Accumulation Potential of Lead and Cadmium Metals in Maize (Zea mays L.) and Effects on Physiological-Morphological Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Heavy Metal Treatments and Experimental Design
2.3. Observations and Measurements
2.3.1. Plant Growth Parameters
2.3.2. Leaf Relative Water Content (LRWC) and Electrolyte Conductivity (EC)
2.3.3. Catalase (CAT), Peroxidase (POD), and Superoxide Dismutase (SOD) Enzyme Activities
2.3.4. Hydrogen Peroxide (H2O2) and Malondialdehyde (MDA)
2.3.5. Accumulation Parameters
2.3.6. Bioconcentration Factor (BCF) and Translocation Factor (TF)
2.3.7. Statistical Analysis
3. Results
3.1. Effects of Heavy Metal Applications on the Growth Characteristic
3.2. Effects of Heavy Metal Applications on Physiological Traits
Leaf Relative Water Content (LRWC), Electrolyte Conductivity
3.3. Effects of Heavy Metal Applications on Biochemical Traits
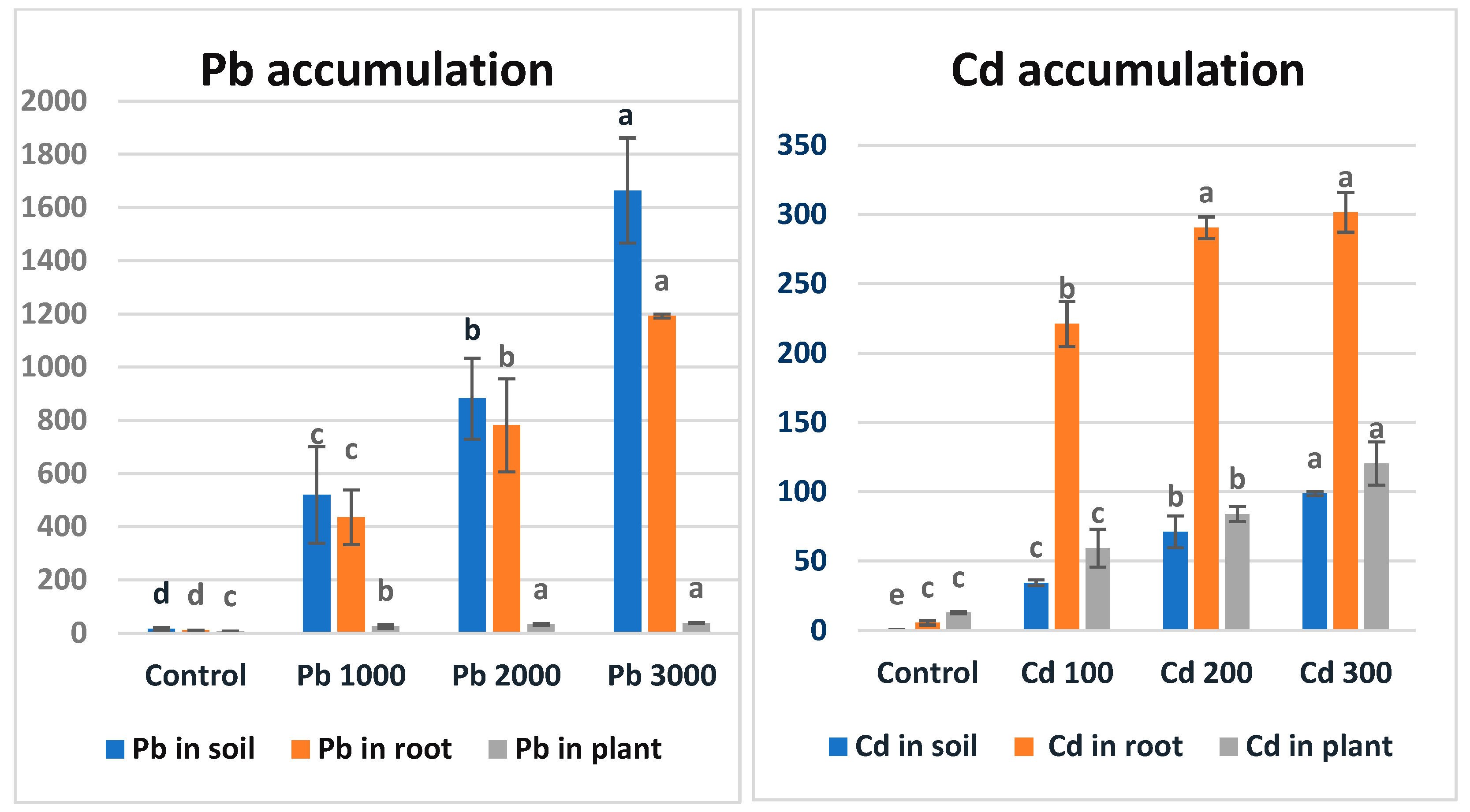
3.4. Pb and Cd Accumulation
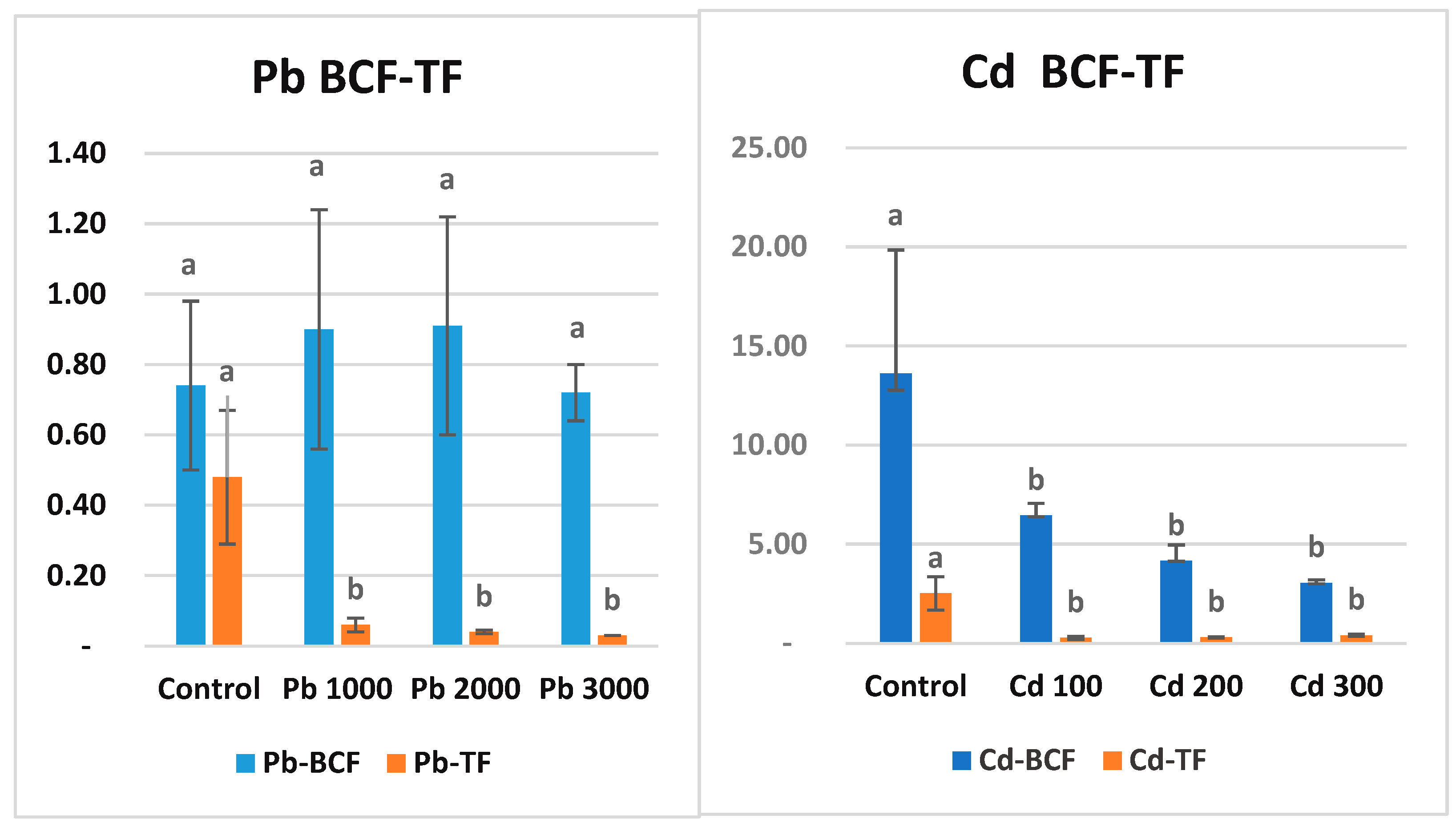
3.5. Bioconcentration Factor and Translocation Factor
4. Discussion
Recommendations
- Phytoremediation Potential:
- -
- Given maize’s ability to accumulate Cd in its roots, further research should explore its potential use in phytoremediation strategies to clean Cd-contaminated soils.
- -
- Developing and utilizing metal-tolerant maize genotypes could enhance the efficiency of phytoremediation practices.
- Agricultural Management:
- -
- Implementing soil amendments and chelating agents could mitigate heavy metal toxicity and improve plant growth and productivity in contaminated soils.
- -
- Regular monitoring and assessment of soil and plant metal concentrations could ensure food safety and minimize health risks.
- Genetic Modification:
- -
- Genetic modification techniques could be explored to develop maize varieties with enhanced heavy metal tolerance and reduced translocation of metals to edible parts.
- -
- The underlying genetic and molecular mechanisms responsible for heavy metal uptake and detoxification in maize should be investigated.
- Antioxidant Defense Mechanisms:
- -
- Research should examine enhancing antioxidant defense mechanisms in maize through breeding or biotechnological approaches to improve plant resilience to oxidative stress caused by heavy metal exposure.
- Environmental and Health Implications:
- -
- Long-term studies should be conducted to assess the environmental and health implications of using maize for phytoremediation and the potential risks associated with heavy metal accumulation in food crops.
- -
- Sustainable agricultural practices that reduce heavy metal contamination and protect soil health should be promoted.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, D.M.; Fu, R.B.; Tong, Y.H.; Shen, D.L.; Guo, X.P. The potential environmental risk implications of heavy metals based on their geochemical and mineralogical characteristics in the size-segregated zinc smelting slags. J. Clean. Prod. 2021, 315, 128199. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, X.; Bai, J.; Shih, K.; Zeng, E.Y.; Cheng, H. Assessing heavy metal pollution in the surface soils of a region that had undergone three decades of intense industrialization and urbanization. Environ. Sci. Pollut. Res. 2013, 20, 6150–6159. [Google Scholar] [CrossRef] [PubMed]
- Nnaji, N.D.; Onyeaka, H.; Miri, T.; Ugwa, C. Bioaccumulation for heavy metal removal: A review. Appl. Sci. 2023, 5, 125. [Google Scholar] [CrossRef]
- Atasoy, N. Atık Sulardan Ağır Metal Giderimi. J. Inst. Sci. Technol. 2024, 14, 1684–1704. [Google Scholar] [CrossRef]
- Clemens, S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 2006, 88, 1707–1719. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Nepovimova, E.; Kuca, K.; Valko, M. Heavy metals: Toxicity and human health effects. Arch. Toxicol. 2024, 99, 153–209. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, G.M.A.; Jasan, R.; Pla, R.; Pignata, M.L. Heavy metals and trace elements in atmospheric fall-out: Their relationship with topsoil and wheat element composition. J. Hazard. Mater. 2012, 213–214, 447–456. [Google Scholar] [CrossRef]
- Yaldız, G.; Şekeroğlu, N. Tıbbi ve aromatik bitkilerin bazı ağır metallere tepkisi. Türk Bilimsel Derlemeler Derg. 2013, 6, 80–84. [Google Scholar]
- Yılmaz, H.Ş. Farklı ağır metal uygulamalarının tane sorgum çeşitlerinde ağır metal birikimi, morfolojik ve yem kalite özelliklerine etkisi. Doctoral Thesis, Bingöl Üniversitesi Tarla Bitkileri Anabilim Dalı, Bingöl, Turkey, 2019. [Google Scholar]
- Sanaei, S.; Sadeghinia, M.; Meftahizade, H.; Ardakani, A.F.; Ghorbanpour, M. Cadmium and lead differentially affect growth, physiology, and metal accumulation in guar (Cyamopsis tetragonoloba L.) genotypes. Environ. Sci. Pollut. Res. 2022, 29, 4180–4192. [Google Scholar] [CrossRef] [PubMed]
- Gallo-Franco, J.J.; Sosa, C.C.; Ghneim-Herrera, T.; Quimbaya, M. Epigenetic control of plant response to heavy metal stress: A new view on aluminum tolerance. Front. Plant Sci. 2020, 11, 602625. [Google Scholar] [CrossRef]
- Noor, I.; Sohail, H.; Sun, J.; Nawaz, M.A.; Li, G.; Hasanuzzaman, M.; Liu, J. Heavy metal and metalloid toxicity in horticultural plants: Tolerance mechanism and remediation strategies. Chemosphere 2022, 303, 135196. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Praveen, S.; Divte, P.R.; Mitra, R.; Kumar, M.; Gupta, C.K.; Singh, B. Metal tolerance in plants: Molecular and physicochemical interface determines the “not so heavy effect” of heavy metals. Chemosphere 2022, 287, 131957. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Tan, Q.; Zhang, F.; Ma, C.; Xiao, J.; Chen, G. Phytoremediation potential evaluation of multiple Salix clones for heavy metals (Cd, Zn and Pb) in flooded soils. Sci. Total Environ. 2022, 813, 152482. [Google Scholar] [CrossRef]
- Çılgın, K. Buğday (Triticum Aestivum L.) ve Arpa (Hordeum Vulgare L.) Bitkilerinde Kadmiyum Stresine Bağlı Genotoksik Etkilerin Belirlenmesi. Master’s Thesis, Marmara Universitesi, Marmara, Turkey, 2022. [Google Scholar]
- Wang, L.; Zheng, B.; Yuan, Y.; Xu, Q.; Chen, P. Transcriptome profiling of Fagopyrum tataricum leaves in response to lead stress. BMC Plant Biol. 2020, 20, 1–14. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, Y.; Gu, H.; Lam, S.S.; Chen, X.; Sonne, C.; Peng, W. A review of phytoremediation of environmental lead (pb) contamination. Chemosphere 2024, 362, 142691. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, T.; Guo, Z.; Xie, H.; Hu, Z.; Ran, H.; Jiang, Z. Spatial heterogeneity and source apportionment of soil metal (loid) s in an abandoned lead/zinc smelter. J. Environ. Sci. 2023, 127, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Yang, F.; Pan, X.; Shao, J. Accumulation of silicon in shoots is required for reducing lead uptake in rice. Crop J. 2023, 11, 1261–1271. [Google Scholar] [CrossRef]
- Collin, S.; Baskar, A.; Geevarghese, D.M.; Ali, M.N.V.S.; Bahubali, P.; Choudhary, R.; Lvov, V.; Tovar, G.I.; Senatov, F.; Koppala, S.; et al. Bioaccumulation of lead (Pb) and its effects in plants: A review. J. Hazard. Mater. Lett. 2022, 3, 100064. [Google Scholar] [CrossRef]
- Pourrut, B.; Ramos, I.; Esteban, E.; Lucena, J.J.; Garate, A. Cadmium uptake and sub-cellular distribution in plants os Lactuca sp. Cd- Mn Interaction. Plant Sci. 2002, 162, 761–767. [Google Scholar] [CrossRef]
- Gupta, D.K.; Nicoloso, F.T.; Schetinger, M.R.C.; Rossato, L.V.; Pereira, L.B.; Castro, G.Y.; Srivastava, S.; Tripathi, R.D. Defense mechanism in hydroponically grown Zea mays seedlings under moderate lead stress. J. Hazard. Mater. 2009, 172, 479–484. [Google Scholar] [CrossRef]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef]
- Qu, F.; Zheng, W. Cadmium Exposure: Mechanisms and Pathways of Toxicity and Implications for Human Health. Toxics 2024, 12, 388. [Google Scholar] [CrossRef] [PubMed]
- Hussain, C.M.; Kecili, R. Modern Environmental Analysis Techniques for Pollutants; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Tüver, G. Farklı Sulama Seviyesi Koşullarında Kadmiyumun Fındık Turpunda (Raphanus sativus L.) Bitki Gelişimi Üzerine Etkisi. Yüksek Lisans Tezi, Atatürk Üniversitesi Bahçe Bitkileri Anabilim Dalı, Erzurum, Turkey, 2021. [Google Scholar]
- Ali, H.; Khan, E. Trophic transfer, bioaccumulation, and biomagnification of non-essential hazardous heavy metals and metalloids in food chains/webs—Concepts and implications for wildlife and human health. Human. Ecol. Risk Assess. An. Int. J. 2018, 25, 1353–1376. [Google Scholar] [CrossRef]
- Simmler, M.; Ciadamidaro, L.; Schulin, R.; Madejon, P.; Reiser, R.; Clucas, L.; Weber, P.; Robinson, B. Lignite reduces the solubility and plant uptake of cadmium in pasturelands. Environ. Sci. Technol. 2013, 47, 495. [Google Scholar] [CrossRef]
- Li, T.; Chang, Q.; Yuan, X.; Li, J.; Ayoko, G.A.; Frost, R.; Che, H.; Zhang, X.; Song, Y.; Song, W. Cadmium transfer from contaminated soils to the human body through rice consumption in southern Jiangsu Province, China Environ. Sci. J. Tegr. Environ. Res. Process. Impacts 2017, 19, 843–850. [Google Scholar] [CrossRef]
- Ahmad, P.; Abd Allah, E.F.; Hashem, A.; Sarwat, M.; Gucel, S. Exogenous application of selenium mitigates cadmium toxicity in Brassica juncea L. (Czern & Cross) by up-regulating antioxidative system and secondary metabolites. J. Plant Growth Regul. 2016, 35, 936–950. [Google Scholar]
- Jali, P.; Pradhan, C.; Das, A.B. Effects of cadmium toxicity in plants: A Review. Artic. Sch. Acad. J. Biosci. 2016, 4, 1074–1081. [Google Scholar]
- Vezza, M.E.; Llanes, A.; Travaglia, C.; Agostini, E.; Talano, M.A. Arsenic stress effects on root water absorption in soybean plants: Physiological and morphological aspects. Plant Physiol. Biochem. 2018, 123, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Aslam, A.; Sheraz, M.; Ali, B.; Ulhassan, Z.; Najeeb, U.; Gill, R.A. Lead toxicity in cereals: Mechanistic insight into toxicity, mode of action, and management. Front. Plant Sci. 2021, 11, 587785. [Google Scholar] [CrossRef] [PubMed]
- Vasilachi, I.C.; Stoleru, V.; Gavrilescu, M. Analysis of heavy metal impacts on cereal crop growth and development in contaminated soils. Agriculture 2023, 13, 1983. [Google Scholar] [CrossRef]
- ATSDR. Konu: Agency for Toxic Substances and Disease Registry (ATSDR)’s Substance Priority List. 2022. Available online: https://www.atsdr.cdc.gov/programs/substance-priority-list.html?CDC_AAref_Val=https://www.atsdr.cdc.gov/spl/index.html (accessed on 7 October 2024).
- Amanjyoti; Singh, J.; Sowdhanya, D.; Rasane, P.; Singh, J.; Ercisli, S.; Ullah, R. Maize. In Cereals and Nutraceuticals; Springer Nature: Singapore, 2024; pp. 47–80. [Google Scholar]
- FAO. Crop Statistics. Food and Agriculture Organization of the United Nations. 2022. Available online: http://www.fao.org/faostat/en/ (accessed on 7 October 2024).
- OECD; FAO. OECD-FAO Agricultural Outlook 2019–2018; FAO and OECD: Washington, DC, USA, 2019. [Google Scholar]
- Figlioli, F.; Sorrentino, M.C.; Memoli, V.; Arena, C.; Maisto, G.; Giordano, S.; Spagnuolo, V. Overall plant responses to Cd and Pb metal stress in maize: Growth pattern, ultrastructure, and photosynthetic activity. Environ. Sci. Pollut. Res. 2019, 26, 1781–1790. [Google Scholar] [CrossRef]
- Jiang, L.; Yi, X.; Xu, B.; Wang, W.; Lai, K. Soil treatment and crop rotation for in situ remediation of heavy metal-contaminated agricultural soil in gold mining areas. Hum. Ecol. Risk Assess. Int. J. 2019, 25, 374–392. [Google Scholar] [CrossRef]
- Wu, Y.; An, T.; Gao, Y.; Kuang, Q.; Liu, S.; Liang, L.; Bingcheng, X.; Suiqi, Z.; Deng, X.; Chen, Y. Genotypic variation in the tolerance to moderate cadmium toxicity among 20 maize genotypes with contrasting root systems. J. Sci. Food Agric. 2023, 103, 2618–2630. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Xiao, J.H.; Li, J.T.; Du, Q.Q.; Zhu, L.W.; Wang, H.; Zhao, H.Y. Accumulation and Transport Characteristics of Cd, Pb, Zn, and As in Different Maize Varieties. Huan Jing Ke Xue=Huanjing Kexue 2022, 43, 4232–4252. [Google Scholar] [CrossRef]
- Sevgi, K.; Leblebici, S. Bitkilerde ağır metal stresine verilen fizyolojik ve moleküler yanıtlar. J. Anatol. Environ. Anim. Sci. 2022, 7, 528–536. [Google Scholar] [CrossRef]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy metal tolerance in plants: Role of transcriptomics, proteomics, metabolomics, and ionomics. Front. Plant Sci. 2016, 6, 1143. [Google Scholar] [CrossRef]
- Li, S.; Han, X.; Lu, Z.; Qiu, W.; Yu, M.; Li, H.; He, Z.; Zhuo, R. MAPK Cascades and Transcriptional Factors: Regulation of Heavy Metal Tolerance in Plants. Int. J. Mol. Sci. 2022, 23, 4463. [Google Scholar] [CrossRef] [PubMed]
- Tan, M. Baklagil ve Buğdaygil Yem Bitkileri Kitabı; Atatürk Üniv. Ziraat Fak. Ders Yayınları: Erzurum, Turkey, 2018. [Google Scholar]
- Ekinci, M.; Yildirim, E.; Ağar, G.; Yüksel, E.A.; Aydin, M.; Örs, S.; Kul, R. Determination of cadmium and/or drought stress effects on some plant phytohormone contents and hormone gene expressions in bean (Phaseolus vulgaris L.). Turk. J. Agric. For. 2023, 47, 402–411. [Google Scholar] [CrossRef]
- Yildirim, E.; Ekinci, M.; Turan, M.; Güleray, A.G.A.R.; Selda, Ö.R.S.; Dursun, A.; Balci, T. Impact of cadmium and lead heavy metal stress on plant growth and physiology of rocket (Eruca sativa L.). Kahramanmaraş Sütçü İmam Üniversitesi Tarım Ve Doğa Derg 2019, 22, 843–850. [Google Scholar] [CrossRef]
- Yildirim, E.; Karlidag, H.; Turan, M. Mitigation of salt stress in strawberry by foliar K, Ca and Mg nutrient supply. Plant Soil Environ. 2009, 55, 213–221. [Google Scholar] [CrossRef]
- Anonymous. 2025. Available online: https://media.licdn.com/dms/document/media/v2/D4D1FAQHCx5U2EMVjyg/feedshare-document-pdf-analyzed/feedshare-document-pdf-analyzed/0/1682363438103?e=1740614400&v=beta&t=WVGRLlAKH8PiAvCFtmuGnBq31QRDB2NlVdJYRZha9kc (accessed on 13 February 2025).
- Turan, M.; Ekinci, M.; Kul, R.; Boynueyri, F.G.; Yildirim, E. Mitigation of salinity stress in cucumber seedlings by exogenous hydrogen sulfide. J. Plant Res. 2022, 135, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Ors, S.; Ekinci, M.; Yildirim, E.; Sahin, U.; Turan, M.; Dursun, A. Interactive effects of salinity and drought stress on photosynthetic characteristics and physiology of tomato (Lycopersicon esculentum L.) seedlings. S. Afr. J. Bot. 2021, 137, 335–339. [Google Scholar] [CrossRef]
- Liu, S.; Dong, Y.; Xu, L.; Kong, J. Effects of foliar applications of nitric oxide and salicylic acid on salt-induced changes in photosynthesis and antioxidative metabolism of cotton seedlings. Plant Growth Regul. 2014, 73, 67–78. [Google Scholar] [CrossRef]
- Sharmin, S.; Lipka, U.; Polle, A.; Eckert, C. The influence of transpiration on foliar accumulation of salt and nutrients under salinity in poplar (Populus × canescens). PLoS ONE 2021, 16, e0253228. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, E.; Ekinci, M.; Turan, M.; Dursun, A.; Kul, R.; Parlakova, F. Roles of glycine betaine in mitigating deleterious effect of salt stress on lettuce (Lactuca sativa L.). Arch. Agron. Soil. Sci. 2015, 61, 1673–1689. [Google Scholar] [CrossRef]
- González, L.; González-Vilar, M. Determination of Relative Water Content. In Handbook of Plant Ecophysiology Techniques; Springer: Dordrecht, The Netherlands, 2001; pp. 207–212. [Google Scholar]
- Ma, H.; Zhao, C.; Zhang, L.; Liu, Z.; Zhang, F.; Wang, H.; Peng, M. Bioavailability, Sources, and Transfer Behavior of Heavy Metals in Soil–Crop Systems from a High Geological Background Area Impacted by Artisanal Zn Smelting in Guizhou Province, Southwest China. Processes 2023, 11, 2538. [Google Scholar] [CrossRef]
- Sahin, U.; Ekinci, M.; Ors, S.; Turan, M.; Yildiz, S.; Yildirim, E. Effects of individual and combined effects of salinity and drought on physiological, nutritional and bio-chemical properties of cabbage (Brassica oleracea var. capitata). Sci. Hortic. 2018, 240, 196–204. [Google Scholar] [CrossRef]
- Rizvi, A.; Khan, M.S. Heavy metal induced oxidative damage and root morphology alterations of maize (Zea mays L.) plants and stress mitigation by metal tolerant nitrogen fixing Azotobacter chroococcum. Ecotoxicol. Environ. Saf. 2018, 157, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, A.; Qin, M.; Elahie, M.; Naeem, M.; Bashir, T.; Yasmin, H.; Younas, M.; Aree, A.; Irfan, M.; Billah, M.; et al. Bacillus pumilus induced tolerance of Maize (Zea mays L.) against Cadmium (Cd) stress. Sci. Rep. 2021, 11, 17196. [Google Scholar] [CrossRef]
- Gidlow, D.A. Lead toxicity. Occup. Med. 2004, 54, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Yerli, C.; Çakmakçı, T.; Şahin, Ü.; Tüfenkçi, Ş. Ağır Metallerin Toprak, Bitki, su ve Insan Sağlığına Etkileri; Bingöl Üniversitesi, Türk Doğa ve Fen Dergisi: Bingöl, Turkey, 2020; Volume 9, pp. 103–114. [Google Scholar]
- Alyemeni, M.N.; Ahanger, M.A.; Wijaya, L.; Alam, P.; Ahmad, P. Contrasting Tolerance Among SoybeanGenotypes Subjected to Different Levels of Cadmium Stress. Pak. J. Bot. 2017, 49, 903–911. [Google Scholar]
- Manousaki, E.; Kalogerakis, N. Phytoextraction of Pb and Cd by the Mediterranean saltbush (Atriplex halimus L.): Metal Uptake in Relation to Salinity. Environ. Sci. Pollut. R. 2009, 16, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Nabi, G.; Ashraf, M. Cadmium-Induced Oxidative Damage in Mustard [Brassica juncea L.) Czern.& Coss.] Plants can be Alleviated by Salicylic Acid. S. Afr. J. Bot. 2011, 77, 36–44. [Google Scholar]
- AbdElgawad, H.; Zinta, G.; Hegab, M.M.; Pandey, R.; Asard, H.; Abuelsoud, W. Yüksek tuzluluk, mısır fidelerinin organlarında farklı oksidatif stres ve antioksidan tepkilere neden olur. Front. Plant Sci. 2016, 7, 276. [Google Scholar]
- Engwa, G.A.; Ferdinand, P.U.; Nwalo, F.N.; Unachukwu, M.N. Mechanism and health effects of heavy metal toxicity in humans. In Poisoning in the Modern World-New Tricks for an Old Dog? IntechOpen: London, UK, 2019; Volume 10, pp. 70–90. [Google Scholar]
- Wang, S.; Shi, X.; Salam, M.M.A.; Chen, G. Integrated study on subcellular localization and chemical speciation of Pb reveals root strategies for Pb sequestration and detoxification in Salix integra. Plant Soil 2021, 467, 197–211. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Flores, L.C. Plant mediated detoxification of mercury and lead. Arab. J. Chem. 2017, 10, S2335–S2342. [Google Scholar] [CrossRef]
- Labrecque, M.; Teodorescu, T.I.; Daigle, S. Effect of wastewater sludge on growth and heavy metal bioaccumulation of two Salix species. Plant Soil 1995, 171, 303–316. [Google Scholar] [CrossRef]
- Kafadar, F.; Saygıdeğer, S. Gaziantep İlinde Organize Sanayi Bölgesi Atık Suları İle Sulanan Bazı Tarım Bitkilerinde Kurşun Miktarlarının Belirlenmesi. Ekoloji 2010, 75, 41–48. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Jia, W.; Lv, S.; Feng, J.; Li, J.; Li, Y.; Li, S. Morphophysiological characteristic analysis demonstrated the potential of sweet sorghum (Sorghum bicolor (L.) Moench) in the phytoremediation of cadmium-contaminated soils. Environ. Sci. Pollut. Res. 2016, 23, 18823–18831. [Google Scholar] [CrossRef]
- Loi, N.N.; Sanzharova, N.I.; Shchagina, N.I.; Mironova, M.P. The effect of cadmium toxicity on the development of Lettuce plants on contaminated sod-podzolic soil. Russ. Agric. Sci. 2018, 44, 49–52. [Google Scholar] [CrossRef]
- Pereira, B.F.F.; Rozane, D.E.; Araújo, S.R.; Barth, G.; Queiroz, R.J.B.; Nogueira, T.A.R.; Malavolta, E. Cadmium availability and accumulation by lettuce and rice. Rev. Bras. De Ciência Do Solo 2011, 35, 645–654. [Google Scholar] [CrossRef]
- John, R.; Ahmad, P.; Gadgil, K.; Sharma, S. Heavy metal toxicity: Effect on plant growth, biochemical parameters and metal accumulation by Brassica juncea L. Int. J. Plant Prod. 2009, 3, 65–76. [Google Scholar]
- Takarina, N.D.; Pin, T.G. Bioconcentration factor (BCF) and translocation factor (TF) of heavy metals in mangrove trees of Blanakan fish farm. Makara J. Sci. 2017, 21, 77–81. [Google Scholar] [CrossRef]
- Sürmen, B.; Kılıç, D.D.; Kutbay, H.G.; Tuna, E.E. Doğal olarak yayılış gösteren Lepidium draba L. türünün fitoremediasyon yönteminde kullanılabilirliğinin araştırılması. Avrupa Bilim. Teknol. Derg. 2019, 17, 491–499. [Google Scholar] [CrossRef]
- McLaughlin, M.J.; Smolders, E.; Degryse, F.; Rietra, R. Uptake of metals from soil into vegetables. In Dealing with Contaminated Sites: From Theory Towards Practical Application; Springer: Berlin/Heidelberg, Germany, 2011; pp. 325–367. [Google Scholar]
- Hidayati, N. Mekanisme fisiologis tumbuhan hiperakumulator logam berat. J. Teknol. Lingkung. 2013, 14, 75–82. (In Indonesian) [Google Scholar] [CrossRef]
- Hidayati, N.; Rini, D.S.R. Assessment on Lead and Cadmium Bioaccumulators for Phytoremediation Contaminated Rice Fields in Bekasi Districts, West Java. In Proceedings of the 9th International Symposium for Sustainable Humanosphere (ISSH): “Integrated Smart Technology and Society for Sustainable Humanosphere, Bogor, Indonesia, 28–29 October 2019. [Google Scholar]
- Wierzbicka, M.H.; Przedpełska, E.; Ruzik, R.; Ouerdane, L.; Połeć-Pawlak, K.; Jarosz, M.; Szakiel, A. Comparison of the toxicity and distribution of cadmium and lead in plant cells. Protoplasma 2007, 231, 99–111. [Google Scholar] [CrossRef]
- Bouida, L.; Rafatullah, M.; Kerrouche, A.; Qutob, M.; Alosaimi, A.M.; Alorfi, H.S.; Hussein, M.A. A review on cadmium and lead contamination: Sources, fate, mechanism, health effects and remediation methods. Water 2022, 14, 3432. [Google Scholar] [CrossRef]
- Mojiri, A. The potential of corn (Zea mays) for phytoremediation of soil contaminated with cadmium and lead. J. Biol. Environ. Sci. 2011, 5, 17–22. [Google Scholar]
- Poniedziałek, M.; Sękara, A.; Jędrszczyk, E.; Ciura, J. Phytoremediation efficiency of crop plants in removing cadmium, lead and zinc from soil. Folia Hortic. 2010, 22, 25–31. [Google Scholar] [CrossRef]
- Azizian, A.; Amin, S.; Maftoun, M.; Emam, Y.; Noshadi, M. Response of Corn to Cadmium and Drought Stress and Its Potential Use for Phytoremediation. J. Agric. Sci. Technol. 2013, 15, 303–310. [Google Scholar]
- Cheng, S.F.; Huang, C.Y.; Lin, Y.C.; Lin, S.C.; Chen, K.L. Phytoremediation of lead using corn in contaminated agricultural land—An in situ study and benefit assessment. Ecotoxicol. Environ. Saf. 2015, 111, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, S.; Zhao, C.; Jiang, X.; Gao, D. Responses of non-structural carbohydrates and biomass in plant to heavy metal treatment. Sci. Total Environ. 2024, 909, 168559. [Google Scholar] [CrossRef] [PubMed]
- Manivasagaperumal, R.; Vijayarengan, P.; Balamurugan, S.; Thiyagarajan, G. Effect of copper on growth, dry matter yield and nutrient content of Vigna radiata (L.) Wilczek. J. Phytol. 2011, 3, 53–62. [Google Scholar]
- Marschner, H. (Ed.) Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Goncharuk, E.A.; Zagoskina, N.V. Heavy metals, their phytotoxicity, and the role of phenolic antioxidants in plant stress responses with focus on cadmium. Molecules 2023, 28, 3921. [Google Scholar] [CrossRef] [PubMed]
- Nagajyoti, P.; Lee, K.; Sreekanth, T. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Ehlert, C.; Maurel, C.; Tardieu, F.; Simonneau, T. Aquaporin-Mediated Reduction in Maize Root Hydraulic Conductivity Impacts Cell Turgor and Leaf Elongation even without Changing Transpiration. Plant Physiol. 2009, 150, 1093–1104. [Google Scholar] [CrossRef]
- Rizvi, A.; Khan, M.S. Heavy metal-mediated toxicity to maize: Oxidative damage, antioxidant defence response and metal distribution in plant organs. Int. J. Environ. Sci. Technol. 2019, 16, 4873–4886. [Google Scholar] [CrossRef]
- Hatamian, M.; Nejad, A.R.; Kafi, M.; Souri, M.K.; Shahbazi, K. Nitrate improves hackberry seedling growth under cadmium application. Heliyon 2020, 6, e03247. [Google Scholar] [CrossRef] [PubMed]
- Poschenrieder, C.; Barceló, J. Water relations in heavy metal stressed plants. In Heavy Metal Stress in Plants; Prasad, M.N.V., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 249–270. [Google Scholar]
- Fan, S.X.; Zhang, N.; Sun, M.H.; Hou, X.D. Screening and stress responsive characteristics of potential hyperaccumulator of Pb, Zn, and Cd compound heavy metals. Huan Jing Ke Xue Huanjing Kexue 2024, 45, 4870–4882. [Google Scholar] [PubMed]
- Phaenark, C.; Seechanhoi, P.; Sawangproh, W. Metal toxicity in Bryum coronatum Schwaegrichen: Impact on chlorophyll content, lamina cell structure, and metal accumulation. Int. J. Phytoremediat. 2024, 26, 1336–1347. [Google Scholar] [CrossRef]
- Kastori, R.; Petrovic, M.; Petrovic, N. Effect of excess lead, cadmium, copper, and zinc on water relations in sunflower. J. Plant Nutr. 2008, 15, 2427–2439. [Google Scholar] [CrossRef]
- Alsokari, S.S.; Aldesuquy, H.S. Synergistic effect of polyamines and waste water on leaf turgidity, heavy metals accumulation in relation to grain yield. J. Appl. Sci. Res. 2011, 70, 376–384. [Google Scholar]
- Pourrut, B. Implication du Stress Oxydatif Dans la Toxicité du Plomb sur une Plante Modèle, Vicia faba. Ph.D. Thesis, l’université de Toulouse, Toulouse, France, 2008; p. 284. [Google Scholar]
- Yildirim, E.; Ekinci, M.; Turan, M.; Ağar, G.; Dursun, A.; Kul, R.; Argin, S. Humic + Fulvic acid mitigated Cd adverse effects on plant growth, physiology and biochemical properties of garden cress. Sci. Rep. 2021, 11, 8040. [Google Scholar] [CrossRef]
- Hussain, I.; Iqbal, M.; Qurat-Ul-Ain, S.; Rasheed, R.; Mahmood, S.; Perveen, A.; Wahid, A. Cadmium dose and exposure-time dependent alterations in growth and physiology of maize (Zea mays). Int. J. Agric. Biol. 2012, 14, 959–964. [Google Scholar]
- Yang, W.W.; Lıu, M.; Cao, M.Z.; Zhang, C.L. Accumulation and transfer of lead (Pb) and cadmium (Cd) on different species of maize. J. Ecol. Rural. Environ. 2014, 30, 774–779. [Google Scholar]
- Sofy, M.R.; Seleiman, M.F.; Alhammad, B.A.; Alharbi, B.M.; Mohamed, H.I. Mini-mizing adverse effects of pb on maize plants by combined treatment with jasmonic, salicylic acids and proline. Agronomy 2020, 10, 699. [Google Scholar] [CrossRef]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Bao, M.; Wang, L.; Khan, I.; Shahzad, B. Cadmium toxicity in Maize (Zea mays L.): Consequences on antioxidative systems, reactive oxygen species and cadmium accumulation. Environ. Sci. Pollut. Res. 2015, 22, 17022–17030. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Hu, C.; Jia, X.; Ren, Y.; Su, D.; He, J. Physiological and biochemical bases of spermidine-induced alleviation of cadmium and lead combined stress in rice. Plant Physiol. Biochem. 2022, 189, 104–114. [Google Scholar] [CrossRef]
- Bamagoos, A.A.; Alharby, H.F.; Abbas, G. Differential uptake and translocation of cadmium and lead by quinoa: A multivariate comparison of physiological and oxidative stress responses. Toxics 2022, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Dong, Y.; Zhu, N.; Jin, H. Foliar application of biosynthetic nano-selenium alleviates the toxicity of Cd, Pb, and Hg in Brassica chinensis by inhibiting heavy metal adsorption and improving antioxidant system in plant. Ecotoxicol. Environ. Saf. 2022, 240, 113681. [Google Scholar] [CrossRef]
- Giannakoula, A.; Therios, I.; Chatzissavvidis, C. Effect of lead and copper on photosynthetic apparatus in citrus (Citrus aurantium L.) plants. The role of antioxidants in oxidative damage as a response to heavy metal stress. Plants 2021, 10, 155. [Google Scholar] [CrossRef]
- Salazar, M.J.; Pignata, M.L. Lead accumulation in plants grown in polluted soils. Screening of native species for phytoremediation. J. Geochem. Explor. Tion 2014, 137, 29–36. [Google Scholar] [CrossRef]
- Lu, Y.; Yao, H.; Shan, D.; Jiang, Y.; Zhang, S.; Yang, J. Heavy metal residues in soil and accumulation in maize at long-term wastewater irrigation area in Tongliao, China. J. Chem. 2015, 2015, 628280. [Google Scholar] [CrossRef]
- Adewole, M.B.; Oyebanji, B.O.; Igbekele, K. Phytoremediation potential of two maize varieties cultivated on metal-particulate-contaminated soil. Ghana J. Agric. Sci. 2019, 54, 38–46. [Google Scholar] [CrossRef]
- Aladesanmi, O.T.; Oroboade, J.G.; Osisiogu, C.P.; Osewole, A.O. Bioaccumulation factor of selected HMs in Zea mays. J. Health Pollut. 2019, 9, 191207. [Google Scholar] [CrossRef]
- Retamal-Salgado, J.; Hirzel, J.; Walter, I.; Matus, I. Bioabsorption and bioaccumulation of cadmium in the straw and grain of maize (Zea mays L.) in growing soils contaminated with cadmium in different environment. Int. J. Environ. Res. Public Health 2017, 14, 1399. [Google Scholar] [CrossRef]
- Rascio, N.; Navari-Izzo, F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef]
- Chen, Z.; Ai, Y.; Fang, C.; Wang, K.; Li, W.; Liu, S.; Li, C.; Xiao, J.; Huang, Z. Distribution and phytoavailability of heavy metal chemical fractions in artificial soil on rock cut slopes alongside railways. J. Hazard. Mater. 2014, 273, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Wahsha, M.; Bini, C.; Argese, E.; Minello, F.; Fontana, S.; Wahsheh, H. Heavy metals accumulation in willows growing on Spolic Technosols from the abandoned Im-perina Valley mine in Italy. J. Geochem. Explor. 2012, 123, 19–24. [Google Scholar] [CrossRef]
- Madanan, M.T.; Shah, I.K.; Varghese, G.K.; Kaushal, R.K. Application of Aztec Marigold (Tagetes erecta L.) for phytoremediation of heavy metal polluted lateritic soil. Environ. Chem. Ecotoxicol. 2021, 3, 17–22. [Google Scholar] [CrossRef]
- Boularbah, A.; Schwartz, C.; Bitton, G.; Aboudrar, W.; Ouhammou, A.; Morel, J.L. Heavy metal contamination from mining sites in South Morocco: 2. Assessment of metal accumulation and toxicity in plants. Chemosphere 2006, 63, 811–817. [Google Scholar] [CrossRef]
- Marchiol, L.; Sacco, P.; Assolari, S.; Zerbi, G. Reclamation of polluted soil: Phytore-mediation potential of crop-related Brassica species. Water Air Soil. Pollut. 2004, 158, 345–356. [Google Scholar] [CrossRef]
- Pasricha, S.; Mathur, V.; Garg, A.; Lenka, S.; Verma, K.; Agarwal, S. Molecular mechanisms underlying heavy metal uptake, translocation and tolerance in hyperaccumulators-an analysis: Heavy metal tolerance in hyperaccumulators. Environ. Chall. 2021, 4, 100197. [Google Scholar] [CrossRef]
- Rezapour, S.; Atashpaz, B.; Moghaddam, S.S.; Kalavrouziotis, I.K.; Damalas, C.A. Cadmium accumulation, translocation factor, and health risk potential in a wastewater-irrigated soil-wheat (Triticum aestivum L.) system. Chemosphere 2019, 231, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Cobbett, C.S. Phytochelatins and their roles in heavy metal detoxification. Plant Physiol. 2000, 123, 825–832. [Google Scholar] [CrossRef]
- Adriano, D.C. Trace Elements in Terrestrial Environments: Biogeochemistry, Bioavailability, and Risks of Metals; Springer: New York, NY, USA, 2001; p. 860. [Google Scholar]
- Ak, A.; Yücel, E. Ecotoxicological effects of heavy metal stress on antioxidant en-zyme levels of triticum aestivum cv. Alpu. Biol. Divers. Conserv. 2011, 4, 19–24. [Google Scholar]
- Bhaduri, A.M.; Fulekar, M.H. Antioxidant Enzyme Responses of Plants to Heavy Metal Stress. Rev. Environ. Sci. Bio-Technol. 2012, 11, 55–69. [Google Scholar] [CrossRef]

| Treatments mg kg−1 | Plant Height (cm) | Plant Fresh Weight (g) | Plant Dry Weight (g) | Root Fresh Weight (g) | Root Dry Weight (g) | Stem Diameter (mm) | Number of Leaves (per Plant) | Leaf Area (cm2/Plant) |
|---|---|---|---|---|---|---|---|---|
| Control | 54.96 ± 1.017 a | 17.27 ± 0.754 a | 4.91 ± 0.046 a | 13.49 ± 1.679 a | 1.28 ± 0.089 a | 8.12 ± 0.448 a | 6.66 ± 0.0667 a | 285.19 ± 2.777 a |
| Pb 1000 | 53.60 ± 2.354 a | 16.05 ± 0.173 a | 4.82 ± 0.069 a | 13.35 ± 1.441 a | 1.25 ± 0.124 a | 6.34 ± 0.246 b | 6.26 ± 0.0667 ab | 304.28 ± 49.023 a |
| Pb 2000 | 49.33 ± 1.212 b | 15.65 ± 1.224 a | 3.83 ± 0.321 b | 11.81 ± 1.247 a | 1.22 ± 0.029 a | 6.27 ± 0.202 b | 6.26 ± 0.0667 ab | 258.57 ± 14.929 a |
| Pb 3000 | 43.80 ± 0.953 c | 14.60 ± 0.678 a | 3.68 ± 0.131 b | 11.18 ± 0.580 a | 1.15 ± 0.816 a | 6.11 ± 0.096 bc | 6.06 ± 0.0667 b | 249.27 ± 19.837 a |
| Cd 100 | 39.26 ± 0.3712 d | 9.83 ± 0.985 b | 3.13 ± 0.550 b | 7.21 ± 0.765 b | 0.77 ± 0.531 b | 5.40 ± 0.178 cd | 5.93 ± 0.0667 b | 230.82 ± 20.141 a |
| Cd 200 | 34.86 ± 1.185 e | 8.19 ± 1.364 bc | 2.12 ± 0.221 c | 7.11 ± 0.556 b | 0.73 ± 0.047 b | 4.79 ± 0.119 d | 5.86 ± 0.133 b | 148.63 ± 20.005 b |
| Cd 300 | 31.93 ± 1.313 e | 6.28 ± 0.416 c | 1.61 ± 0.121 c | 5.92 ± 0.003 b | 0.60 ± 0.011 b | 3.92 ± 0.258 e | 5.33 ± 0.333 c | 116.15 ± 11.787 b |
| p | ** | ** | ** | ** | ** | ** | * | ** |
| Treatments (mg kg−1) | Chlorophyll Reading Value (SPAD) | EC (%) | LRWC (%) | CAT-(EU/gFW) | POD-(EU/gFW) | SOD-(EU/gFW) | H2O2 (mmol kg−1) | MDA-(nmol g−1) |
|---|---|---|---|---|---|---|---|---|
| Control | 46.28 ± 0.227 a | 10.76 ± 0.509 c | 81.48 ± 0.239 a | 0.02 ± 0.001 b | 8.21 ± 0.001 d | 76.72 ± 0.341 c | 98.69 ± 1.008 e | 1.75 ± 0.033 f |
| Pb 1000 | 46.14 ± 0.682 a | 12.58 ± 0.737 c | 70.58 ± 0.398 c | 0.02 ± 0.002 b | 7.34 ± 0.016 e | 82.56 ± 0.828 b | 146.66 ± 1.118 b | 1.90 ± 0.086 f |
| Pb 2000 | 45.01 ± 0.724 ab | 14.80 ± 0.655 bc | 65.05 ± 1.544 de | 0.008 ± 0.000 c | 10.98 ± 0.179 b | 82.76 ± 2.146 b | 147.29 ± 0.454 ab | 2.20 ± 0.036 e |
| Pb 3000 | 43.76 ± 0.283 b | 15.22 ± 0.052 bc | 62.32 ± 0.516 e | 0.017 ± 0.001 b | 7.36 ± 0.084 e | 82.13 ± 1.815 b | 152.57 ± 0.238 a | 2.46 ± 0.040 d |
| Cd 100 | 38.97 ± 0.377 c | 17.51 ± 3.291 ab | 75.40 ± 1.076 b | 0.013 ± 0.001 bc | 14.06 ± 0.414 a | 87.00 ± 0.716 a | 115.82 ± 2.788 d | 5.68 ± 0.136 c |
| Cd 200 | 37.04 ± 0.203 d | 18.64 ± 0.192 ab | 72.68 ± 0.664 bc | 0.015 ± 0.002 b | 8.86 ± 0.126 c | 80.53 ± 0.249 bc | 118.25 ± 0.158 d | 6.11 ± 0.073 b |
| Cd 300 | 36.09 ± 0.456 d | 20.93 ± 1.761 a | 66.41 ± 1.772 d | 0.032 ± 0.002 a | 7.13 ± 0.093 e | 89.70 ± 1.735 a | 125.68 ± 3.682 c | 6.46 ± 0.019 a |
| p | ** | * | ** | ** | ** | ** | ** | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elik, Ü.; Gül, Z. Accumulation Potential of Lead and Cadmium Metals in Maize (Zea mays L.) and Effects on Physiological-Morphological Characteristics. Life 2025, 15, 310. https://doi.org/10.3390/life15020310
Elik Ü, Gül Z. Accumulation Potential of Lead and Cadmium Metals in Maize (Zea mays L.) and Effects on Physiological-Morphological Characteristics. Life. 2025; 15(2):310. https://doi.org/10.3390/life15020310
Chicago/Turabian StyleElik, Ümit, and Zeynep Gül. 2025. "Accumulation Potential of Lead and Cadmium Metals in Maize (Zea mays L.) and Effects on Physiological-Morphological Characteristics" Life 15, no. 2: 310. https://doi.org/10.3390/life15020310
APA StyleElik, Ü., & Gül, Z. (2025). Accumulation Potential of Lead and Cadmium Metals in Maize (Zea mays L.) and Effects on Physiological-Morphological Characteristics. Life, 15(2), 310. https://doi.org/10.3390/life15020310





