Applications and Effectiveness of 3D Printing in Various Ankle Surgeries: A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Study Selection
2.3. Data Collection
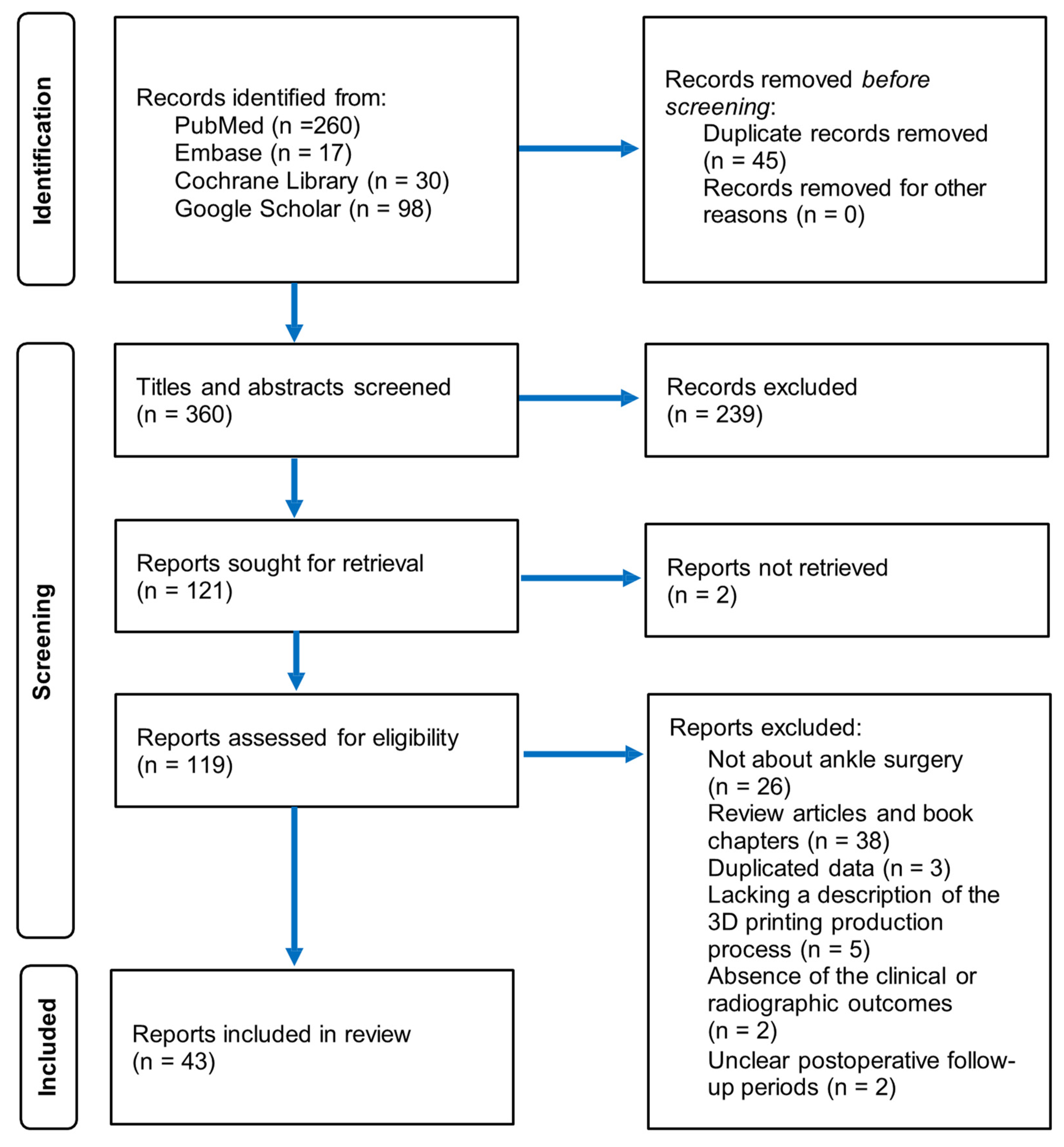
3. Results
3.1. Study Selection and Characteristics
3.2. Talar Replacement
3.3. Total Ankle Arthroplasty
3.4. Arthrodesis
3.5. Supramalleolar Osteotomy
3.6. Materials
4. Summary
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Adams, S.B.; Danilkowicz, R.M. Talonavicular joint-sparing 3D printed navicular replacement for osteonecrosis of the navicular. Foot Ankle Int. 2021, 42, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Angthong, C. Anatomic total talar prosthesis replacement surgery and ankle arthroplasty: An early case series in Thailand. Orthop. Rev. 2014, 6, 5486. [Google Scholar]
- Jastifer, J.R.; Gustafson, P.A. Three-Dimensional Printing Technology in Foot and Ankle Surgery. In 3D Printing in Orthopaedic Surgery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 95–104. [Google Scholar]
- Kadakia, R.J.; Wixted, C.M.; Kelly, C.N.; Hanselman, A.E.; Adams, S.B. From patient to procedure: The process of creating a custom 3D-printed medical device for foot and ankle pathology. Foot Ankle Spec. 2021, 14, 271–280. [Google Scholar] [CrossRef]
- West, T.A.; Rush, S.M. Total talus replacement: Case series and literature review. J. Foot Ankle Surg. 2021, 60, 187–193. [Google Scholar] [CrossRef]
- Jennison, T.; Dalgleish, J.; Sharpe, I.; Davies, M.; Goldberg, A. Total talus replacements. Foot Ankle Orthop. 2023, 8, 24730114221151068. [Google Scholar] [CrossRef]
- Scott, D.; Steele, J.; Fletcher, A.; Parekh, S. Clinical and Radiographic Outcomes of 3D Printed Total Talus Arthroplasty. Foot Ankle Orthop. 2019, 4, 2473011419S2473000376. [Google Scholar] [CrossRef]
- Lin, Y.; He, P.; Yang, G.; Wang, F.; Jia, L.; Fan, H.; Yang, L.; Tang, H.; Duan, X. Efficacy of 3D-printed customized titanium implants and its clinical validation in foot and ankle surgery. Int. J. Bioprinting 2023, 10, 0125. [Google Scholar] [CrossRef]
- Kadakia, R.J.; Akoh, C.C.; Chen, J.; Sharma, A.; Parekh, S.G. The 3D Printed Total Talus Replacement: A Novel Treatment Option for Avascular Necrosis of the Talus. Foot Ankle Orthop. 2020, 5, 2473011420S2473000274. [Google Scholar] [CrossRef]
- Luo, W.; Zhang, H.; Han, Q.; Li, Z.; Zhong, Z.; Jia, G.; Liu, Y.; Chang, F.; Wang, J. Total talar replacement with custom-made vitallium prosthesis for talar avascular necrosis. Front. Bioeng. Biotechnol. 2022, 10, 916334. [Google Scholar] [CrossRef]
- Preston, N.L.; Wilson, M.; Hewitt, E.A. Salvage arthrodesis of a failed total ankle replacement using a custom 3D-printed cage implant: A case report and review of the literature. Proc. Singap. Healthc. 2018, 27, 277–281. [Google Scholar] [CrossRef]
- Berlet, G.C.; Penner, M.J.; Lancianese, S.; Stemniski, P.M.; Obert, R.M. Total ankle arthroplasty accuracy and reproducibility using preoperative CT scan-derived, patient-specific guides. Foot Ankle Int. 2014, 35, 665–676. [Google Scholar] [CrossRef]
- Kanzaki, N.; Chinzei, N.; Yamamoto, T.; Yamashita, T.; Ibaraki, K.; Kuroda, R. Clinical outcomes of total ankle arthroplasty with total talar prosthesis. Foot Ankle Int. 2019, 40, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, H.; Taniguchi, A.; Morita, S.; Takakura, Y.; Tanaka, Y. Total ankle arthroplasty incorporating a total talar prosthesis: A comparative study against the standard total ankle arthroplasty. Bone Jt. J. 2019, 101, 443–446. [Google Scholar] [CrossRef]
- Constantino, J.; Perler, A. The partial talus replacement (PTR)–An alternative treatment for severe osteochondral lesions of the talus utilizing a custom 3D printed talar hemiarthroplasty implant: A case report and technique guide. J. Int. Foot Ankle Found. 2023, 2. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, B.; Wang, G.; Wang, J.; Liu, B.; Zhu, L.; Xu, Q. Partial talar replacement with a novel 3D printed prosthesis. Comput. Assist. Surg. 2023, 28, 2198106. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.; Parekh, S. Outcomes following 3D printed total talus arthroplasty. Foot Ankle Orthop. 2018, 3, 2473011418S2473000419. [Google Scholar] [CrossRef]
- Han, Y.; Wang, S.; Kaken, H.; Zhao, W.; Julaiti, T.; Wang, L. Effectiveness analysis of 3D printing combined guide plate technology in ankle joint replacement for the management of talus osteonecrosis and late traumatic ankle arthritis: A case report. Foot Ankle Surg. Tech. Rep. Cases 2022, 2, 100201. [Google Scholar] [CrossRef]
- Kadakia, R.J.; Wixted, C.M.; Allen, N.B.; Hanselman, A.E.; Adams, S.B. Clinical applications of custom 3D printed implants in complex lower extremity reconstruction. 3d Print. Med. 2020, 6, 29. [Google Scholar] [CrossRef]
- Mazzotti, A.; Arceri, A.; Zielli, S.; Bonelli, S.; Viglione, V.; Faldini, C. Patient-specific instrumentation in total ankle arthroplasty. World J. Orthop. 2022, 13, 230. [Google Scholar] [CrossRef]
- Zhang, C.; Cao, J.; Zhu, H.; Fan, H.; Yang, L.; Duan, X. Endoscopic treatment of symptomatic foot and ankle bone cyst with 3D printing application. BioMed Res. Int. 2020, 2020, 8323658. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Tanaka, Y.; Maegawa, N.; Shinohara, Y.; Taniguchi, A.; Kumai, T.; Takakura, Y. Total talar replacement following collapse of the talar body as a complication of total ankle arthroplasty: A case report. JBJS 2010, 92, 2115–2120. [Google Scholar] [CrossRef] [PubMed]
- Magnan, B.; Facci, E.; Bartolozzi, P. Traumatic loss of the talus treated with a talar body prosthesis and total ankle arthroplasty: A case report. JBJS 2004, 86, 1778–1782. [Google Scholar] [CrossRef]
- Giannini, S.; Cadossi, M.; Mazzotti, A.; Ramponi, L.; Belvedere, C.; Leardini, A. Custom-made total talonavicular replacement in a professional rock climber. J. Foot Ankle Surg. 2016, 55, 1271–1275. [Google Scholar] [CrossRef] [PubMed]
- Gadkari, K.P.; Anderson, J.G.; Bohay, D.R.; Maskill, J.D.; Padley, M.A.; Behrend, L.A. An eleven-year follow-up of a custom talar prosthesis after open talar extrusion in an adolescent patient: A case report. JBJS Case Connect. 2013, 3, e118. [Google Scholar] [CrossRef]
- Taniguchi, A.; Takakura, Y.; Tanaka, Y.; Kurokawa, H.; Tomiwa, K.; Matsuda, T.; Kumai, T.; Sugimoto, K. An alumina ceramic total talar prosthesis for osteonecrosis of the talus. Jbjs 2015, 97, 1348–1353. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Yasui, T.; Isawa, K.; Tanaka, S.; Tanaka, Y.; Takakura, Y. Total talar replacement for idiopathic necrosis of the talus: A case report. J. Foot Ankle Surg. 2016, 55, 1292–1296. [Google Scholar] [CrossRef]
- Ruatti, S.; Corbet, C.; Boudissa, M.; Kerschbaumer, G.; Milaire, M.; Merloz, P.; Tonetti, J. Total talar prosthesis replacement after talar extrusion. J. Foot Ankle Surg. 2017, 56, 905–909. [Google Scholar] [CrossRef]
- Tonogai, I.; Hamada, D.; Yamasaki, Y.; Wada, K.; Takasago, T.; Tsutsui, T.; Goto, T.; Sairyo, K. Custom-Made Alumina Ceramic Total Talar Prosthesis for Idiopathic Aseptic Necrosis of the Talus: Report of Two Cases. Case Rep. Orthop. 2017, 2017, 8290804. [Google Scholar] [CrossRef]
- Fang, X.; Liu, H.; Xiong, Y.; Zhang, W.; Luo, Y.; Wu, F.; Zhou, Y.; Song, L.; Yu, Z.; Tu, C. Total talar replacement with a novel 3D printed modular prosthesis for tumors. Ther. Clin. Risk Manag. 2018, 5, 1897–1905. [Google Scholar] [CrossRef]
- Chiu, S.-Y.; Wan, K.-W. Use of three-dimensional printing techniques in the management of a patient suffering from traumatic loss of the talus. J. Foot Ankle Surg. 2019, 58, 176–183. [Google Scholar] [CrossRef]
- Tracey, J.; Arora, D.; Gross, C.E.; Parekh, S.G. Custom 3D-printed total talar prostheses restore normal joint anatomy throughout the hindfoot. Foot Ankle Spec. 2019, 12, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Angthong, C.; Rajbhandari, P. Total talar prosthesis with and without ankle ligament reconstruction using the three-dimensional computer-aided design and computer numerical control manufacturing techniques. Orthop. Rev. 2020, 12, 8844. [Google Scholar] [CrossRef] [PubMed]
- Katsui, R.; Takakura, Y.; Taniguchi, A.; Tanaka, Y. Ceramic artificial talus as the initial treatment for comminuted talar fractures. Foot Ankle Int. 2020, 41, 79–83. [Google Scholar] [CrossRef]
- Abramson, M.; Hilton, T.; Hosking, K.; Campbell, N.; Dey, R.; McCollum, G. Total talar replacements short-medium term case series, South Africa 2019. J. Foot Ankle Surg. 2021, 60, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Broughton, K.K.; Chien, B.; Stenquist, D.; Williams, C.; Miller, C.P.; Kwon, J.Y. 3-D generated anatomic custom talar cement spacers: Case reports, technical tips and literature review. 3d Print. Med. 2021, 7, 30. [Google Scholar] [CrossRef]
- Huang, J.; Xie, F.; Tan, X.; Xing, W.; Zheng, Y.; Zeng, C. Treatment of osteosarcoma of the talus with a 3D-printed talar prosthesis. J. Foot Ankle Surg. 2021, 60, 194–198. [Google Scholar] [CrossRef]
- Mu, M.D.; Yang, Q.d.; Chen, W.; Tao, X.; Zhang, C.k.; Zhang, X.; Xie, M.M.; Tang, K.L. Three dimension printing talar prostheses for total replacement in talar necrosis and collapse. Int. Orthop. 2021, 2313–2321. [Google Scholar] [CrossRef]
- Morita, S.; Taniguchi, A.; Miyamoto, T.; Kurokawa, H.; Takakura, Y.; Takakura, Y.; Tanaka, Y. The long-term clinical results of total talar replacement at 10 years or more after surgery. JBJS 2022, 104, 790–795. [Google Scholar] [CrossRef]
- Strand, G.; Juels, C.; Nowak, J. Custom total talus replacement as a salvage option for failed total ankle arthroplasty: A prospective report of two cases. Foot Ankle Surg. Tech. Rep. Cases 2022, 2, 100113. [Google Scholar] [CrossRef]
- He, X.; Lu, M.; Zou, C.; Li, Z.; Gong, T.; Kenmegne, G.R.; Wang, Y.; Luo, Y.; Zhou, Y.; Min, L. Three-dimensional printed custom-made modular talus prosthesis in patients with talus malignant tumor resection. J. Orthop. Surg. Res. 2024, 19, 273. [Google Scholar] [CrossRef]
- Wang, J.E.-H.; Day, J.; McCann, J.; Cooper, P. Early results of combined total ankle total talus replacement in the revision setting. Foot Ankle Surg. 2024, 30, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Dekker, T.; Steele, J.; Hamid, K. The use of patient-specific 3d printed titanium implants for complex foot and ankle limb salvage, deformity correction, and arthrodesis procedures. Foot Ankle Orthop. 2017, 2, 2473011417S2473000018. [Google Scholar] [CrossRef]
- Bejarano-Pineda, L.; Sharma, A.; Adams, S.B.; Parekh, S.G. Three-dimensional printed cage in patients with tibiotalocalcaneal arthrodesis using a retrograde intramedullary nail: Early outcomes. Foot Ankle Spec. 2021, 14, 401–409. [Google Scholar] [CrossRef]
- Akoh, C.C.; Chen, J.; Adams, S.B. Total ankle total talus replacement using a 3D printed talus component: A case report. J. Foot Ankle Surg. 2020, 59, 1306–1312. [Google Scholar] [CrossRef]
- Shnol, H.; LaPorta, G.A. 3D printed total talar replacement: A promising treatment option for advanced arthritis, avascular osteonecrosis, and osteomyelitis of the ankle. Clin. Podiatr. Med. Surg. 2018, 35, 403–422. [Google Scholar] [CrossRef]
- Harnroongroj, T.; Harnroongroj, T. The talar body prosthesis: Results at ten to thirty-six years of follow-up. JBJS 2014, 96, 1211–1218. [Google Scholar] [CrossRef]
- Taniguchi, A.; Takakura, Y.; Sugimoto, K.; Hayashi, K.; Ouchi, K.; Kumai, T.; Tanaka, Y. The use of a ceramic talar body prosthesis in patients with aseptic necrosis of the talus. J. Bone Jt. Surg. Br. Vol. 2012, 94, 1529–1533. [Google Scholar] [CrossRef]
- Belvedere, C.; Cadossi, M.; Mazzotti, A.; Giannini, S.; Leardini, A. Fluoroscopic and gait analyses for the functional performance of a custom-made total talonavicular replacement. J. Foot Ankle Surg. 2017, 56, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Wagener, J.; Gross, C.; Schweizer, C.; Lang, T.H.; Hintermann, B. Custom-made total ankle arthroplasty for the salvage of major talar bone loss. Bone Jt. J. 2017, 99, 231–236. [Google Scholar] [CrossRef]
- Belvedere, C.; Siegler, S.; Fortunato, A.; Caravaggi, P.; Liverani, E.; Durante, S.; Ensini, A.; Konow, T.; Leardini, A. New comprehensive procedure for custom-made total ankle replacements: Medical imaging, joint modeling, prosthesis design, and 3D printing. J. Orthop. Res. ® 2019, 37, 760–768. [Google Scholar] [CrossRef]
- Gagne, O.J.; Veljkovic, A.; Townshend, D.; Younger, A.; Wing, K.J.; Penner, M.J. Intraoperative assessment of the axial rotational positioning of a modern ankle arthroplasty tibial component using preoperative patient-specific instrumentation guidance. Foot Ankle Int. 2019, 40, 1160–1165. [Google Scholar] [CrossRef]
- Faldini, C.; Mazzotti, A.; Belvedere, C.; Durastanti, G.; Panciera, A.; Geraci, G.; Leardini, A. A new ligament-compatible patient-specific 3D-printed implant and instrumentation for total ankle arthroplasty: From biomechanical studies to clinical cases. J. Orthop. Traumatol. 2020, 21, 16. [Google Scholar] [CrossRef] [PubMed]
- Doty, J.F.; Dunson, J.; Duff, J.R. Single-Center, Early Experience with the First 3D-Printed Surface, Fixed Bearing, Total Ankle Arthroplasty: A Minimum of 2-Year Follow-Up. Foot Ankle Orthop. 2024, 9, 2473011424S2473000091. [Google Scholar] [CrossRef]
- Gross, C.E.; Scott, D.; Friscia, D.A.; Ellis, S.J.; Owens, R.F. A Prospective Evaluation of a 3D-Printed 4th-Generation Total Ankle Prosthesis with 1-Year Follow-Up. Foot Ankle Orthop. 2024, 9, 2473011424S2473000044. [Google Scholar] [CrossRef]
- Cody, E.A.; Scott, D.J.; Easley, M.E. Total ankle arthroplasty: A critical analysis review. JBJS Rev. 2018, 6, e8. [Google Scholar] [CrossRef]
- Hsu, A.R.; Ellington, J.K. Patient-specific 3-dimensional printed titanium truss cage with tibiotalocalcaneal arthrodesis for salvage of persistent distal tibia nonunion. Foot Ankle Spec. 2015, 8, 483–489. [Google Scholar] [CrossRef]
- Steele, J.R.; Kadakia, R.J.; Cunningham, D.J.; Dekker, T.J.; Kildow, B.J.; Adams, S.B. Comparison of 3D printed spherical implants versus femoral head allografts for tibiotalocalcaneal arthrodesis. J. Foot Ankle Surg. 2020, 59, 1167–1170. [Google Scholar] [CrossRef]
- Bejarano-Pineda, L.; Adams, S.B.; Parekh, S.G. 3D Printed Cage in Patients with Tibiotalocalcaneal Arthrodesis Using a Retrograde Intramedullary Nail: Early Outcomes. Foot Ankle Orthop. 2019, 4, 2473011419S2473000104. [Google Scholar] [CrossRef]
- Ramhamadany, E.; Chadwick, C.; Davies, M.B. Treatment of severe avascular necrosis of the talus using a novel keystone-shaped 3D-printed titanium truss implant. Foot Ankle Orthop. 2021, 6, 24730114211043516. [Google Scholar] [CrossRef]
- Antounian, F.; Avagyan, H.; Ghaltaghchyan, T.; Holovenko, Y.; Khachatryan, H.; Aghayan, M. Designing and additive manufacturing of talus implant for post-traumatic talus avascular necrosis: A case study. J. Orthop. Surg. Res. 2024, 19, 501. [Google Scholar] [CrossRef]
- Kim, M.S.; Mann, T.; Kelly, C.; Palmer, R.C.; Abar, B.; Zhang, H.; Cush, G.J. Mid-term outcomes of lower limb salvage with 3D-printed ankle cages. Foot Ankle Surg. Tech. Rep. Cases 2024, 4, 100413. [Google Scholar] [CrossRef]
- Duan, X.; He, P.; Fan, H.; Zhang, C.; Wang, F.; Yang, L. Application of 3D-Printed Personalized Guide in Arthroscopic Ankle Arthrodesis. BioMed Res. Int. 2018, 2018, 3531293. [Google Scholar] [CrossRef]
- Liang, H.; Wang, J.; Yang, Y.; Niu, T.; Du, Z.; Zang, J.; Wei, R.; Yan, T.; Tang, X.; Guo, W. Reconstruction With a 3D-Printed Megaprosthesis With Ankle Arthrodesis After Distal Tibial Tumor Resection. Foot Ankle Int. 2022, 43, 1450–1459. [Google Scholar] [CrossRef]
- Duan, X.j.; Fan, H.q.; Wang, F.y.; He, P.; Yang, L. Application of 3D-printed customized guides in subtalar joint arthrodesis. Orthop. Surg. 2019, 11, 405–413. [Google Scholar] [CrossRef]
- Gross, C.; Erickson, B.J.; Adams, S.B.; Parekh, S.G. Ankle arthrodesis after failed total ankle replacement: A systematic review of the literature. Foot Ankle Spec. 2015, 8, 143–151. [Google Scholar] [CrossRef]
- Jeng, C.L.; Campbell, J.T.; Tang, E.Y.; Cerrato, R.A.; Myerson, M.S. Tibiotalocalcaneal arthrodesis with bulk femoral head allograft for salvage of large defects in the ankle. Foot Ankle Int. 2013, 34, 1256–1266. [Google Scholar] [CrossRef] [PubMed]
- Gross, C.E.; Lewis, J.S.; Adams, S.B.; Easley, M.; DeOrio, J.K.; Nunley, J.A. Secondary arthrodesis after total ankle arthroplasty. Foot Ankle Int. 2016, 37, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Ferkel, R.D.; Hewitt, M. Long-term results of arthroscopic ankle arthrodesis. Foot Ankle Int. 2005, 26, 275–280. [Google Scholar] [CrossRef]
- Duan, X.; Yang, L.; Yin, L. Arthroscopic arthrodesis for ankle arthritis without bone graft. J. Orthop. Surg. Res. 2016, 11, 154. [Google Scholar] [CrossRef]
- Faict, S.; Burssens, A.; Kristian, B. Correction of Ankle Varus Deformity using Patient Specific Dome Shaped Osteotomy Guides Designed on Weight Bearing CT. Foot Ankle Orthop. 2022, 7, 2473011421S2473000192. [Google Scholar] [CrossRef]
- Zhang, C.; Lin, Y.; Yang, L.; Duan, X. 3D printing-assisted supramalleolar osteotomy for ankle osteoarthritis. ACS Omega 2022, 7, 42191–42198. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Chen, C.; Wang, C.; Li, X.; Fu, S.; Wang, J.; Wu, C.; Liu, F.; Gu, W.; Song, G. Open wedge supramalleolar osteotomy versus 3D printing patient-specific guides for varus ankle osteoarthritis: A retrospective case control study. 2023. Available online: https://www.researchsquare.com/article/rs-2687547/v1 (accessed on 23 March 2023).
- Saltzman, C.L.; Salamon, M.L.; Blanchard, G.M.; Huff, T.; Hayes, A.; Buckwalter, J.A.; Amendola, A. Epidemiology of ankle arthritis: Report of a consecutive series of 639 patients from a tertiary orthopaedic center. Iowa Orthop. J. 2005, 25, 44. [Google Scholar] [PubMed]
- Kim, H.-J.; Park, J.; Shin, J.-Y.; Park, I.-H.; Park, K.-H.; Kyung, H.-S. More accurate correction can be obtained using a three-dimensional printed model in open-wedge high tibial osteotomy. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 3452–3458. [Google Scholar] [CrossRef]
- Becker, A.S.; Myerson, M.S. The indications and technique of supramalleolar osteotomy. Foot Ankle Clin. 2009, 14, 549–561. [Google Scholar] [CrossRef]
- Butler, J.J.; Azam, M.T.; Weiss, M.B.; Kennedy, J.G.; Walls, R.J. Supramalleolar osteotomy for the treatment of ankle osteoarthritis leads to favourable outcomes and low complication rates at mid-term follow-up: A systematic review. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Cong, B.; Zhang, H. Innovative 3D printing technologies and advanced materials revolutionizing orthopedic surgery: Current applications and future directions. Front. Bioeng. Biotechnol. 2025, 13, 1542179. [Google Scholar] [CrossRef]
- Liang, W.; Zhou, C.; Zhang, H.; Bai, J.; Jiang, B.; Jiang, C.; Ming, W.; Zhang, H.; Long, H.; Huang, X. Recent advances in 3D printing of biodegradable metals for orthopaedic applications. J. Biol. Eng. 2023, 17, 56. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S. 3D Printing Technologies and Materials for Prosthetics and Orthotics. In 3D Printing in Prosthetics and Orthotics: Innovations and Opportunities; Springer: Berlin/Heidelberg, Germany, 2024; pp. 13–34. [Google Scholar]
- Rodriguez Colon, R.; Nayak, V.V.; Parente, P.E.; Leucht, P.; Tovar, N.; Lin, C.C.; Rezzadeh, K.; Hacquebord, J.H.; Coelho, P.G.; Witek, L. The presence of 3D printing in orthopedics: A clinical and material review. J. Orthop. Res. ® 2023, 41, 601–613. [Google Scholar] [CrossRef]
- Hintermann, B.; Ruiz, R. Indications: Contraindications for Total Ankle Replacement. In Total Ankle Replacement: A Practical Guide to Surgical Management; Springer: Berlin/Heidelberg, Germany, 2024; pp. 59–74. [Google Scholar]
- Liu, S.; Wang, Y.; Zhang, M.; Wei, P.; Li, Y.; Wang, T.; Meng, Q. A comparative study of modern total ankle replacement and ankle arthrodesis for ankle osteoarthritis at different follow-up times: A systematic review and meta-analysis. Int. Orthop. 2023, 47, 1493–1510. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A. Additive manufacturing applications in orthopaedics: A review. J. Clin. Orthop. Trauma 2018, 9, 202–206. [Google Scholar] [CrossRef]
- Tack, P.; Victor, J.; Gemmel, P.; Annemans, L. 3D-printing techniques in a medical setting: A systematic literature review. Biomed. Eng. Online 2016, 15, 115. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.C. 3D-printed patient-specific applications in orthopedics. Orthop. Res. Rev. 2016, 14, 57–66. [Google Scholar] [CrossRef] [PubMed]
| Author | Published Year | Study Design | No. of Cases | Ankle Pathology | Surgery Type | Outcomes | Mean Follow-Up Period (Months) | 3D Printing Use (Material) |
|---|---|---|---|---|---|---|---|---|
| Kuldeep et al. [25] | 2013 | Case report | 1 | Open talar extrusion | TTR | AOFAS score (75), ankle ROM (25°), radiographs (prosthesis was in a slightly PF but stable position) | 132 | Customized implant (cobalt–chrome) |
| Taniguchi et al. [26] | 2015 | Retrospective | 55 | Talar AVN | TTR | JSSF score (improved from 43.1 to 89.4), AOS (score for “pain at its worst” improved from a mean of 6.1 to 2.0), improved ankle DF | 52.8 | Customized implant (alumina ceramic) |
| Yukari et al. [27] | 2015 | Case report | 1 | Idiopathic necrosis of the talus | TTR | AOFAS score (improved from 45 to 90), ankle ROM (20° of DF and 40° of PF), radiographs (stable position in ankle mortise, no degenerative or destructive changes in the surrounding bones) | 24 | Customized implant (alumina ceramic) |
| Sebastient et al. [28] | 2017 | Case report | 1 | Total dislocation | TTR | AOFAS score (improved from 11 to 77), SF-36 (improved from 17 to 82), ankle ROM (10° of DF and 40° of PF), radiographs (no periprosthetic delineation and no premature loosening of the implant) | 24 | Customized implant (cobalt–chrome) |
| Ichiro et al. [29] | 2017 | Case report | 2 | Idiopathic necrosis of the talus | TTR | JSSF score (improved from 22 and 29 to 90 and 95, respectively), VAS (improved from 9 and 8 to 0 and 0), ankle ROM (10° of DF and 30° of PF, respectively), radiographs (prosthesis was appropriately positioned in the ankle with no degenerative or destructive changes in the surrounding bones) | 24 | Customized implant (alumina ceramic) |
| Joseph et al. [32] | 2018 | Retrospective | 14 | Talar AVN | TTR | Radiographic parameters (talar height and talar tilt angle were significantly improved, Meary’s angle correction was observed in cavus and planus foot deformity) | 5 | Customized implant (cobalt–chrome) |
| Xiang et al. [30] | 2018 | Case report | 1 | Mesenchymal sarcoma of the talus | TTR | MSTS score (26), AOFAS score (91), ankle ROM (10° of DF, 30° of PF, 5° of eversion, and 10° of inversion), radiographs (prosthesis and the screws were in a stable position, no abnormalities were observed in the surrounding bones) | 6 | Customized implant (UHMWPE, titanium alloy) |
| Daniel et al. [17] | 2019 | Retrospective | 15 | Talar AVN | TTR | FAOS and VAS (significant improvements), coronal and sagittal alignment on weight-bearing radiographs (no significant difference) | 12.8 | Customized implant (cobalt–chrome) |
| Shin et al. [31] | 2019 | Case report | 1 | Traumatic loss of the talus | TTR | Walk independently with mild pain for 15 min with a crutch occasionally, ankle ROM (5° of DF, 10° of PF), radiographs (no visible erosion of the left tibia, navicular, or calcaneus, no fracture of the talus cement spacer) | 14 | Customized implant (antibiotic-loaded cement spacer) |
| Ryuhei et al. [34] | 2019 | Retrospective | 6 | Comminuted talar dome fracture or talar body defects | TTR | AOFAS score (78.8), ankle ROM (10° of DF, 31° of PF) | 16.4 | Customized implant (alumina ceramic) |
| Jihui et al. [37] | 2020 | Case report | 1 | Osteoblastic osteosarcoma of the talus | TTR | MSTS (93%), TESS (93), walk normally without support | 24 | Customized implant (titanium) |
| Rishin et al. [9] | 2020 | Retrospective | 27 | Talar AVN | TTR | FAOS concerning pain, symptoms, and quality of life and VAS (significant improvements), ankle ROM (insignificantly improved), 3 complications requiring reoperation | 22.2 | Customized implant (cobalt–chromium or cobalt–chromium with titanium nitride coating) |
| Chayanin et al. [33] | 2020 | Retrospective | 5 | Severe talar loss or damage | TTR | VAS (82.3), SF-36 (83.38), mild subsidence (1 patient), periprosthetic fracture (1 patient, a mild displaced calcaneal fracture) | 17.8 | Customized implant (four cases of stainless steel, one case of titanium) |
| Abramson et al. [35] | 2021 | Retrospective | 8 | 2 (complex, irreparable trauma), 4 (post-traumatic AVN with symptomatic collapse), 2 (primary bone neoplasms) | TTR | AOFAS score (79.25), SF-36 (83.25), no revision surgeries, radiological changes in minor tibial wear (1 patient, symptom-free) | 23 | Customized implant (cobalt–chrome) |
| Mi dou et al. [38] | 2021 | Retrospective | 9 | Talar AVN | TTR | AOFAS scores and VAS (significant improvements), ankle ROM (insignificantly improved), radiographs (no degenerative arthritis or prosthetic dislocation; talar prosthesis was placed in the original anatomical position), radiographic parameters (talar height and Meary’s angle were significantly improved) | 23.17 | Customized implant (titanium alloy for talar structure, cobalt–chromium–molybdenum alloy for articular facet) |
| Kimberly et al. [36] | 2021 | Case report | 2 | Talus fracture nonunion (failed TTR), infected subtalar arthrodesis nonunion | TTR | WB as tolerated in a regular shoe, 3/10 daily pain in both patients, ankle ROM (10° and 6° of DF, 25° and 20° of PF, 15° and 10° of eversion, and 15° and 10° of inversion, respectively) | 11, 4 | Customized implant (antibiotic-loaded cement spacer) |
| Wenbin et al. [10] | 2022 | Retrospective | 3 | Talar AVN | TTR | AOFAS score (88.5), radiographs (no signs of prosthesis loosening or serious degenerative change in the surrounding area of the joint, small osteophytes on the tibial side and navicular side) | 53 | Customized implant (vitallium alloy) |
| Morita et al. [39] | 2022 | Retrospective | 19 | Talar AVN | TTR | AOS and JSSF score (significantly improved), median postoperative ankle ROM (45°) | 152 | Customized implant (alumina ceramic) |
| Xuanhong et al. [41] | 2024 | Retrospective | 6 | Malignant tumor of the talus | TTR | MSTS-93 score (26.8), AOFAS score (88.5), ankle ROM (9.2° of DF, 32.5° of PF), radiographs (no aseptic loosening, fracture/dislocation of the prosthesis, or screws loosening) | 54.8 | Customized implant (UHMWPE part and titanium alloy part) |
| Giannini et al. [24] | 2015 | Case report | 1 | Severe osteoarthritis of both the ankle and the talonavicular joints, secondary to talar AVN | Total talonavicular replacement | VAS (1), AOFAS score (81), Tegner activity (9), ankle ROM (11° of DF, 7° of PF), radiographs (no signs of radiolucency around the implant) | 30 | Customized implant (cobalt–chrome) |
| Magnan et al. [23] | 2004 | Case report | 1 | Open total medial dislocation of the talus | TAA plus total talus prosthesis | No pain or limp during normal activities, ankle ROM (5° of DF, 30° of PF), radiographs (good alignment of both component, no loosening or periprosthetic radiolucent line) | 28 | Customized implant (titanium) |
| Shinji et al. [22] | 2010 | Case report | 1 | Failed TAA | TAA plus total talus prosthesis | JSSF score (65), no pain during normal activities, and was able to walk without aids, ankle ROM (15° of DF, 10° of PF), radiographs (no evidence of loosening) | 24 | Customized implant (alumina ceramic) |
| Kurokawa et al. [14] | 2018 | Retrospective | 10 (combined), 12 (standard) | Ankle arthritis with severe talar collapse | TAA plus total talus prosthesis | JSSF score (postoperative score was significantly higher in the combined TAA group), AOS (no significant differences between the two groups in terms of pre-and postoperative function and postoperative pain) | 58 (combined), 64 (standard) | Customized implant (alumina ceramic) |
| Kanzaki et al. [13] | 2019 | Retrospective | 22 | Ankle arthritis with severe talar collapse | TAA plus total talus prosthesis | JSSF score and SAFE-Q score (significantly improved), ankle ROM (significantly improved) | 34.9 | Customized implant (alumina ceramic) |
| Strand et al. [40] | 2022 | Prospective | 2 | Failed TAA | TAA plus total talus prosthesis | AOFAS score, VAS, and SF-36 improved postoperatively, improved ankle ROM, radiographs (no evidence of loosening or peri-cystic changes) | 24 | Customized implant (cobalt–chrome) |
| Wang et al. [42] | 2024 | Retrospective | 19 | Failed TAA | TAA plus total talus prosthesis | PROMIS (all domains significantly improved) | 37.9 | Customized implant (titanium) |
| Author | Published Year | Study Design | No. of Cases | Ankle Pathology | Surgery Type | Outcomes | Mean Follow-Up Period (Months) | 3D Printing Use |
|---|---|---|---|---|---|---|---|---|
| Belvedere et al. [51] | 2018 | Cadaveric study | 3 | Normal ankle joint | TAA | Mean manufacturing errors (smaller than 0.08 mm), consistent ankle motion patterns, mobility, and stability (compared well with the original natural conditions) | N-S | Customized implant |
| Gagne et al. [52] | 2019 | Prospective | 22 | Ankle OA | TAA | Compared to the PSI and conventional methods (average difference was −0.46), 50% (11/22) were within 2 degrees of the target, and 77% (17/22) were within 4 degrees of the target | N-S | PSI (guide) |
| Faldini et al. [53] | 2020 | Case report | 1 | Ankle OA | TAA | Clinical abilities (restored without pain), VAS, AOFAS score, SF-36 (improved), gait analysis (quasi-physiological pattern of rotation, normal muscle activation time), radiographs (stable prosthesis with no signs of radiolucency around the implant) | 4 | Customized implant and PSI (guide) |
| Gross et al. [55] | 2024 | Prospective (multicenter) | 91 | Ankle OA | TAA | Improved in all PROM domains (AOS, PROMIS Global Physical Heath, FAOS symptom scores) | 12 | Customized implant |
| Doty et al. [54] | 2024 | Retrospective | 30 | Ankle OA | TAA | VAS, PROMIS physical scores (improved), high implant survival rate (90%) | 26 | Customized implant |
| Author | Published Year | Study Design | No. of Cases | Ankle Pathology | Surgery Type | Outcomes | Mean Follow-Up Period (Months) | 3D Printing Use |
|---|---|---|---|---|---|---|---|---|
| Hsu et al. [57] | 2015 | Case report | 1 | Persistent distal tibial nonunion with large bony defect | TTC arthrodesis | Minimal pain, ambulation, and work independency, no wound complication | 12 | Customized implant |
| Steele et al. [58] | 2020 | Retrospective | 8 (3D spherical implant), 7 (femoral head allograft) | Severe ankle bone defects | TTC arthrodesis | The proportion of total fused articulations and the number of patients who successfully fused all three articulations were notably higher in the 3D sphere group. In contrast, the rate of graft resorption was significantly elevated in the femoral head allograft group. | 23, 30 | Customized implant |
| Lorena et al. [59] | 2020 | Retrospective | 7 | Ankle OA with segmental bone defects | TTC arthrodesis | Participants reported performing daily activities without pain. Both the AOFAS score and VAS showed significant improvement. Six patients achieved over 50% bony bridging, while one patient underwent below-knee amputation due to a recurrence of chronic osteomyelitis. | 21 | Customized implant |
| Eamon et al. [60] | 2021 | Retrospective | 3 | End-stage talar AVN | TTC arthrodesis | AOFAS score (improved), radiographs (satisfactory radiological union) | 32 | Customized implant |
| Antounian et al. [61] | 2024 | Case report | 1 | Post-traumatic talar AVN and failed ankle arthrodesis | TTC arthrodesis | Patients could walk without canes or crutches and did so without pain. Radiographs indicated that the custom-designed implant fit the bony defect area. | 24 | Customized implant |
| Kim et al. [62] | 2024 | Retrospective | 113 | Charcot arthropathy or end-stage ankle OA | TTC arthrodesis | Mean NRS pain (improved from 6.6 to 2.0), 11 patients were able to ambulate independently | 44.6 | Customized implant |
| Duan et al. [63] | 2018 | Retrospective | 15 (PSI), 14 (control) | Ankle OA | Ankle arthrodesis (arthroscopic) | The duration required to drill the Kirschner wires into the correct position was notably shorter in the PSI group. There were no significant differences in fusion time or AOFAS scores, and neither groups experienced obvious complications. | 25.2 | PSI (guide) |
| Liang et al. [64] | 2022 | Retrospective | 13 | Distal tibia tumor (wide or marginal resection) | Ankle arthrodesis | Mean MSTS-93 score (28.0 ± 1.5), 1 case of periprosthetic infection after paronychia | 26.8 | Customized implant |
| Duan et al. [65] | 2019 | Retrospective | 14 (PSI), 16 (control) | Subtalar OA | Subtalar arthrodesis | The duration for positioning the Kirschner wires was notably shorter in the PSI group. There were two instances of re-drilling in the PSI group compared to eight in the control group. The AOFAS scores showed no significant differences, and radiographic fusion was confirmed in all cases. | 24 | PSI (guide) |
| Author | Published Year | Study Design | No. of Cases | Ankle Pathology | Surgery Type | Outcomes | Mean Follow-Up Period (Months) | 3D Printing Use |
|---|---|---|---|---|---|---|---|---|
| Faict et al. [71] | 2021 | Retrospective | 5 | Ankle OA | SMO | EFAS score, FAOS, and VAS (significantly improved), radiographs (healing of the osteotomy site was confirmed on WBCT), radiographic parameters (improvement of the TAS, tibiotalar angle, and hindfoot angle) | 40.8 | PSI (guide) |
| Wang et al. [73] | 2022 | Retrospective | 11 (PSI), 17 (control) | Ankle OA | SMO | Mean operating time, postoperative hospital stay, number of fluoroscopy examinations, and albumin reduction (significantly lower in the PSI group compared to the control group) | 33.4 | PSI (guide plate) |
| Zhang et al. [72] | 2022 | Retrospective | 7 (PSI), 9 (control) | Ankle OA | SMO | Mean operating time, intraoperative blood loss, and intraoperative fluoroscopy time (significantly lower in the PSI group compared to the control group) | 13.9 | Customized implant (cage), PSI (guide) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-J.; Choi, J.Y.; Lee, J.-M.; Seok, H.-G.; Park, C.H. Applications and Effectiveness of 3D Printing in Various Ankle Surgeries: A Narrative Review. Life 2025, 15, 473. https://doi.org/10.3390/life15030473
Park J-J, Choi JY, Lee J-M, Seok H-G, Park CH. Applications and Effectiveness of 3D Printing in Various Ankle Surgeries: A Narrative Review. Life. 2025; 15(3):473. https://doi.org/10.3390/life15030473
Chicago/Turabian StylePark, Jeong-Jin, Jun Young Choi, Jung-Min Lee, Hyun-Gyu Seok, and Chul Hyun Park. 2025. "Applications and Effectiveness of 3D Printing in Various Ankle Surgeries: A Narrative Review" Life 15, no. 3: 473. https://doi.org/10.3390/life15030473
APA StylePark, J.-J., Choi, J. Y., Lee, J.-M., Seok, H.-G., & Park, C. H. (2025). Applications and Effectiveness of 3D Printing in Various Ankle Surgeries: A Narrative Review. Life, 15(3), 473. https://doi.org/10.3390/life15030473







