Regulatory Mechanisms of Phytohormones in Thiocyanate-Exposed Rice Plants: Integrating Multi-Omics Profiling with Mathematical Modeling
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experiment Design
2.2. Measurements of Phytohormones
2.3. Identification of Genes Activated in Different Hormonal Pathways
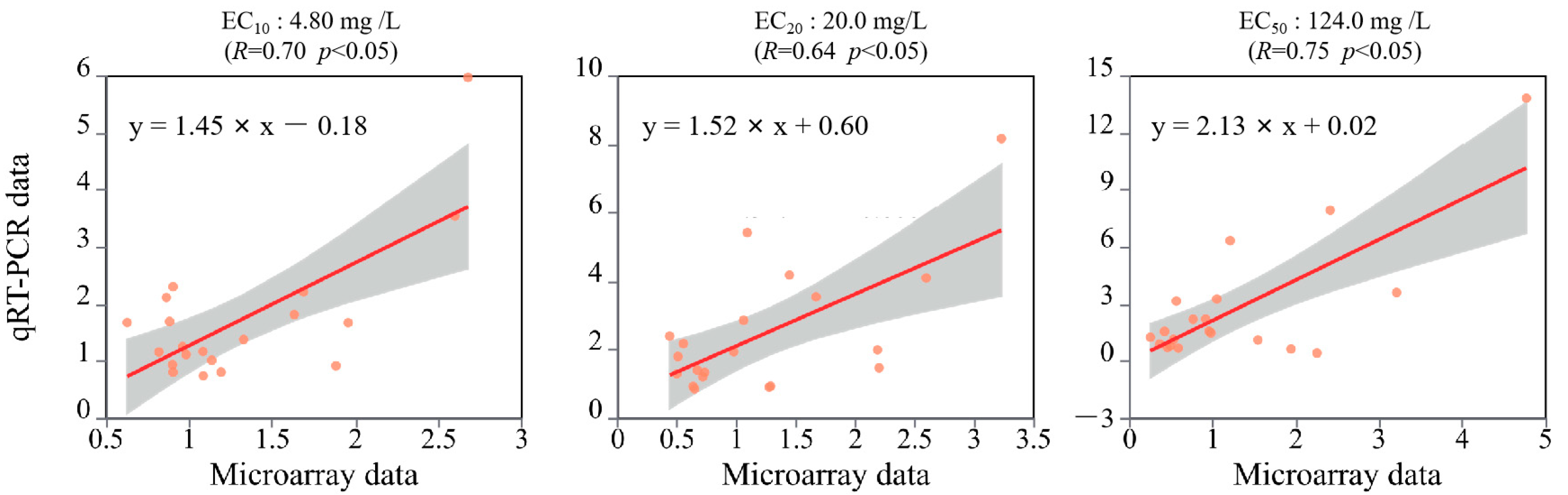
2.4. Data Analysis
3. Results
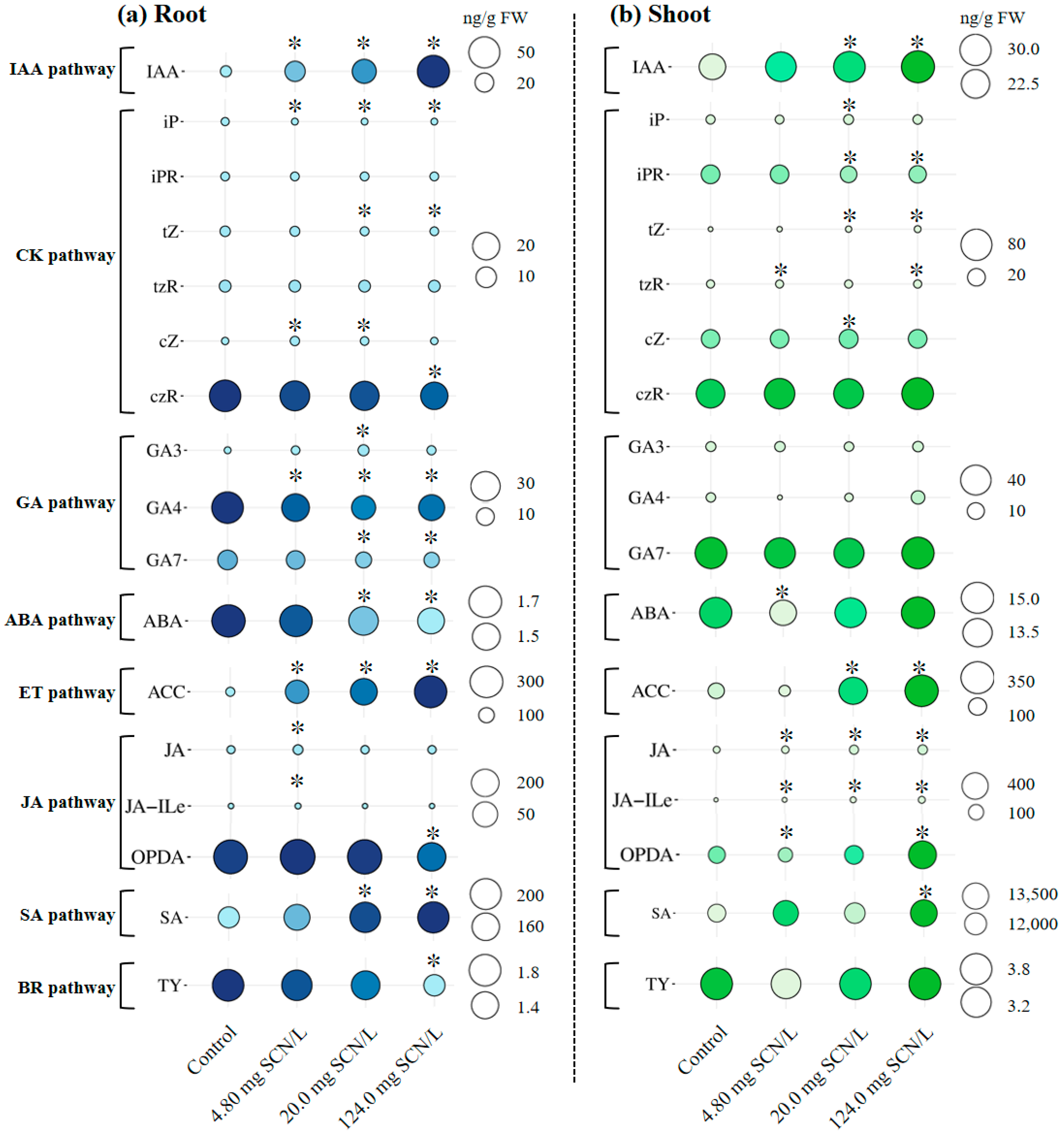
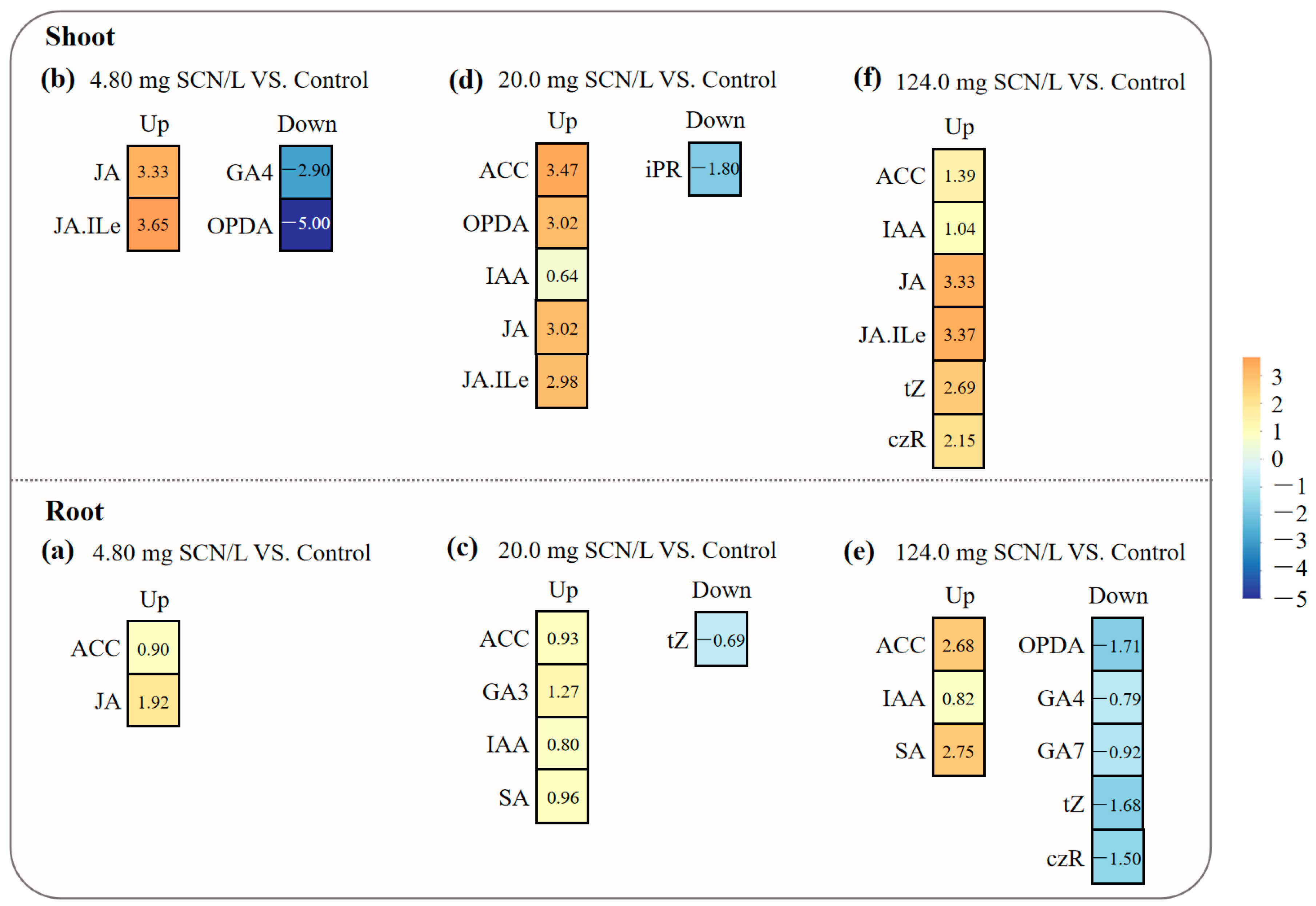
3.1. Concentrations of Phytohormones in SCN−-Treated Rice Plants
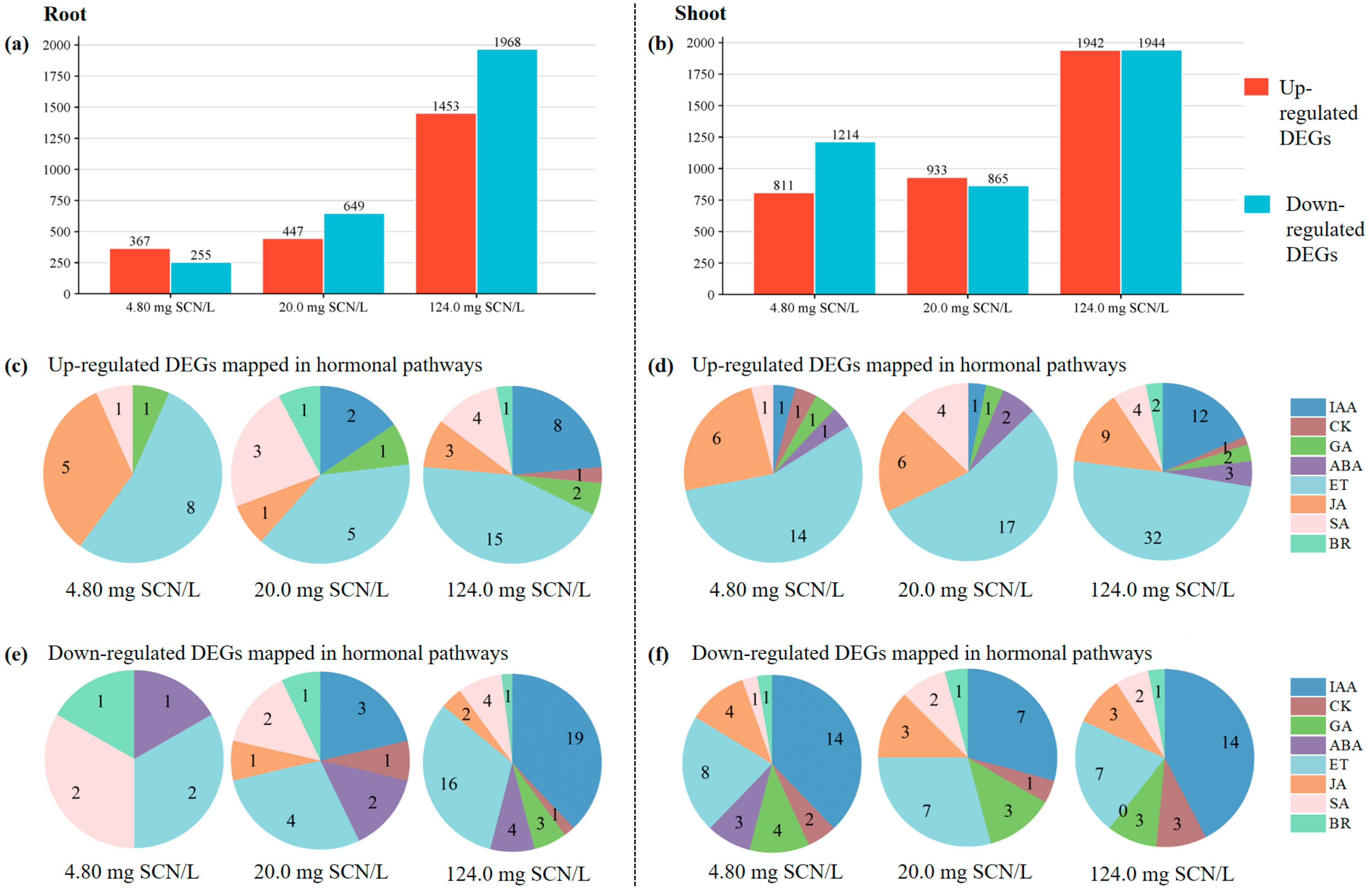
3.2. Identification of DEGs Activated in the Phytohormone Pathways
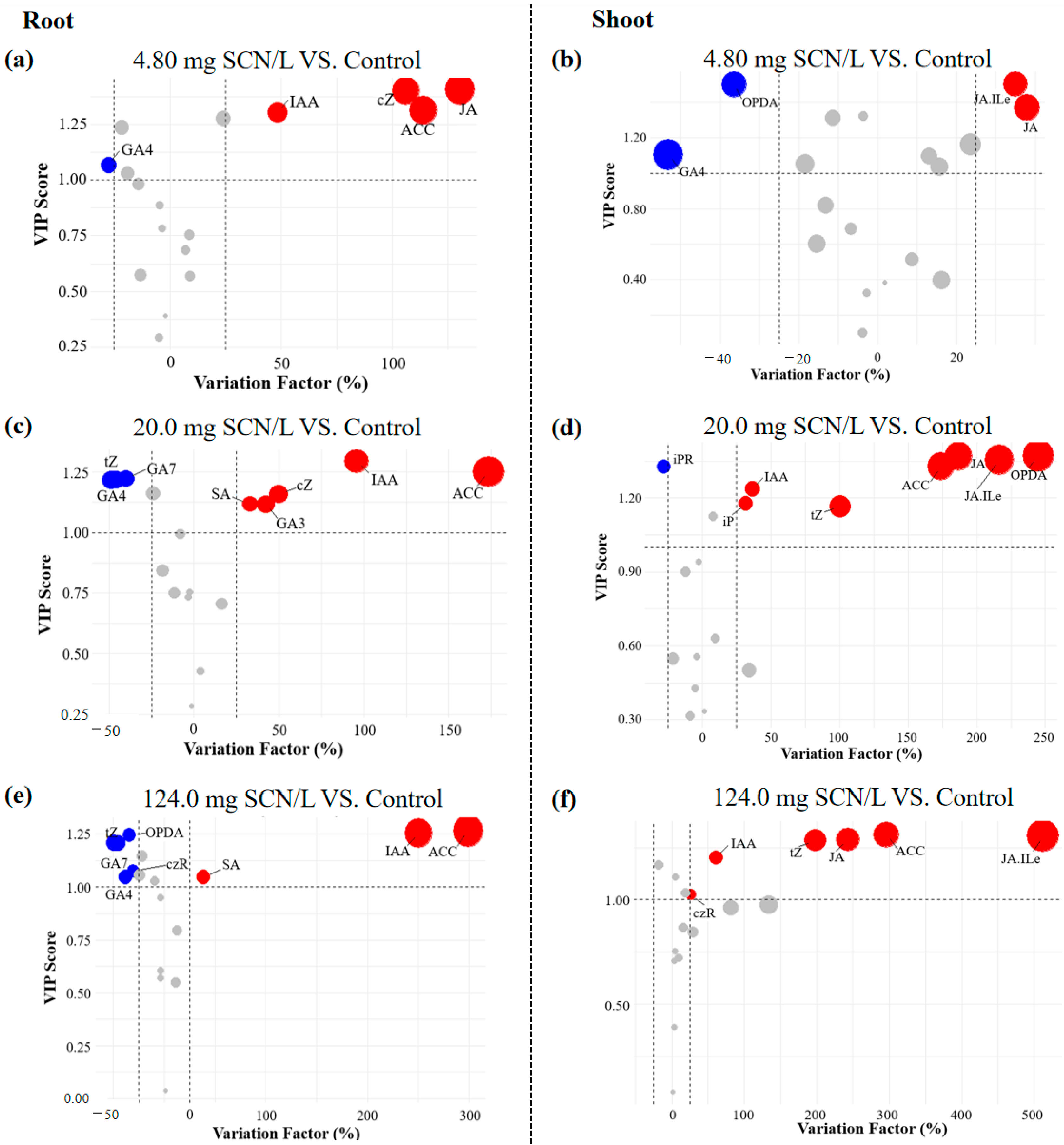
3.3. Selection of Significantly Altered Phytohormones
3.4. Estimation of the Total Weighted Contribution Score of Hormonal Pathways
3.5. Selection of the Sensitive Phytohormones
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Katayama, Y.; Hashimoto, K.; Nakayama, H.; Mino, H.; Nojiri, M.; Ono, T.A.; Nyunoya, H.; Yohda, M.; Takio, K.; Odaka, M. Thiocyanate hydrolyase is a cobalt-containing metalloenzyme with a cysteine-sulfinic acid ligand. J. Am. Chem. Soc. 2006, 128, 728–729. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.S.; Chung, J.S. Biodegradation of thiocyanate in biofilm reactor using fluidized-carriers. Process Chem. 2006, 41, 701–707. [Google Scholar] [CrossRef]
- Dash, R.R.; Gaur, A.; Balomajumder, C. Cyanide in industrial wastewaters and its removal: A review on biotreatment. J. Hazard. Mater. 2009, 163, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Combarros, R.G.; Collado, S.; Laca, A.; Díaz, M. Understanding the simultaneous biodegradation of thiocyanate and salicylic acid by Paracoccus thiocyanatus and Pseudomonas putida. Int. J. Environ. Sci. Technol. 2016, 13, 649–662. [Google Scholar] [CrossRef]
- Rossinioliva, S.; Abreu, M.M.; Santos, E.S.; Leidi, E.O. Soil-plant system and potential human health risk of Chinese cabbage and oregano growing in soils from Mn- and Fe-abandoned mines: Microcosm assay. Environ. Geochem. Health 2020, 42, 4073–4086. [Google Scholar] [CrossRef]
- Gould, W.D.; King, M.; Mohapatra, B.R.; Cameron, R.; Kapoor, A.; Koren, D.W. A critical review on destruction of thiocyanate in mining effluents. Miner. Eng. 2012, 34, 38–47. [Google Scholar] [CrossRef]
- Sun, Z.; Xie, X.; Wang, P.; Hu, Y.; Cheng, H. Heavy metal pollution caused by small-scale metal ore mining activities: A case study from a polymetallic mine in South China. Sci. Total. Environ. 2018, 639, 217–227. [Google Scholar] [CrossRef]
- Lin, Y.J.; Yu, X.Z.; Li, Y.H.; Yang, L. Inhibition of the mitochondrial respiratory components (Complex I and Complex III) as stimuli to induce oxidative damage in Oryza sativa L. under thiocyanate exposure. Chemosphere 2020, 243, 125472. [Google Scholar] [CrossRef]
- Yu, X.Z.; Lin, Y.J.; Shen, P.P.; Zhang, Q.; Gupta, D.K. Molecular evidences on transport of thiocyanate into rice seedlings and assimilation by 13C and 15N labelling and gene expression analyses. Int. Biodeterior. Biodegrad. 2019, 139, 11–17. [Google Scholar] [CrossRef]
- Yu, X.Z.; Zhang, F.Z. Effects of exogenous thiocyanate on mineral nutrients, antioxidative responses and free amino acids in rice seedlings. Ecotoxicology 2013, 22, 752–760. [Google Scholar] [CrossRef]
- Lin, Y.J.; Yu, X.Z.; Zhang, Q. Transcriptome analysis of Oryza sativa in responses to different concentrations of thiocyanate. Environ. Sci. Pollut. Res. 2019, 26, 11696–11709. [Google Scholar] [CrossRef]
- Yang, L.; Feng, Y.X.; Lin, Y.J.; Yu, X.Z. Comparative effects of sodium hydrosulfide and proline on functional repair in rice chloroplast through the D1 protein and thioredoxin system under simulated thiocyanate pollution. Chemosphere 2021, 284, 131389. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.X.; Yang, L.; Lin, Y.J.; Song, Y.; Yu, X.Z. Merging the occurrence possibility into gene co-expression network deciphers the importance of exogenous 2-oxoglutarate in improving the growth of rice seedlings under thiocyanate stress. Front. Plant Sci. 2023, 14, 1086098. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Du, K.; Xie, J.; Sun, G.; Wang, P.; Chen, X.; Cao, Z.; Wang, B.; Chao, Q.; Li, X.; et al. Activated malate circulation contributes to the manifestation of light-dependent mosaic symptoms. Cell Rep. 2023, 42, 112333. [Google Scholar] [CrossRef]
- Erb, M.; Kliebenstein, D.J. Plant secondary metabolites as defenses, regulators, and primary metabolites: The blurred functional trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Zulffqar, F.; Akram, N.A.; Ashraf, M. Osmoprotection in plants under abiotic stresses: New insights into a classical phenomenon. Planta 2020, 251, 3. [Google Scholar] [CrossRef]
- Kiba, T.; Kudo, T.; Kojima, M.; Sakakibara, H. Hormonal control of nitrogen acquisition: Roles of auxin, abscisic acid, and cytokinin. J. Exp. Bot. 2011, 62, 1399–1409. [Google Scholar] [CrossRef]
- Li, Z.; Ahammed, G.J. Hormonal regulation of anthocyanin biosynthesis for improved stress tolerance in plants. Plant Physiol. Biochem. 2023, 201, 107835. [Google Scholar] [CrossRef]
- Leng, Y.; Li, Y.; Ma, Y.H.; He, L.F.; Li, S.W. Abscisic acid modulates differential physiological and biochemical responses of roots, stems, and leaves in mung bean seedlings to cadmium stress. Environ. Sci. Pollut. Res. 2021, 28, 6030–6043. [Google Scholar] [CrossRef]
- Lymperopoulos, P.; Msanne, J.; Rabara, R. Phytochrome and phytohormones: Working in tandem for plant growth and development. Front. Plant Sci. 2018, 9, 1037. [Google Scholar] [CrossRef]
- Rahman, S.U.; Li, Y.; Hussain, S.; Hussain, B.; Khan, W.; Riaz, L.; Ashraf, M.N.; Muhammad Khaliq, A.; Du, Z.; Cheng, H. Role of phytohormones in heavy metal tolerance in plants: A review. Ecol. Indic. 2023, 146, 109844. [Google Scholar] [CrossRef]
- Jamla, M.; Khare, T.; Joshi, S.; Patil, S.; Penna, S.; Kumar, V. Omics approaches for understanding heavy metal responses and tolerance in plants. Curr. Plant Biol. 2021, 27, 100213. [Google Scholar] [CrossRef]
- Saini, S.; Kaur, N.; Pati, P.K. Phytohormones: Key players in the modulation of heavy metal stress tolerance in plants. Ecotoxicol. Environ. Saf. 2021, 223, 112578. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, M.; Ciarkowska, A.; Jakubowska, A. The auxin conjugate indole-3- acetyl-aspartate affects responses to cadmium and salt stress in Pisum sativum L. J. Plant Physiol. 2016, 191, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Huang, D.; Niu, D.; Deng, J.; Ma, F.; Liu, C. Overexpression of auxin response gene MdIAA24 enhanced cadmium tolerance in apple (Malus domestica). Ecotoxicol. Environ. Saf. 2021, 225, 112734. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, A.; Ali, S.; Yasmeen, T.; Arif, M.S.; Zubair, M.; Rizwan, M.; Alhaithloul, H.A.S.; Alayaff, A.A.; Soliman, M.H. Effect of gibberellic acid on growth, photosynthesis and antioxidant defense system of wheat under zinc oxide nanoparticle stress. Environ. Pollut. 2019, 254, 113109. [Google Scholar] [CrossRef]
- Ahmad, P.; Raja, V.; Ashraf, M.; Wijaya, L.; Bajguz, A.; Alyemeni, M.N. Jasmonic acid (JA) and gibberellic acid (GA3) mitigated Cd-toxicity in chickpea plants through restricted Cd uptake and oxidative stress management. Sci. Rep. 2021, 11, 19768. [Google Scholar] [CrossRef]
- Chen, H.; Yang, R.; Zhang, X.; Chen, Y.; Xia, Y.; Xu, X. Foliar application of gibberellin inhibits the cadmium uptake and xylem transport in lettuce (Lactuca sativa L.). Sci. Hortic. 2021, 288, 110410. [Google Scholar] [CrossRef]
- Saltveit, M.E. Synthesis and metabolism of phenolic compounds. Fruit Veget. Phytochem. Chem. Human Health 2017, 2, 115. [Google Scholar]
- Bali, S.; Kaur, P.; Kohli, S.K.; Ohri, P.; Thukral, A.K.; Bhardwaj, R.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Jasmonic acid induced changes in physio-biochemical attributes and ascorbate-glutathione pathway in Lycopersicon esculentum under lead stress at different growth stages. Sci. Total Environ. 2018, 645, 1344–1360. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F. The role of salicylic acid and gibberellin signaling in plant responses to abiotic stress with an emphasis on heavy metals. Plant Signal. Behav. 2020, 15, 1777372. [Google Scholar] [CrossRef] [PubMed]
- Betti, C.; Della Rovere, F.; Piacentini, D.; Fattorini, L.; Falasca, G.; Altamura, M.M. Jasmonates, ethylene and brassinosteroids control adventitious and lateral rooting as stress avoidance responses to heavy metals and metalloids. Biomolecules 2021, 11, 77. [Google Scholar] [CrossRef]
- Esmaielzadeh, S.; Fallah, H.; Niknejad, Y.; Mahmoudi, M.; Tari, D.B. Methyl jasmonate increases aluminum tolerance in rice by augmenting the antioxidant defense system, maintaining ion homeostasis, and increasing nonprotein thiol compounds. Environ. Sci. Pollut. Res. 2022, 29, 46708–46720. [Google Scholar] [CrossRef]
- Alam, P.; Kaur Kohli, S.; Al Balawi, T.; Altalayan, F.H.; Alam, P.; Ashraf, M.; Bhardwaj, R.; Ahmad, P. Foliar application of 24-Epibrassinolide improves growth, ascorbate-glutathione cycle, and glyoxalase system in brown mustard (Brassica juncea (L.) Czern.) under cadmium toxicity. Plants 2020, 9, 1487. [Google Scholar] [CrossRef]
- Basit, F.; Chen, M.; Ahmed, T.; Shahid, M.; Noman, M.; Liu, J.; An, J.; Hashem, A.; Fahad Al-Arjani, A.B.; Alqarawi, A.A. Seed priming with brassinosteroids alleviates chromium stress in rice cultivars via improving ROS metabolism and antioxidant defense response at biochemical and molecular levels. Antioxidants 2021, 10, 1089. [Google Scholar] [CrossRef] [PubMed]
- Tadaiesky, L.B.; da Silva, B.R.; Batista, B.L.; Lobato, A.K.D.S. Brassinosteroids trigger tolerance to iron toxicity in rice. Physiol. Plant. 2021, 171, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.X.; Lin, Y.J.; Tian, P.; Yu, X.Z. Proline interacts with Ca2+-dependent signaling to enhance chromium tolerance in rice by manipulating nitrate reductase and sucrose phosphate synthase. Int. J. Biol. Macromol. 2023, 253, 126655. [Google Scholar] [CrossRef]
- Kang, Y.; Lin, Y.J.; Ullah, A.; Zhang, F.F.; Yu, X.Z. Trivalent chromium stress trigger accumulation of secondary metabolites in rice plants: Integration of biochemical and transcriptomic analysis. Environ. Technol. Innov. 2024, 36, 103802. [Google Scholar] [CrossRef]
- Mahieu, B.; Qannari, E.M.; Jaillais, B. Extension and significance testing of Variable Importance in Projection (VIP) indices in Partial Least Squares Regression and Principal Components Analysis. Chemometr. Intell. Lab. Syst. 2023, 242, 104986. [Google Scholar] [CrossRef]
- Xiao, H.; Yang, X.; Huang, X.; Luo, H.; Liu, Y.; Li, J.; Dang, X.; Wang, S. Quality evaluation of Schisandra propinqua var. Sinensis based on HPLC fingerprint and multi-component content determination. Northwest Pharm. J. 2022, 37, 1–7. (In Chinese) [Google Scholar]
- Chong, L.G.; Jun, C.H. Performance of some variable selection methods when multicollinearity is present. Chemometr. Intell. Lab. Syst. 2005, 78, 103–112. [Google Scholar] [CrossRef]
- Li, C.Z.; Ullah, A.; Kang, Y.; Yu, X.Z. Amino acid metabolic shifts in rice: Responses to elevated CO2, cyanide, and nitrogen sources. Chem. Biol. Technol. Agric. 2024, 11, 170. [Google Scholar] [CrossRef]
- Li, J.; Tao, X.; Bu, J.; Ying, T.; Mao, L.; Luo, Z. Global transcriptome profiling analysis of ethylene-auxin interaction during tomato fruit ripening. Postharvest Biol. Technol. 2017, 130, 28–38. [Google Scholar] [CrossRef]
- Růžička, K.; Ljung, K.; Vanneste, S.; Podhorská, R.; Beeckman, T.; Friml, J.; Benková, E. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 2007, 19, 2197–2212. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, A.N.; Yun, J.; Likhacheva, A.V.; Alonso, J.M. Multilevel interactions between ethylene and auxin in Arabidopsis roots. Cell 2007, 19, 2169–2185. [Google Scholar]
- Wang, Y.; Wang, N.; Xu, H.; Jiang, S.; Fang, H.; Su, M.; Zhang, Z.; Zhang, T.; Chen, X. Auxin regulates anthocyanin biosynthesis through the Aux/IAA–ARF signaling pathway in apple. Hortic. Res. 2018, 5, 59. [Google Scholar] [CrossRef]
- Rowe, J.H.; Topping, J.F.; Liu, J.; Lindsey, K. Abscisic acid regulates root growth under osmotic stress conditions via an interacting hormonal network with cytokinin, ethylene, and auxin. New Phytol. 2016, 211, 225–239. [Google Scholar] [CrossRef]
- Li, W.; Ma, M.; Feng, Y.; Li, H.; Wang, Y.; Ma, Y.; Li, M.; An, F.; Guo, H. EIN2-directed translational regulation of ethylene signaling in Arabidopsis. Plant Cell 2015, 163, 670–683. [Google Scholar] [CrossRef]
- Ling, Q.L.; Feng, Y.X.; Lu, C.J.; Lin, Y.J.; Yu, X.Z. Genetic variation and gene expression of anthocyanin synthesis and transport related enzymes in Oryza sativa against thiocyanate. Plant Physiol. Biochem. 2021, 160, 18–26. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, H.; Zhou, J.; Smith, S.M.; Li, J. Malate circulation: Linking Chloroplast metabolism to mitochondrial ROS. Trends Plant Sci. 2020, 25, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Aro, E.; Suorsa, M.; Rokka, A.; Allahverdiyeva, Y.; Paakkarinen, V.; Saleem, A.; Rintamaki, E. Dynamics of photosystem II: A proteomic approach to thylakoid protein complexes. J. Exp. Bot. 2024, 56, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Alencar, V.T.C.B.; Lobo, A.K.M.; Carvalho, F.E.L.; Silveira, J.A.G. High ammonium supply impairs photosynthetic efffciency in rice exposed to excess light. Photosynth. Res. 2019, 140, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.J.; Feng, Y.X.; Li, Y.H.; Yu, G.; Yu, X.Z. Fuzzy synthetic evaluation of the impact of plant growth regulators on the root phenotype traits of rice seedlings under thiocyanate stress. Plant Physiol. Biochem. 2021, 158, 182–189. [Google Scholar] [CrossRef]
- Hedden, P.; Thomas, S.G. Gibberellin biosynthesis and its regulation. Biochem. J. 2012, 444, 11–25. [Google Scholar] [CrossRef]
- Hedden, P. The current status of research on gibberellin biosynthesis. Plant Cell Physiol. 2020, 61, 1832–1849. [Google Scholar] [CrossRef]
- Achard, P.; Gong, F.; Cheminant, S.; Alioua, M.; Hedden, P.; Genschik, P. The cold-inducible CBF1 factor–dependent signaling pathway modulates the accumulation of the growth-repressing DELLA proteins via its effect on gibberellin metabolism. Plant Cell 2008, 20, 2117–2129. [Google Scholar] [CrossRef]
- Colebrook, E.H.; Thomas, S.G.; Phillips, A.L.; Hedden, P. The role of gibberellin signaling in plant responses to abiotic stress. J. Exp. Biol. 2014, 217, 67–75. [Google Scholar] [CrossRef]
- Sharma, L.; Priya, M.; Kaushal, N.; Bhandhari, K.; Chaudhary, S.; Dhankher, O.P.; Prasad, P.V.V.; Siddique, K.H.M.; Nayyar, H. Plant growth-regulating molecules as thermoprotectants: Functional relevance and prospects for improving heat tolerance in food crops. J. Exp. Bot. 2020, 71, 569–594. [Google Scholar] [CrossRef]

| Comparison Groups | Variables in Shoots | Variables in Roots | ||||
|---|---|---|---|---|---|---|
| R2x | R2y | Q2 | R2x | R2y | Q2 | |
| SCN− (4.80 mg/L) vs. Control | 0.636 | 0.999 | 0.838 | 0.741 | 0.984 | 0.909 |
| SCN− (20.0 mg/L) vs. Control | 0.635 | 0.995 | 0.874 | 0.761 | 0.997 | 0.921 |
| SCN− (124.0 mg/L) vs. Control | 0.684 | 0.998 | 0.950 | 0.749 | 0.999 | 0.963 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, Y.; Li, C.; Yu, X. Regulatory Mechanisms of Phytohormones in Thiocyanate-Exposed Rice Plants: Integrating Multi-Omics Profiling with Mathematical Modeling. Life 2025, 15, 486. https://doi.org/10.3390/life15030486
Kang Y, Li C, Yu X. Regulatory Mechanisms of Phytohormones in Thiocyanate-Exposed Rice Plants: Integrating Multi-Omics Profiling with Mathematical Modeling. Life. 2025; 15(3):486. https://doi.org/10.3390/life15030486
Chicago/Turabian StyleKang, Yi, Chengzhi Li, and Xiaozhang Yu. 2025. "Regulatory Mechanisms of Phytohormones in Thiocyanate-Exposed Rice Plants: Integrating Multi-Omics Profiling with Mathematical Modeling" Life 15, no. 3: 486. https://doi.org/10.3390/life15030486
APA StyleKang, Y., Li, C., & Yu, X. (2025). Regulatory Mechanisms of Phytohormones in Thiocyanate-Exposed Rice Plants: Integrating Multi-Omics Profiling with Mathematical Modeling. Life, 15(3), 486. https://doi.org/10.3390/life15030486






