Therapeutic Challenges and Emerging Strategies for T674I and PTPN11 Mutations in a FIP1L1-PDGFRA-Positive Myeloproliferative Neoplasm: A Case Report
Abstract
1. Introduction
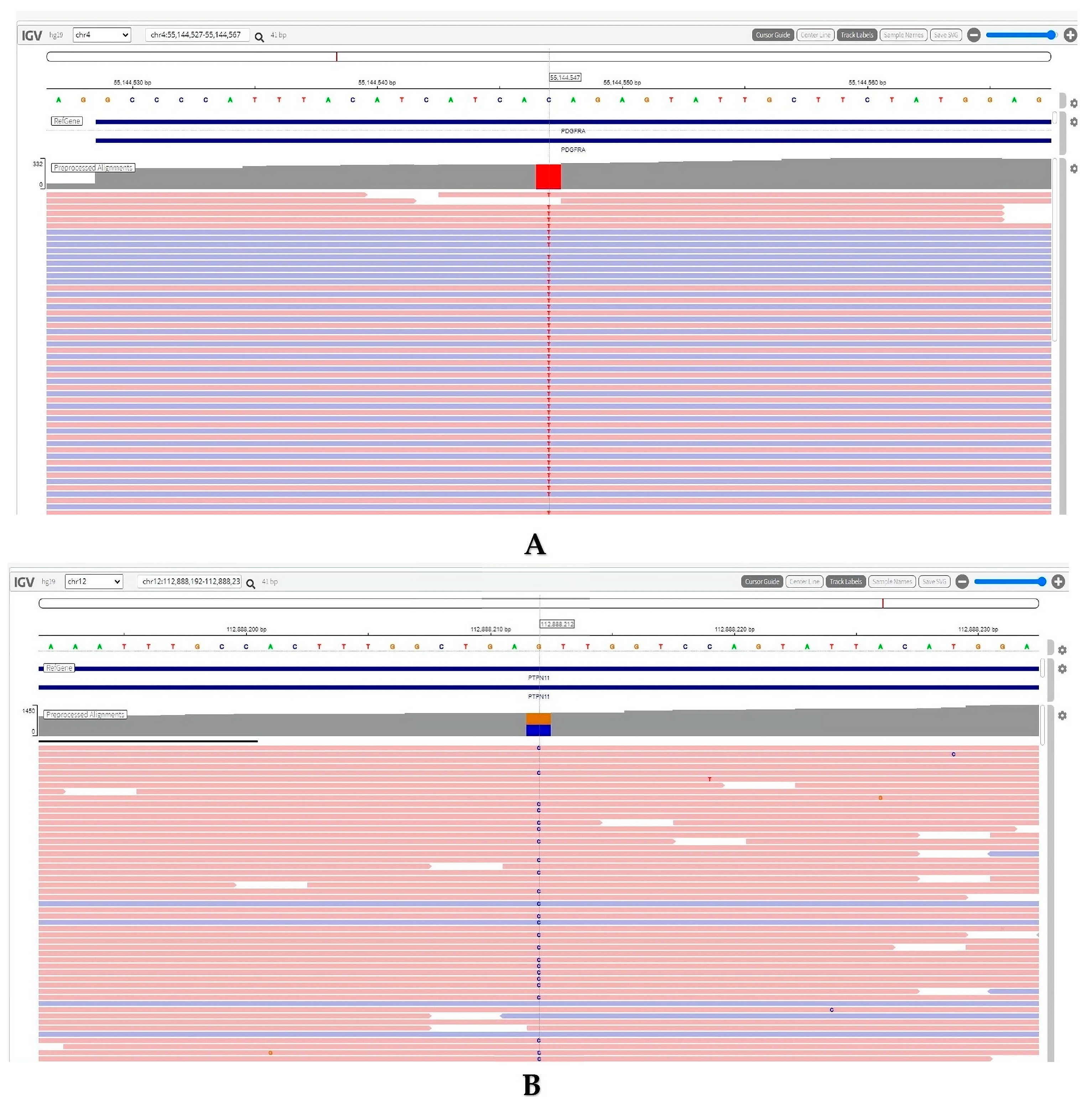
2. Detailed Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ARA-C | Cytarabine |
| allo-HSCT | Allogeneic Hematopoietic Stem Cell Transplantation |
| B symptoms | Fever, night sweats, and weight loss |
| M/LN-eo | Myeloid and lymphoid neoplasms with eosinophilia |
| MPN | Myeloproliferative neoplasm |
| CT | Computed Tomography |
| FDG | Fluorodeoxyglucose |
| FIP1L1-PDGFRA | Fusion of FIP1-Like 1 and Platelet-Derived Growth Factor Receptor Alpha |
| FLAG-IDA | Fludarabine, Cytarabine, and Idarubicin regimen |
| FISH | Fluorescence In Situ Hybridization |
| FLU | Fludarabine |
| GVHD | Graft-Versus-Host Disease |
| Hb | Hemoglobin |
| HES | Hypereosinophilic Syndrome |
| HLA | Human Leukocyte Antigen |
| MCV | Mean Corpuscular Volume |
| MEL | Melphalan |
| MRI | Magnetic Resonance Imaging |
| PET | Positron Emission Tomography |
| PTPN11 | Protein Tyrosine Phosphatase Non-Receptor Type 11 |
| RBC | Red Blood Cell |
| RDW | Red Cell Distribution Width |
| SUVmax | Maximum Standardized Uptake Value |
| TBI | Total Body Irradiation |
| TKI | Tyrosine Kinase Inhibitor |
| WBC | White Blood Cell |
References
- Crane, M.M.; Chang, C.M.; Kobayashi, M.G.; Weller, P.F. Incidence of myeloproliferative hypereosinophilic syndrome in the United States and an estimate of all hypereosinophilic syndrome incidence. J. Allergy Clin. Immunol. 2010, 126, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Chusid, M.J.; Dale, D.C.; West, B.C.; Wolff, S.M. The hypereosinophilic syndrome: Analysis of fourteen cases with review of the literature. Medicine 1975, 54, 1–27. [Google Scholar] [PubMed]
- Simon, H.-U.; Rothenberg, M.E.; Bochner, B.S.; Weller, P.F.; Wardlaw, A.J.; Wechsler, M.E.; Rosenwasser, L.J.; Roufosse, F.; Gleich, G.J.; Klion, A.D. Refining the definition of hypereosinophilic syndrome. J. Allergy Clin. Immunol. 2010, 126, 45–49. [Google Scholar] [CrossRef]
- Gotlib, J. World Health Organization-defined eosinophilic disorders: 2017 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2017, 92, 1243–1259. [Google Scholar] [CrossRef]
- Rohmer, J.; Couteau-Chardon, A.; Trichereau, J.; Panel, K.; Gesquiere, C.; Ben Abdelali, R.; Bidet, A.; Bladé, J.; Cayuela, J.; Cony-Makhoul, P.; et al. Epidemiology, clinical picture and long-term outcomes of FIP1L1-PDGFRA-positive myeloid neoplasm with eosinophilia: Data from 151 patients. Am. J. Hematol. 2020, 95, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Klion, A. Hypereosinophilic syndrome: Approach to treatment in the era of precision medicine. Hematol. Am. Soc. Hematol. Educ. Program 2018, 2018, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.-Q.; Qin, T.-J.; Xu, Z.-F.; Zhang, Y.; Ai, X.-F.; Li, B.; Zhang, H.-L.; Fang, L.-W.; Pan, L.-J.; Hu, N.-B.; et al. Long-term outcomes of imatinib in patients with FIP1L1/PDGFRA associated chronic eosinophilic leukemia: Experience of a single center in China. Oncotarget 2016, 7, 33229–33236. [Google Scholar] [CrossRef] [PubMed]
- Kanumuri, R.; Kumar Pasupuleti, S.; Burns, S.S.; Ramdas, B.; Kapur, R. Targeting SHP2 phosphatase in hematological malignancies. Expert Opin. Ther. Targets 2022, 26, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.; Kalaitzidis, D.; Usenko, T.; Kutok, J.L.; Yang, W.; Mohi, M.G.; Neel, B.G. Leukemogenic Ptpn11 causes fatal myeloproliferative disorder via cell-autonomous effects on multiple stages of hematopoiesis. Blood 2009, 113, 4414–4424. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.A.; Orazi, A.; Gotlib, J.; Reiter, A.; Tzankov, A.; Hasserjian, R.P.; Arber, D.A.; Tefferi, A. The international consensus classification of eosinophilic disorders and systemic mastocytosis. Am. J. Hematol. 2023, 98, 1286–1306. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Augi, T.; Alilovic, K.; Trinchant, N.M.; Sinha, E.; Contreras, J.; Samuel, M.; Desai, P.; Kluk, M.; Smith, B.N.; et al. PTPN11 mutations Confer Adverse Outcomes and Therapy Resistance in Older Patients with Acute Myeloid Leukemia (AML). Blood 2022, 140 (Suppl. 1), 3208–3209. [Google Scholar] [CrossRef]
- Metzgeroth, G.; Erben, P.; Martin, H.; Mousset, S.; Teichmann, M.; Walz, C.; Klippstein, T.; Hochhaus, A.; Cross, N.C.P.; Hofmann, W.-K.; et al. Limited clinical activity of nilotinib and sorafenib in FIP1L1-PDGFRA positive chronic eosinophilic leukemia with imatinib-resistant T674I mutation. Leukemia 2012, 26, 162–164. [Google Scholar] [CrossRef] [PubMed]
- Lierman, E.; Folens, C.; Stover, E.H.; Mentens, N.; Van Miegroet, H.; Scheers, W.; Boogaerts, M.; Vandenberghe, P.; Marynen, P.; Cools, J. Sorafenib is a potent inhibitor of FIP1L1-PDGFRalpha and the imatinib-resistant FIP1L1-PDGFRalpha T674I mutant. Blood 2006, 108, 1374–1376. [Google Scholar] [CrossRef] [PubMed]
- Lierman, E.; Michaux, L.; Beullens, E.; Pierre, P.; Marynen, P.; Cools, J.; Vandenberghe, P. FIP1L1-PDGFRalpha D842V, a novel panresistant mutant, emerging after treatment of FIP1L1-PDGFRalpha T674I eosinophilic leukemia with single agent sorafenib. Leukemia 2009, 23, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Lierman, E.; Smits, S.; Appleby, N.; Conneally, E.; Michaux, L.; Vandenberghe, P. FIP1L1-PDGFRalpha p.T674I-D842L: A Novel and Ponatinib Resistant Compound Mutation in FIP1L1-PDGFRalpha Positive Leukemia. Hemasphere 2019, 3, e182. [Google Scholar] [CrossRef]
- Nguyen, L.; Saha, A.; Kuykendall, A.; Zhang, L. Clinical and Therapeutic Intervention of Hypereosinophilia in the Era of Molecular Diagnosis. Cancers 2024, 16, 1383. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, M.E.; Akuthota, P.; Jayne, D.; Khoury, P.; Klion, A.; Langford, C.A.; Merkel, P.A.; Moosig, F.; Specks, U.; Cid, M.C.; et al. Mepolizumab or Placebo for Eosinophilic Granulomatosis with Polyangiitis. N. Engl. J. Med. 2017, 376, 1921–1932. [Google Scholar] [CrossRef] [PubMed]
- Kuang, F.L.; De Melo, M.S.; Makiya, M.; Kumar, S.; Brown, T.; Wetzler, L.; Ware, J.M.; Khoury, P.; Collins, M.H.; Quezado, M.; et al. Benralizumab Completely Depletes Gastrointestinal Tissue Eosinophils and Improves Symptoms in Eosinophilic Gastrointestinal Disease. J. Allergy Clin. Immunol. Pract. 2022, 10, 1598–1605.e2. [Google Scholar] [CrossRef] [PubMed]
- Al-Riyami, A.Z.; Hudoba, M.; Young, S.; Forrest, D. Sorafenib is effective for imatinib-resistant FIP1L1/PDGFRA T674I mutation-positive acute myeloid leukemia with eosinophilia. Leuk. Lymphoma 2013, 54, 1788–1790. [Google Scholar] [CrossRef] [PubMed]
- McLornan, D.P.; Gras, L.; Martin, I.; Sirait, T.; Schroeder, T.; Blau, I.W.; Kuball, J.; Byrne, J.; Collin, M.; Stadler, M.; et al. Outcome of allogeneic haematopoietic cell transplantation in eosinophilic disorders: A retrospective study by the chronic malignancies working party of the EBMT. Br. J. Haematol. 2022, 198, 209–213. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özkan, S.G.; Kimiaei, A.; Safaei, S.; Karkucak, M.; Yenerel, M.N.; Öztürkmen, A.Y.; Alp, B.; Özkan, H.A. Therapeutic Challenges and Emerging Strategies for T674I and PTPN11 Mutations in a FIP1L1-PDGFRA-Positive Myeloproliferative Neoplasm: A Case Report. Life 2025, 15, 505. https://doi.org/10.3390/life15030505
Özkan SG, Kimiaei A, Safaei S, Karkucak M, Yenerel MN, Öztürkmen AY, Alp B, Özkan HA. Therapeutic Challenges and Emerging Strategies for T674I and PTPN11 Mutations in a FIP1L1-PDGFRA-Positive Myeloproliferative Neoplasm: A Case Report. Life. 2025; 15(3):505. https://doi.org/10.3390/life15030505
Chicago/Turabian StyleÖzkan, Sıdıka Gülkan, Ali Kimiaei, Seyedehtina Safaei, Mutlu Karkucak, Mustafa Nuri Yenerel, Aslı Yüksel Öztürkmen, Burak Alp, and Hasan Atilla Özkan. 2025. "Therapeutic Challenges and Emerging Strategies for T674I and PTPN11 Mutations in a FIP1L1-PDGFRA-Positive Myeloproliferative Neoplasm: A Case Report" Life 15, no. 3: 505. https://doi.org/10.3390/life15030505
APA StyleÖzkan, S. G., Kimiaei, A., Safaei, S., Karkucak, M., Yenerel, M. N., Öztürkmen, A. Y., Alp, B., & Özkan, H. A. (2025). Therapeutic Challenges and Emerging Strategies for T674I and PTPN11 Mutations in a FIP1L1-PDGFRA-Positive Myeloproliferative Neoplasm: A Case Report. Life, 15(3), 505. https://doi.org/10.3390/life15030505





