Can Serum GFAP and UCH-L1 Replace CT in Assessing Acute Ischemic Stroke Severity?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Blood Sample Collection and Processing
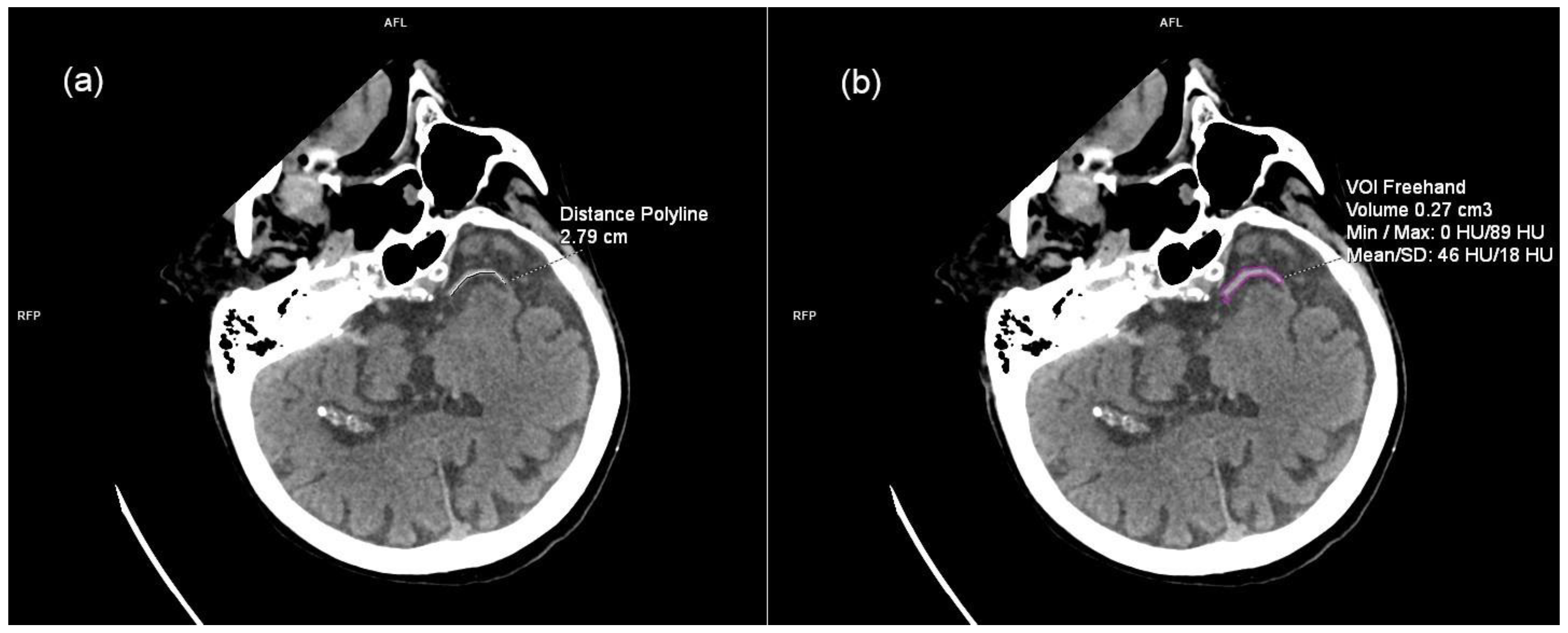
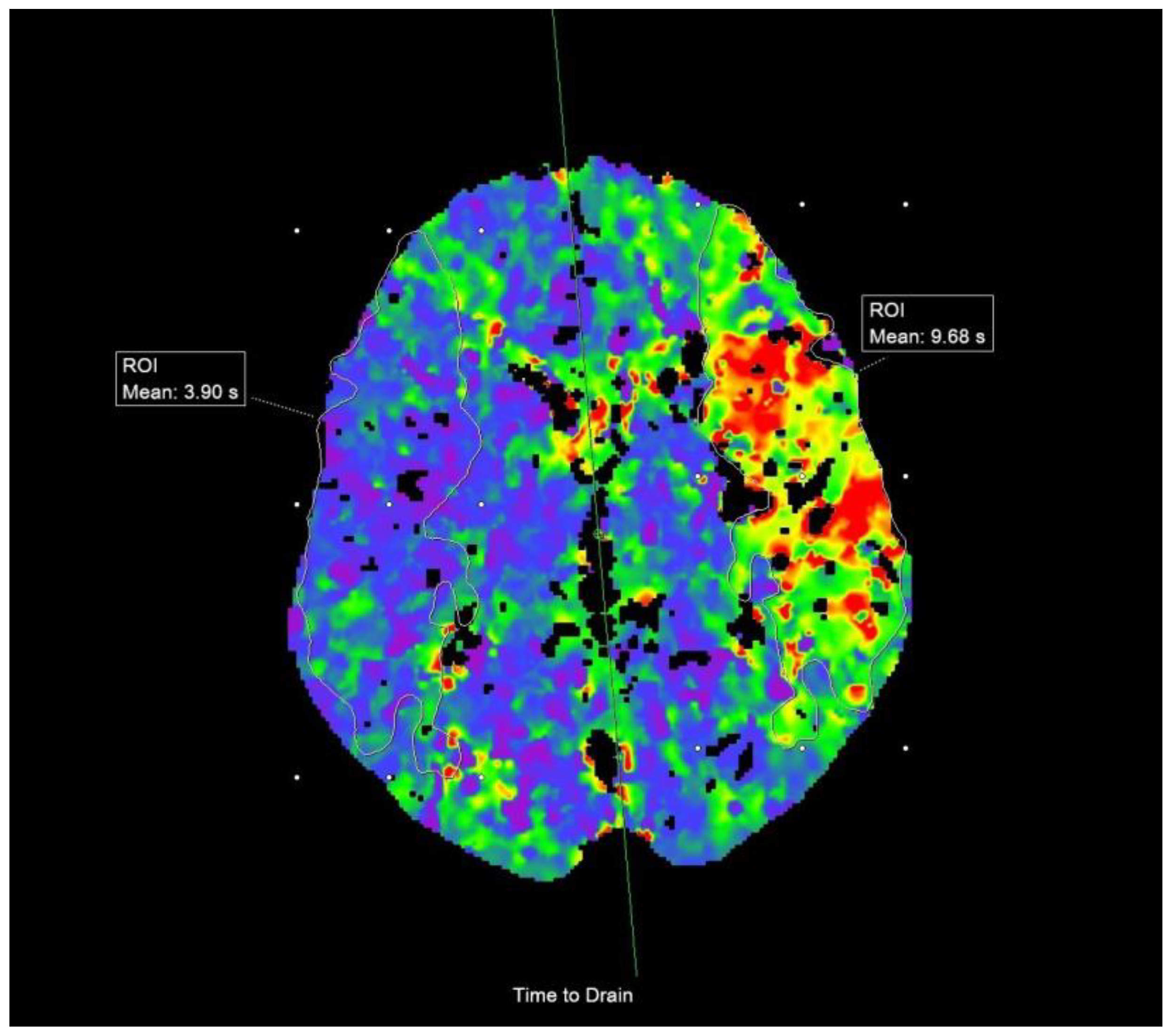
2.3. Imaging Data and Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HS | Hemorrhagic Stroke |
| AIS | Acute Ischemic Stroke |
| IVT | Intravenous Thrombolysis |
| MT | Mechanical Thrombectomy |
| CT | Computed Tomography |
| MRI | Magnetic Resonance Imaging |
| GFAP | Glial Fibrillary Acidic Protein |
| UCH-L1 | Ubiquitin C-terminal Hydrolase L1 |
| LVO | Large Vessel Occlusion |
| SVO | Small Vessel Occlusion |
| ICA | Internal Carotid Artery |
| MCA | Middle Cerebral Artery |
| ACA | Anterior Cerebral Artery |
| BA | Basilar Artery |
| WUS | Wake up Stroke |
| HAS | Hyperdense Artery Sign |
| CBS | Clot Burden Score |
| CTA | Computed Tomography Angiography |
| CS | Collateral Score |
| CTP | Computed Tomography Perfusion |
| mTICI | Modified Treatment in Cerebral Ischemia |
| NIHSS | National Institutes of Health Stroke Scale |
| mRS | Modified Ranking Scale |
| VOI | Volume of Interest |
| CBF | Cerebral Blood Flow |
| CBV | Cerebral Blood Volume |
| MTT | Mean Transit Time |
| TTD | Time-to-Drain |
| ROI | Region of Interest |
References
- George, M.G.; Fischer, L.; Koroshetz, W.; Bushnell, C.; Frankel, M.; Foltz, J.; Thorpe, P.G. CDC Grand Rounds: Public Health Strategies to Prevent and Treat Strokes. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 479–481. [Google Scholar] [CrossRef] [PubMed]
- Sacco, R.L.; Kasner, S.E.; Broderick, J.P.; Caplan, L.R.; Connors, J.J.; Culebras, A.; Elkind, M.S.; George, M.G.; Hamdan, A.D.; Higashida, R.T.; et al. An updated definition of stroke for the 21st century: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 2064–2089. [Google Scholar] [CrossRef] [PubMed]
- Chugh, C. Acute Ischemic Stroke: Management Approach. Indian J. Crit. Care Med. 2019, 23, S140–S146. [Google Scholar] [PubMed]
- Hacke, W.; Kaste, M.; Bluhmki, E.; Brozman, M.; Dávalos, A.; Guidetti, D.; Larrue, V.; Lees, K.R.; Medeghri, Z.; Machnig, T.; et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N. Engl. J. Med. 2008, 359, 1317–1329. [Google Scholar] [CrossRef]
- Turc, G.; Bhogal, P.; Fischer, U.; Khatri, P.; Lobotesis, K.; Mazighi, M.; Schellinger, P.D.; Toni, D.; de Vries, J.; White, P.; et al. European Stroke Organisation (ESO)—European Society for Minimally Invasive Neurological Therapy (ESMINT) Guidelines on Mechanical Thrombectomy in Acute Ischemic Stroke. J. Neurointerv. Surg. 2023, 15, e8. [Google Scholar] [CrossRef]
- Brunkhorst, R.; Pfeilschifter, W.; Foerch, C. Astroglial proteins as diagnostic markers of acute intracerebral hemorrhage pathophysiological background and clinical findings. Transl. Stroke Res. 2010, 1, 246–251. [Google Scholar] [CrossRef]
- Eng, L.F.; Ghirnikar, R.S.; Lee, Y.L. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000). Neurochem. Res. 2000, 25, 1439–1451. [Google Scholar] [CrossRef]
- Bishop, P.; Rocca, D.; Henley, J.M. Ubiquitin C-terminal hydrolase L1 (UCH-L1): Structure, distribution and roles in brain function and dysfunction. Biochem. J. 2016, 473, 2453–2462. [Google Scholar] [CrossRef]
- FDA. Authorizes Marketing of First Blood Test to Aid in the Evaluation of Concussion in Adults. Available online: https://www.fda.gov/news-events/press-announcements/fda-authorizes-marketing-first-blood-test-aid-evaluation-concussion-adults (accessed on 8 January 2025).
- Perry, L.A.; Lucarelli, T.; Penny-Dimri, J.C.; McInnes, M.D.; Mondello, S.; Bustamante, A.; Montaner, J.; Foerch, C.; Kwan, P.; Davis, S.; et al. Glial fibrillary acidic protein for the early diagnosis of intracerebral hemorrhage: Systematic review and meta-analysis of diagnostic test accuracy. Int. J. Stroke 2019, 14, 390–399. [Google Scholar] [CrossRef]
- Ren, C.; Kobeissy, F.; Alawieh, A.; Li, N.; Li, N.; Zibara, K.; Zoltewicz, S.; Guingab-Cagmat, J.; Larner, S.F.; Ding, Y.; et al. Assessment of Serum UCH-L1 and GFAP in Acute Stroke Patients. Sci. Rep. 2016, 6, 24588. [Google Scholar] [CrossRef]
- Kraljević, I.; Sablić, S.; Marinović Guić, M.; Budimir Mršić, D.; Štula, I.; Dolić, K.; Benzon, B.; Košta, V.; Čaljkušić, K.; Marčić, M.; et al. The Importance of Increased Serum GFAP and UCH-L1 Levels in Distinguishing Large Vessel from Small Vessel Occlusion in Acute Ischemic Stroke. Biomedicines 2024, 12, 608. [Google Scholar] [CrossRef]
- Morotti, A.; Paciaroni, M.; Zini, A.; Silvestrelli, G.; Del Zotto, E.; Caso, V.; Dell’Acqua, M.L.; Simone, A.M.; Lanari, A.; Costa, P.; et al. Risk Profile of Symptomatic Lacunar Stroke Versus Nonlobar Intracerebral Hemorrhage. Stroke 2016, 47, 2141–2143. [Google Scholar] [CrossRef] [PubMed]
- Lakomkin, N.; Dhamoon, M.; Carroll, K.; Singh, I.P.; Tuhrim, S.; Lee, J.; Fifi, J.T.; Mocco, J. Prevalence of large vessel occlusion in patients presenting with acute ischemic stroke: A 10-year systematic review of the literature. J. Neurointerv. Surg. 2019, 11, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Conforto, A.B.; Liew, S.L.; Luft, A.R.; Kitago, T.; Bernhardt, J.; Arenillas, J.F. Editorial: Understanding stroke recovery to improve outcomes: From acute care to chronic rehabilitation. Front. Neurol. 2022, 13, 1021033. [Google Scholar] [CrossRef]
- Fink, J.N.; Kumar, S.; Horkan, C.; Linfante, I.; Selim, M.H.; Caplan, L.R.; Schlaug, G. The stroke patient who woke up: Clinical and radiological features, including diffusion and perfusion MRI. Stroke 2002, 33, 988–993. [Google Scholar] [CrossRef]
- Rexrode, K.M.; Madsen, T.E.; Yu, A.Y.X.; Carcel, C.; Lichtman, J.H.; Miller, E.C. The Impact of Sex and Gender on Stroke. Circ. Res. 2022, 130, 512–528. [Google Scholar] [CrossRef]
- Nichols, N.R.; Day, J.R.; Laping, N.J.; Johnson, S.A.; Finch, C.E. GFAP mRNA increases with age in rat and human brain. Neurobiol. Aging 1993, 14, 421–429. [Google Scholar] [CrossRef]
- Papa, L.; Brophy, G.M.; Alvarez, W.; Hirschl, R.; Cress, M.; Weber, K.; Giordano, P. Sex differences in time course and diagnostic accuracy of GFAP and UCH-L1 in trauma patients with mild traumatic brain injury. Sci. Rep. 2023, 13, 11833. [Google Scholar] [CrossRef]
- Somford, D.M.; Nederkoorn, P.J.; Rutgers, D.R.; Kappelle, L.J.; Mali, W.P.; van der Grond, J. Proximal and distal hyperattenuating middle cerebral artery signs at CT: Different prognostic implications. Radiology 2002, 223, 667–671. [Google Scholar] [CrossRef]
- Puetz, V.; Dzialowski, I.; Hill, M.D.; Subramaniam, S.; Sylaja, P.N.; Krol, A.; O’Reilly, C.; Hudon, M.E.; Hu, W.Y.; Coutts, S.B.; et al. Intracranial thrombus extent predicts clinical outcome, final infarct size and hemorrhagic transformation in ischemic stroke: The clot burden score. Int. J. Stroke 2008, 3, 230–236. [Google Scholar] [CrossRef]
- Tan, I.Y.; Demchuk, A.M.; Hopyan, J.; Zhang, L.; Gladstone, D.; Wong, K.; Martin, M.; Symons, S.P.; Fox, A.J.; Aviv, R.I. CT angiography clot burden score and collateral score: Correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR Am. J. Neuroradiol. 2009, 30, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Katyal, A.; Bhaskar, S.M.M. Value of pre-intervention CT perfusion imaging in acute ischemic stroke prognosis. Diagn. Interv. Radiol. 2021, 27, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, K.; Bhagavan, S.; Bains, N.; French, B.; Siddiq, F.; Gomez, C.R.; Qureshi, A.I. Current Endovascular Treatment of Acute Ischemic Stroke. Mo. Med. 2020, 117, 480–489. [Google Scholar]
- Tsang, C.O.A.; Cheung, I.H.W.; Lau, K.K.; Brinjikji, W.; Kallmes, D.F.; Krings, T. Outcomes of Stent Retriever versus Aspiration-First Thrombectomy in Ischemic Stroke: A Systematic Review and Meta-Analysis. AJNR Am. J. Neuroradiol. 2018, 39, 2070–2076. [Google Scholar] [CrossRef]
- Ringheanu, V.M.; Tekle, W.G.; Preston, L.; Sarraj, A.; Hassan, A.E. Higher number of stent-retriever thrombectomy passes significantly increases risk of mass effect, poor functional outcome, and mortality. Interv. Neuroradiol. 2023, 29, 674–682. [Google Scholar] [CrossRef]
- Chamorro, Á.; Blasco, J.; López, A.; Amaro, S.; Román, L.S.; Llull, L.; Renú, A.; Rudilosso, S.; Laredo, C.; Obach, V.; et al. Complete reperfusion is required for maximal benefits of mechanical thrombectomy in stroke patients. Sci. Rep. 2017, 7, 11636. [Google Scholar] [CrossRef]
- Florijn, B.W.; Leontien van der Bent, M.; Nguyen, T.M.T.; Quax, P.H.A.; Wermer, M.J.H.; Yaël Nossent, A.; Kruyt, N.D. Non-coding RNAs versus protein biomarkers to diagnose and differentiate acute stroke: Systematic review and meta-analysis. J. Stroke Cerebrovasc. Dis. 2023, 32, 107388. [Google Scholar] [CrossRef]
- Herrmann, M.; Vos, P.; Wunderlich, M.T.; de Bruijn, C.H.; Lamers, K.J. Release of glial tissue-specific proteins after acute stroke: A comparative analysis of serum concentrations of protein S-100B and glial fibrillary acidic protein. Stroke 2000, 31, 2670–2677. [Google Scholar] [CrossRef]
- Pujol-Calderón, F.; Zetterberg, H.; Portelius, E.; Löwhagen Hendén, P.; Rentzos, A.; Karlsson, J.E.; Höglund, K.; Blennow, K.; Rosengren, L.E. Prediction of Outcome After Endovascular Embolectomy in Anterior Circulation Stroke Using Biomarkers. Transl. Stroke Res. 2022, 13, 65–76. [Google Scholar] [CrossRef]
- National Institute of Neurological Disorders and Stroke (U.S.). NIH Stroke Scale. Available online: https://www.ninds.nih.gov/health-information/public-education/know-stroke/health-professionals/nih-stroke-scale (accessed on 8 January 2025).
- Zihni, E.; McGarry, B.L.; Kelleher, J.D. Moving Toward Explainable Decisions of Artificial Intelligence Models for the Prediction of Functional Outcomes of Ischemic Stroke Patients. In Digital Health; Linwood, S.L., Ed.; Chapter 6; Exon Publications: Brisbane, QLD, Australia, 2022. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Establishing and Verifying an Extended Measuring Interval through Specimen Dilution and Spiking. In CLSI Guideline EP34; CLSI: Berwyn, PA, USA, 2018; Volume 1, pp. 1–11. [Google Scholar]
- Day, I.N.; Thompson, R.J. UCHL1 (PGP 9.5): Neuronal biomarker and ubiquitin system protein. Prog. Neurobiol. 2010, 90, 327–362. [Google Scholar] [CrossRef]
- Tongaonkar, P.; Chen, L.; Lambertson, D.; Ko, B.; Madura, K. Evidence for an interaction between ubiquitin-conjugating enzymes and the 26S proteasome. Mol. Cell Biol. 2000, 20, 4691–4698. [Google Scholar] [CrossRef] [PubMed]
- Papa, L.; Akinyi, L.; Liu, M.C.; Pineda, J.A.; Tepas, J.J., 3rd; Oli, M.W.; Zheng, W.; Robinson, G.; Robicsek, S.A.; Gabrielli, A.; et al. Ubiquitin C-terminal hydrolase is a novel biomarker in humans for severe traumatic brain injury. Crit. Care Med. 2010, 38, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Papa, L.; Lewis, L.M.; Silvestri, S.; Falk, J.L.; Giordano, P.; Brophy, G.M.; Demery, J.A.; Liu, M.C.; Mo, J.; Akinyi, L.; et al. Serum levels of ubiquitin C-terminal hydrolase distinguish mild traumatic brain injury from trauma controls and are elevated in mild and moderate traumatic brain injury patients with intracranial lesions and neurosurgical intervention. J. Trauma Acute Care Surg. 2012, 72, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Vasile, F.; Dossi, E.; Rouach, N. Human astrocytes: Structure and functions in the healthy brain. Brain Struct. Funct. 2017, 222, 2017–2029. [Google Scholar] [CrossRef]
- Fawcett, J.W.; Asher, R.A. The glial scar and central nervous system repair. Brain Res. Bull. 1999, 49, 377–391. [Google Scholar] [CrossRef]
- Wilhelmsson, U.; Bushong, E.A.; Price, D.L.; Smarr, B.L.; Phung, V.; Terada, M.; Ellisman, M.H.; Pekny, M. Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc. Natl. Acad. Sci. USA 2006, 103, 17513–17518. [Google Scholar] [CrossRef]
- d’Esterre, C.D.; Boesen, M.E.; Ahn, S.H.; Pordeli, P.; Najm, M.; Minhas, P.; Davari, P.; Fainardi, E.; Rubiera, M.; Khaw, A.V.; et al. Time-Dependent Computed Tomographic Perfusion Thresholds for Patients With Acute Ischemic Stroke. Stroke 2015, 46, 3390–3397. [Google Scholar] [CrossRef]
- Najm, M.; Al-Ajlan, F.S.; Boesen, M.E.; Hur, L.; Kim, C.K.; Fainardi, E.; Hill, M.D.; Demchuk, A.M.; Goyal, M.; Lee, T.Y.; et al. Defining CT Perfusion Thresholds for Infarction in the Golden Hour and With Ultra-Early Reperfusion. Can. J. Neurol. Sci. 2018, 45, 339–342. [Google Scholar] [CrossRef]
- Chemmanam, T.; Campbell, B.C.; Christensen, S.; Nagakane, Y.; Desmond, P.M.; Bladin, C.F.; Parsons, M.W.; Levi, C.R.; Barber, P.A.; Donnan, G.A.; et al. Ischemic diffusion lesion reversal is uncommon and rarely alters perfusion-diffusion mismatch. Neurology 2010, 75, 1040–1047. [Google Scholar] [CrossRef]
- Dvorak, F.; Haberer, I.; Sitzer, M.; Foerch, C. Characterisation of the diagnostic window of serum glial fibrillary acidic protein for the differentiation of intracerebral haemorrhage and ischaemic stroke. Cerebrovasc. Dis. 2009, 27, 37–41. [Google Scholar] [CrossRef]
- Liu, M.C.; Akinyi, L.; Scharf, D.; Mo, J.; Larner, S.F.; Muller, U.; Oli, M.W.; Zheng, W.; Kobeissy, F.; Papa, L.; et al. Ubiquitin C-terminal hydrolase-L1 as a biomarker for ischemic and traumatic brain injury in rats. Eur. J. Neurosci. 2010, 31, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Pinnschmidt, H.; Königsberg, A.; Schlemm, E.; Boutitie, F.; Ebinger, M.; Endres, M.; Fiebach, J.B.; Fiehler, J.; Galinovic, I.; et al. Estimating nocturnal stroke onset times by magnetic resonance imaging in the WAKE-UP trial. Int. J. Stroke 2022, 17, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Fitzgerald, S.; Gil, S.M.; Mereuta, O.M.; Douglas, A.; Pandit, A.; Brennan, P.; Power, S.; Alderson, J.; O’Hare, A.; et al. Correlation between acute ischaemic stroke clot length before mechanical thrombectomy and extracted clot area: Impact of thrombus size on number of passes for clot removal and final recanalization. Eur. Stroke J. 2021, 6, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Moftakhar, P.; English, J.D.; Cooke, D.L.; Kim, W.T.; Stout, C.; Smith, W.S.; Dowd, C.F.; Higashida, R.T.; Halbach, V.V.; Hetts, S.W. Density of thrombus on admission CT predicts revascularization efficacy in large vessel occlusion acute ischemic stroke. Stroke 2013, 44, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Di Donna, A.; Muto, G.; Giordano, F.; Muto, M.; Guarnieri, G.; Servillo, G.; De Mase, A.; Spina, E.; Leone, G. Diagnosis and management of tandem occlusion in acute ischemic stroke. Eur. J. Radiol. Open 2023, 11, 100513. [Google Scholar] [CrossRef]
- Anogianakis, G.; Daios, S.; Topouzis, N.; Barmpagiannos, K.; Kaiafa, G.; Myrou, A.; Ztriva, E.; Tsankof, A.; Karlafti, E.; Anogeianaki, A.; et al. Current Trends in Stroke Biomarkers: The Prognostic Role of S100 Calcium-Binding Protein B and Glial Fibrillary Acidic Protein. Life 2024, 14, 1247. [Google Scholar] [CrossRef]
- Pego-Pérez, E.R.; Fernández-Rodríguez, I.; Pumar-Cebreiro, J.M. National Institutes of Health Stroke Scale, modified Rankin Scale, and modified Thrombolysis in Cerebral Infarction as autonomy predictive tools for stroke patients. Rev. Neurosci. 2019, 30, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Surjawan, Y.; As’ad, S.; Ranakusuma, T.A.S.; Wijaya, A. GFAP and S100B Protein Are Associated with Discharged NIHSS of Anterior Circulation Ischemic Stroke. Indones. Biomed. J. 2012, 4, 7–112. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, L.; Bai, X. The diagnostic efficacy of serum UCH-L1, GFAP and CyPA on ACI and its influence on neurological function and prognosis of patients. Arch. Clin. Psychiatry 2023, 50, 8–13. [Google Scholar]
- Yigit, I.; Atescelik, M.; Yilmaz, M.; Goktekin, M.C.; Gurger, M.; Ilhan, N. Investigation of UCH-L1 levels in ischemic stroke, intracranial hemorrhage and metabolic disorder induced impaired consciousness. Am. J. Emerg. Med. 2017, 35, 1895–1898. [Google Scholar] [CrossRef]

| n = 52 | |
|---|---|
| Women (n, %) | 34 (65.4%) |
| Arterial hypertension (n, %) | 35 (67.3%) |
| Diabetes mellitus (n, %) | 9 (17.3%) |
| Atrial fibrillation (n, %) | 25 (48.1%) |
| Hyperlipidemia (n, %) | 11 (21.2%) |
| Known malignancy (n, %) | 6 (11.5%) |
| Median (IQR), n = 52 | |
|---|---|
| Leukocytes (×109/L) | 8.4 (6.9–11.68) |
| Erythrocytes (×1012/L) | 4.6 (4.05–4.97) |
| Hemoglobin (g/L) | 137 (127.3–149.8) |
| Hematocrit (L/L) | 0.4 (0.38–0.44) |
| Thrombocytes (×109/L) | 211 (160.8–273.5) |
| PT (s) | 1.05 (0.85–1.16) |
| Glucose (mmol/L) | 7.15 (6.33–8.68) |
| Urea (mmol/L) | 7 (5.43–9.43) |
| Creatinine (µmol/L) | 83 (72–101.5) |
| AST (U/L) | 22 (18–27) |
| ALT (U/L) | 16 (13–27.75) |
| GGT (U/L) | 23.5 (16–39) |
| LDH (U/L) | 180.5 (34.25–230.5) |
| CRP (mg/L) | 3.45 (1.53–13.38) |
| Na+ (mmol/L) | 141 (140–142.8) |
| K+ (mmol/L) | 4 (3.43–4.28) |
| Cl− (mmol/L) | 103 (101–106) |
| CT Parameter | p Value | R Value | |
|---|---|---|---|
| GFAP (n = 51) | thrombus length * | 0.586 | 0.077 |
| thrombus volume * | 0.834 | 0.029 | |
| thrombus density * | 0.428 | 0.112 | |
| CBS # | 0.157 | −0.199 | |
| CS # | 0.489 | −0.098 | |
| ischemic core volume * | 0.177 | 0.190 | |
| ischemic penumbra volume * | 0.428 | 0.112 | |
| CBF * | 0.789 | −0.038 | |
| CBV * | 0.536 | 0.088 | |
| MTT * | 0.310 | −0.144 | |
| TTD * | 0.742 | −0.047 | |
| UCH-L1 (n = 52) | thrombus length * | 0.999 | <0.001 |
| thrombus volume * | 0.866 | −0.024 | |
| thrombus density * | 0.710 | −0.053 | |
| CBS # | 0.291 | −0.149 | |
| CS # | 0.318 | −0.141 | |
| ischemic core volume * | 0.679 | 0.059 | |
| ischemic penumbra volume * | 0.708 | −0.053 | |
| CBF * | 0.296 | −0.148 | |
| CBV * | 0.907 | −0.017 | |
| MTT * | 0.130 | −0.213 | |
| TTD * | 0.341 | −0.135 |
| No. of Patients Unfit for MT | Cause |
|---|---|
| 5 | unfavorable core/penumbra ratio |
| 3 | extremely tortuous vascular anatomy for catheter manipulation |
| 2 | extensive carotid thrombosis |
| 1 | complete recanalization of the occluded vessel after only IVT |
| 1 | too low NIHSS (3) |
| 1 | substantial calcifications of the arteries at the puncture site |
| 1 | shortage of material needed for the procedure |
| 1 | no data |
| GFAP (pg/mL) (Median, IQR) | p Value | UCH-L1 (pg/mL) (Median, IQR) | p Value | ||
|---|---|---|---|---|---|
| all patients | good mRS (n = 12) | 129.7 (59.48–252.4) | 0.005 * | 288.2 (191–413.5) | 0.001 * |
| poor mRS (n = 39) | 493 (73.1–2046) | 591.1 (309.7–889.4) | |||
| successful recanalization | good mRS (n = 10) | 104.6 (56.2–203.8) | 0.007 * | 312.8 (183.8–481.6) | 0.004 # |
| poor mRS (n = 22) | 943.9 (137.8–3599) | 692.3 (328.4–1179) |
| GFAP | UCH-L1 | ||||
|---|---|---|---|---|---|
| CT Parameter | p Value | R Value | p Value | R Value | |
| good mRS (n = 12) | thrombus length * | 0.011 | 0.705 | 0.992 | 0.003 |
| thrombus volume * | 0.042 | 0.593 | 0.899 | −0.041 | |
| thrombus density * | 0.636 | 0.153 | 0.955 | −0.018 | |
| CBS # | 0.372 | −0.282 | 0.899 | −0.042 | |
| CS # | 0.713 | −0.122 | 0.766 | −0.099 | |
| ischemic core volume * | 0.773 | −0.093 | 0.007 | 0.727 | |
| ischemic penumbra volume * | 0.347 | −0.298 | 0.849 | −0.061 | |
| CBF * | 0.581 | −0.178 | 0.812 | −0.078 | |
| CBV * | 0.573 | 0.181 | 0.979 | −0.008 | |
| MTT * | 0.271 | −0.346 | 0.599 | −0.169 | |
| TTD * | 0.978 | 0.009 | 0.486 | 0.223 | |
| poor mRS (n = 39) | thrombus length * | 0.762 | 0.050 | 0.793 | −0.044 |
| thrombus volume * | 0.979 | −0.004 | 0.654 | −0.074 | |
| thrombus density * | 0.415 | 0.134 | 0.724 | −0.059 | |
| CBS # | 0.158 | −0.230 | 0.304 | 0.169 | |
| CS # | 0.818 | −0.038 | 0.617 | −0.083 | |
| ischemic core volume * | 0.349 | 0.154 | 0.782 | −0.046 | |
| ischemic penumbra volume * | 0.467 | 0.119 | 0.689 | −0.066 | |
| CBF * | 0.826 | −0.036 | 0.328 | −0.161 | |
| CBV * | 0.812 | 0.039 | 0.512 | −0.108 | |
| MTT * | 0.699 | −0.064 | 0.573 | −0.093 | |
| TTD * | 0.973 | 0.006 | 0.569 | −0.094 | |
| GFAP (pg/mL) (Median, IQR) | p Value | UCH-L1 (pg/mL) (Median, IQR) | p Value | ||
|---|---|---|---|---|---|
| sex | male (n = 34) | 147.7 (71.1–1602) | 0.871 * | 569.1 (296.1−849.9) | 0.345 * |
| female (n = 18) | 250.7 (61.1−1064) | 395.3 (283.5−628.1) | |||
| symptom onset | WUS (n = 10) | 132.3 (72.9−810.8) | 0.708 # | 709.1 (253.8−1276) | 0.080 * |
| within 6 h (n = 42) | 189.4 (66.9−1602) | 429.7 (291.7−701.2) | |||
| NIHSS | favorable (n = 14) | 207.9 (92.2−2175) | 0.102 * | 551.6 (290.6−977.2) | 0.194 * |
| unfavorable (n = 19) | 92.8 (57.4−319.8) | 354.3 (273.4−698.5) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kraljević, I.; Marinović Guić, M.; Budimir Mršić, D.; Dolić, K.; Čaljkušić, K.; Benzon, B.; Šupe Domić, D.; Lovrić Kojundžić, S. Can Serum GFAP and UCH-L1 Replace CT in Assessing Acute Ischemic Stroke Severity? Life 2025, 15, 495. https://doi.org/10.3390/life15030495
Kraljević I, Marinović Guić M, Budimir Mršić D, Dolić K, Čaljkušić K, Benzon B, Šupe Domić D, Lovrić Kojundžić S. Can Serum GFAP and UCH-L1 Replace CT in Assessing Acute Ischemic Stroke Severity? Life. 2025; 15(3):495. https://doi.org/10.3390/life15030495
Chicago/Turabian StyleKraljević, Ivan, Maja Marinović Guić, Danijela Budimir Mršić, Krešimir Dolić, Krešimir Čaljkušić, Benjamin Benzon, Daniela Šupe Domić, and Sanja Lovrić Kojundžić. 2025. "Can Serum GFAP and UCH-L1 Replace CT in Assessing Acute Ischemic Stroke Severity?" Life 15, no. 3: 495. https://doi.org/10.3390/life15030495
APA StyleKraljević, I., Marinović Guić, M., Budimir Mršić, D., Dolić, K., Čaljkušić, K., Benzon, B., Šupe Domić, D., & Lovrić Kojundžić, S. (2025). Can Serum GFAP and UCH-L1 Replace CT in Assessing Acute Ischemic Stroke Severity? Life, 15(3), 495. https://doi.org/10.3390/life15030495









