular adaptation in older adults. Eur. J. Appl. Physiol. 2017, 117, 1181–1194. [Google Scholar] [CrossRef]
- de Almeida, S.S.; Teixeira, E.L.; Merege, C.A.A.; Brucki, S.M.D.; Painelli, V.D. Acute Effects of Resistance and Functional-Task Exercises on Executive Function of Obese Older Adults: Two Counterbalanced, Crossover, Randomized Exploratory Studies. Sport Exerc. Perform. Psychol. 2021, 10, 102–113. [Google Scholar] [CrossRef]
- de Sá Souza, H.; de Melo, C.M.; Piovezan, R.D.; Miranda, R.E.E.P.C.; Carneiro-Junior, M.A.; Silva, B.M.; Thomatieli-Santos, R.V.; Tufik, S.; Poyares, D.; D’Almeida, V. Resistance Training Improves Sleep and Anti-Inflammatory Parameters in Sarcopenic Older Adults: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2022, 19, 16322. [Google Scholar] [CrossRef]
- Dinh, H.C.; Bautmans, I.; Beyer, I.; Onyema, O.O.; Liberman, K.; De Dobbeleer, L.; Renmans, W.; Vander Meeren, S.; Jochmans, K.; Delaere, A.; et al. Six weeks of strength endurance training decreases circulating senescence-prone T-lymphocytes in cytomegalovirus seropositive but not seronegative older women. Immun. Ageing 2019, 16, 17. [Google Scholar] [CrossRef]
- Flor-Rufino, C.; Barrachina-Igual, J.; Pérez-Ros, P.; Pablos-Monzó, A.; Martínez-Arnau, F.M. Resistance training of peripheral muscles benefits respiratory parameters in older women with sarcopenia: Randomized controlled trial. Arch. Gerontol. Geriatr. 2023, 104, 104799. [Google Scholar] [CrossRef] [PubMed]
- Gadelha, A.B.; Paiva, F.M.L.; Gauche, R.; de Oliveira, R.J.; Lima, R.M. Effects of resistance training on sarcopenic obesity index in older women: A randomized controlled trial. Arch. Gerontol. Geriatr. 2016, 65, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Ghasemikaram, M.; Chaudry, O.; Nagel, A.M.; Uder, M.; Jakob, F.; Kemmler, W.; Kohl, M.; Engelke, K. Effects of 16 months of high intensity resistance training on thigh muscle fat infiltration in elderly men with osteosarcopenia. GeroScience 2021, 43, 607–617. [Google Scholar] [CrossRef]
- Kalapotharakos, V.I.; Diamantopoulos, K.; Tokmakidis, S.P. Effects of resistance training and detraining on muscle strength and functional performance of older adults aged 80 to 88 years. Aging Clin. Exp. Res. 2010, 22, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.X.; Bo, L.; Zhu, H.W.; Chen, B.Y.; Wu, Z.; Du, H.D.; Huo, X.P. Effects of lower limb resistance exercise on muscle strength, physical fitness, and metabolism in pre-frail elderly patients: A randomized controlled trial. BMC Geriatr. 2021, 21, 447. [Google Scholar] [CrossRef]
- Nagai, K.; Miyamato, T.; Okamae, A.; Tamaki, A.; Fujioka, H.; Wada, Y.; Uchiyama, Y.; Shinmura, K.; Domen, K. Physical activity combined with resistance training reduces symptoms of frailty in older adults: A randomized controlled trial. Arch. Gerontol. Geriatr. 2018, 76, 41–47. [Google Scholar] [CrossRef]
- Perkin, O.J.; McGuigan, P.M.; Stokes, K.A. Exercise Snacking to Improve Muscle Function in Healthy Older Adults: A Pilot Study. J. Aging Res. 2019, 2019, 7516939. [Google Scholar] [CrossRef]
- Rabelo, H.T.; Bezerra, L.A.; Terra, D.F.; Lima, R.M.; Silva, M.A.F.; Leite, T.K.; De Oliveira, R.J. Effects of 24 weeks of progressive resistance training on knee extensors peak torque and fat-free mass in older women. J. Strength Cond. Res. 2011, 25, 2298–2303. [Google Scholar] [CrossRef]
- Ramirez-Campillo, R.; Alvarez, C.; Garcìa-Hermoso, A.; Celis-Morales, C.; Ramirez-Velez, R.; Gentil, P.; Izquierdo, M. High-speed resistance training in elderly women: Effects of cluster training sets on functional performance and quality of life. Exp. Gerontol. 2018, 110, 216–222. [Google Scholar] [CrossRef]
- Ribeiro, A.S.; Picoloto, A.; Nunes, J.P.; Bezerra, E.S.; Schoenfeld, B.J.; Cyrino, E.S. Effects of Different Resistance Training Loads on the Muscle Quality Index in Older Women. J. Strength Cond. Res. 2022, 36, 1445–1449. [Google Scholar] [CrossRef] [PubMed]
- Saeterbakken, A.H.; Bårdstu, H.B.; Brudeseth, A.; Andersen, V. Effects of Strength Training on Muscle Properties, Physical Function, and Physical Activity among Frail Older People: A Pilot Study. J. Aging Res. 2018, 2018, 8916274. [Google Scholar] [CrossRef] [PubMed]
- Schulte, J.N.; Yarasheski, K.E. Effects of resistance training on the rate of muscle protein synthesis in frail elderly people. Int. J. Sport Nutr. Exerc. Metab. 2001, 11, S111–S118. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.W.; Jung, S.W.; Kim, S.W.; Lee, J.M.; Jung, H.C.; Song, J.K. Effects of 16 weeks of resistance training on muscle quality and muscle growth factors in older adult women with sarcopenia: A randomized controlled trial. Int. J. Environ. Res. Public Health 2021, 18, 6762. [Google Scholar] [CrossRef]
- Silva, A.C.; Pereira, M.A.; Peixoto, L.M.; Rosse, I.C.; Júnior, J.B.F.; de Oliveira, E.C.; Becker, L.K.; Coelho, D.B. 12 weeks of resistance training with progressive intensity improves the diagnostic parameters of sarcopenia in individuals of advanced age. Geriatr. Nurs. 2023, 54, 60–65. [Google Scholar] [CrossRef]
- Stoever, K.; Heber, A.; Eichberg, S.; Brixius, K. Influences of Resistance Training on Physical Function in Older, Obese Men and Women With Sarcopenia. J. Geriatr. Phys. Ther. 2018, 41, 20–27. [Google Scholar] [CrossRef]
- Banitalebi, E.; Ghahfarrokhi, M.M.; Dehghan, M. Effect of 12-weeks elastic band resistance training on MyomiRs and osteoporosis markers in elderly women with Osteosarcopenic obesity: A randomized controlled trial. BMC Geriatr. 2021, 21, 433. [Google Scholar] [CrossRef]
- Chang, M.C.; Lee, A.Y.; Kwak, S.; Kwak, S.G. Effect of Resistance Exercise on Depression in Mild Alzheimer Disease Patients With Sarcopenia. Am. J. Geriatr. Psychiatry 2020, 28, 587–589. [Google Scholar] [CrossRef]
- Yuenyongchaiwat, K.; Akekawatchai, C. Beneficial effects of walking-based home program for improving cardio-respiratory performance and physical activity in sarcopenic older people: A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2022, 58, 838–844. [Google Scholar] [CrossRef]
- Sun, R.; Wan, J.; Tang, J.; Deng, Y.; Zhang, M.; Liu, C.; Li, J.; Zhang, Q. Effectiveness of resistance training on body composition, muscle strength, and biomarker in sarcopenic older adults: A meta-analysis of randomized controlled trials. Arch. Gerontol. Geriatr. 2025, 128, 105595. [Google Scholar] [CrossRef]
- Xavier, L.N.; do Nascimento, V.B. Professional Narratives about Older Adults and Health Services Responsive to Fall-Inducing Frailty. Int. J. Environ. Res. Public Health 2023, 20, 6975. [Google Scholar] [CrossRef]
- Borde, R.; Hortobágyi, T.; Granacher, U. Dose-Response Relationships of Resistance Training in Healthy Old Adults: A Systematic Review and Meta-Analysis. Sports Med. 2015, 45, 1693–1720. [Google Scholar] [CrossRef] [PubMed]
- Beijersbergen, C.M.; Granacher, U.; Vandervoort, A.A.; DeVita, P.; Hortobágyi, T. The biomechanical mechanism of how strength and power training improves walking speed in old adults remains unknown. Ageing Res. Rev. 2013, 12, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Rivera, F.B.; Escolano, B.T.; Nifas, F.M.; Choi, S.; Carado, G.P.; Lerma, E.; Vijayaraghavan, K.; Yu, M.G. Interrelationship of Sarcopenia and Cardiovascular Diseases: A Review of Potential Mechanisms and Management. J. ASEAN Fed. Endocr. Soc. 2024, 39, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Mancus, G.C.; Yuen, H.K.; Watson, J.H.; Lake, M.L.; Jenkins, G.R. Changes in cortisol and dehydroepiandrosterone levels immediately after urban park visits. Int. J. Environ. Health Res. 2023, 33, 206–218. [Google Scholar] [CrossRef]
- Momma, H.; Kawakami, R.; Honda, T.; Sawada, S.S. Muscle-strengthening activities are associated with lower risk and mortality in major non-communicable diseases: A systematic review and meta-analysis of cohort studies. Br. J. Sports Med. 2022, 56, 755–763. [Google Scholar] [CrossRef]
- Merz, K.E.; Thurmond, D.C. Role of Skeletal Muscle in Insulin Resistance and Glucose Uptake. Compr. Physiol. 2020, 10, 785–809. [Google Scholar] [CrossRef]
- Salimans, L.; Liberman, K.; Njemini, R.; Kortekaas Krohn, I.; Gutermuth, J.; Bautmans, I. The effect of resistance exercise on the immune cell function in humans: A systematic review. Exp. Gerontol. 2022, 164, 111822. [Google Scholar] [CrossRef]
- Strasser, B.; Siebert, U.; Schobersberger, W. Resistance Training in the Treatment of the Metabolic Syndrome. Sports Med. 2010, 40, 397–415. [Google Scholar] [CrossRef]
- Kristensen, J.; Franklyn-Miller, A. Resistance training in musculoskeletal rehabilitation: A systematic review. Br. J. Sports Med. 2012, 46, 719–726. [Google Scholar] [CrossRef]
- McMaster, D.; Cronin, J.; McGuigan, M. Forms of Variable Resistance Training. Strength Cond. J. 2009, 31, 50–64. [Google Scholar] [CrossRef]
- Hayes, E.J.; Stevenson, E.; Sayer, A.A.; Granic, A.; Hurst, C. Recovery from Resistance Exercise in Older Adults: A Systematic Scoping Review. Sports Med. Open 2023, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T. Selected Methods of Resistance Training for Prevention and Treatment of Sarcopenia. Cells 2022, 11, 1389. [Google Scholar] [CrossRef] [PubMed]

| Framework | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population |
|
|
| Intervention | Any RT program > 4 weeks
|
|
| Comparison |
| |
| Outcomes | Any of the outcomes related to sarcopenia risk
| Psychological outcomes that were not part of the objective |
| Study design | Intervention trials
| Observational trials
|
| Author (Year) | Study Design | Country | Participants | Intervention | Outcome Measures | Key Findings |
|---|---|---|---|---|---|---|
| Abreu et al., 2014 [17] | Non-randomized trial | USA |
|
| Senior Fitness Test
|
|
| Adnan et al., 2021 [18] | Non-randomized trial | Malaysia |
|
|
|
|
| Aragao-Santos et al., 2019 [20] | Non-randomized controlled trial | Brazil |
|
|
| Both interventions increased power, strength, and workability |
| Baggen et al., 2018 [21] | Non-randomized trial | Belgium |
|
|
|
|
| Balachandran et al., 2014 [22] | Non-randomized trial | USA |
|
|
|
|
| Banitalebi et al., 2021 [47] | Randomized controlled trial | Iran |
|
|
|
|
| Binder et al., 2005 [23] | Randomized controlled trial | USA |
|
|
|
|
| Candow et al., 2011 [24] | Randomized controlled trial | Canada |
|
|
| Before training, compared to young adults
|
| Cebrià I Iranzo et al., 2018 [25] | Randomized controlled trial | Spain |
|
|
|
|
| Chang and Chiu, 2020 [26] | Non-randomized trial | Taiwan |
|
|
|
|
| Chun-De et al., 2017 [27] | Randomized controlled trial | Taiwan |
|
|
|
|
| Conlon et al., 2017 [28] | Randomized controlled trial | Australia |
|
|
|
|
| de Almeida et al., 2021 [29] | Randomized controlled trial | Brazil |
| Study 1
| Cognitive function
| Study 1
|
| de Sá Souza et al., 2022 [30] | Randomized controlled trial | Brazil |
|
|
|
|
| Dinh et al., 2019 [31] | Randomized controlled trial | Belgium |
|
|
|
|
| Flor-Rufino et al., 2023 [32] | Randomized controlled trial | Spain |
|
|
|
|
| Gadelha et al., 2016 [33] | Randomized controlled trial | Brazil |
|
|
|
|
| Ghasemikaram et al., 2021 [34] | Randomized controlled trial (FROST) | Germany |
|
|
|
|
| Heo and Jee, 2024 [10] | Randomized controlled trial | Korea |
|
|
|
|
| Kalapotharakos et al., 2010 [35] | Randomized controlled trial | Greece |
|
|
| RT and RDT at 8th week
|
| Lai et al., 2021 [36] | Randomized controlled trial | China |
|
|
|
|
| Nagai et al., 2018 [37] | Randomized controlled trial | Japan |
|
|
|
|
| Perkin et al., 2019 [38] | Non-randomized trial | UK |
|
|
|
|
| Perreault et al., 2016 [9] | Non-randomized trial | Canada |
|
|
|
|
| Rabelo et al., 2011 [39] | Randomized controlled trial | Brazil |
|
|
| RT vs. CG
|
| Ramirez-Campillo et al., 2018 [40] | Randomized controlled trial | Chile |
|
|
|
|
| Ribeiro et al., 2022 [41] | Non-randomized controlled trial | Brazil |
|
|
|
|
| Saeterbakken et al., 2018 [42] | Non-randomized trial | Norway |
|
|
|
|
| Schulte and Yarasheski, 2001 [43] | Non-randomized trial | USA |
|
|
|
|
| Seo et al., 2021 [44] | Non-randomized controlled trial | South Korea |
|
|
|
|
| Silva et al., 2023 [45] | Non-randomized trial | Brazil |
|
|
|
|
| Stoever et al., 2018 [46] | Non-randomized controlled trial | Germany |
| Sarcopenia group
|
|
|
| Van Roie et al., 2013 [16] | Non-randomized controlled trial | Belgium |
|
|
|
|
| Vezzoli et al., 2019 [1] | Randomized controlled trial | Italy |
|
|
|
|
| Vikberg et al., 2019 [15] | Randomized controlled, parallel-group, 2-arm trial | Sweden |
|
|
|
|
| Yuenyongchaiwat et al., 2023 [14] | Randomized controlled trial | Thailand |
|
|
|
|
| Dose of the RT Program | Site of Training | |
| Access to Traditional Gyms | Only Home-Based Programs | |
| Type | Circuit training, progressive | Conventional, progressive |
| Equipment | Machine plates, barbells with incremental weights | Body weights, TheraBands, medicine balls, TRX |
| Intensity | 60–85% of 1-RM, 60–90% of maximal voluntary contraction | Not specific, sometimes based on progressive elastic resistance (different colors) |
| Volume | 8–15 reps/set, 2–3 sets/muscle, eight larger muscles | |
| Progression | 1st two weeks 55–65% 1-RM 12–15 reps, two sets, 3–4 weeks, 65–75% 1-RM, 2–3 sets, 10–12 reps, 5–6 weeks 75–85% 1-RM 8–10 reps/set, 6–8 reps/set, three sets at 6–8 weeks. In the 8th week, new 1-RM test | 1st two weeks, 12–15 reps, two sets progressing to 6–8 reps, three sets at 6–8 weeks, thereby progressing the number of reps and sets as per the individual’s ability |
| Duration per single session | 30–50 min with at least 5 min of warm-up and cool down with stretches | |
| Duration for clinically meaningful change | Eight weeks (6 weeks to 1 year) | 12 weeks (10 weeks to 2 years) |
| Adjunct | Balance and flexibility: Tai-Chi and Yoga | Balance exercises—body support exercises |
| Nutritional supplements | Protein supplements (reinforcing protein synthesis) | Dearth of evidence |
| Group exercise | Not possible | A group of 6–8 members, chair-based or traditional group exercises |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Govindasamy, K.; Rao, C.R.; Chandrasekaran, B.; Parpa, K.; Granacher, U. Effects of Resistance Training on Sarcopenia Risk Among Healthy Older Adults: A Scoping Review of Physiological Mechanisms. Life 2025, 15, 688. https://doi.org/10.3390/life15050688
Govindasamy K, Rao CR, Chandrasekaran B, Parpa K, Granacher U. Effects of Resistance Training on Sarcopenia Risk Among Healthy Older Adults: A Scoping Review of Physiological Mechanisms. Life. 2025; 15(5):688. https://doi.org/10.3390/life15050688
Chicago/Turabian StyleGovindasamy, Karuppasamy, Chythra R. Rao, Baskaran Chandrasekaran, Koulla Parpa, and Urs Granacher. 2025. "Effects of Resistance Training on Sarcopenia Risk Among Healthy Older Adults: A Scoping Review of Physiological Mechanisms" Life 15, no. 5: 688. https://doi.org/10.3390/life15050688
APA StyleGovindasamy, K., Rao, C. R., Chandrasekaran, B., Parpa, K., & Granacher, U. (2025). Effects of Resistance Training on Sarcopenia Risk Among Healthy Older Adults: A Scoping Review of Physiological Mechanisms. Life, 15(5), 688. https://doi.org/10.3390/life15050688









