Assessment of Intraspecific Variability in the Forest Dormouse (Dryomys nitedula) and Woolly Dormouse (Dryomys laniger) from Türkiye and Adjacent Regions Based on Mitochondrial DNA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling, DNA Extraction, and Amplification
2.2. Phylogenetic Analyses, Molecular Dating, and Genetic Structure Assessments
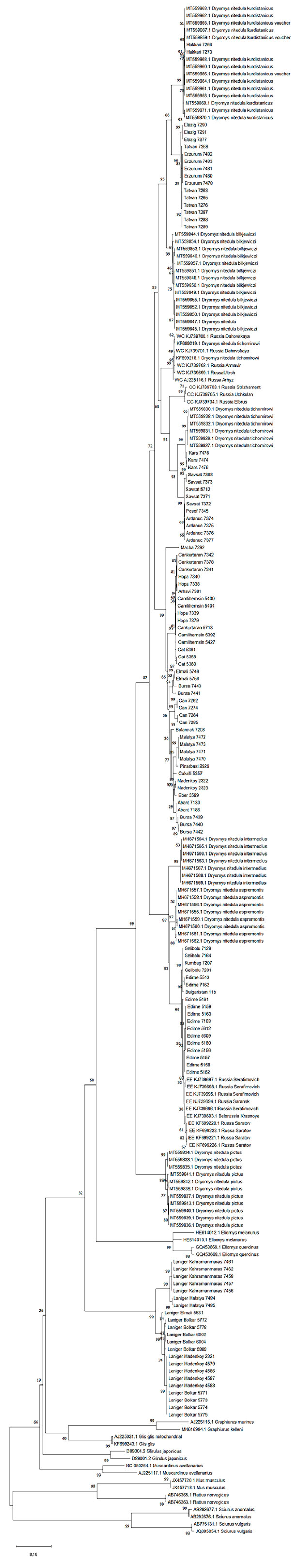
3. Results
3.1. Phylogeny
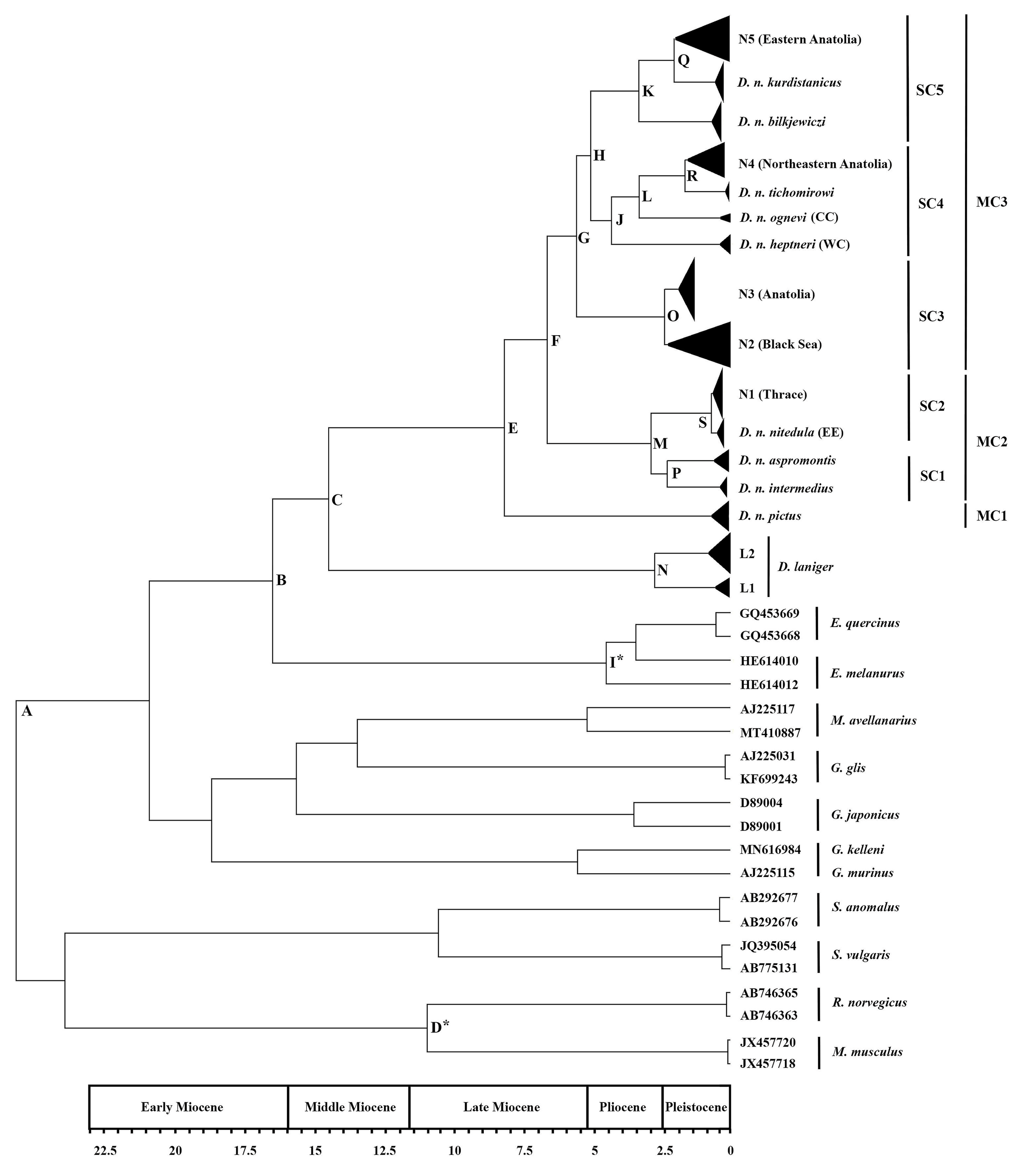
3.2. Divergence Time
| Nodes | Description | NA (Myr) | %95 HPD (Myr) | BPP | K2P (%) |
|---|---|---|---|---|---|
| A | Sciuridae + Mus + Rattus/Gliridae | 27.88 | 22.68–34.24 | 1 | – |
| B | Eliomys/Dryomys | 17.87 | 14.1–22.15 | 1 | – |
| C | D. laniger/D. nitedula | 15.68 | 12.28–19.49 | 0.72 | 20.3 |
| D * | Mus/Rattus | 11.82 | 11.04–12.58 | 1 | – |
| E | D. n. pictus (MC1)/D. nitedula (MC2 + MC3) | 8.82 | 6.79–11.12 | 1 | 12.9 |
| F | MC2/MC3 | 7.15 | 5.56–8.96 | 0.99 | 10.4 |
| G | SC3/SC4 | 6.00 | 4.64–7.46 | 0.99 | – |
| H | SC4/SC5 | 5.45 | 4.21–6.79 | 0.88 | – |
| I * | E. melanurus/E. quercinus | 4.84 | 3.57–6.14 | 1 | – |
| J | WC/CC + D. n. tichomirowi + N4 (northeastern Anatolia) | 4.64 | 3.55–5.86 | 0.99 | 7.19 |
| K | D. n. bilkjewiczi/D. n. kurdistanicus + N5 (eastern Anatolia) | 3.57 | 2.67–4.63 | 1 | 5.47 |
| L | CC/D. n. tichomirowi + N4 (northeastern Anatolia) | 3.56 | 2.67–4.59 | 1 | 6.94 |
| M | SC1/SC2 | 3.09 | 2.07–4.36 | 1 | 5.49 |
| N | L1–L2 | 2.94 | 2.01–3.96 | 1 | 5.57 |
| O | N2 (Black Sea)/N3 (central Anatolia) | 2.56 | 1.89–3.31 | 1 | 4.01 |
| P | D. n. intermedius/D. n. aspromontis | 2.46 | 1.51–3.58 | 0.61 | 4.27 |
| Q | D. n. kurdistanicus + N5 (eastern Anatolia) | 2.12 | 1.57–2.8 | 1 | 4.90 |
| R | D. n. tichomirowi/N4 (northeastern Anatolia) | 1.77 | 1.24–2.36 | 1 | 4.72 |
| S | EE/N1 (Thrace) | 0.74 | 0.47–1.06 | 1 | 1.15 |
3.3. Genetic Diversity
4. Discussion
4.1. Divergence of MC1
4.2. Divergence of MC2
4.3. Divergence of MC3
4.4. Divergence of Dryomys laniger
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CYTB | Cytochrome b |
| MC1 | Major clade 1 |
| MC2 | Major clade 2 |
| MC3 | Major clade 3 |
| SC1 | Subclade 1 |
| SC2 | Subclade 2 |
| SC3 | Subclade 3 |
| SC4 | Subclade 4 |
| SC5 | Subclade 5 |
| N1 | Thracian lineage of D. nitedula |
| N2 | Black Sea lineage of D. nitedula |
| N3 | West and central Anatolia lineage of D. nitedula |
| N4 | Northeastern Anatolia lineage of D. nitedula |
| N5 | Eastern Anatolia lineage of D. nitedula |
| L1 | Western lineage of D. laniger |
| L2 | Eastern lineage of D. laniger |
| CTAB | Cetyltrimethylammonium bromide DNA isolation method |
| PCR | Polymerase chain reaction |
| TAE | Tris–Acetate–EDTA buffer |
| MCMC | Markov Chain Monte Carlo algorithm |
| ML | Maximum Likelihood |
| BI | Bayesian Inference |
| BIC | Bayesian Information Criterion |
| AICc | Corrected Akaike Information Criterion |
| TN93 + G + I | Tamura Nei 1993 nucleotide substitution model with Gamma distribution Invariant sites |
| HKY + G + I | Hasegawa–Kishino–Yano 1985 nucleotide substitution model with Gamma distribution Invariant |
| Mya | Million years ago |
| K–2P | Kimura–2-Parameter |
| WC | West Caucasian haplogroup |
| CC | Central Caucasian haplogroup |
| EE | Eastern Europe haplogroup |
Appendix A
| Map Number | Species | Lineage | Locality | Sample Size | Coordinates | |
|---|---|---|---|---|---|---|
| 1 | D. nitedula | N1 | Bulgaria | 1 | 42°41′51″ N | 23°19′18″ E |
| 2 | D. nitedula | N1 | Edirne | 11 | 40°46′33″ N | 26°26′22″ E |
| 3 | D. nitedula | N1 | Edirne | 2 | 41°39′23″ N | 26°33′19″ E |
| 4 | D. nitedula | N1 | Kumbağ (Tekirdağ) | 1 | 40°52′25″ N | 27°25′18″ E |
| 5 | D. nitedula | N1 | Gelibolu (Çanakkale) | 3 | 40°25′3″ N | 26°40′19″ E |
| 6 | D. nitedula | N3 | Çan (Çanakkale) | 4 | 39°58′36″ N | 27°5′34″ E |
| 7 | D. nitedula | N3 | Abant (Bolu) | 2 | 40°34′41″ N | 31°16′30″ E |
| 8 | D. nitedula | N3 | Bursa | 5 | 40°8′37″ N | 29°5′57″ E |
| 9 | D. nitedula | N3 | Eber (Afyon) | 1 | 38°36′19″ N | 31°7′30″ E |
| 10 | D. nitedula | N3 | Madenköy (Niğde) | 2 | 37°26′43″ N | 34°37′30″ E |
| 11 | D. nitedula | N3 | Pınarbaşı (Kayseri) | 1 | 38°42′13″ N | 36°26′21″ E |
| 12 | D. nitedula | N3 | Elmalı (Antalya) | 2 | 36°43′2″ N | 29°52′24″ E |
| 13 | D. nitedula | N3 | Çakallı (Samsun) | 1 | 41°8′17″ N | 36°7′1″ E |
| 14 | D. nitedula | N3 | Bulancak (Giresun) | 1 | 40°49′54″ N | 38°14′36″ E |
| 15 | D. nitedula | N3 | Malatya | 4 | 38°32′39″ N | 37°30′58″ E |
| 16 | D. nitedula | N2 | Çat (Rize) | 3 | 40°52′3″ N | 40°56′5″ E |
| 17 | D. nitedula | N2 | Çamlıhemşin (Rize) | 4 | 41°3′4″ N | 41°1′1″ E |
| 18 | D. nitedula | N2 | Maçka (Trabzon) | 1 | 40°41′33″ N | 39°39′39″ E |
| 19 | D. nitedula | N2 | Arhavi (Artvin) | 1 | 41°18′13″ N | 41°24′34″ E |
| 20 | D. nitedula | N2 | Hopa (Artvin) | 8 | 41°23′13″ N | 41°30′34″ E |
| 21 | D. nitedula | N4 | Ardanuç (Artvin) | 4 | 41°7′9″ N | 42°7′21″ E |
| 22 | D. nitedula | N4 | Şavşat (Artvin) | 5 | 41°22′29″ N | 42°22′54″ E |
| 23 | D. nitedula | N4 | Posof (Ardahan) | 1 | 41°29′58″ N | 42°45′11″ E |
| 24 | D. nitedula | N4 | Kars | 3 | 40°17′26″ N | 42°36′19″ E |
| 25 | D. nitedula | N5 | Erzurum | 5 | 39°55′53″ N | 40°43′42″ E |
| 26 | D. nitedula | N5 | Tatvan (Bitlis) | 7 | 38°27′26″ N | 42°18′55″ E |
| 27 | D. nitedula | N5 | Elazığ | 3 | 38°36′52″ N | 39°32′49″ E |
| 28 | D. nitedula | N5 | Hakkari | 2 | 37°36′45″ N | 43°51′15″ E |
| 29 | D. laniger | L1 | Elmalı (Antalya) | 1 | 36°46′33″ N | 29°55′11″ E |
| 30 | D. laniger | L1 | Madenköy (Niğde) | 5 | 37°26′17″ N | 34°36′55″ E |
| 31 | D. laniger | L1 | Bolkar (Niğde) | 9 | 37°24′40″ N | 34°34′10″ E |
| 32 | D. laniger | L2 | Kahramanmaraş | 5 | 37°55′49″ N | 36°35′53″ E |
| 33 | D. laniger | L2 | Malatya | 2 | 38°36′52″ N | 37°34′45″ E |
| Taxon/Clade | H | F | Locality and Voucher Number/ Accession No. of GenBank Samples | MN | Accession No./Reference |
|---|---|---|---|---|---|
| D. n. wingei (N1) | 1 | 1 | Bulgaria (11b) | 1 | PV216972 |
| D. n. wingei (N1) | 2 | 5 | Edirne (5156, 5160, 5609, 5612, 7163) | 2 | PV216973 |
| D. n. wingei (N1) | 3 | 2 | Edirne (5157, 5162) | 2 | PV216974 |
| D. n. wingei (N1) | 4 | 1 | Edirne (5158) | 2 | PV216975 |
| D. n. wingei (N1) | 5 | 1 | Edirne (5159) | 2 | PV216976 |
| D. n. wingei (N1) | 6 | 1 | Edirne (5161) | 2 | PV216977 |
| D. n. wingei (N1) | 7 | 1 | Edirne (5163) | 2 | PV216978 |
| D. n. wingei (N1) | 8 | 2 | Edirne (5543, 7162) | 3 | PV216979 |
| D. n. wingei (N1) | 9 | 3 | Kumbağ–Tekirdağ (7207), Gelibolu–Çanakkale (7219, 7164) | 4, 5 | PV216980 |
| D. n. wingei (N1) | 10 | 1 | Gelibolu–Çanakkale (7201) | 5 | PV216981 |
| D. n. phrygius (N3) | 11 | 2 | Çan–Çanakkale (7262, 7274) | 6 | PV216982 |
| D. n. phrygius (N3) | 12 | 2 | Çan–Çanakkale (7264, 7285) | 6 | PV216983 |
| D. n. phrygius (N3) | 13 | 2 | Abant–Bolu (7130, 7186) | 7 | PV216984 |
| D. n. phrygius (N3) | 14 | 1 | Bursa (7419) | 8 | PV216985 |
| D. n. phrygius (N3) | 15 | 2 | Bursa (7440, 7442) | 8 | PV216986 |
| D. n. phrygius (N3) | 16 | 1 | Bursa (7441) | 8 | PV216987 |
| D. n. phrygius (N3) | 17 | 1 | Bursa (7443) | 8 | PV216988 |
| D. n. phrygius (N3) | 18 | 1 | Eber–Afyon (5589) | 9 | PV216989 |
| D. n. phrygius (N3) | 19 | 2 | Madenköy–Niğde (2322, 2323) | 10 | PV216990 |
| D. n. phrygius (N3) | 20 | 1 | Pınarbaşı–Kayseri (2929) | 11 | PV216991 |
| D. n. phrygius (N3) | 21 | 2 | Elmalı–Antalya (5749, 5756) | 12 | PV216992 |
| D. n. phrygius (N3) | 22 | 1 | Çakallı–Samsun (5357) | 13 | PV216993 |
| D. n. phrygius (N3) | 23 | 1 | Bulancak–Giresun (7208) | 14 | PV216994 |
| D. n. phrygius (N3) | 24 | 4 | Malatya (7470, 7471, 7472, 7473) | 15 | PV216995 |
| A potential new taxon (N2) | 25 | 2 | Çat–Rize (5358, 5360) | 16 | PV216996 |
| A potential new taxon (N2) | 26 | 1 | Çat–Rize (5361) | 16 | PV216997 |
| A potential new taxon (N2) | 27 | 1 | Çamlıhemşin–Rize (5392) | 17 | PV216998 |
| A potential new taxon (N2) | 28 | 2 | Çamlıhemşin–Rize (5400, 5404) | 17 | PV216999 |
| A potential new taxon (N2) | 29 | 1 | Çamlıhemşin–Rize (5427) | 17 | PV217000 |
| A potential new taxon (N2) | 30 | 1 | Maçka–Trabzon (7282) | 18 | PV217001 |
| A potential new taxon (N2) | 31 | 2 | Arhavi–Artvin (7381), Hopa–Artvin (7338) | 19, 20 | PV217002 |
| A potential new taxon (N2) | 32 | 3 | Hopa–Artvin (7339, 7379), Cankurtaran–Artvin (5713) | 20 | PV217003 |
| A potential new taxon (N2) | 33 | 4 | Hopa–Artvin (7340), Cankurtaran–Artvin (7341, 7342, 7378) | 20 | PV217004 |
| D. n. tichomirowi (N4) | 34 | 4 | Ardanuç–Artvin (7374, 7375, 7376, 7377) | 21 | PV217005 |
| D. n. tichomirowi (N4) | 35 | 2 | Şavşat–Artvin (5712, 7371) | 22 | PV217006 |
| D. n. tichomirowi (N4) | 36 | 1 | Şavşat–Artvin (7368) | 22 | PV217007 |
| D. n. tichomirowi (N4) | 37 | 1 | Şavşat–Artvin (7372) | 22 | PV217008 |
| D. n. tichomirowi (N4) | 38 | 1 | Şavşat–Artvin (7373) | 22 | PV217009 |
| D. n. tichomirowi (N4) | 39 | 1 | Posof–Ardahan (7345) | 23 | PV217010 |
| D. n. tichomirowi (N4) | 40 | 2 | Kars (7474, 7476) | 24 | PV217011 |
| D. n. tichomirowi (N4) | 41 | 1 | Kars (7475) | 24 | PV217012 |
| D. n. pictus (N5) | 42 | 1 | Erzurum (7478) | 25 | PV217013 |
| D. n. pictus (N5) | 43 | 4 | Erzurum (7480, 7481, 7482, 7483) | 25 | PV217014 |
| D. n. pictus (N5) | 44 | 6 | Tatvan–Bitlis (7263, 7265, 7276, 7287, 7288, 7289) | 26 | PV217015 |
| D. n. pictus (N5) | 45 | 1 | Tatvan–Bitlis (7268) | 26 | PV217016 |
| D. n. pictus (N5) | 46 | 3 | Elazığ (7277, 7290, 7291) | 27 | PV217017 |
| D. n. pictus (N5) | 47 | 2 | Hakkari (7266, 7273) | 28 | PV217018 |
| D. n. nitedula (EE) | 48 | 1 | Belorussia (KJ739693) | – | [18] |
| D. n. nitedula (EE) | 49 | 1 | Russia (KF699220) | – | [18] |
| D. n. nitedula (EE) | 50 | 1 | Russia (KF699221) | – | [18] |
| D. n. nitedula (EE) | 51 | 1 | Russia (KF699223) | – | [18] |
| D. n. nitedula (EE) | 52 | 1 | Russia (KF699226) | – | [18] |
| D. n. nitedula (EE) | 53 | 1 | Russia (KJ739694) | – | [18] |
| D. n. nitedula (EE) | 54 | 1 | Russia (KJ739695) | – | [18] |
| D. n. nitedula (EE) | 55 | 1 | Russia (KJ739696) | – | [18] |
| D. n. nitedula (EE) | 56 | 1 | Russia (KJ739697) | – | [18] |
| D. n. nitedula (EE) | 57 | 1 | Russia (KJ739698) | – | [18] |
| D. n. heptneri WC | 58 | 1 | Russia (KJ739699) | – | [18] |
| D. n. heptneri WC | 59 | 1 | Russia (KJ739700) | – | [18] |
| D. n. heptneri WC | 60 | 2 | Russia (KJ739701, KF699218) | – | [18] |
| D. n. heptneri WC | 61 | 1 | Russia (KJ739702) | – | [18] |
| D. n. heptneri WC | 62 | 1 | Russia (AJ225116) | – | [18] |
| D. n. ognevi (CC) | 63 | 1 | Russia (KJ739703) | – | [18] |
| D. n. ognevi (CC) | 64 | 1 | Russia (KJ739704) | – | [18] |
| D. n. ognevi (CC) | 65 | 1 | Russia 8KJ739705) | – | [18] |
| D. n. aspromontis | 66 | 4 | S Italy (MH671555, MH671556, MH671557, MH671558) | – | [20] |
| D. n. aspromontis | 67 | 2 | S Italy, (MH671559, MH671560) | – | [20] |
| D. n. aspromontis | 68 | 2 | S Italy, (MH671561 MH671562) | – | [20] |
| D. n. intermedius | 69 | 4 | NE Italy, (MH671563, MH671564, MH671565, MH671566) | – | [20] |
| D. n. intermedius | 70 | 3 | NE Italy (MH671567, MH671568, MH671569) | – | [20] |
| D. n. pictus | 71 | 1 | Yazd–Iran (MT559843) | – | [19] |
| D. n. pictus | 72 | 2 | Yazd–Iran (MT559842, MT559838) | – | [19] |
| D. n. pictus | 73 | 1 | Yazd–Iran (MT559841) | – | [19] |
| D. n. pictus | 74 | 3 | Yazd–Iran (MT559840, MT559839, MT559836) | – | [19] |
| D. n. pictus | 75 | 1 | Yazd–Iran (MT559837) | – | [19] |
| D. n. pictus | 76 | 3 | Isfahan–Iran (MT559835, MT559834, MT559833) | – | [19] |
| D. n. tichomirowi | 77 | 3 | Zanjan–Iran (MT559832, MT559830, MT559828) | – | [19] |
| D. n. tichomirowi | 78 | 3 | Eastern Azerbaijan–Iran (MT559831, MT559829, MT559827) | – | [19] |
| D. n. heptneri | 79 | 1 | Russia (KF699219) | – | [19] |
| D. n. kurdistanicus | 80 | 1 | Karmanshah–Iran (MT559871) | – | [19] |
| D. n. kurdistanicus | 81 | 1 | Kurdistan–Iran (MT559870) | – | [19] |
| D. n. kurdistanicus | 82 | 1 | Kurdistan–Iran (MT559869) | – | [19] |
| D. n. kurdistanicus | 83 | 2 | Kurdistan–Iran (MT559868, MT559860) | – | [19] |
| D. n. kurdistanicus | 84 | 1 | Kurdistan–Iran (MT559867) | – | [19] |
| D. n. kurdistanicus | 85 | 4 | Kurdistan–Iran (MT559866, MT559864, MT559861, MT559858) | – | [19] |
| D. n. kurdistanicus | 86 | 3 | Kurdistan–Iran (MT559865, MT559863, MT559862) | – | [19] |
| D. n. kurdistanicus | 87 | 1 | Kurdistan–Iran (MT559859) | – | [19] |
| D. n. bilkjewiczi | 88 | 1 | Northern Khorasan–Iran (MT559857) | – | [19] |
| D. n. bilkjewiczi | 89 | 1 | Northern Khorasan–Iran (MT559856) | – | [19] |
| D. n. bilkjewiczi | 90 | 5 | Northern Khorasan–Iran (MT559855, MT559852, MT559850, MT559847), Razavi Khorasan–Iran (MT559845) | – | [19] |
| D. n. bilkjewiczi | 91 | 1 | Northern Khorasan–Iran (MT559854) | – | [19] |
| D. n. bilkjewiczi | 92 | 1 | Northern Khorasan–Iran (MT559853) | – | [19] |
| D. n. bilkjewiczi | 93 | 2 | Northern Khorasan–Iran (MT559851, MT559848) | – | [19] |
| D. n. bilkjewiczi | 94 | 1 | Northern Khorasan–Iran (MT559849) | – | [19] |
| D. n. bilkjewiczi | 95 | 1 | Northern Khorasan–Iran (MT559846) | – | [19] |
| D. n. bilkjewiczi | 96 | 1 | Razavi Khorasan–Iran (MT559844) | – | [19] |
| D. laniger (L1) | 97 | 1 | Elmalı–Antalya (5631) | 29 | PV217019 |
| D. laniger (L1) | 98 | 9 | Madenköy–Niğde (2321, 4579, 4586, 4587, 4588), Bolkar–Niğde (5771, 5773, 5774, 5775) | 30, 31 | PV217020 |
| D. laniger (L1) | 99 | 1 | Bolkar–Niğde (5772) | 31 | PV217021 |
| D. laniger (L1) | 100 | 1 | Bolkar–Niğde (5778) | 31 | PV217022 |
| D. laniger (L1) | 101 | 1 | Bolkar–Niğde (5989) | 31 | PV217023 |
| D. laniger (L1) | 102 | 2 | Bolkar–Niğde (6002, 6004) | 31 | PV217024 |
| D. laniger (L2) | 103 | 5 | Kahramanmaraş (7456, 7457, 7458, 7461, 7462) | 32 | PV217025 |
| D. laniger (L2) | 104 | 2 | Malatya (7484, 7485) | 33 | PV217026 |
| M. musculus | 105 | JX457720 | – | – | |
| M. musculus | 106 | JX457718 | – | – | |
| R. norvegicus | 107 | AB746365 | – | – | |
| R. norvegicus | 108 | AB746363 | – | – | |
| G. japonicus | 109 | D89004 | – | – | |
| G. japonicus | 110 | D89001 | – | – | |
| G. murinus | 111 | AJ225115 | – | – | |
| G. kelleni | 112 | MN616984 | – | – | |
| E. melanurus | 113 | HE614012 | – | – | |
| E. melanurus | 114 | HE614010 | – | – | |
| E. quercinus | 115 | GQ453669 | – | – | |
| E. quercinus | 116 | GQ453668 | – | – | |
| G. glis | 117 | AJ225031 | – | – | |
| G. glis | 118 | KF699243 | – | – | |
| S. anomalus | 119 | AB292677 | – | – | |
| S. anomalus | 120 | AB292676 | – | – | |
| S. vulgaris | 121 | AB775131 | – | – | |
| S. vulgaris | 122 | JQ395054 | – | – | |
| M. avellanarius | 123 | MT410887 | – | – | |
| M. avellanarius | 124 | AJ225117 | – | – |
References
- Kryštufek, B.; Vohralík, V. Mammals of Turkey and Cyprus. Rodentia I: Sciuridae, Dipodidae, Gliridae, Arvicolinae; Zgodovinsko Društvo za Južno Primorsko: Koper, Slovenia, 2005. [Google Scholar]
- Wilson, D.E.; Reeder, D.A.M. (Eds.) Mammal Species of the World: A Taxonomic and Geographic Reference; Johns Hopkins University Press: Baltimore, MD, USA, 2005; Volume 2, 142p. [Google Scholar]
- Yiğit, N.; Çolak, E.; Sözen, M.; Karataş, A. Rodents of Türkiye; Meteksan Yayıncılık: Ankara, Türkiye, 2006; 154p. [Google Scholar]
- Yiğit, N.; Çolak, E.; Çolak, R.; Özlük, A.; Gül, N.; Çam, P.; Saygılı, F. Biometric and Allozymic Variation in the Genus Dryomys (Rodentia: Gliridae) in Turkey. Acta Zool. Bulgarica 2011, 63, 67–75. [Google Scholar]
- Helvacı, Z. Climate Change Impacts on the Distribution of Dryomys laniger (woolly dormouse) in Türkiye: A Data-driven Approach. Mammalia 2024, 88, 554–566. [Google Scholar] [CrossRef]
- Kankılıç, T.; Şeker, P.S.; Erdik, A.C.; Kankılıç, T.; Selvi, E.; Yiğit, N.; Çolak, E. Determination of Genetic Variations in the Genus Dryomys Thomas, 1906 (Rodentia: Gliridae) Distributed in Turkey Using NADH Dehydrogenase 1 (ND1) Gene. Mitochondrial DNA Part A 2017, 29, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Doğramacı, S.; Kefelioğlu, H. Türkiye Dryomys nitedula (Mammalia: Rodentia) Türünün Karyotipi. Turk. J. Zool. 1990, 14, 316–328. [Google Scholar]
- Filippucci, M.G.; Kryštufek, B.; Simson, S.; Kurtonur, C.; Özkan, B. Allozymic and Biometric Variation in Dryomys nitedula (Pallas, 1778). Hystrix 1994, 6, 127–140. [Google Scholar] [CrossRef]
- Markov, G. Cranial Epigenetic Polymorphism and Population Differentiation of the Forest Dormouse (Dryomys nitedula Pall., 1779) in Bulgaria. Acta Zool. Acad. Sci. Hungaricae 2003, 49 (Suppl. S1), 109–115. [Google Scholar]
- Yiğit, N.; Çolak, E.; Çolak, R.; Özkan, B.; Özkurt, Ş. On the Turkish Populations of Dryomys nitedula (Pallas, 1779) and Dryomys laniger (Felten and Storch, 1968) (Mammalia: Rodentia). Acta Zool. Acad. Sci. Hung. 2003, 49, 147–158. [Google Scholar]
- Arslan, A.; Kankılıç, T.; Yorulmaz, T.; Kankılıç, T.; Zima, J. Comparison of the Chromosome Banding Patterns in Dryomys laniger and D. nitedula from Turkey. Turk. J. Zool. 2016, 40, 363–368. [Google Scholar] [CrossRef]
- Markov, G.; Çolak, E.; Yiğit, N.; Dimitrov, H. Epigenetic Variation and Population Uniqueness of the Forest Dormouse (Dryomys nitedula) as Revealed by Craniological Nonmetric Traits. C. R. L’Acad. Bulg. Sci. 2017, 70, 381–386. [Google Scholar]
- Markov, G.G.; Colak, E.; Yigit, N.; Kocheva, M.A.; Gospodinova, M.K.; Dimitrov, H. Population Epigenetic Diversity versus Subspecies Detachment of the Forest Dormouse, Dryomys nitedula (Pallas, 1778) (Rodentia: Gliridae), in a Long Distance Transect in Eurasia: Implication for its Conservation. Acta Zool. Bulgarica 2017, 69 (Suppl. S8), 199–204. [Google Scholar]
- Kankılıç, T.; Şeker, P.S.; Aydın, B.; Altunbaş, D.; Selvi, E.; Yiğit, N.; Çolak, E. Nuclear and organelle genes based phylogeny of Dryomys (Gliridae, Rodentia, Mammalia) from Turkey. Acta Zool. Acad. Sci. Hung. 2019, 65, 399–413. [Google Scholar] [CrossRef]
- Kankılıç, T.; Şeker, P.S.; Selvi, E.; Özkan, B.; Yiğit, N.; Çolak, E. G-banded Karyotypes of Some Species in Gliridae (Mammalia: Rodentia) from Turkey. Adıyaman Univ. J. Sci. 2021, 11, 59–72. [Google Scholar] [CrossRef]
- Ognev, S.I. Mammals of the USSR and Adjacent Countries; USSR Academy of Sciences: Moscow, Russia, 1948. [Google Scholar]
- Grigoryeva, O.O.; Balakirev, A.E.; Stakheev, V.V.; Krivonogov, D.M.; Andreychev, A.V.; Oparin, M.L.; Orlov, V.N. Mitochondrial Phylogeography and Taxonomy of the Forest Dormouse Dryomys nitedula (Pallas, 1778) (Gliridae, Rodentia) in the Western Caucasus with Description of New Subspecies D. n. heptneri subsp. nov. Biota’s Conservation in State Nature Reserve “Utrish”. Sci. Trans. 2014, 3, 332–344. (In Russian) [Google Scholar]
- Grigoryeva, O.; Krivonogov, D.; Balakirev, A.; Stakheev, V.; Andreychev, A.; Orlov, V. Phylogeography of the Forest Dormouse Dryomys nitedula (Gliridae, Rodentia) in Russian Plain and the Caucasus. Folia Zool. 2015, 64, 361–364. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Kami, H.G.; Ghorbani, F.; Khajeh, A.; Olsson, U. Cryptic Lineage Diversity Within Forest Dormice (Mammalia: Dryomys nitedula) Revealed by Deep Genetic Divergence Among Different Subspecies on the Iranian Plateau and in Adjacent Areas. Mamm. Biol. 2021, 101, 21–34. [Google Scholar] [CrossRef]
- Bisconti, R.; Aloise, G.; Siclari, A.; Fava, V.; Provenzano, M.; Arduino, P.; Chiocchio, A.; Nascetti, G.; Canestrelli, D. Forest Dormouse (Dryomys nitedula) populations in southern Italy belong to a deeply divergent evolutionary lineage: Implications for taxonomy and conservation. Hystrix 2018, 29, 75–79. [Google Scholar] [CrossRef]
- Harrison, D.L.; Bates, P.J.J. The Mammals of Arabia, 2nd ed.; Harrison Zoological Museum Publication: Kent, UK, 1991. [Google Scholar]
- Mursaloğlu, B. New Records for Turkish Rodents (Mammalia). Commun. (Sér. C) Fac. Sci. Univ. Ankara 1973, 17, 213–219. [Google Scholar]
- İbiş, O.; Selçuk, A.Y.; Teber, S.; Baran, M.; Koepfli, K.-P.; Kefelioğlu, H.; Tez, C. Mitogenomic Analysis of Glirids (Gliridae) and Squirrels (Sciuridae) from Türkiye: Evolutionary and Taxonomic Implications Within the Suborder Sciuromorpha. Ecol. Evol. 2025, 15, e70956. [Google Scholar] [CrossRef]
- Ellerman, J.R.; Morrison-Scott, T.C.S. Checklist of Palaearctic and Indian Mammals 1758 to 1946; British Museum (Natural History): London, UK, 1951; pp. 1–810. [Google Scholar]
- Corbet, G.B. The Mammals of the Palaearctic Region: A Taxonomic Review; British Museum (Natural History) and Cornell University Press: London, UK, 1978. [Google Scholar]
- Çetintaş, O. Kaya Yediuyuru Dryomys laniger (Mammalia: Rodentia)’in Genetik Çeşitliliği ve Yayılış Alanının Belirlenmesi. Ph.D. Thesis, Zonguldak Bülent Ecevit University, Zonguldak, Türkiye, 2021. [Google Scholar]
- Bilgin, R. Back to the suture: The Distribution of Intraspecific Genetic Diversity in and Around Anatolia. Int. J. Mol. Sci. 2011, 12, 4080–4103. [Google Scholar] [CrossRef]
- Hewitt, G.M. Some Genetic Consequences of Ice Ages, and Their Role in Divergence and Speciation. Biol. J. Linn. Soc. 1996, 58, 247–276. [Google Scholar] [CrossRef]
- Taberlet, P.; Fumagalli, L.; Wust-Saucy, A.G.; Cossons, J.F. Comparative Phylogeography and Post-glacial Colonization Routes in Europe. Mol. Ecol. 1998, 7, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, G.M. Post-glacial Re-colonization of European biota. Biol. J. Linnean Soc. 1999, 68, 87–112. [Google Scholar] [CrossRef]
- Avise, J.C. Phylogeography: The History and Formation of Species; Harvard University Press: Cambridge, MA, USA, 2000. [Google Scholar]
- Castresana, J. Cytochrome b Phylogeny and the Taxonomy of Great Apes and Mammals. Mol. Biol. Evol. 2001, 18, 465–471. [Google Scholar] [CrossRef]
- Tobe, S.S.; Kitchener, A.C.; Linacre, A.M.T. Reconstructing Mammalian Phylogenies: A Detailed Comparison of the Cytochrome b and Cytochrome Oxidase Subunit I Mitochondrial Genes. PLoS ONE 2010, 5, e14156. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.J.; Doyle, J.L. Isolation of Plant DNA from Fresh Tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Irwin, D.M.; Kocher, T.D.; Wilson, A.C. Evolution of the Cytochrome-b Gene of Mammals. J. Mol. Evol. 1991, 32, 128–144. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A User-friendly Biological Sequence Alignment, Editor, and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis Across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Drummond, A.J.; Suchard, M.A.; Xie, D.; Rambaut, A. Bayesian Phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012, 29, 1969–1973. [Google Scholar] [CrossRef]
- Montgelard, C.; Matthee, C.A.; Robinson, T.J. Molecular Systematics of Dormice (Rodentia: Gliridae) and the Radiation of Graphiurus in Africa. Proc. R. Soc. B 2003, 270, 1947–1955. [Google Scholar] [CrossRef]
- Benton, M.J.; Donoghue, P.C. Paleontological Evidence to Date the Tree of Life. Mol. Biol. Evol. 2007, 24, 26–53. [Google Scholar] [CrossRef] [PubMed]
- Voloch, C.M.; Schrago, C.G. Impact of the Partitioning Scheme on Divergence Times Inferred from Mammalian Genomic Data Sets. Evol. Bioinform. Online 2012, 8, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. FigTree, Version 1.2. Available online: http://tree.bio.ed.ac.uk/software/FigTree/ (accessed on 20 July 2013).
- Bendelt, H.; Froster, P.; Rohl, A. Median Joining Network for Inferring Intraspecific Phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sanchez-Gracia, A. DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E.L. Arlequin suite ver 3.5: A New Series of Programs to Perform Population Genetics Analyses Under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef]
- Lavocat, R. Le Gisement de Vertèbres Miocènes de Beni Mellal (Maroc). Étude Systématique de la Faune de Mammifères et Conclusions Générales. Notes Mem. Serv. Géol. Maroc 1961, 155, 29–94, 52–67, 109–144. [Google Scholar]
- Franzen, J.F.; Storch, G. Die Unterpliozane (Turolische) Wirbeltierfauna von Darn-Dürkheim, Rheinhessen (SW-Deutschland). 1. Entdeckung, Geologie, Mammalia: Carnivora, Proboscidea, Rodentia. Senck. Leth. 1975, 56, 233–303. [Google Scholar]
- Daams, R.; De Bruijn, H. A Classification of the Gliridae (Rodentia) on the Basis of Dental Morphology. Hystrix 1995, 6, 3–50. [Google Scholar]
- Demirsoy, A. Genel Zoocoğrafya ve Türkiye Zoocoğrafyası “Hayvan Coğrafyası”; Meteksan A.S.: Ankara, Türkiye, 2008; p. 630. [Google Scholar]
- Bradley, R.D.; Baker, R.J. A Test of the Genetic Species Concept: Cytochrome-b Sequences and Mammals. J. Mammal. 2001, 82, 960–973. [Google Scholar] [CrossRef]
- Baker, R.J.; Bradley, R.D. Speciation in Mammals and the Genetic Species Concept. J. Mammal. 2006, 87, 643–662. [Google Scholar] [CrossRef]
- Johns, G.C.; Avise, J.C. A Comparative Summary of Genetic Distances in the Vertebrates from the Mitochondrial Cytochrome b Gene. Mol. Biol. Evol. 1998, 15, 1481–1490. [Google Scholar] [CrossRef] [PubMed]
- Krijgsman, W.; Hilgen, F.J.; Raffi, I.; Sierro, F.J. Chronology, Causes, and Progression of the Messinian Salinity Crisis. Nature 1999, 400, 652–655. [Google Scholar] [CrossRef]
- Duggen, S.; Hoernle, K.; Bogaard, P.V.; Rupke, L.; Morgan, J.P. Deep roots of the Messinian salinity crisis. Nature 2003, 422, 602–605. [Google Scholar] [CrossRef] [PubMed]
- Popov, S.V.; Shcherba, I.G.; Ilyina, L.B.; Nevesskaya, L.A.; Paramonova, N.P.; Khondkarian, S.O.; Magyar, I. Late Miocene to Pliocene Palaeogeography of the Paratethys and its Relation to the Mediterranean. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006, 238, 91–106. [Google Scholar] [CrossRef]
- Kryštufek, B.; Bryja, J.; Buzan, E.V. Mitochondrial Phylogeography of the European Ground Squirrel, Spermophilus citellus, Yields Evidence on Refugia for Steppic Taxa in the Southern Balkans. Heredity 2009, 103, 129–135. [Google Scholar] [CrossRef]
- Neumann, K.; Michaux, J.R.; Maak, S.; Jansman, H.A.H.; Kayser, A.; Mundt, G.; Gattermann, R. Genetic Spatial Structure of European Common Hamsters (Cricetus cricetus)—A Result of Repeated Range Expansion and Demographic Bottlenecks. Mol. Ecol. 2005, 14, 1473–1483. [Google Scholar] [CrossRef]
- Stöck, M.; Dufresnes, C.; Litvinchuk, S.N.; Lymberakis, P.; Biollay, S.; Berroneau, M.; Borzée, A.; Ghali, K.; Ogielska, M.; Perrin, N. Cryptic Diversity Among Western Palearctic Tree Frogs: Postglacial Range Expansions, Range Limits, and Secondary Contacts of Three European Tree Frog Lineages (Hyla arborea Group). Mol. Phylogenet. Evol. 2012, 65, 1–9. [Google Scholar] [CrossRef]
- Dubey, S.; Zaitsev, M.; Cosson, J.-F.; Abdukadier, A.; Vogel, P. Pliocene and Pleistocene diversification and multiple refugia in a Eurasian shrew (Crocidura suaveolens group). Mol. Phylogenet. Evol. 2006, 38, 635–647. [Google Scholar] [CrossRef]
- Hewitt, G.M. Quaternary phylogeography: The roots of hybrid zones. Genetica 2011, 139, 617–638. [Google Scholar] [CrossRef]
- Norton, K.P.; Abbühl, L.; Schlunegger, F. Glacial conditioning as an erosional driving force in the Central Alps. Geology 2010, 38, 655–658. [Google Scholar] [CrossRef]
- Sosdian, S.; Rosenthal, Y. Deep-Sea Temperature and Ice Volume Changes Across the Pliocene-Pleistocene Climate Transitions. Science 2009, 325, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, G.M. The Genetic Legacy of the Quaternary Ice Ages. Nature 2000, 405, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, G.M. Genetic Consequences of Climatic Oscillations in the Quaternary. Philos. Trans. R. Soc. Lond. B 2004, 359, 183–195. [Google Scholar] [CrossRef]
- Helvacı, Z.; Renaud, S.; Ledevin, R.; Adriaens, D.; Michaux, J.; Çolak, R.; Kankılıç, T.; Kandemir, İ.; Yiğit, N.; Çolak, E. Morphometric and genetic structure of the edible dormouse (Glis glis): A consequence of forest fragmentation in Turkey. Biol. J. Linn. Soc. 2012, 107, 611–623. [Google Scholar] [CrossRef]
- Sağlam, İ.; Küçükyıldırım, S.; Çağlar, S.S. Diversification of Montane Species via Elevation Shifts: The Case of the Kaçkar Cricket Phonochorion (Orthoptera). J. Zool. Syst. Evol. Res. 2014, 53, 177–189. [Google Scholar] [CrossRef]
- Demir, T.; Yeşilnacar, İ.; Westaway, R. River terrace sequences in Turkey: Sources of evidence for lateral variations in regional uplift. Proc. Geol. Assoc. 2004, 115, 289–311. [Google Scholar] [CrossRef]
- Doğan, U. Fluvial response to climate change during and after the Last Glacial Maximum in Central Anatolia, Turkey. Quat. Int. 2010, 222, 221–229. [Google Scholar] [CrossRef]
- Bridgland, D.R.; Demir, T.; Seyrek, A.; Daoud, M.; Romieh, M.A.; Westaway, R. River terrace development in the NE Mediterranean region (Syria and Turkey): Patterns in relation to crustal type. Quat. Sci. Rev. 2017, 166, 307–323. [Google Scholar] [CrossRef]
- Trifonov, V.G. Collision and Mountain Building. Geotectonics 2016, 50, 1–20. [Google Scholar] [CrossRef]
- Şeker, P.S.; Selvi, E.; Kankılıç, T.; Çolak, E. Geographical Distribution Pattern of Mitochondrial DNA Cytochrome b Diversity in Populations of Arvicola amphibius (Linnaeus, 1758) (Mammalia: Rodentia) in Turkey as Determined by PCR-RFLP. Acta Zool. Bulg. 2018, 70, 19–30. [Google Scholar]
- Mutun, S. Intraspecific Genetic Variation and Phylogeography of the Oak Gallwasp Andricus caputmedusae (Hymenoptera: Cynipidae): Effects of the Anatolian Diagonal. Acta Zool. Acad. Sci. Hungaricae 2010, 56, 153–172. [Google Scholar]
- Gür, H. The Anatolian Diagonal Revisited: Testing the Ecological Basis of a Biogeographic Boundary. Zool. Middle East 2016, 62, 189–199. [Google Scholar] [CrossRef]
- Çıplak, B. Biogeography of Anatolia: The marker group Orthoptera. Mem. Soc. Entomol. Ital. 2004, 82, 357–372. [Google Scholar]
- van Riemsdijk, I.; Arntzen, J.W.; Bogaerts, S.; Franzen, M.; Litvinchuk, S.N.; Olgun, K.; Wielstra, B. The Near East as a Cradle of Biodiversity: A Phylogeography of Banded Newts (Genus Ommatotriton) Reveals Extensive Inter- and Intraspecific Genetic Differentiation. Mol. Phylogenet. Evol. 2017, 114, 73–81. [Google Scholar] [CrossRef]
- Yiğit, N.; Çetintürk, D.; Çolak, E. Phylogenetic Assessment of Voles of the Guentheri Group (Mammalia: Microtus) in Turkish Thrace and Western Anatolia. Eur. Zool. J. 2017, 84, 252–260. [Google Scholar] [CrossRef]
- Jablonski, D.; Kukushkin, O.V.; Avcı, A.; Bunyatova, S.; Kumlutaş, Y.; Ilgaz, Ç.; Polyakova, E.; Shiryaev, K.; Tuniyev, B.; Jandzik, D. The biogeography of Elaphe sauromates (Pallas, 1814), with a description of a new rat snake species. PeerJ Life Environ. 2019, 7, e6944. [Google Scholar] [CrossRef]
- Seyrek, A.; Westaway, R.; Pringle, M.; Yurtmen, S.; Demir, T.; Rowbotham, G. Timing of the Quaternary Elazığ Volcanism, Eastern Turkey, and its Significance for Constraining Landscape Evolution and Surface Uplift. Turkish J. Earth Sci. 2008, 17, 497–541. Available online: https://journals.tubitak.gov.tr/earth/vol17/iss3/5 (accessed on 13 April 2025).
- Demir, T.; Seyrek, A.; Westaway, R.; Bridgland, D.; Beck, A. Late Cenozoic surface uplift revealed by incision by the River Euphrates at Birecik, southeast Turkey. Quat. Int. 2008, 186, 132–163. [Google Scholar] [CrossRef]
- Westaway, R.; Demir, T.; Seyrek, A.; Beck, A. Kinematics of Active Left-Lateral Faulting in SE Turkey from Offset Pleistocene River Gorges: Improved Constraint on the Rate and History of Relative Motion Between the Turkish and Arabian Plates. J. Geol. Soc. 2006, 163, 149–164. [Google Scholar] [CrossRef]
- Kaymakçı, N.; İnceöz, M.; Ertepınar, P. 3D-Architecture and Neogene Evolution of the Malatya Basin: Inferences for the Kinematics of the Malatya and Ovacık Fault Zones. Turk. J. Earth Sci. 2006, 15, 123–154. [Google Scholar]
- Koç, A.; Kaymakçı, N. Kinematics of Sürgü Fault Zone (Malatya, Turkey): A Remote Sensing Study. J. Geodyn. 2013, 65, 292–307. [Google Scholar] [CrossRef]


| Taxon/Clade | N | S | NH | H ± SD | π ± SD | |
|---|---|---|---|---|---|---|
| D. nitedula (Türkiye) | All | 88 | 218 | 47 | 0.981 ± 0.005 | 0.06928 ± 0.00190 |
| N1 | 18 | 23 | 10 | 0.902 ±0.050 | 0.00789 ± 0.00078 | |
| N2 | 17 | 57 | 9 | 0.912 ±0.042 | 0.00968 ± 0.00454 | |
| N3 | 23 | 77 | 14 | 0.953 ± 0.025 | 0.02391 ± 0.00152 | |
| N4 | 13 | 43 | 8 | 0.897 ± 0.067 | 0.01627 ± 0.00455 | |
| N5 | 17 | 56 | 6 | 0.816 ± 0.061 | 0.01519 ± 0.00558 | |
| N2 + N3 + N4 + N5 | 70 | 189 | 37 | 0.976 ± 0.006 | 0.05910 ± 0.00222 | |
| D. n. nitedula (Russia) | EE | 10 | 12 | 9 | 0.978 ± 0.054 | 0.00579 ± 0.00082 |
| D. n. heptneri (Caucasia) | WC | 5 | 10 | 5 | 1.000 ± 0.01600 | 0.00466 ± 0.00076 |
| D. n. ognevi (Caucasia) | CC | 3 | 8 | 3 | 1.000 ± 0.07407 | 0.00567 ± 0.00189 |
| D. n. aspromontis (Italy) | 8 | 3 | 3 | 0.714 ± 0.123 | 0.00305 ± 0.00080 | |
| D. n. intermedius (Italy) | 7 | 1 | 2 | 0.571 ± 0.119 | 0.00135 ± 0.00028 | |
| D. n. pictus (Iran) | 11 | 18 | 6 | 0.873 ± 0.071 | 0.00759 ± 0.00110 | |
| D. n. tichomirowi (Iran) | 6 | 1 | 2 | 0.600 ± 0.129 | 0.00064 ± 0.00014 | |
| D. n. heptneri (Russia) | 2 | 3 | 2 | 1.000 ± 0.500 | 0.00337 ± 0.00169 | |
| D. n. kurdistanicus (Iran) | 14 | 13 | 7 | 0.879 ± 0.058 | 0.00401 ± 0.00086 | |
| D. n. bilkjewiczi (Iran) | 14 | 22 | 8 | 0.857 ± 0.077 | 0.00497 ± 0.00108 | |
| D. laniger (Türkiye) | All | 22 | 64 | 8 | 0.792 ± 0.069 | 0.02714 ± 0.00369 |
| L1 | 15 | 21 | 6 | 0.648 ± 0.134 | 0.00567 ± 0.00127 | |
| L2 | 7 | 9 | 2 | 0.476 ± 0.171 | 0.00455 ± 0.00164 | |
| Clade | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. N1 | 0.0104 | 0.0112 | 0.0113 | 0.0111 | 0.0025 | 0.0111 | 0.0120 | 0.0095 | 0.0116 | 0.0130 | 0.0103 | 0.0113 | 0.0112 | |
| 2. N3 | 0.0983 | 0.0053 | 0.0088 | 0.0091 | 0.0108 | 0.0088 | 0.0105 | 0.0157 | 0.0164 | 0.0115 | 0.0085 | 0.0101 | 0.0085 | |
| 3. N2 | 0.1045 | 0.0401 | 0.0091 | 0.0097 | 0.0120 | 0.0097 | 0.0105 | 0.0158 | 0.0171 | 0.0125 | 0.0092 | 0.0103 | 0.0095 | |
| 4. N4 | 0.1119 | 0.0876 | 0.0859 | 0.0093 | 0.0114 | 0.0091 | 0.0082 | 0.0180 | 0.0190 | 0.0120 | 0.0054 | 0.0105 | 0.0091 | |
| 5. N5 | 0.1097 | 0.0874 | 0.0904 | 0.0932 | 0.0119 | 0.0093 | 0.0101 | 0.0143 | 0.0149 | 0.0119 | 0.0094 | 0.0066 | 0.0067 | |
| 6. D. n. nitedula | 0.0115 | 0.0993 | 0.1070 | 0.1085 | 0.1127 | 0.0117 | 0.0121 | 0.0149 | 0.0177 | 0.0139 | 0.0104 | 0.0124 | 0.0117 | |
| 7. D. n. heptneri | 0.0987 | 0.0768 | 0.0812 | 0.0780 | 0.0830 | 0.1005 | 0.0093 | 0.0160 | 0.0174 | 0.0118 | 0.0074 | 0.0102 | 0.0089 | |
| 8. D. n. ognevi | 0.1173 | 0.1008 | 0.0960 | 0.0686 | 0.0902 | 0.1138 | 0.0730 | 0.0185 | 0.0202 | 0.0134 | 0.0079 | 0.0110 | 0.0100 | |
| 9. D. n. aspromontis | 0.0445 | 0.1011 | 0.0964 | 0.1233 | 0.0826 | 0.0562 | 0.0934 | 0.1187 | 0.0101 | 0.0189 | 0.0164 | 0.0158 | 0.0158 | |
| 10. D. n. intermedius | 0.0568 | 0.0985 | 0.0997 | 0.1289 | 0.0858 | 0.0712 | 0.1048 | 0.1299 | 0.0427 | 0.0202 | 0.0181 | 0.0157 | 0.0171 | |
| 11. D. n. pictus | 0.1348 | 0.1185 | 0.1268 | 0.1258 | 0.1292 | 0.1412 | 0.1213 | 0.1395 | 0.1247 | 0.1340 | 0.0119 | 0.0129 | 0.0123 | |
| 12. D. n. tichomirowi | 0.1028 | 0.0867 | 0.0898 | 0.0472 | 0.0970 | 0.1014 | 0.0616 | 0.0707 | 0.1124 | 0.1254 | 0.1302 | 0.0100 | 0.0087 | |
| 13. D. n. kurdistanicus | 0.1112 | 0.0937 | 0.0899 | 0.0989 | 0.0490 | 0.1183 | 0.0886 | 0.0964 | 0.0954 | 0.0839 | 0.1361 | 0.0997 | 0.0078 | |
| 14. D. n. bilkjewiczi | 0.1018 | 0.0761 | 0.0811 | 0.0801 | 0.0523 | 0.1026 | 0.0696 | 0.0827 | 0.0924 | 0.1022 | 0.1242 | 0.0780 | 0.0575 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çolak, E.; Markov, G.; Selvi, E.; Kankılıç, T.; Şeker, P.S.; Kocheva, M.A.; Gospodinova, M.K.; Çolak, R.; Dimitrov, H.; Yiğit, N. Assessment of Intraspecific Variability in the Forest Dormouse (Dryomys nitedula) and Woolly Dormouse (Dryomys laniger) from Türkiye and Adjacent Regions Based on Mitochondrial DNA. Life 2025, 15, 660. https://doi.org/10.3390/life15040660
Çolak E, Markov G, Selvi E, Kankılıç T, Şeker PS, Kocheva MA, Gospodinova MK, Çolak R, Dimitrov H, Yiğit N. Assessment of Intraspecific Variability in the Forest Dormouse (Dryomys nitedula) and Woolly Dormouse (Dryomys laniger) from Türkiye and Adjacent Regions Based on Mitochondrial DNA. Life. 2025; 15(4):660. https://doi.org/10.3390/life15040660
Chicago/Turabian StyleÇolak, Ercüment, Georgi Markov, Engin Selvi, Teoman Kankılıç, Perinçek Seçkinozan Şeker, Maria A. Kocheva, Milena K. Gospodinova, Reyhan Çolak, Hristo Dimitrov, and Nuri Yiğit. 2025. "Assessment of Intraspecific Variability in the Forest Dormouse (Dryomys nitedula) and Woolly Dormouse (Dryomys laniger) from Türkiye and Adjacent Regions Based on Mitochondrial DNA" Life 15, no. 4: 660. https://doi.org/10.3390/life15040660
APA StyleÇolak, E., Markov, G., Selvi, E., Kankılıç, T., Şeker, P. S., Kocheva, M. A., Gospodinova, M. K., Çolak, R., Dimitrov, H., & Yiğit, N. (2025). Assessment of Intraspecific Variability in the Forest Dormouse (Dryomys nitedula) and Woolly Dormouse (Dryomys laniger) from Türkiye and Adjacent Regions Based on Mitochondrial DNA. Life, 15(4), 660. https://doi.org/10.3390/life15040660







