Expression of Dlx-5 and HLX Proteins in Odontogenic Cysts
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Characteristics and Collection of Cyst Samples
2.2. Routine Histological Tissue Processing and Staining
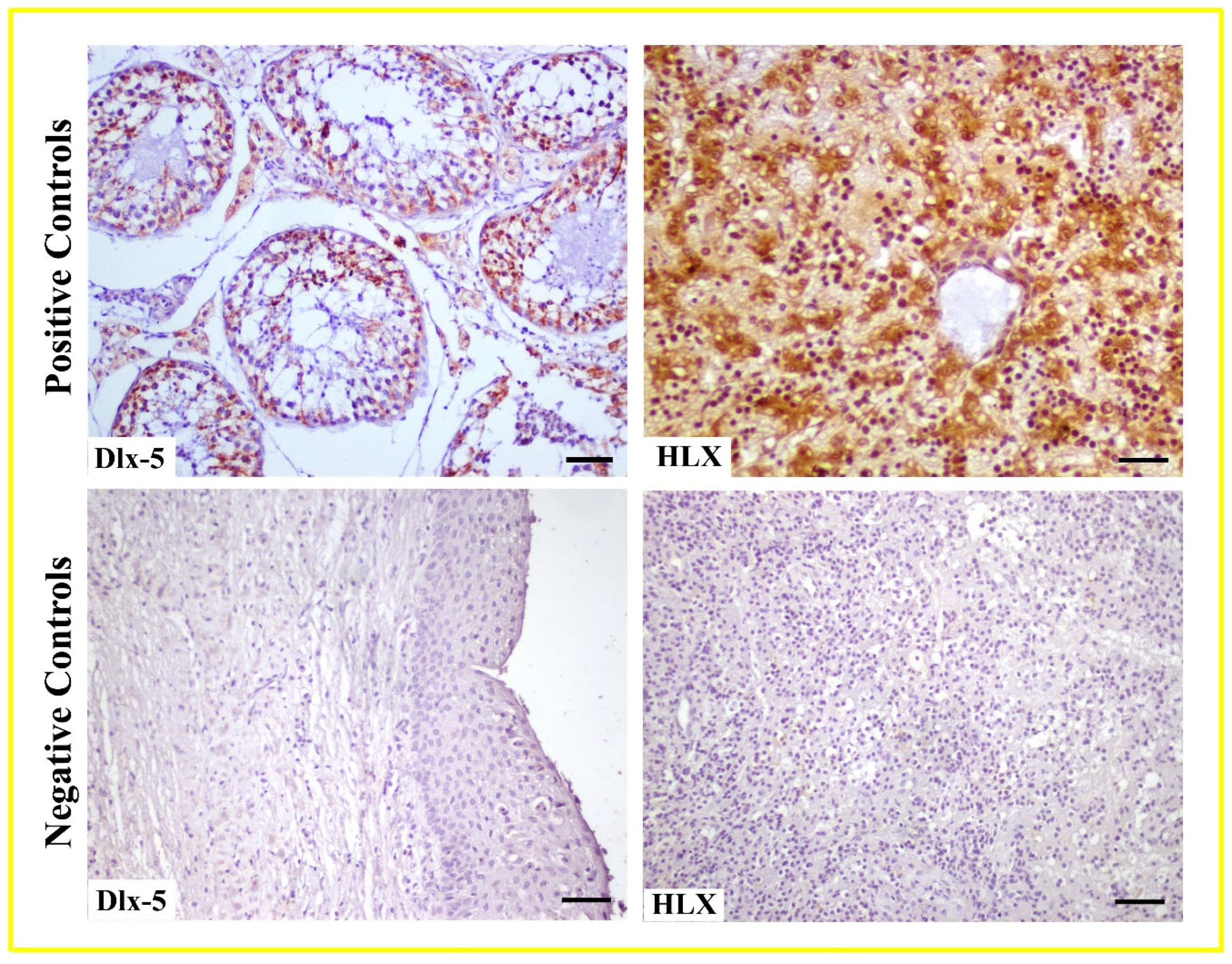
2.3. Immunohistochemical Staining
2.4. SemiQuantitative Evaluation
3. Results
3.1. Patient Details
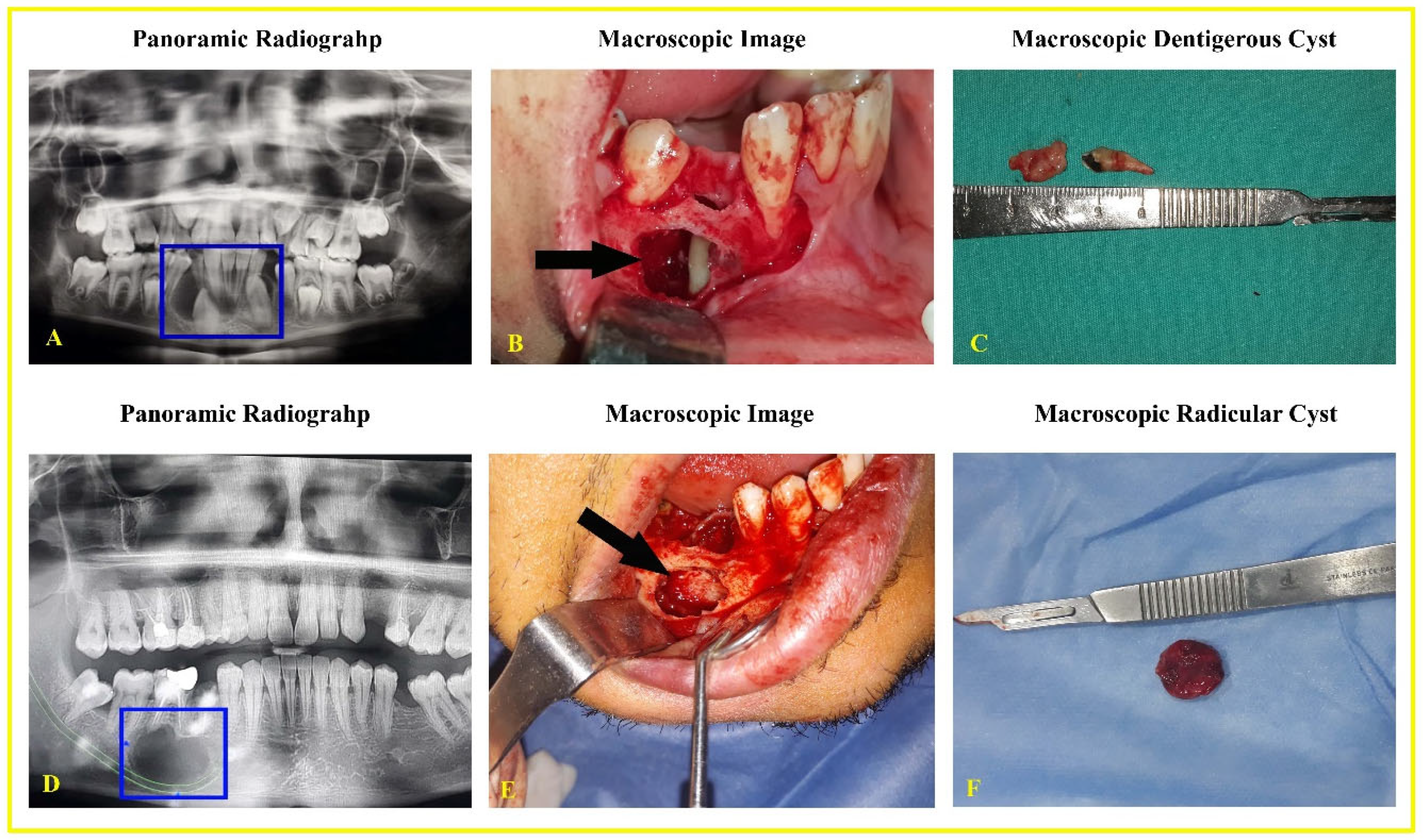
3.2. Macroscopic and Radiographic Findings
3.3. Histopathological and Immunohistochemical Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- High, A.S.; Robinson, P.A.; Klein, C.E. Discrimination of para keratinised odontogenic keratocyst from other odontogenic and non-odontogenic cyst types by expression of a 38kd cell surface glycoprotein. J. Oral. Pathol. Med. 1993, 22, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Stoelinga, P.J. Long-term follow-up on keratocysts treated according to a defined protocol. Int. J. Oral. Maxillofac. Surg. 2001, 30, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Myoung, H.; Hong, S.P.; Hong, S.D.; Lee, J.I.; Lim, C.Y.; Choung, P.H.; Lee, J.-H.; Choi, J.-Y.; Seo, B.-M.; Kim, M.-J. Odontogenic keratocyst: Review of 256 cases for recurrence and clinicopathologic parameters. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2001, 91, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Regezi, J.A.; Sciubba, J.J.; Jordan, R.C.K. Oral Pathology, 5th ed.; Saunders-Elsiver: Philadelphia, PA, USA, 2008; pp. 237–238. [Google Scholar]
- Yazdani, J.; Kahnamouii, S.S. Developmental odontogenic cysts of jaws: A clinical study of 245 cases. J. Dent. Res. Dent. Clin. Dent. Prospect. 2009, 3, 64–66. [Google Scholar]
- Scholl, R.J.; Kellet, H.M.; Neumann, D.P.; Lurie, A.G. Cysts and cystic lesions of the mandible: Clinical and radiologic-histopathologic review. Radiographics 1999, 19, 1107–1124. [Google Scholar] [CrossRef]
- Darling, M.R.; Wehrli, B.M.; Ciavarro, C.; Daley, T.D. Peri-coronal radiolucency in the posterior mandible. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2008, 105, 139–143. [Google Scholar] [CrossRef]
- Johnson, N.R.; Gannon, O.M.; Savage, N.W.; Batstone, M.D. Frequency of odontogenic cysts and tumors: A systematic review. J. Invest. Clin. Dent. 2014, 5, 9–14. [Google Scholar] [CrossRef]
- Brown, S.J.; Conn, B.I. Odontogenic cysts: Classification, histological features and a practical approach to common diagnostic problems. Diagn. Histopathol. 2022, 28, 253–266. [Google Scholar] [CrossRef]
- Jones, A.V.; Craig, G.T.; Franklin, C.D. Range and demographics of odontogenic cysts diagnosed in a UK population over a 30-year period. J. Oral. Pathol. Med. 2006, 35, 500–507. [Google Scholar] [CrossRef]
- Aşır, F.; Özalp, Z.; Yülek, Ö.U.; Erdemci, F.; Korak, T.; Taş, F. CITED1 expression in odontogenic cysts. BMC Oral. Health 2024, 12, 782. [Google Scholar] [CrossRef]
- Gorlin, R.J. Potentialities of oral epithelium manifest by mandibular dentigerous cysts. Oral. Surg. Oral. Med. Oral. Pathol. 1957, 10, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.A. Diagnosing the most common odontogenic cystic and osseous lesions of the jaws for the practicing pathologist. Mod. Pathol. 2017, 30, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Nayyer, N.V.; Macluskey, M.; Keys, W. Odontogenic Cysts—An Overview. Dent. Update 2015, 42, 548–555. [Google Scholar] [CrossRef][Green Version]
- Probst, F.A.; Probst, M.; Pautke, C.; Kaltsi, E.; Otto, S.; Schiel, S.; Troeltzsch, M.; Ehrenfeld, M.; Cornelius, C.P.; Müller-Lisse, U.G. Magnetic resonance imaging: A useful tool to distinguish between keratocystic odontogenic tumors and odontogenic cysts. Br. J. Oral. Maxillofac. Surg. 2015, 53, 217–222. [Google Scholar] [CrossRef]
- Yalçin, B.K.; Berberoğlu, H.K.; Aralaşmak, A.; Köseoğlu, B.G.; Çakarer, S.; Tekkesin, M.S.; Kula, O. Evaluation of CT and MRI imaging results of radicular cysts, odontogenic keratocysts, and dentigerous cysts and their contribution to the differential diagnosis. Curr. Med. Imaging 2022, 18, 1447–1452. [Google Scholar]
- Cassetta, M.; Di-Carlo, S.; Pranno, N.; Stagnitti, A.; Pompa, V.; Pompa, G. The use of high-resolution magnetic resonance on a 3.0-T system in the diagnosis and surgical planning of intraosseous lesions of the jaws: Preliminary results of a retrospective study. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 2021–2028. [Google Scholar]
- Srinivasan, K.; Seith Bhalla, A.; Sharma, R.; Kumar, A.; Roychoudhury, A.; Bhutia, O. Diffusion-weighted imaging in the evaluation of odontogenic cysts and tumors. Br. J. Radiol. 2012, 85, 864–870. [Google Scholar] [CrossRef]
- Fujita, M.; Matsuzaki, H.; Yanagi, Y.; Hara, M.; Katase, N.; Hisatomi, M.; Unetsubo, T.; Konouchi, H.; Nagatsuka, H.; Asaumi, J.I. Diagnostic value of MRI for odontogenic tumors. Dentomaxillofacial Radiol. 2013, 42, 20120265. [Google Scholar] [CrossRef]
- Hii, E.P.W.; Ramanathan, A.; Pandarathodiyil, A.K.; Wong, G.R.; Sekhar, E.V.S.; Binti Talib, R.; Zaini, Z.M.; Zain, R.B. Homeobox genes in odontogenic lesions: A Scoping Review. Head. Neck Pathol. 2023, 17, 218–232. [Google Scholar] [CrossRef]
- Chung, I.H.; Han, J.; Iwata, J.; Chai, Y. Msx1 and Dlx5 function synergistically to regulate frontal bone development. Genesis 2010, 48, 645–655. [Google Scholar] [CrossRef]
- Zhao, Z.; Stock, D.; Buchanan, A.; Weiss, K. Expression of Dlx genes during the development of the murine dentition. Dev. Genes. Evol. 2000, 210, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Hentsch, B.; Lyons, I.; Li, R.; Hartley, L.; Lints, T.J.; Adams, J.M.; Harvey, R.P. Hlx Homeo box gene Is essential for an inductive tissue interaction that drives expansion of embryonic liver and gut. Genes. Dev. 1996, 10, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Prahst, C.; Kasaai, B.; Moraes, F.; Jahnsen, E.D.; Larrivee, B.; Villegas, D.; Pardanaud, L.; Zhang, F.; Zaun, H.C. The H2.0-like homeobox transcription factor modulates yolk sac vascular remodeling in mouse embryos. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1468–1476. [Google Scholar] [CrossRef]
- Lints, T.J.; Hartley, L.; Parsons, L.M.; Harvey, R.P. Mesoderm-Specific expression of the divergent homeobox gene Hlx during murine embryogenesis. Dev. Dyn. 1996, 205, 457–470. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Guo, Q.Y.; Zhu, M.; Chen, B.G.; Wang, L.Y.; Zhang, D.Q.; Zhang, L.; Shao, Y.P.; Luo, W.D. HLX affects cell cycle and proliferation in AML cells via the JAK/STAT signaling pathway. Oncol. Lett. 2020, 20, 1888–1896. [Google Scholar] [CrossRef]
- Al Sheddi, M.A. Odontogenic cysts. A clinicopathological study. Saudi Med. J. 2012, 33, 304–308. [Google Scholar]
- Lezcano, M.R.; Enz, N.; Affur, M.C.; Gili, M.A. Histological characteristics of bovine dentin using Masson’s Trichrome staining. Rev. Cient. Odontol. 2023, 11, e176. [Google Scholar]
- Devkato, B.; Sasaki, M.; Takahashi, K.; Matsuzaki, S.; Matsui, M.; Haneda, S.; Takahashi, M.; Osawa, T.; Miyake, Y. Postnatal developmental changes in immunohistochemical localization of a-smooth muscle actin (SMA) and vimentin in bovine testis. J. Reprod. Dev. 2006, 52, 43e9. [Google Scholar]
- Gomes, I.P.; Bastos, V.C.; Guimarães, L.M.; Gomes, C.C. The molecular basis of odontogenic cysts and tumours. J. Oral. Pathol. Med. 2023, 52, 351–356. [Google Scholar] [CrossRef]
- Tsuneki, M.; Yamazaki, M.; Cheng, J.; Maruyama, S.; Kobayashi, T.; Saku, T. Combined immunohistochemistry for the differential diagnosis of cystic jaw lesions: Its practical use in surgical pathology. Histopathology 2010, 57, 806–813. [Google Scholar] [CrossRef]
- Saku, T.; Shibata, Y.; Koyama, Z.; Cheng, J.; Okabe, H.; Yeh, Y. Lectinhistochemistry of cystic jaw lesions: An aid for differential diagnosis between cystic ameloblastoma and odontogenic cysts. J. Oral. Pathol. Med. 1991, 20, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.M.; Odell, E.W.; Speight, P.; Takata, T. Odontogenic tumors, WHO 2005: Where do we go from here? Head Neck Pathol. 2014, 8, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Marijanovic, I.; Kronenberg, M.S.; Erceg, I.; Stover, M.L.; Velonis, D.; Mina, M.; Heinrich, J.G.; Harris, S.E.; Upholt, W.B.; et al. Expression and function of Dlx genes in the osteoblast lineage. Dev. Biol. 2008, 316, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Testa, J.R. DLX Genes: Roles in Development and Cancer. Cancers 2021, 13, 3005. [Google Scholar] [CrossRef]
- Duverger, O.; Morasso, M.I. Role of homeobox genes in the patterning, specification, and differentiation of ectodermal appendages in mammals. J. Cell Physiol. 2008, 216, 337–346. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, J.; Chen, Y.; Zhang, W. Dlx5 promotes cancer progression through regulation of CCND1 in oral squamous cell carcinoma (OSCC). Biochem. Cell Biol. 2021, 99, 424–434. [Google Scholar] [CrossRef]
- Sun, S.; Yang, F.; Zhu, Y.; Zhang, S. KDM4A promotes the growth of non-small cell lung cancer by mediating the expression of Myc via DLX5 through the Wnt/beta-catenin signaling pathway. Life Sci. 2020, 262, 118508. [Google Scholar] [CrossRef]
- Tan, Y.; Cheung, M.; Pei, J.; Menges, C.W.; Godwin, A.K.; Testa, J.R. Upregulation of DLX5 promotes ovarian cancer cell proliferation by enhancing IRS-2-AKT signaling. Cancer Res. 2010, 70, 9197–9206. [Google Scholar] [CrossRef]
- Morini, M.; Astigiano, S.; Gitton, Y.; Emionite, L.; Mirisola, V.; Levi, G.; Barbieri, O. Mutually exclusive expression of DLX2 and DLX5/6 is associated with the metastatic potential of the human breast cancer cell line MDA-MB-231. BMC Cancer 2010, 10, 649. [Google Scholar] [CrossRef]
- Seifert, A.; Werheid, D.F.; Knapp, S.M.; Tobiasch, E. Role of Hox genes in stem cell differentiation. World J. Stem Cells 2015, 7, 583–595. [Google Scholar] [CrossRef]
- Zheng, W.P.; Zhao, Q.; Zhao, X.; Li, B.; Hubank, M.; Schatz, D.G.; Flavell, R.A. Up-regulation of Hlx in immature Th cells induces IFN-gamma expression. J. Immunol. 2004, 172, 114–122. [Google Scholar] [CrossRef]
- Xu, Y.; Gao, J.; Su, Z.; Dai, X.; Li, Y.; Liu, Y.; Chen, J.; Tong, J.; Zhang, Y.; Wu, C.; et al. Downregulation of Hlx closely related to the decreased expressions of T-bet and Runx3 in patients with gastric cancer may be associated with a pathological event leading to the imbalance of Th1/Th2. Clin. Dev. Immunol. 2012, 949821. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Watanabe, M.; Inoue, N.; Inaoka, C.; Akamizu, T.; Tatsumi, K.I.; Hidaka, Y.; Iwatani, Y. Functional polymorphisms in TBX21 and HLX are associated with development and prognosis of Graves’ disease. Autoimmunity 2012, 45, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, L.; Wang, K.; Huo, J.; Zhang, S.; Zhang, X. The potential dual role of h2.0-like homeobox in the tumorigenesis and development of colorectal cancer and its prognostic value. Can. J. Gastroenterol. Hepatol. 2023, 9, 5521544. [Google Scholar]
- Pandolfi, A.; Steidl, U. HLX in AML: Novel prognostic and therapeutic target. Oncotarget 2012, 11, 1059–1060. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ramanathan, A.; Srijaya, T.C.; Sukumaran, P.; Zain, R.B.; Abu Kasim, N.H. Homeobox genes and tooth development: Understanding the biological pathways and applications in regenerative dental science. Arch. Oral. Biol. 2018, 85, 23–39. [Google Scholar] [CrossRef]
- Lin, L.M.; Huang, G.T.; Rosenberg, P.A. Proliferation of epithelial cell rests, formation of apical cysts, and regression of apical cysts after periapical wound healing. J. Endod. 2007, 33, 908–916. [Google Scholar] [CrossRef]
- Kiss, C. Cell to cell interactions. Endod. Top. 2004, 8, 88–103. [Google Scholar] [CrossRef]
- Nair, P.; Sundqvist, G.; Sjogren, U. Experimental evidence supports the abscess theory of the development of radicular cysts. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2008, 106, 294–303. [Google Scholar] [CrossRef]
- Pai, P.; Sukumar, S. HOX genes and the NF-κB pathway: A convergence of developmental biology, inflammation and cancer biology. BBA Cancer 2020, 1874, 188450. [Google Scholar] [CrossRef]


| Parameters | ||
|---|---|---|
| Age, mean (min–max) | Radicular cyst | 34.20 (26–69) |
| Dentigerous cyst | 42.00 (32–63) | |
| Gender, n (%) | Male | 40 (50%) |
| Female | 40 (50%) | |
| Positions on the jaw, n (%) | Maxillary | 20 (25%) |
| Mandible | 60 (75%) | |
| Surgery | Marsupialization | 20 (12.50%) |
| Enucleation | 80 (87.50%) |
| Cells | Dlx-5 | HLX | ||
|---|---|---|---|---|
| Radicular | Dentigerous | Radicular | Dentigerous | |
| Epithelial | ++/+++ | ++/+++ | +/++ | +/++ |
| Connective tissue | + | ++/+++ | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ateş, S.; Topaloğlu, U.; Akbalik, M.E.; Keleş Karagözoğlu, Ş. Expression of Dlx-5 and HLX Proteins in Odontogenic Cysts. Life 2025, 15, 301. https://doi.org/10.3390/life15020301
Ateş S, Topaloğlu U, Akbalik ME, Keleş Karagözoğlu Ş. Expression of Dlx-5 and HLX Proteins in Odontogenic Cysts. Life. 2025; 15(2):301. https://doi.org/10.3390/life15020301
Chicago/Turabian StyleAteş, Sinan, Uğur Topaloğlu, Mehmet Erdem Akbalik, and Şeyma Keleş Karagözoğlu. 2025. "Expression of Dlx-5 and HLX Proteins in Odontogenic Cysts" Life 15, no. 2: 301. https://doi.org/10.3390/life15020301
APA StyleAteş, S., Topaloğlu, U., Akbalik, M. E., & Keleş Karagözoğlu, Ş. (2025). Expression of Dlx-5 and HLX Proteins in Odontogenic Cysts. Life, 15(2), 301. https://doi.org/10.3390/life15020301






