Non-Invasive Spectroscopic Determination of the Skin and Blood Carotenoids of Term and Preterm Infants in the First Month of Life and the Influence of Free Radical-Mediated Diseases
Abstract
1. Introduction
2. Materials and Methods
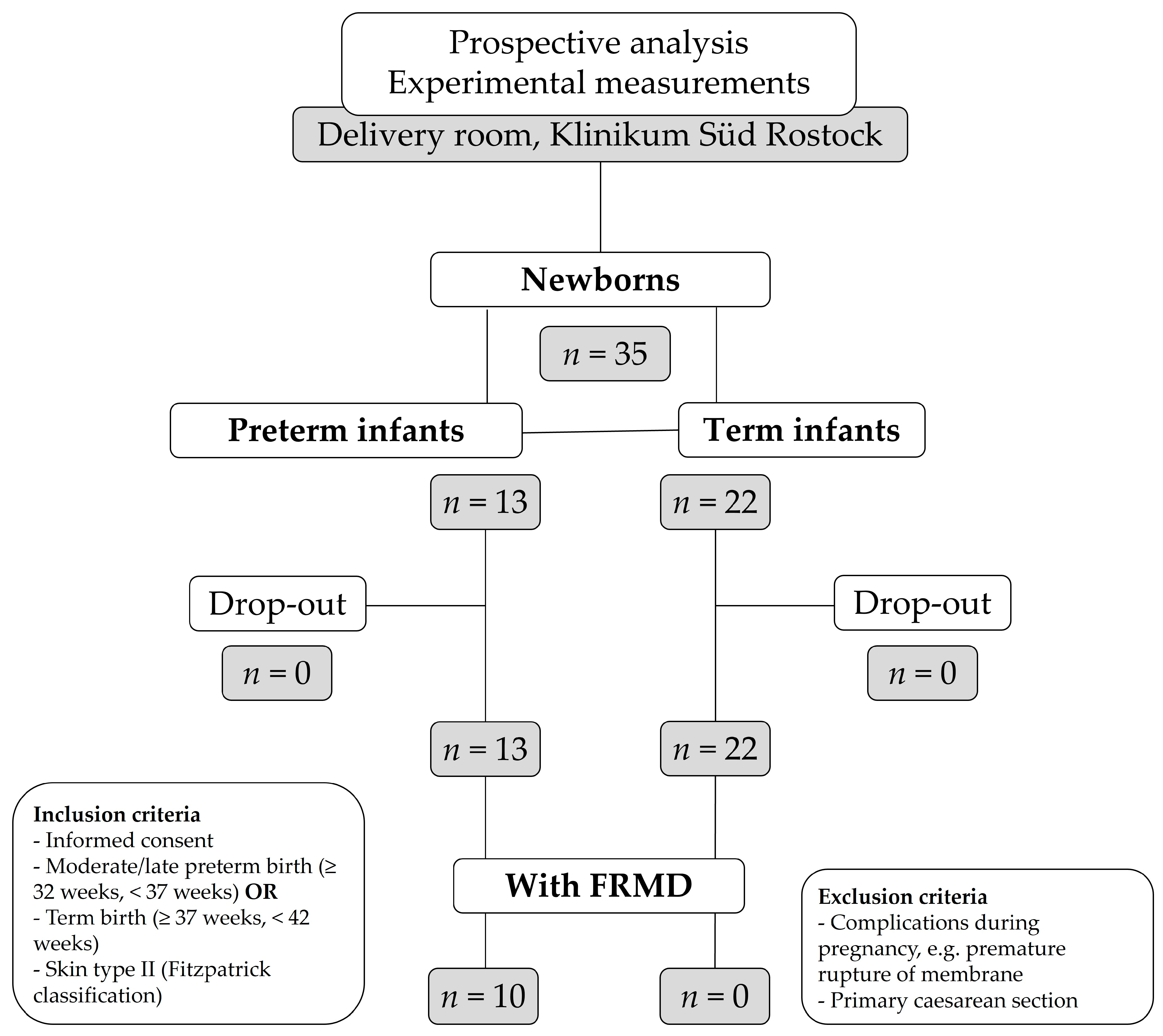
2.1. Patients
2.2. Ethics
2.3. Study Protocol
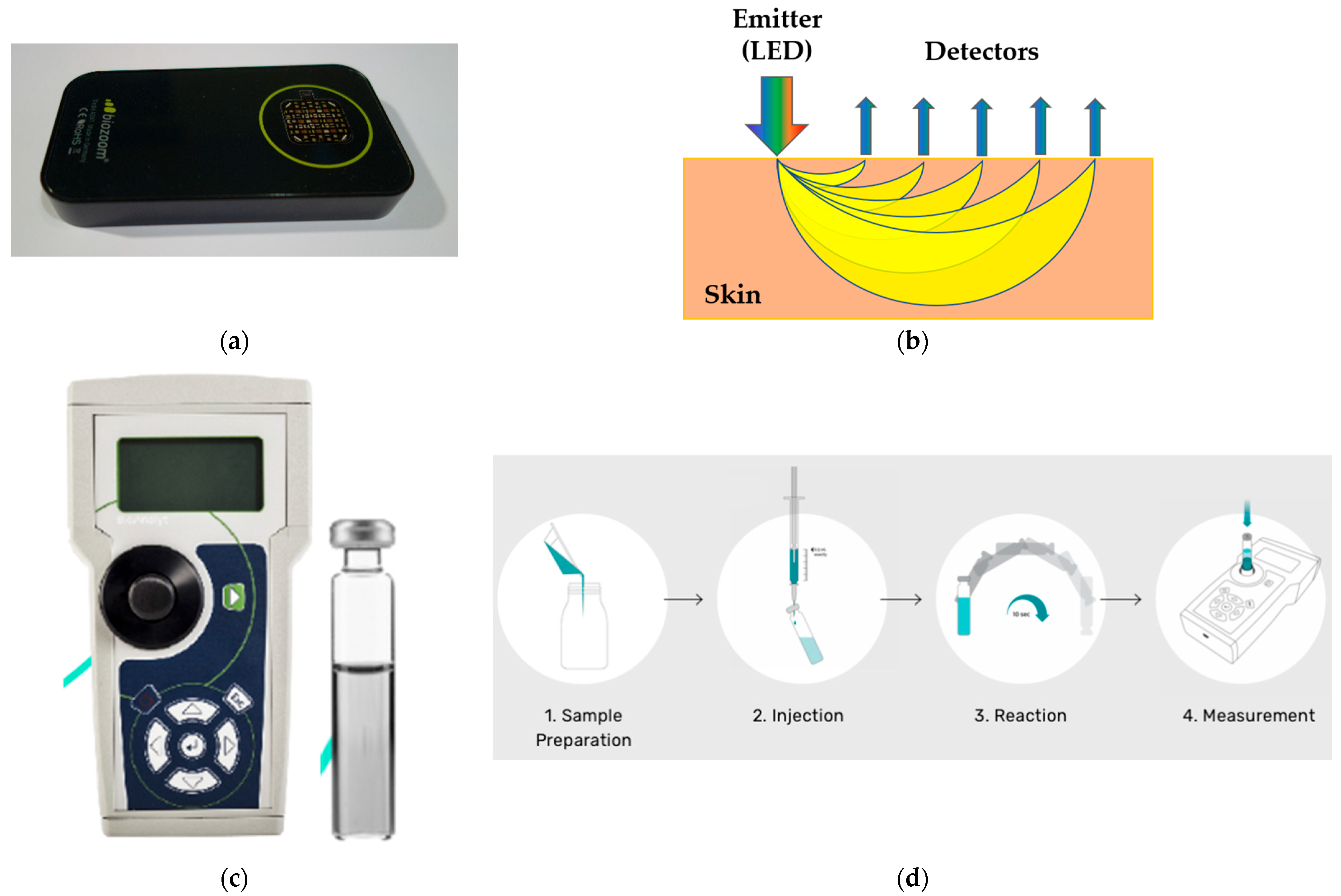
2.4. Validation Experiments
2.4.1. Skin Measurement
2.4.2. Blood Measurement
2.5. Statistics
3. Results
3.1. Validation
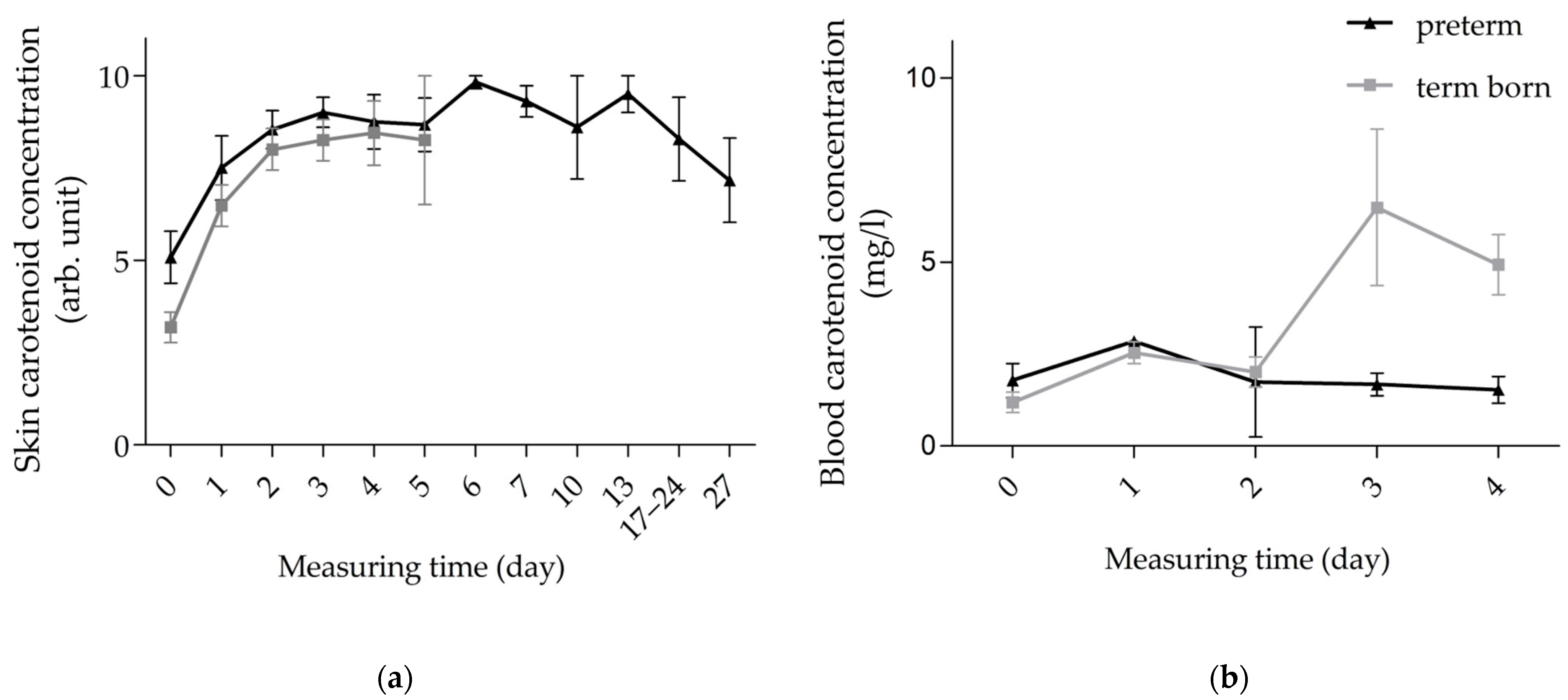
3.2. Kinetics of Carotenoids in Skin and Blood of Preterm vs. Term Infants
3.3. Kinetics of Carotenoids in Skin and Blood of FRMD vs. Non-FRMD Infants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Darvin, M.E.; Lademann, J.; von Hagen, J.; Lohan, S.B.; Kolmar, H.; Meinke, M.C.; Jung, S. Carotenoids in Human Skin In Vivo: Antioxidant and Photo-Protectant Role against External and Internal Stressors. Antioxidants 2022, 11, 1451. [Google Scholar] [CrossRef] [PubMed]
- Sarker, A.H.; Watanabe, S.; Seki, S.; Akiyama, T.; Okada, S. Oxygen Radical-Induced Single-Strand DNA Breaks and Repair of the Damage in a Cell-Free System. Mutat. Res./DNA Repair 1995, 337, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Perrone, S.; Manti, S.; Petrolini, C.; Dell’Orto, V.G.; Boscarino, G.; Ceccotti, C.; Bertini, M.; Buonocore, G.; Esposito, S.M.R.; Gitto, E. Oxygen for the Newborn: Friend or Foe? Children 2023, 10, 579. [Google Scholar] [CrossRef] [PubMed]
- Lembo, C.; Buonocore, G.; Perrone, S. Oxidative Stress in Preterm Newborns. Antioxidants 2021, 10, 1672. [Google Scholar] [CrossRef]
- Falsaperla, R.; Lombardo, F.; Filosco, F.; Romano, C.; Saporito, M.A.N.; Puglisi, F.; Piro, E.; Ruggieri, M.; Pavone, P. Oxidative Stress in Preterm Infants: Overview of Current Evidence and Future Prospects. Pharmaceuticals 2020, 13, 145. [Google Scholar] [CrossRef]
- Offord, E.A.; Gautier, J.-C.; Avanti, O.; Scaletta, C.; Runge, F.; Krämer, K.; Applegate, L.A. Photoprotective Potential of Lycopene, β-Carotene, Vitamin E, Vitamin C and Carnosic Acid in UVA-Irradiated Human Skin Fibroblasts. Free. Radic. Biol. Med. 2002, 32, 1293–1303. [Google Scholar] [CrossRef]
- Steenvoorden, P.T.; Beijersbergen, G.M.J.; Van Henegouwen, D. Protection against UV-Induced Systemic Immunosuppression in Mice by a Single Topical Application of the Antioxidant Vitamins C and E. Int. J. Radiat. Biol. 1999, 75, 747–755. [Google Scholar] [CrossRef]
- Niki, E. Antioxidants in Relation to Lipid Peroxidation. Chem. Phys. Lipids 1987, 44, 227–253. [Google Scholar] [CrossRef]
- Valgimigli, L.; Pratt, D.A. Antioxidants in Chemistry and Biology. In Encyclopedia of Radicals in Chemistry, Biology and Materials; Major Reference Works; Wiley Online Library: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Foy, C.J.; Passmore, A.P.; Vahidassr, M.D.; Young, I.S.; Lawson, J.T. Plasma Chain-Breaking Antioxidants in Alzheimer’s Disease, Vascular Dementia and Parkinson’s Disease. QJM Int. J. Med. 1999, 92, 39–45. [Google Scholar] [CrossRef]
- Lademann, J.; Patzelt, A.; Schanzer, S.; Richter, H.; Meinke, M.C.; Sterry, W.; Zastrow, L.; Doucet, O.; Vergou, T.; Darvin, M.E. Uptake of Antioxidants by Natural Nutrition and Supplementation: Pros and Cons from the Dermatological Point of View. Ski. Pharmacol. Physiol. 2011, 24, 269–273. [Google Scholar] [CrossRef]
- Thiele, J.J.; Schroeter, C.; Hsieh, S.N.; Podda, M.; Packer, L. The Antioxidant Network of the Stratum Corneum. Curr. Probl. Dermatol. 2001, 29, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Haag, S.F.; Bechtel, A.; Darvin, M.E.; Klein, F.; Groth, N.; Schafer-Korting, M.; Bittl, R.; Lademann, J.; Sterry, W.; Meinke, M.C. Comparative Study of Carotenoids, Catalase and Radical Formation in Human and Animal Skin. Ski. Pharmacol. Physiol. 2010, 23, 306–312. [Google Scholar] [CrossRef]
- Masaki, H. Role of Antioxidants in the Skin: Anti-Aging Effects. J. Dermatol. Sci 2010, 58, 85–90. [Google Scholar] [CrossRef]
- Al Senaidy, A.M. Plasma α- and γ-Tocopherol Have Different Pattern during Normal Human Pregnancy. Mol. Cell. Biochem. 1996, 154, 71–75. [Google Scholar] [CrossRef]
- Böhles, H. Antioxidative Vitamins in Prematurely and Maturely Born Infants. Int. J. Vitam. Nutr. Res. 1997, 67, 321–328. [Google Scholar]
- Gopinathan, V.; Miller, N.J.; Milner, A.D.; Rice-Evans, C.A. Bilirubin and Ascorbate Antioxidant Activity in Neonatal Plasma. FEBS Lett. 1994, 349, 197–200. [Google Scholar] [CrossRef]
- Jain, S.K.; Wise, R.; Bocchini, J.J., Jr. Vitamin E and Vitamin E-Quinone Levels in Red Blood Cells and Plasma of Newborn Infants and Their Mothers. J. Am. Coll. Nutr. 1996, 15, 44–48. [Google Scholar] [CrossRef]
- Sun, A.; Tian, L.; Xiong, X.; Kuchan, M.; Dai, X.; Sun, H.; Wang, H.; Li, X.; Zhang, L.; Zhao, Y.; et al. Carotenoids in Maternal and Cord Blood, Breast Milk and Their Association with Maternal Dietary Intake: A Longitudinal Study in Shanghai, China. Br. J. Nutr. 2024, 131, 1041–1052. [Google Scholar] [CrossRef]
- Addo, E.K.; Allman, S.J.; Arunkumar, R.; Gorka, J.E.; Harrison, D.Y.; Varner, M.W.; Bernstein, P.S. Systemic Effects of Prenatal Carotenoid Supplementation in the Mother and Her Child: The Lutein and Zeaxanthin in Pregnancy (L-ZIP) Randomized Trial —Report Number 1. J. Nutr. 2023, 153, 2205–2215. [Google Scholar] [CrossRef]
- Sun, H.; Wu, T.; Mao, Y.; Tian, F.; Cai, X.; Kuchan, M.J.; Zhang, L.; Zhao, Y.; Chen, J. Carotenoid Profile in Breast Milk and Maternal and Cord Plasma: A Longitudinal Study in Southwest China. Br. J. Nutr. 2021, 126, 1281–1287. [Google Scholar] [CrossRef]
- Tian, L.; Wang, L.; Li, F.; Sun, A.; Ni, M.; Sun, H.; Wang, H.; Li, X.; Zhao, Y.; Zhang, L.; et al. Carotenoid Profile in Maternal and Cord Plasma and Its Trends in Breast Milk during Lactation: A Comparative Study among Three Cities in Northern China. Food Funct. 2025, 16, 1000–1015. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, Y.; Stroh, R.; Moran, N.E. Systematic Review of Carotenoid Concentrations in Human Milk and Infant Blood. Nutr. Rev. 2022, 80, 2029–2050. [Google Scholar] [CrossRef] [PubMed]
- Barbas, C.; Herrera, E. Lipid Composition and Vitamin E Content in Human Colostrum and Mature Milk. J. Physiol. Biochem. 1998, 54, 167–173. [Google Scholar] [PubMed]
- Macias, C.; Schweigert, F.J. Changes in the Concentration of Carotenoids, Vitamin A, Alpha-Tocopherol and Total Lipids in Human Milk throughout Early Lactation. Ann. Nutr. Metab. 2001, 45, 82–85. [Google Scholar] [CrossRef]
- Minda, H.; Kovács, A.; Funke, S.; Szász, M.; Burus, I.; Molnár, S.; Marosvölgyi, T.; Decsi, T. Changes of Fatty Acid Composition of Human Milk during the First Month of Lactation: A Day-to-Day Approach in the First Week. Ann. Nutr. Metab. 2004, 48, 202–209. [Google Scholar] [CrossRef]
- Gitto, E.; Pellegrino, S.; Gitto, P.; Barberi, I.; Reiter, R.J. Oxidative Stress of the Newborn in the Pre- and Postnatal Period and the Clinical Utility of Melatonin. J. Pineal Res. 2009, 46, 128–139. [Google Scholar] [CrossRef]
- Walsh, S.W.; Wang, Y. Secretion of Lipid Peroxides by the Human Placenta. Am. J. Obstet. Gynecol. 1993, 169, 1462–1466. [Google Scholar] [CrossRef]
- Lademann, H.; Gerber, B.; Olbertz, D.M.; Darvin, M.E.; Stauf, L.; Ueberholz, K.; Heinrich, V.; Lademann, J.; Briese, V. Non-Invasive Spectroscopic Determination of the Antioxidative Status of Gravidae and Neonates. Ski. Pharmacol. Physiol. 2015, 28, 189–195. [Google Scholar] [CrossRef]
- Saugstad, O.D. Bronchopulmonary Dysplasia—Oxidative Stress and Antioxidants. Semin. Neonatol. 2003, 8, 39–49. [Google Scholar] [CrossRef]
- Shoji, H.; Koletzko, B. Oxidative Stress and Antioxidant Protection in the Perinatal Period. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 324. [Google Scholar] [CrossRef]
- Szabó, M.; Vásárhelyi, B.; Balla, G.; Szabó, T.; Machay, T.; Tulassay, T. Acute Postnatal Increase of Extracellular Antioxidant Defence of Neonates: The Role of Iron Metabolism. Acta Paediatr. 2001, 90, 1167–1170. [Google Scholar] [CrossRef] [PubMed]
- Perrone, S.; Lembo, C.; Giordano, M.; Petrolini, C.; Cannavò, L.; Gitto, E. Molecular Mechanisms of Oxidative Stress-Related Neonatal Jaundice. J. Biochem. Mol. Toxicol. 2023, 37, e23349. [Google Scholar] [CrossRef] [PubMed]
- Saugstad, O.D. Hypoxanthine as an Indicator of Hypoxia: Its Role in Health and Disease through Free Radical Production. Pediatr. Res. 1988, 23, 143–150. [Google Scholar] [CrossRef]
- Warner, B.B.; Wispe, J.R. Free Radical-Mediated Diseases in Pediatrics. Semin. Perinatol. 1992, 16, 47–57. [Google Scholar]
- Perrone, S.; Tataranno, M.L.; Negro, S.; Longini, M.; Marzocchi, B.; Proietti, F.; Iacoponi, F.; Capitani, S.; Buonocore, G. Early Identification of the Risk for Free Radical-Related Diseases in Preterm Newborns. Early Hum. Dev. 2010, 86, 241–244. [Google Scholar] [CrossRef]
- Lawn, J.E.; Kerber, K.; Enweronu-Laryea, C.; Cousens, S. 3.6 Million Neonatal Deaths—What Is Progressing and What Is Not? Semin. Perinatol. 2010, 34, 371–386. [Google Scholar] [CrossRef]
- Cortese, F.; Scicchitano, P.; Gesualdo, M.; Filaninno, A.; De Giorgi, E.; Schettini, F.; Laforgia, N.; Ciccone, M.M. Early and Late Infections in Newborns: Where Do We Stand? A Review. Pediatr. Neonatol. 2016, 57, 265–273. [Google Scholar] [CrossRef]
- Knoop, K.A.; Coughlin, P.E.; Floyd, A.N.; Ndao, I.M.; Hall-Moore, C.; Shaikh, N.; Gasparrini, A.J.; Rusconi, B.; Escobedo, M.; Good, M.; et al. Maternal Activation of the EGFR Prevents Translocation of Gut-Residing Pathogenic Escherichia Coli in a Model of Late-Onset Neonatal Sepsis. Proc. Natl. Acad. Sci. USA 2020, 117, 7941–7949. [Google Scholar] [CrossRef]
- Perez, E.M.; Weisman, L.E. Novel Approaches to the Prevention and Therapy of Neonatal Bacterial Sepsis. Clin. Perinatol. 1997, 24, 213–229. [Google Scholar] [CrossRef]
- Hardy, P.; Dumont, I.; Bhattacharya, M.; Hou, X.; Lachapelle, P.; Varma, D.R.; Chemtob, S. Oxidants, Nitric Oxide and Prostanoids in the Developing Ocular Vasculature: A Basis for Ischemic Retinopathy. Cardiovasc. Res. 2000, 47, 489–509. [Google Scholar] [CrossRef]
- De Curtis, M.; Paone, C.; Vetrano, G.; Romano, G.; Paludetto, R.; Ciccimarra, F. A Case Control Study of Necrotizing Enterocolitis Occurring over 8 Years in a Neonatal Intensive Care Unit. Eur. J. Pediatr. 1987, 146, 398–400. [Google Scholar] [CrossRef] [PubMed]
- Platt, M.J. Outcomes in Preterm Infants. Public Health 2014, 128, 399–403. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, V.O.; Pereira, R.A.; Amantéa, S.L.; Rhoden, C.R.; Colvero, M.O. Neonatal Diseases and Oxidative Stress in Premature Infants: An Integrative Review. J. Pediatr. 2022, 98, 455–462. [Google Scholar] [CrossRef]
- Flieger, J.; Raszewska-Famielec, M.; Radzikowska-Büchner, E.; Flieger, W. Skin Protection by Carotenoid Pigments. Int. J. Mol. Sci. 2024, 25, 1431. [Google Scholar] [CrossRef]
- Meinke, M.C.; Darvin, M.E.; Vollert, H.; Lademann, J. Bioavailability of Natural Carotenoids in Human Skin Compared to Blood. Eur. J. Pharm. Biopharm. 2010, 76, 269–274. [Google Scholar] [CrossRef]
- Kohen, R. Skin Antioxidants: Their Role in Aging and in Oxidative Stress—New Approaches for Their Evaluation. Biomed. Pharmacother. 1999, 53, 181–192. [Google Scholar] [CrossRef]
- Chung, H.-Y.; Ferreira, A.L.A.; Epstein, S.; Paiva, S.A.; Castaneda-Sceppa, C.; Johnson, E.J. Site-Specific Concentrations of Carotenoids in Adipose Tissue: Relations with Dietary and Serum Carotenoid Concentrations in Healthy Adults123. Am. J. Clin. Nutr. 2009, 90, 533–539. [Google Scholar] [CrossRef]
- Haag, S.F.; Taskoparan, B.; Darvin, M.E.; Groth, N.; Lademann, J.; Sterry, W.; Meinke, M.C. Determination of the Antioxidative Capacity of the Skin In Vivo Using Resonance Raman and Electron Paramagnetic Resonance Spectroscopy. Exp. Dermatol. 2011, 20, 483–487. [Google Scholar] [CrossRef]
- Talwar, D.; Ha, T.K.; Cooney, J.; Brownlee, C.; O’Reilly, D.S. A Routine Method for the Simultaneous Measurement of Retinol, Alpha-Tocopherol and Five Carotenoids in Human Plasma by Reverse Phase HPLC. Clin. Chim. Acta 1998, 270, 85–100. [Google Scholar] [CrossRef]
- Baydas, G.; Karatas, F.; Gursu, M.F.; Bozkurt, H.A.; Ilhan, N.; Yasar, A.; Canatan, H. Antioxidant Vitamin Levels in Term and Preterm Infants and Their Relation to Maternal Vitamin Status. Arch. Med. Res. 2002, 33, 276–280. [Google Scholar] [CrossRef]
- Cavia-Saiz, M.; Arnaez, J.; Cilla, A.; Puente, L.; Garcia-Miralles, L.C.; Muñiz, P. Biomarkers of Oxidative Stress in Healthy Infants within the First Three Days after Birth. Antioxidants 2023, 12, 1249. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Garcia, H.; Vilchis-Gil, J.; Garcia-Roca, P.; Klünder-Klünder, M.; Gomez-Lopez, J.; Granados-Riveron, J.T.; Sanchez-Urbina, R. Dietary and Antioxidant Vitamins Limit the DNA Damage Mediated by Oxidative Stress in the Mother–Newborn Binomial. Life 2022, 12, 1012. [Google Scholar] [CrossRef] [PubMed]
- Perrone, S.; Alagna, M.G.; Cori, M.S.; Santacroce, A.; Negro, S.; Tataranno, M.L.; Longini, M.; Felici, C.; Proietti, F.; Picardi, A.; et al. Free radical-related diseases: The predictive value of biomarkers in the umbilical cord blood. J. Siena Acad. Sci. 2013, 5, 49–52. [Google Scholar] [CrossRef]
- Darvin, M.E.; Meinke, M.C.; Sterry, W.; Lademann, J. Optical Methods for Noninvasive Determination of Carotenoids in Human and Animal Skin. J. Biomed. Opt. 2013, 18, 61230. [Google Scholar] [CrossRef]
- Alaluf, S.; Heinrich, U.; Stahl, W.; Tronnier, H.; Wiseman, S. Dietary Carotenoids Contribute to Normal Human Skin Color and UV Photosensitivity. J. Nutr. 2002, 132, 399–403. [Google Scholar] [CrossRef]
- Cannavale, C.N.; Keye, S.A.; Rosok, L.M.; Martell, S.G.; Holthaus, T.A.; Raine, L.R.; Mullen, S.P.; Holscher, H.D.; Hillman, C.H.; Kramer, A.F.; et al. Macular Pigment Optical Density and Skin Carotenoids in a Childhood Sample. J. Nutr. 2023, 153, 3144–3151. [Google Scholar] [CrossRef]
- Whitehead, R.D.; Re, D.; Xiao, D.; Ozakinci, G.; Perrett, D.I. You Are What You Eat: Within-Subject Increases in Fruit and Vegetable Consumption Confer Beneficial Skin-Color Changes. PLoS ONE 2012, 7, e32988. [Google Scholar] [CrossRef]
- Darvin, M.E.; Sandhagen, C.; Koecher, W.; Sterry, W.; Lademann, J.; Meinke, M.C. Comparison of Two Methods for Noninvasive Determination of Carotenoids in Human and Animal Skin: Raman Spectroscopy versus Reflection Spectroscopy. J. Biophotonics 2012, 5, 550–558. [Google Scholar] [CrossRef]
- Darvin, M.E.; Gersonde, I.; Albrecht, H.; Gonchukov, S.A.; Sterry, W.; Lademann, J. Determination of Beta Carotene and Lycopene Concentrations in Human Skin Using Resonance Raman Spectroscopy. Laser Phys. 2005, 15, 295–299. [Google Scholar]
- Darvin, M.E.; Magnussen, B.; Lademann, J.; Kocher, W. Multiple Spatially Resolved Reflection Spectroscopy for In Vivo Determination of Carotenoids in Human Skin and Blood. Laser Phys. Lett. 2016, 13, 095601. [Google Scholar] [CrossRef]
- Ermakov, I.V.; Ermakova, M.R.; Bernstein, P.S.; Chan, G.M.; Gellermann, W. Resonance Raman Based Skin Carotenoid Measurements in Newborns and Infants. J. Biophotonics 2013, 6, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain During Pregnancy: Reexamining the Guidelines; Rasmussen, K.M., Yaktine, A.L., Eds.; National Academies Press: Washington, DC, USA, 2009. [Google Scholar] [CrossRef]
- Hedderson, M.M.; Gunderson, E.P.; Ferrara, A. Gestational Weight Gain and Risk of Gestational Diabetes Mellitus. Obstet. Gynecol. 2010, 115, 597. [Google Scholar] [CrossRef] [PubMed]
- Gibson, K.S.; Waters, T.P.; Catalano, P.M. Maternal Weight Gain in Women Who Develop Gestational Diabetes Mellitus. Obstet. Gynecol. 2012, 119, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Egan, A.M.; Dennedy, M.C.; Al-Ramli, W.; Heerey, A.; Avalos, G.; Dunne, F. ATLANTIC-DIP: Excessive Gestational Weight Gain and Pregnancy Outcomes in Women with Gestational or Pregestational Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2014, 99, 212–219. [Google Scholar] [CrossRef]
- Ren, M.; Li, H.; Cai, W.; Niu, X.; Ji, W.; Zhang, Z.; Niu, J.; Zhou, X.; Li, Y. Excessive Gestational Weight Gain in Accordance with the IOM Criteria and the Risk of Hypertensive Disorders of Pregnancy: A Meta-Analysis. BMC Pregnancy Childbirth 2018, 18, 281. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Levine, L.D.; Durnwald, C.P.; Elovitz, M.A.; Srinivas, S.K. Excessive Weight Gain and Hypertensive Disorders of Pregnancy in the Obese Patient. J. Matern.-Fetal Neonatal Med. 2015, 28, 964–968. [Google Scholar]
- Maier, J.T.; Schalinski, E.; Gauger, U.; Hellmeyer, L. Antenatal Body Mass Index (BMI) and Weight Gain in Pregnancy—Its Association with Pregnancy and Birthing Complications. J. Perinat. Med. 2016, 44, 397–404. [Google Scholar] [CrossRef]
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.; Boyle, J.A.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; Rode, L.; et al. Association of Gestational Weight Gain with Maternal and Infant Outcomes: A Systematic Review and Meta-Analysis. JAMA 2017, 317, 2207–2225. [Google Scholar] [CrossRef]
- Rubin, L.P.; Chan, G.M.; Barrett-Reis, B.M.; Fulton, A.B.; Hansen, R.M.; Ashmeade, T.L.; Oliver, J.S.; Mackey, A.D.; Dimmit, R.A.; Hartmann, E.E.; et al. Effect of Carotenoid Supplementation on Plasma Carotenoids, Inflammation and Visual Development in Preterm Infants. J. Perinatol. 2012, 32, 418–424. [Google Scholar] [CrossRef]
- Chan, G.M.; Chan, M.M.; Gellermann, W.; Ermakov, I.; Ermakova, M.; Bhosale, P.; Bernstein, P.; Rau, C. Resonance Raman Spectroscopy and the Preterm Infant Carotenoid Status. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 556. [Google Scholar] [CrossRef]
- Addo, E.K.; Gorka, J.E.; Allman, S.J.; Harrison, D.Y.; Sharifzadeh, M.; Hoffman, R.O.; Hartnett, M.E.; Varner, M.W.; Bernstein, P.S. Ocular Effects of Prenatal Carotenoid Supplementation in the Mother and Her Child: The Lutein and Zeaxanthin in Pregnancy (L-ZIP) Randomized Trial—Report Number 2. Ophthalmol. Sci. 2024, 4, 100537. [Google Scholar] [CrossRef] [PubMed]
- Thapa, R.; Addo, E.K.; Ruit, S.; Bernstein, P.S. Assessment of Skin Carotenoid Measurement as a Means to Detect Vitamin A Deficiency in Children and Pregnant Women of Nepal. J. Nutr. 2023, 153, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, T.B. The Validity and Practicality of Sun-Reactive Skin Types I through VI. Arch. Dermatol. 1988, 124, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Longini, M.; Perrone, S.; Vezzosi, P.; Marzocchi, B.; Kenanidis, A.; Centini, G.; Rosignoli, L.; Buonocore, G. Association between Oxidative Stress in Pregnancy and Preterm Premature Rupture of Membranes. Clin. Biochem. 2007, 40, 793–797. [Google Scholar] [CrossRef]
- iCheck Carotene. Available online: https://www.bioanalyt.com/product/carotene/ (accessed on 18 January 2024).
- Islam, K.M.S.; Schweigert, F.J. Comparison of Three Spectrophotometric Methods for Analysis of Egg Yolk Carotenoids. Food Chem. 2015, 172, 233–237. [Google Scholar] [CrossRef]
- Maeter, H. Das Kutan Messbare Antioxidative Potenzial von Schwangeren und Neugeborenen; Universität Rostock: Rostock, Germany, 2012. [Google Scholar]
- Moran, N.E.; Chang, J.; Stroh, R.; Zaidi, Y.; Hason, N.; Musaad, S.; O’Connor, T. Noninvasive Reflection Spectroscopy Measurement of Skin Carotenoid Score in Infants Is Feasible and Reliable. J. Nutr. 2022, 152, 2966–2977. [Google Scholar] [CrossRef]
- Chiou, Y.B.; Blume-Peytavi, U. Stratum Corneum Maturation: A Review of Neonatal Skin Function. Ski. Pharmacol. Appl. Ski. Physiol. 2004, 17, 57–66. [Google Scholar] [CrossRef]
- Kusari, A.; Han, A.M.; Virgen, C.A.; Matiz, C.; Rasmussen, M.; Friedlander, S.F.; Eichenfield, D.Z. Evidence-Based Skin Care in Preterm Infants. Pediatr. Dermatol. 2019, 36, 16–23. [Google Scholar] [CrossRef]
- Behrman, R.E.; Kliegman, R.M.; Jenson, H.B. Nelson Textbook of Pediatrics; W.B. Saunders Company: Philadelphia, PA, USA, 1983; ISBN 978-0-7216-1736-7. [Google Scholar]
- Ishisaka, A.; Fujiwara, N.; Mukai, R.; Nishikawa, M.; Ikushiro, S.; Murakami, A. Flavonoids in Breast Milk and Their Absorption, Metabolism, and Bioactivity in Infants. Biosci. Biotechnol. Biochem. 2025, 89, 165–173. [Google Scholar] [CrossRef]
- Porzig, S. Untersuchung zum Antioxidativen Status von Kühen und Deren Neugeborenen Kälbern. PhD Thesis, Ludwig-Maximilians-Universität München, München, Germany, 2004. [Google Scholar]
- Surjowardojo, P.; Muarifah, H.; Fithron, Z.U.R.; Rifa’i, R. Performance of Carotenoid Contents in Colostrum of Dairy Cows during the Initial to Five Days of Lactation. BIO Web Conf. 2023, 81, 00048. [Google Scholar] [CrossRef]
- Ri, J.-S.; Choe, C.-S.; Choe, S.-H.; Jong, K.-H.; Hong, S.-N.; Schleusener, J.; Lademann, J.; Darvin, M.E. Lycopene, but Not Zeaxanthin, Serves as a Skeleton for the Formation of an Orthorhombic Organization of Intercellular Lipids within the Lamellae in the Stratum Corneum: Molecular Dynamics Simulations of the Hydrated Ceramide NS Bilayer Model. Biochim. Biophys. Acta (BBA)—Biomembr. 2023, 1865, 184081. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Caspers, P.; van der Pol, J.A.; Richter, H.; Sterry, W.; Lademann, J.; Darvin, M.E. Kinetics of Carotenoid Distribution in Human Skin in Vivo after Exogenous Stress: Disinfectant and wIRA-Induced Carotenoid Depletion Recovers from Outside to Inside. J. Biomed. Opt. 2011, 16, 035002. [Google Scholar] [CrossRef]
- Harpin, V.A.; Rutter, N. Sweating in Preterm Babies. J. Pediatr. 1982, 100, 614–619. [Google Scholar] [CrossRef]
- Lowes, D.A.; Webster, N.R.; Murphy, M.P.; Galley, H.F. Antioxidants That Protect Mitochondria Reduce Interleukin-6 and Oxidative Stress, Improve Mitochondrial Function, and Reduce Biochemical Markers of Organ Dysfunction in a Rat Model of Acute Sepsis. Br. J. Anaesth. 2013, 110, 472–480. [Google Scholar] [CrossRef]
- Prauchner, C.A. Oxidative Stress in Sepsis: Pathophysiological Implications Justifying Antioxidant Co-Therapy. Burns 2017, 43, 471–485. [Google Scholar] [CrossRef]
- Berger, M.M.; Chioléro, R.L. Antioxidant Supplementation in Sepsis and Systemic Inflammatory Response Syndrome. Crit. Care Med. 2007, 35, S584. [Google Scholar] [CrossRef]
- Victor, V.M.; Esplugues, J.V.; Hernandez-Mijares, A.; Rocha, M. Oxidative Stress and Mitochondrial Dysfunction in Sepsis: A Potential Therapy with Mitochondria-Targeted Antioxidants. Infect. Disord.-Drug Targets Disord. 2009, 9, 376–389. [Google Scholar] [CrossRef]


| Pre-Pregnancy BMI [kg/m2] | Pre-Pregnancy Class | Total Weight Gain Range [kg] |
|---|---|---|
| <18.5 | underweight | 12.5–18.0 |
| 18.5–24.9 | normal weight | 11.5–16.0 |
| 25.0–29.9 | overweight | 7.0–11.5 |
| ≥30.0 | all classes of obesity | 5.0–9.0 |
| Measuring Time | Day After Birth | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 10 | 13 | 17–24 | 27 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Skin | |||||||||||||
| Total | n | 35 | 29 | 33 | 29 | 21 | 16 | 11 | 10 | 5 | 2 | 7 | 6 |
| Preterm | 13 | 8 | 13 | 13 | 12 | 12 | 11 | 10 | 5 | 2 | 7 | 6 | |
| Term | 22 | 21 | 20 | 16 | 9 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Blood | |||||||||||||
| Total | n | 33 | 3 | 8 | 9 | 6 | |||||||
| Preterm | 13 | 1 | 2 | 5 | 4 | ||||||||
| Term | 20 | 2 | 6 | 4 | 2 | ||||||||
| Term Infants | Preterm Infants | Missing Information | |||
|---|---|---|---|---|---|
| n = 22 | n = 13 | p-Value | n (%) | ||
| female | n (%) | 14 (63.6) | 10 (76.9) | ns b | 0 |
| gestational age [weeks + days] | mean ± SD | 40 + 1 ± 1 + 2 | 34 + 4 ± 1 + 5 | *** a | 0 |
| birth weight [gram] | 3544 ± 374 | 2178 ± 414 | *** a | 1 ti | |
| head circumference [cm] | 35.4 ± 1.6 | 31.4 ± 2.16 | *** a | 3 ti 3 pi | |
| substitution of vitamins | n (%) | ||||
| pre-natal | 19 (86.4) | 6 (46.2) | ns b | 2 ti 4 pi | |
| post-natal | 0 (0.0) | 4 (30.8) | * b | 1 ti | |
| substitution of iron | |||||
| pre-natal | 15 (68.2) | 4 (30.7) | ns b | 2 ti 4 pi | |
| post-natal | 0 (0.0) | 6 (46.2) | ** b | 1 ti | |
| diet | |||||
| human breast milk | 19 (86.4) | 12 (92.3) | ns b | 0 | |
| formula milk | 6 (27.3) | 11 (84.6) | ** b | 0 | |
| pathological maternal weight gain during pregnancy | 13 (59.1) | 2 (15.4) | * b | 0 | |
| maternal pre-existing conditions | 10 (45.5) | 3 (23.1) | ns b | 0 | |
| FRMD | 0 | 10 (76.9) | *** b | 1 ti | |
| hyperbilirubinemia | 0 (0.0) | 9 (69.2) | *** b | 1 ti | |
| respiratory distress | 0 (0.0) | 3 (23.1) | * b | 1 ti | |
| sepsis | 0 (0.0) | 2 (15.4) | ns b | 1 ti |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lademann, H.; Darvin, M.E.; Häfke, A.; Lademann, J.; Wagner, L.; Däbritz, J.; Olbertz, D.M. Non-Invasive Spectroscopic Determination of the Skin and Blood Carotenoids of Term and Preterm Infants in the First Month of Life and the Influence of Free Radical-Mediated Diseases. Life 2025, 15, 534. https://doi.org/10.3390/life15040534
Lademann H, Darvin ME, Häfke A, Lademann J, Wagner L, Däbritz J, Olbertz DM. Non-Invasive Spectroscopic Determination of the Skin and Blood Carotenoids of Term and Preterm Infants in the First Month of Life and the Influence of Free Radical-Mediated Diseases. Life. 2025; 15(4):534. https://doi.org/10.3390/life15040534
Chicago/Turabian StyleLademann, Hanne, Maxim E. Darvin, Anna Häfke, Jürgen Lademann, Laura Wagner, Jan Däbritz, and Dirk M. Olbertz. 2025. "Non-Invasive Spectroscopic Determination of the Skin and Blood Carotenoids of Term and Preterm Infants in the First Month of Life and the Influence of Free Radical-Mediated Diseases" Life 15, no. 4: 534. https://doi.org/10.3390/life15040534
APA StyleLademann, H., Darvin, M. E., Häfke, A., Lademann, J., Wagner, L., Däbritz, J., & Olbertz, D. M. (2025). Non-Invasive Spectroscopic Determination of the Skin and Blood Carotenoids of Term and Preterm Infants in the First Month of Life and the Influence of Free Radical-Mediated Diseases. Life, 15(4), 534. https://doi.org/10.3390/life15040534







