Cerebral Inflammation in an Animal Ischemia–Reperfusion Model Comparing Histidine-Tryptophan-α-Ketoglutarate and Del Nido Cardioplegia
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Anesthesia Management
2.2. Surgical Technique, Extracorporeal Circulation, and Perfusion
2.3. Study Design
2.4. Randomization and Blinding
2.5. Porcine Brain Preparation
2.6. RNA Isolation and Quantitative Real-Time PCR
2.7. Immunohistochemical Analysis and Evaluation
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Statistical Analysis
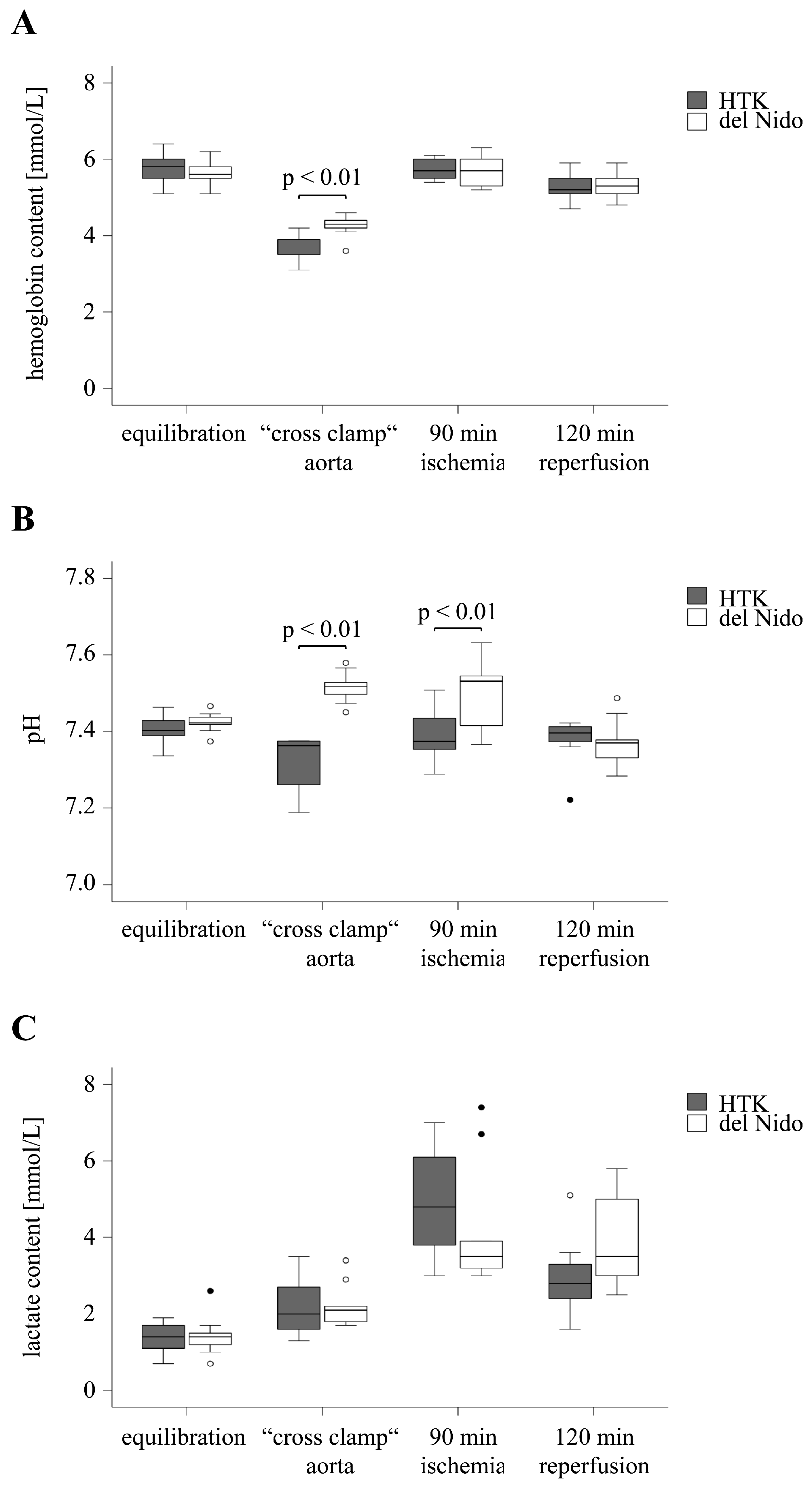
3. Results
3.1. Hemodynamic Parameters
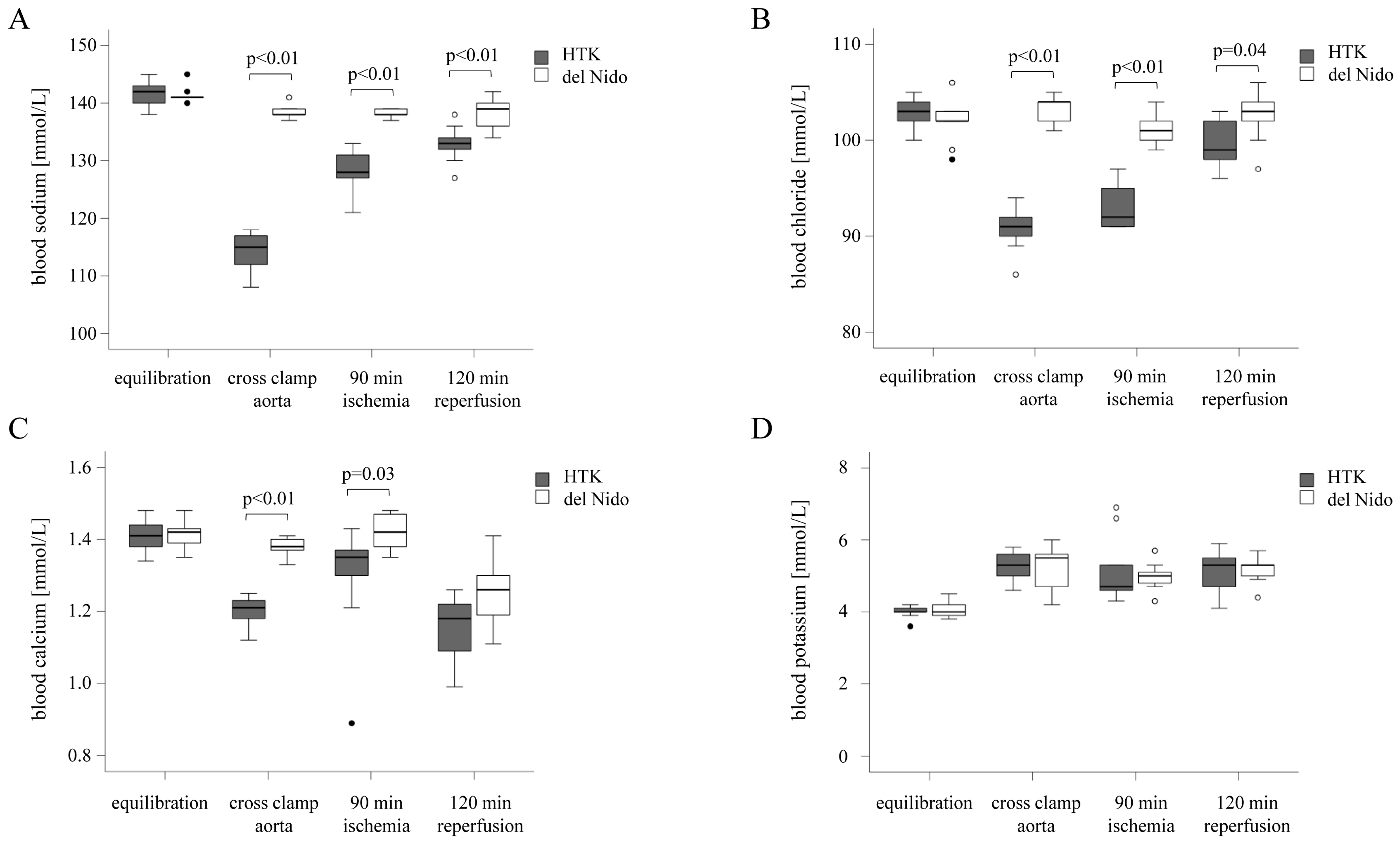
3.2. Analysis of the Electrolyte Balance
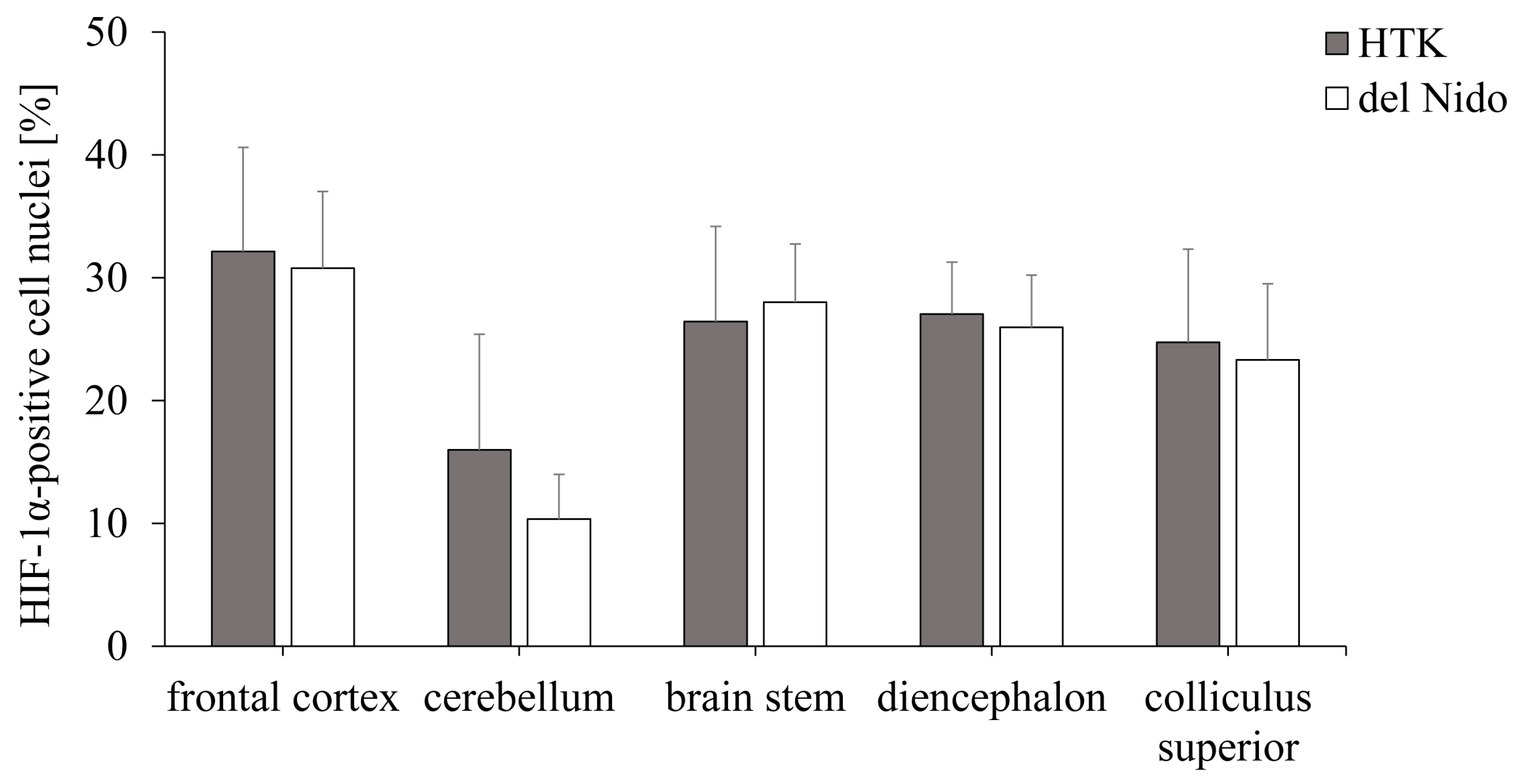
3.3. Analysis of the Hypoxia-Induced Transcription Factor HIF-1α
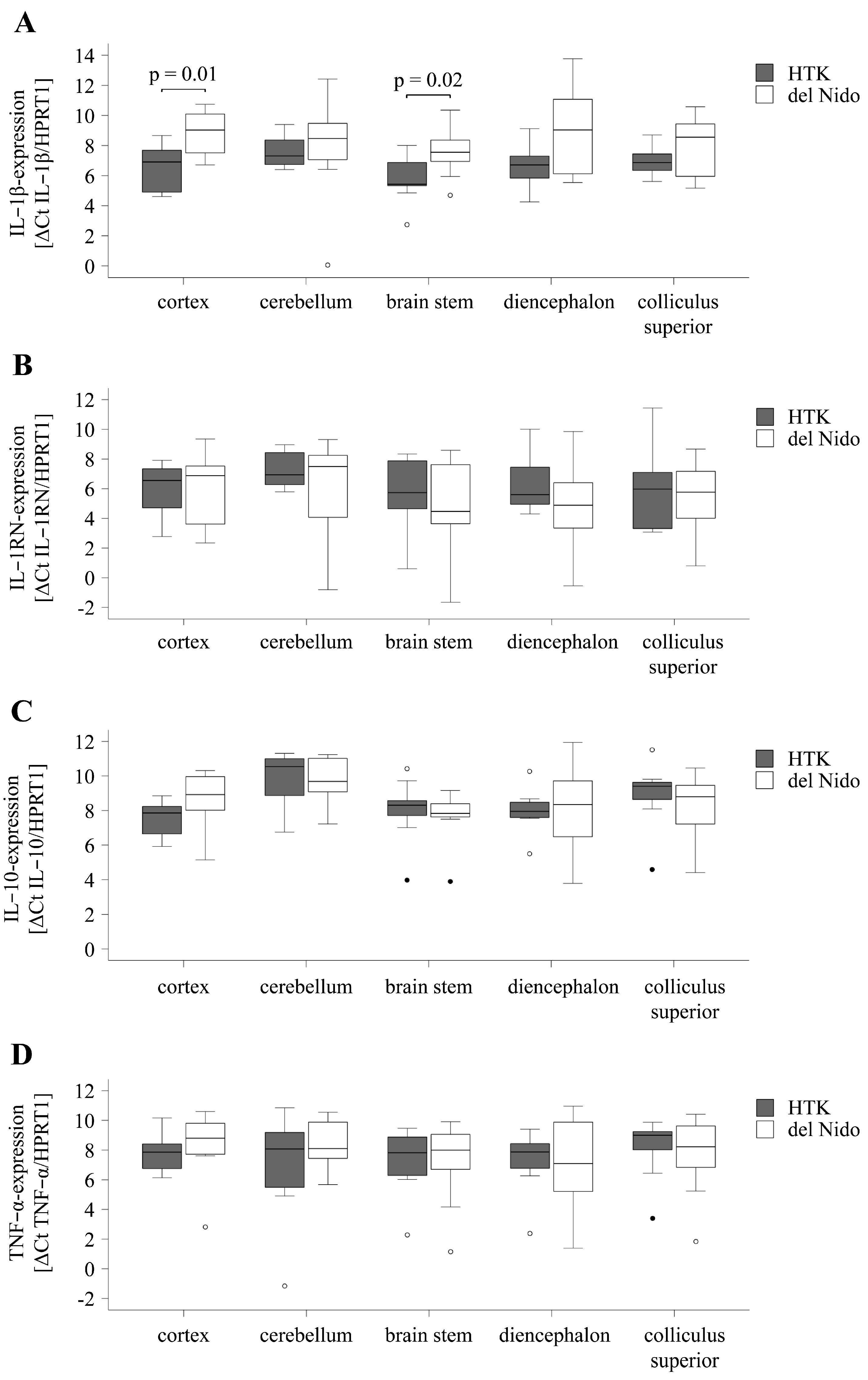
3.4. Cytokine Expression in the Brain
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CPB | cardiopulmonary bypass |
| DEPC | diethylpyrocarbonate |
| ELISA | Enzyme-linked immunosorbent assay |
| HIF-1 | hypoxia-inducible factor-1α |
| HPRT-1 | hypoxanthine-guanine phosphoribosyltransferase-1 |
| HTK | histidine-tryptophan-α-ketoglutarate |
| IL | interleukin |
| IL-1RN | IL-1-receptor antagonist |
| IRI | ischemia–reperfusion injury |
| PBS | phosphate-buffered saline |
| RT-qPCR | quantitative real-time PCR analysis |
| TBS | tris-buffered saline |
| TNF-α | tumor-necrosis factor-α |
References
- Hogue, C.W.; Gottesman, R.F.; Stearns, J. Mechanisms of cerebral injury from cardiac surgery. Crit. Care Clin. 2008, 24, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Stanley, M.E.; Sellke, F.W. Neurocognitive decline in cardiac surgery patients: What do we know? J. Thorac. Cardiovasc. Surg. 2023, 166, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Lomivorotov, V.V.; Moroz, G.; Abubakirov, M.; Osinsky, R.; Landoni, G. Volatile and intravenous anesthetics for brain protection in cardiac surgery: Does the choice of anesthesia matter? J. Cardiothorac. Vasc. Anesth. 2022, 36, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Miniksar, Ö.H.; Çiçekçioğlu, F.; Kılıç, M.; Honca, M.; Miniksar, D.Y.; Gocmen, A.Y.; Kaçmaz, O.; Öz, H. Decreased brain-derived neurotrophic factor levels may predict early perioperative neurocognitive disorder in patients undergoing coronary artery bypass surgery: A prospective observational pilot study. J. Clin. Anesth. 2021, 71, 110235. [Google Scholar] [CrossRef]
- Gorvitovskaia, A.Y.; Scrimgeour, L.A.; Potz, B.A.; Sellke, N.C.; Ehsan, A.; Sodha, N.R.; Sellke, F.W. Lower preoperative hematocrit, longer hospital stay, and neurocognitive decline after cardiac surgery. Surgery 2020, 168, 147–154. [Google Scholar] [CrossRef]
- Glöckner, A.; Ossmann, S.; Ginther, A.; Kang, J.; Borger, M.A.; Hoyer, A.; Dieterlen, M.-T. Relevance and Recommendations for the Application of Cardioplegic Solutions in Cardiopulmonary Bypass Surgery in Pigs. Biomedicines 2021, 9, 1279. [Google Scholar] [CrossRef]
- Abrahamov, D.; Levran, O.; Naparstek, S.; Refaeli, Y.; Kaptson, S.; Abu Salah, M.; Ishai, Y.; Sahar, G. Blood-brain barrier disruption after cardiopulmonary bypass: Diagnosis and correlation to cognition. Ann. Thorac. Surg. 2017, 104, 161–169. [Google Scholar] [CrossRef]
- Li, Y.; Lin, H.; Zhao, Y.; Li, Z.; Liu, D.; Wu, X.; Ji, B.; Gao, B. Del Nido Cardioplegia for Myocardial Protection in Adult Cardiac Surgery: A Systematic Review and Meta-Analysis. ASAIO J. 2018, 64, 360–367. [Google Scholar] [CrossRef]
- Reynolds, A.C.; Asopa, S.; Modi, A.; King, N. HTK versus multidose cardioplegias for myocardial protection in adult cardiac surgery: A meta-analysis. J. Card. Surg. 2021, 36, 1334–1343. [Google Scholar] [CrossRef]
- Hildebrandt, L.; Dieterlen, M.-T.; Klaeske, K.; Haunschild, J.; Saeed, D.; Eifert, S.; Borger, M.A.; Jawad, K. Myostatin/AKT/FOXO Signaling Is Altered in Human Non-Ischemic Dilated Cardiomyopathy. Life 2022, 12, 1418. [Google Scholar] [CrossRef]
- Hoyer, A.; Bergh, F.T.; Klaeske, K.; Lehmann, S.; Misfeld, M.; Borger, M.; Dieterlen, M.-T. Custodiol-N™ cardioplegia lowers cerebral inflammation and activation of hypoxia-inducible factor-1α. Interact. Cardiovasc. Thorac. Surg. 2019, 28, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, A.; Dieterlen, M.T.; Kang, J.; Oetzel, H.; Wiesner, K.; Klaeske, K.; Kiefer, P.; Oßmann, S.; Ginther, A.; Kostelka, M.; et al. Comparison of Del Nido and histidine-tryptophan-ketoglutarate cardioplegia solutions: An animal study with prolonged ischaemia. Front. Cardiovasc. Med. 2024, 11, 1457770. [Google Scholar] [CrossRef] [PubMed]
- Günday, M.; Bingöl, H. Is crystalloid cardioplegia a strong predictor of intra-operative hemodilution? J. Cardiothorac. Surg. 2014, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Soliman, R.; Saad, D.; Abukhudair, W.; Abdeldayem, S. The neurocognitive outcomes of hemodilution in adult patients undergoing coronary artery bypass grafting using cardiopulmonary bypass. Ann. Card. Anaesth. 2022, 25, 133–140. [Google Scholar] [CrossRef]
- Lindner, G.; Zapletal, B.; Schwarz, C.; Wisser, W.; Hiesmayr, M.; Lassnigg, A. Acute hyponatremia after cardioplegia by histidine-tryptophane-ketoglutarate—A retrospective study. J. Cardiothorac. Surg. 2012, 7, 52. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, L.; An, C.; Wang, R.; Yang, L.; Yu, W.; Li, P.; Gao, Y. The blood brain barrier in cerebral ischemic injury—Disruption and repair. Brain Hemorrhages 2020, 1, 34–53. [Google Scholar] [CrossRef]
- Castilla-Guerra, L.; del Carmen Fernández-Moreno, M.; López-Chozas, J.M.; Fernández-Bolaños, R. Electrolytes disturbances and seizures. Epilepsia 2006, 47, 1990–1998. [Google Scholar] [CrossRef]
- Crestanello, J.A.; Phillips, G.; Firstenberg, M.S.; Sai-Sudhakar, C.; Sirak, J.; Higgins, R.; Abraham, W.T. Postoperative hyponatremia predicts an increase in mortality and in-hospital complications after cardiac surgery. J. Am. Coll. Surg. 2013, 216, 1135–1143. [Google Scholar] [CrossRef]
- Kim, J.-T.; Park, Y.-H.; Chang, Y.-E.; Byon, H.-J.; Kim, H.-S.; Kim, C.-S.; Lim, H.-G.; Kim, W.-H.; Lee, J.-R.; Kim, Y.-J. The effect of cardioplegic solution-induced sodium concentration fluctuation on postoperative seizure in pediatric cardiac patients. Ann. Thorac. Surg. 2011, 91, 1943–1948. [Google Scholar] [CrossRef]
- Cui, C.; Jiang, X.; Wang, Y.; Li, C.; Lin, Z.; Wei, Y.; Ni, Q. Cerebral Hypoxia-Induced Molecular Alterations and Their Impact on the Physiology of Neurons and Dendritic Spines: A Comprehensive Review. Cell Mol. Neurobiol. 2024, 44, 58. [Google Scholar] [CrossRef]
- McGettrick, A.F.; O’Neill, L.A.J. The Role of HIF in Immunity and Inflammation. Cell Metab. 2020, 32, 524–536. [Google Scholar] [CrossRef]
- Heck-Swain, K.L.; Li, J.; Ruan, W.; Yuan, X.; Wang, Y.; Koeppen, M.; Eltzschig, H.K. Myeloid hypoxia-inducible factor HIF1A provides cardio-protection during ischemia and reperfusion via induction of netrin-1. Front. Cardiovasc. Med. 2022, 9, 970415. [Google Scholar] [CrossRef] [PubMed]
- Shi, H. Hypoxia inducible factor 1 as a therapeutic target in ischemic stroke. Curr. Med. Chem. 2009, 16, 4593–4600. [Google Scholar] [CrossRef]
- Mukandala, G.; Tynan, R.; Lanigan, S.; O’Connor, J.J. The Effects of Hypoxia and Inflammation on Synaptic Signaling in the CNS. Brain Sci. 2016, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Amantea, D.; Nappi, G.; Bernardi, G.; Bagetta, G.; Corasaniti, M.T. Post-ischemic brain damage: Pathophysiology and role of inflammatory mediators. FEBS J. 2009, 276, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Clausen, B.H.; Wirenfeldt, M.; Høgedal, S.S.; Frich, L.H.; Nielsen, H.H.; Schrøder, H.D.; Østergaard, K.; Finsen, B.; Kristensen, B.W.; Lambertsen, K.L. Characterization of the TNF and IL-1 systems in human brain and blood after ischemic stroke. Acta Neuropathol. Commun. 2020, 8, 81. [Google Scholar] [CrossRef]
- Catană, M.G.; Popențiu, I.A.; Văleanu, M.; Roman-Filip, C.; Mihăilă, R.G. IL-1 Beta-A Biomarker for Ischemic Stroke Prognosis and Atherosclerotic Lesions of the Internal Carotid Artery. Medicina 2023, 59, 1790. [Google Scholar] [CrossRef]
- Elsaafien, K.; Sloan, J.M.; Evans, R.G.; Cochrane, A.D.; Marino, B.; McCall, P.R.; Hood, S.G.; Yao, S.T.; Korim, W.S.; Bailey, S.R.; et al. Associations Between Systemic and Cerebral Inflammation in an Ovine Model of Cardiopulmonary Bypass. Anesth Analg. 2023, 136, 802–813. [Google Scholar] [CrossRef]
- Leal, M.C.; Casabona, J.C.; Puntel, M.; Pitossi, F.J. Interleukin-1β and tumor necrosis factor-α: Reliable targets for protective therapies in Parkinson’s Disease? Front. Cell Neurosci. 2013, 7, 53. [Google Scholar] [CrossRef]
- Yang, T.T.; Lin, C.; Hsu, C.T.; Wang, T.F.; Ke, F.Y.; Kuo, Y.M. Differential distribution and activation of microglia in the brain of male C57BL/6J mice. Brain Struct. Funct. 2013, 218, 1051–1060. [Google Scholar] [CrossRef]
- Wu, D.M.; Liu, J.P.; Liu, J.; Ge, W.H.; Wu, S.Z.; Zeng, C.J.; Liang, J.; Liu, K.; Lin, Q.; Hong, X.W.; et al. Immune pathway activation in neurons triggers neural damage after stroke. Cell Rep. 2023, 42, 113368. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Hu, S.; Li, Y.; Sun, Y.; Xiong, X.; Hu, X.; Chen, J.; Qiu, S. Interleukins and Ischemic Stroke. Front. Immunol. 2022, 13, 828447. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.; Lénárt, N.; Hill, L.; Toms, L.; Coutts, G.; Martinecz, B.; Császár, E.; Nyiri, G.; Papaemmanouil, A.; Waisman, A.; et al. Interleukin-1 mediates ischaemic brain injury via distinct actions on endothelial cells and cholinergic neurons. Brain Behav. Immun. 2019, 76, 126–138. [Google Scholar] [CrossRef]
- Squiccimarro, E.; Lorusso, R.; Consiglio, A.; Labriola, C.; Haumann, R.G.; Piancone, F.; Speziale, G.; Whitlock, R.P.; Paparella, D. Impact of Inflammation After Cardiac Surgery on 30-Day Mortality and Machine Learning Risk Prediction. J. Cardiothorac. Vasc. Anesth. 2024; in press. [Google Scholar] [CrossRef]
- Villa, A.; Gelosa, P.; Castiglioni, L.; Cimino, M.; Rizzi, N.; Pepe, G.; Lolli, F.; Marcello, E.; Sironi, L.; Vegeto, E.; et al. Sex-Specific Features of Microglia from Adult Mice. Cell Rep. 2018, 23, 3501–3511. [Google Scholar] [CrossRef] [PubMed]
- Stanley, M.E.; Kant, S.; Raker, C.; Sabe, S.; Sodha, N.R.; Ehsan, A.; Sellke, F.W. Effect of Patient Sex on Neurocognitive Decline after Cardiac Surgery. J. Am. Coll. Surg. 2023, 236, 1112–1124. [Google Scholar] [CrossRef]
- Kang, J.; Hoyer, A.; Dieterlen, M.T.; Oetzel, H.; Otto, W.; Ginther, A.; Pfannmüller, B.; Misfeld, M.; Noack, T.; Kiefer, P.; et al. Comparison of modified Del Nido and Custodiol® cardioplegia in minimally invasive mitral valve surgery. Eur. J. Cardiothorac. Surg. 2024, 65, ezae161. [Google Scholar] [CrossRef]
| HTK Solution | Jonosteril®-Based Del Nido Solution * | |
|---|---|---|
| Na+ [mmol/L] | 15 | 142 |
| K+ [mmol/L] | 9 | 28 |
| Cl− [mmol/L] | 50 | 129 |
| Mg2+ [mmol/L] | 4 | 9 |
| Ca2+ [mmol/L] | 0.015 | 1.564 |
| histidine [mmol/L] | 198 | 0 |
| tryptophan [mmol/L] | 2 | 0 |
| mannitol [mmol/L] | 30 | 17 |
| α-ketoglutarate [mmol/L] | 1 | 0 |
| lidocaine [mmol/L] | 0 | 0.26 |
| Target Gene | Forward Primer 5′-3′ | Reverse Primer 5′-3′ |
|---|---|---|
| HIF-1α | cacacagaaatggccttgtgaa | tgttcatagttctccccctgc |
| HPRT1 | ggacttgaatcatgtttgtg | cagatgtttccaaactcaac |
| IL-10 | ggcgctgtcatcaatttctgc | ggctttgtagacacccctct |
| IL-1β | cgtgcaatgatgactttgtctgt | tcatgcagaacaccacttctct |
| IL-1RN | gtcctgttgttgcatggtcac | ggtctctttcccaaggggtg |
| TNF-α | gcccccagaaggaagagttt | gacattggctacaacgtggg |
| Equilibration Period | 120 min Reperfusion | |||||
|---|---|---|---|---|---|---|
| HTK n = 9 | Del Nido n = 9 | p Value | HTK n = 9 | Del Nido n = 9 | p Value | |
| HR [bpm] | 94.9 ± 16.7 | 94.9 ± 18.6 | 0.99 | 123.5 ± 18.2 | 112.4 ± 14.1 | 0.18 |
| RRsys [mmHg] | 93.3 ± 18.7 | 97.1 ± 20 | 0.69 | 73.9 ± 14.4 | 79.9 ± 10.7 | 0.34 |
| RRdias [mmHg] | 50.9 ± 11.4 | 52.6 ± 10.2 | 0.75 | 41.1 ± 3.0 | 44.1 ± 4.5 | 0.14 |
| MAP [mmHg] | 67.6 ± 14.3 | 69.3 ± 13.9 | 0.81 | 54.1 ± 6.7 | 59.1 ± 5.8 | 0.12 |
| CVP [mmHg] | 12.5 ± 4.0 | 13.3 ± 0.9 | 0.58 | 17.8 ± 1.8 | 16.1 ± 1.8 | 0.08 |
| CO [L/min] | 6.0 ± 1.1 | 6.5 ± 1.1 | 0.36 | 5.3 ± 1.4 | 5.4 ± 1.4 | 0.85 |
| SVRI [dyn*s*cm−5*m2] | 961 ± 258 | 877 ± 114 | 0.42 | 760 ± 281 | 830 ± 128 | 0.53 |
| PAsys [mmHg] | 29 ± 8.4 | 30.4 ± 9.2 | 0.74 | 40.8 ± 10.6 | 42.7 ± 13.2 | 0.75 |
| PAdias [mmHg] | 21.1 ± 5.6 | 23.2 ± 4.8 | 0.42 | 26.4 ± 6.8 | 26.6 ± 7.5 | 0.96 |
| PAmean [mmHg] | 25.1 ± 6.0 | 26.8 ± 6.6 | 0.60 | 33.8 ± 9.1 | 33.7 ± 9.4 | 0.99 |
| HI [(L/min)/m2] | 4.7 ± 0.8 | 5.1 ± 0.7 | 0.30 | 4.1 ± 1.0 | 4.3 ± 1.2 | 0.77 |
| HTK (n = 9) | Del Nido (n = 9) | p-Value | |
|---|---|---|---|
| sodium [mmol/L] | |||
| equilibration | 141.4 ± 2.2 | 141.4 ± 1.4 | 1.00 |
| aorta cross-clamp | 114.3 ± 3.3 | 138.3 ± 1.2 | <0.01 |
| 90 min ischemia | 128.6 ± 3.7 | 138.1 ± 0.8 | <0.01 |
| 120 min reperfusion | 133.0 ± 3.2 | 138.2 ± 2.9 | <0.01 |
| chloride [mmol/L] | |||
| equilibration | 102.9 ± 1.7 | 102.0 ± 2.4 | 0.37 |
| aorta cross-clamp | 90.8 ± 2.3 | 103.1 ± 1.5 | <0.01 |
| 90 min ischemia | 92.9 ± 2.2 | 101.3 ± 1.9 | <0.01 |
| 120 min reperfusion | 99.7 ± 2.5 | 102.3 ± 2.6 | 0.04 |
| calcium [mmol/L] | |||
| equilibration | 1.41 ± 0.04 | 1.42 ± 0.04 | 0.77 |
| aorta cross-clamp | 1.20 ± 0.05 | 1.38 ± 0.03 | 0.01 |
| 90 min ischemia | 1.29 ± 0.16 | 1.42 ± 0.05 | 0.03 |
| 120 min reperfusion | 1.15 ± 0.09 | 1.24 ± 0.09 | 0.06 |
| potassium [mmol/L] | |||
| equilibration | 4.0 ± 0.2 | 4.1 ± 0.2 | 0.53 |
| aorta cross-clamp | 5.3 ± 0.4 | 5.3 ± 0.6 | 0.93 |
| 90 min ischemia | 5.2 ± 0.9 | 5.0 ± 0.4 | 0.57 |
| 120 min reperfusion | 5.1 ± 0.6 | 5.2 ± 0.4 | 0.85 |
| Brain Region | HTK (n = 9) | Del Nido (n = 9) | p-Value |
|---|---|---|---|
| frontal cortex | 2.0 ± 1.1 | 2.5 ± 1.4 | 0.44 |
| cerebellum | 1.4 ± 1.0 | 1.1 ± 1.4 | 0.58 |
| brain stem | 1.9 ± 1.2 | 1.2 ± 1.3 | 0.25 |
| diencephalon | 1.9 ± 1.2 | 1.5 ± 1.5 | 0.55 |
| colliculus superior | 2.2 ± 1.2 | 1.8 ± 1.8 | 0.63 |
| TNF-α-Positive Area [‰] | HTK (n = 9) | Del Nido (n = 9) | p-Value |
|---|---|---|---|
| frontal cortex | 1.2 ± 0.4 | 0.7 ± 0.2 | 0.30 |
| cerebellum | 0.8 ± 0.2 | 1.1 ± 0.4 | 0.43 |
| brain stem | 1.8 ± 1.2 | 1.9 ± 0.6 | 0.95 |
| diencephalon | 0.8 ± 0.4 | 1.4 ± 0.6 | 0.45 |
| colliculus superior | 0.3 ± 0.1 | 0.5 ± 0.3 | 0.57 |
| Cytokine | HTK (n = 9) | Del Nido (n = 9) | p-Value |
|---|---|---|---|
| IL-1β [pg/mg protein] | |||
| frontal cortex | 3.4 ± 0.7 | 2.1 ± 0.7 | 0.23 |
| cerebellum | 14.4 ± 5.4 | 17.9 ± 8.7 | 0.74 |
| brain stem | 33.8 ± 14.5 | 33.0 ± 12.9 | 0.97 |
| diencephalon | 9.6 ± 2.5 | 13.7 ± 6.3 | 0.55 |
| colliculus superior | 7.6 ± 1.2 | 18.9 ± 11.8 | 0.37 |
| IL-1RN [ng/mg protein] | |||
| frontal cortex | 1.9 ± 0.3 | 2.4 ± 0.5 | 0.33 |
| cerebellum | 4.5 ± 1.1 | 9.3 ± 2.9 | 0.16 |
| brain stem | 13.4 ± 3.4 | 15.9 ± 3.3 | 0.61 |
| diencephalon | 9.1 ± 2.0 | 7.3 ± 2.6 | 0.60 |
| colliculus superior | 7.4 ± 2.6 | 14.6 ± 6.5 | 0.33 |
| IL-10 [pg/mg protein] | |||
| frontal cortex | 9.5 ± 4.0 | 10.0 ± 3.3 | 0.91 |
| cerebellum | 45.7 ± 11.1 | 41.8 ± 9.8 | 0.80 |
| brain stem | 81.0 ± 14.1 | 100.7 ± 17.6 | 0.40 |
| diencephalon | 33.3 ± 6.4 | 37.8 ± 11.0 | 0.73 |
| colliculus superior | 34.7 ± 7.1 | 64.6 ± 22.8 | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klaeske, K.; Dieterlen, M.-T.; Kang, J.; Detzer, Z.; Ginther, A.; Ossmann, S.; Borger, M.A.; Kiefer, P.; Hoyer, A.A. Cerebral Inflammation in an Animal Ischemia–Reperfusion Model Comparing Histidine-Tryptophan-α-Ketoglutarate and Del Nido Cardioplegia. Life 2025, 15, 451. https://doi.org/10.3390/life15030451
Klaeske K, Dieterlen M-T, Kang J, Detzer Z, Ginther A, Ossmann S, Borger MA, Kiefer P, Hoyer AA. Cerebral Inflammation in an Animal Ischemia–Reperfusion Model Comparing Histidine-Tryptophan-α-Ketoglutarate and Del Nido Cardioplegia. Life. 2025; 15(3):451. https://doi.org/10.3390/life15030451
Chicago/Turabian StyleKlaeske, Kristin, Maja-Theresa Dieterlen, Jagdip Kang, Zoe Detzer, André Ginther, Susann Ossmann, Michael A. Borger, Philipp Kiefer, and Alexandro A. Hoyer. 2025. "Cerebral Inflammation in an Animal Ischemia–Reperfusion Model Comparing Histidine-Tryptophan-α-Ketoglutarate and Del Nido Cardioplegia" Life 15, no. 3: 451. https://doi.org/10.3390/life15030451
APA StyleKlaeske, K., Dieterlen, M.-T., Kang, J., Detzer, Z., Ginther, A., Ossmann, S., Borger, M. A., Kiefer, P., & Hoyer, A. A. (2025). Cerebral Inflammation in an Animal Ischemia–Reperfusion Model Comparing Histidine-Tryptophan-α-Ketoglutarate and Del Nido Cardioplegia. Life, 15(3), 451. https://doi.org/10.3390/life15030451







