Brain Functional Connectivity Significantly Improves After Surgical Eradication of Porto-Systemic Shunting in Pediatric Patients
Abstract
1. Background
2. Material and Methods
2.1. Patient Population and Selection Criteria
2.2. Study
2.3. Statistical Analysis
2.4. Imaging Analysis
2.5. Data and Functionnal Image Pre-Processing
2.6. Seed Selection and Functional Connectivity Analysis
2.7. Behavioral Correlations
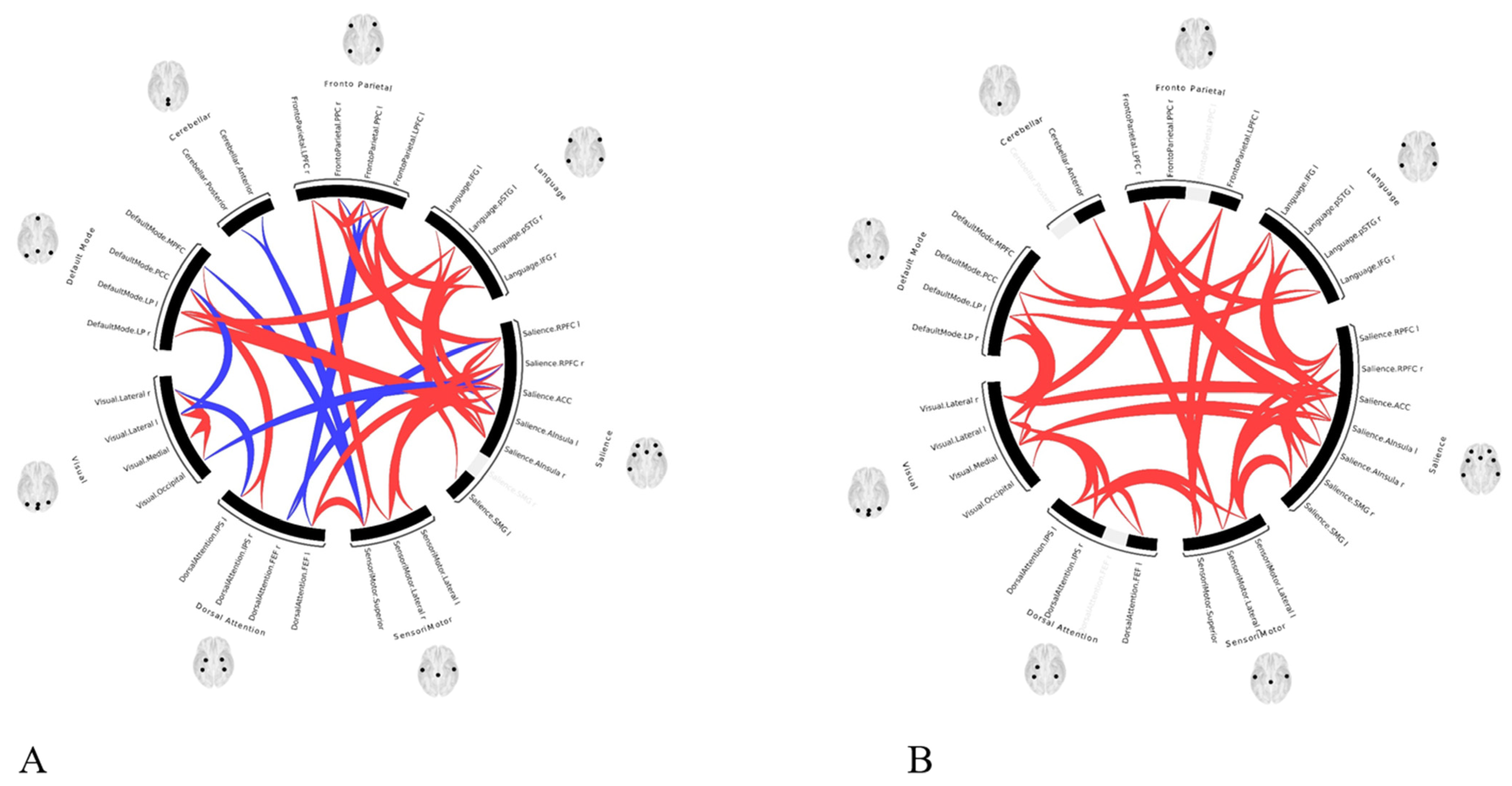
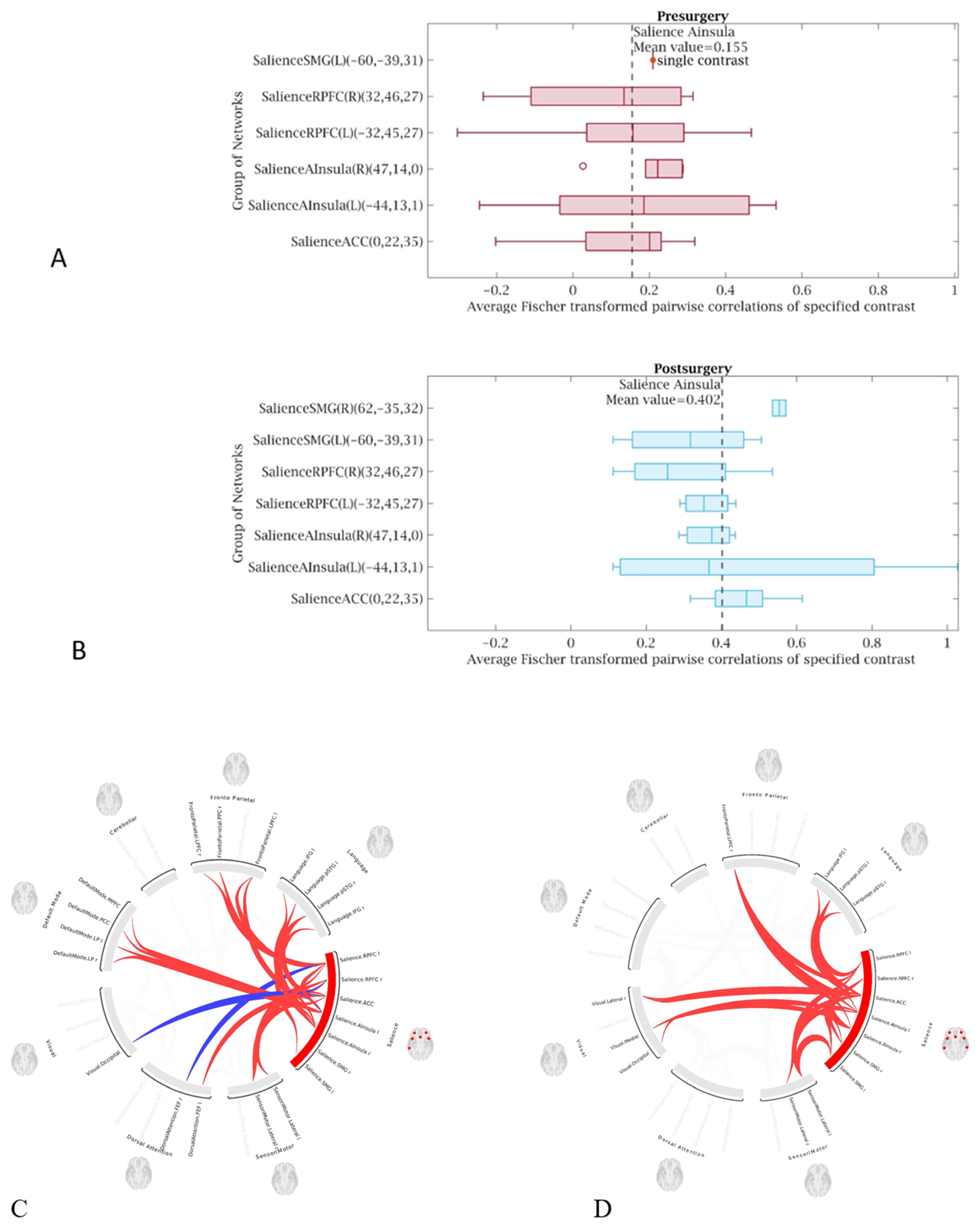
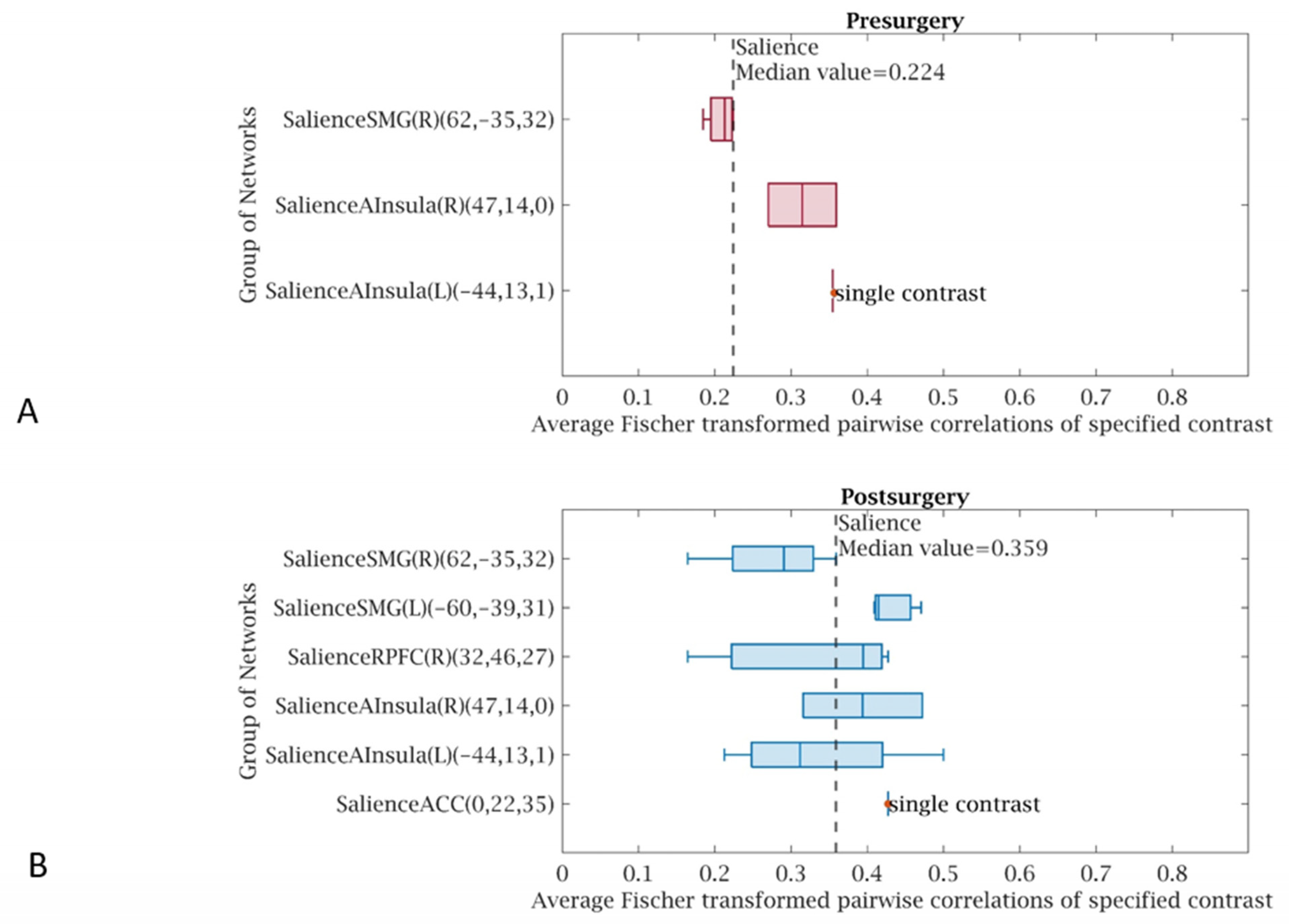
3. Results
4. Discussion
- 1.
- Pre-intervention rest-fMRI showed low functional connectivity in all patients, though three of four were asymptomatic and were as young as 7 to 9 years old. It suggests that the brain functional alteration starts very early in the story of PSS or portal hypertension, much earlier than it has ever been thought.
- 2.
- Pre-intervention low functional connectivity was evidenced even in children with a normal ammonia blood level and normal liver function: this suggests that other factors or metabolites—not identified clearly until now—may play a role in the physio-pathogenesis of HE.
- 3.
- The functional connectivity did improve significantly after the correction of PSS, in all four patients—each case showing improvement compared to their own preoperative examination. This may impose revisiting our management strategies and opting for early cures rather than palliative or conservative management.
- 4.
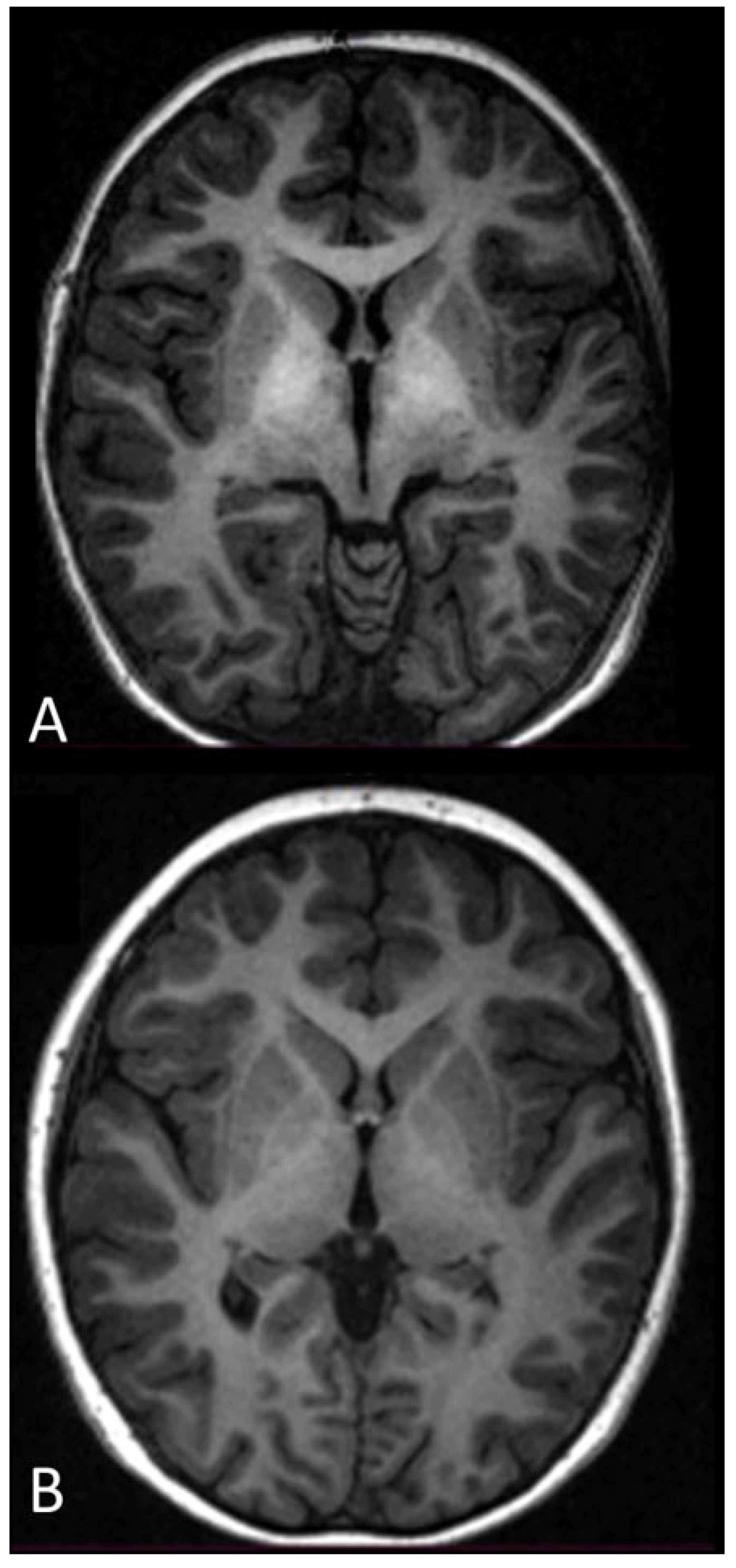
- Though the phenomenon was slower, MRI evidenced the disappearance of the hyperintensity of the globus pallidus on T1-weighted sequences (in two cases that had follow-up >1 year). This needs further studying.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AM | Abernethy malformation |
| AUROC | Area under the receiver operating characteristic curve |
| fMRI | Functional magnetic resonance imaging |
| FSE | Fast spin echo |
| FOV | Field of view |
| FEW | Family-wise error |
| HE | Hepatic encephalopathy |
| LDLT | Living-donor liver transplantation |
| MELD | Model for end-stage liver disease |
| MHE | Minimal hepatic encephalopathy |
| MR | Magnetic resonance |
| MRB | Meso-Rex bypass |
| MRI | Magnetic resonance imaging |
| OVP | Obstruction of the portal vein |
| PSS | Porto-systemic shunting |
| rest-fMRI | Resting-state functional magnetic resonance imaging |
| ROI | Region of interest |
| ROC | Receiver operating characteristic |
| RSN | Resting-state network |
| SD | Standard deviation |
| TFCE | Threshold-free cluster enhancement |
References
- Häussinger, D.; Dhiman, R.K.; Felipo, V.; Görg, B.; Jalan, R.; Kircheis, G.; Merli, M.; Montagnese, S.; Romero-Gomez, M.; Schnitzler, A.; et al. Hepatic encephalopathy. Nat. Rev. Dis. Primers 2022, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Lu, K. Cellular Pathogenesis of Hepatic Encephalopathy: An Update. Biomolecules 2023, 13, 396. [Google Scholar] [CrossRef] [PubMed]
- Ridola, L.; Faccioli, J.; Nardelli, S.; Gioia, S.; Riggio, O. Hepatic Encephalopathy: Diagnosis and Management. J. Transl. Int. Med. 2020, 8, 210–219. [Google Scholar] [CrossRef]
- Qi, R.; Zhang, L.J.; Zhong, J.; Wu, S.; Zhang, Z.; Zhong, Y.; Ni, L.; Zheng, G.; Jiao, Q.; Wu, X.; et al. Dynamic changes of intrinsic brain activity in cirrhotic patients after transjugular intrahepatic portosystemic shunt: A resting-state FMRI study. PLoS ONE 2012, 7, e46681. [Google Scholar] [CrossRef]
- Häussinger, D.; Butz, M.; Schnitzler, A.; Görg, B. Pathomechanisms in hepatic encephalopathy. Biol. Chem. 2021, 402, 1087–1102. [Google Scholar] [CrossRef] [PubMed]
- López-Cervantes, M.; Quintanar-Stephano, A.; Alcauter-Solórzano, S.; Hernández-Pando, R.; Aguilar-Roblero, R.; Gasca-Martínez, D.; Ortíz, J.J.; Vázquez-Martínez, O.; Ximénez-Camilli, C.; Díaz-Muñoz, M. Cerebellar spongiform degeneration is accompanied by metabolic, cellular, and motor disruption in male rats with portacaval anastomosis. J. Neurosci. Res. 2021, 99, 2287–2304. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y. Longitudinal Intrinsic Brain Activity Changes in Cirrhotic Patients before and One Month after Liver Transplantation. Korean J. Radiol. 2017, 18, 370–377. [Google Scholar] [CrossRef]
- Hopp, A.E.; Dirks, M.; Petrusch, C.; Goldbecker, A.; Tryc, A.B.; Barg-Hock, H.; Strassburg, C.; Klempnauer, J.; Weissenborn, K.; Pflugrad, H. Hepatic Encephalopathy Is Reversible in the Long Term After Liver Transplantation. Liver Transpl. 2019, 25, 1661–1672. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Pastor, A.; Llansola, M.; Montoliu, C.; Malaguarnera, M.; Balzano, T.; Taoro-Gonzalez, L.; García-García, R.; Mangas-Losada, A.; Izquierdo-Altarejos, P.; Arenas, Y.M.; et al. Peripheral inflammation induces neuroinflammation that alters neurotransmission and cognitive and motor function in hepatic encephalopathy: Underlying mechanisms and therapeutic implications. Acta Physiol. 2019, 226, e13270. [Google Scholar] [CrossRef] [PubMed]
- Aldridge, D.R.; Tranah, E.J.; Shawcross, D.L. Pathogenesis of hepatic encephalopathy: Role of ammonia and systemic inflammation. J. Clin. Exp. Hepatol. 2015, 5 (Suppl. S1), S7–S20. [Google Scholar] [CrossRef] [PubMed]
- Sepehrinezhad, A.; Moghaddam, N.G.; Shayan, N.; Sahab Negah, S. Correlation of ammonia and blood laboratory parameters with hepatic encephalopathy: A systematic review and meta-analysis. PLoS ONE 2024, 19, e0307899. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Reichert, A.S. Rapid metabolic and bioenergetic adaptations of astrocytes under hyperammonemia—A novel perspective on hepatic encephalopathy. Biol. Chem. 2021, 402, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, A. Pathophysiology of Hepatic Encephalopathy. Clin. Liver Dis. 2020, 24, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Drews, L.; Zimmermann, M.; Westhoff, P.; Brilhaus, D.; Poss, R.E.; Bergmann, L.; Wiek, C.; Brenneisen, P.; Piekorz, R.P.; Mettler-Altmann, T.; et al. Ammonia inhibits energy metabolism in astrocytes in a rapid and glutamate dehydrogenase 2-dependent manner. Dis. Model. Mech. 2020, 13, dmm047134. [Google Scholar] [CrossRef] [PubMed]
- Sepehrinezhad, A.; Zarifkar, A.; Namvar, G.; Shahbazi, A.; Williams, R. Astrocyte swelling in hepatic encephalopathy: Molecular perspective of cytotoxic edema. Metab. Brain Dis. 2020, 35, 559–578. [Google Scholar] [CrossRef] [PubMed]
- Llansola, M.; Montoliu, C.; Agusti, A.; Hernandez-Rabaza, V.; Cabrera-Pastor, A.; Gomez-Gimenez, B.; Malaguarnera, M.; Dadsetan, S.; Belghiti, M.; Garcia-Garcia, R.; et al. Interplay between glutamatergic and GABAergic neurotransmission alterations in cognitive and motor impairment in minimal hepatic encephalopathy. Neurochem. Int. 2015, 88, 15–19. [Google Scholar] [CrossRef]
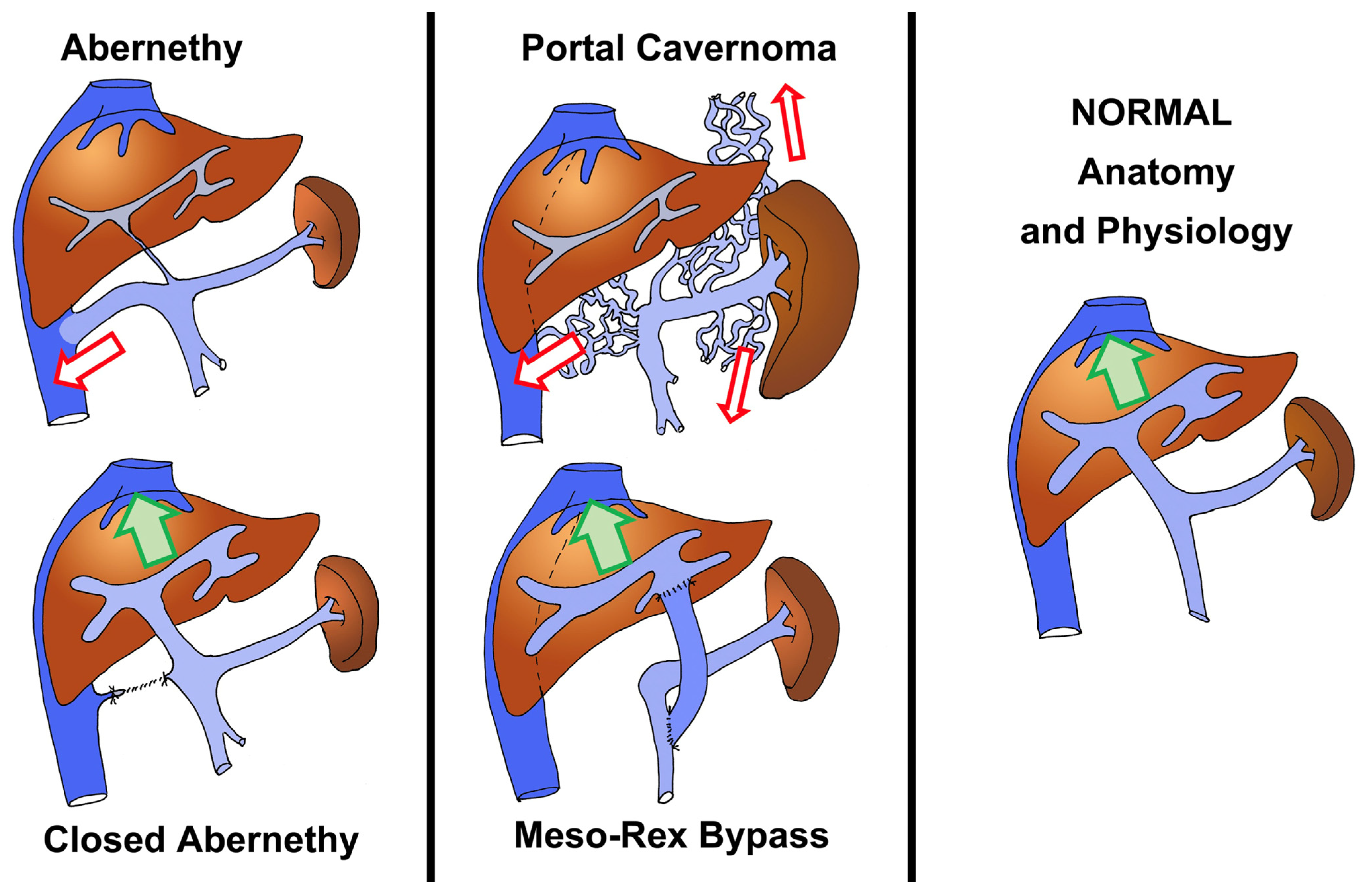
- Elkrief, L.; Houssel-Debry, P.; Ackermann, O.; Franchi-Abella, S.; Branchereau, S.; Valla, D.; Hillaire, S.; Dutheil, D.; Plessier, A.; Hernandez-Gea, V.; et al. Portal cavernoma or chronic non cirrhotic extrahepatic portal vein obstruction. Clin. Res. Hepatol. Gastroenterol. 2020, 44, 491–496. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, B.C.; Puri, V.; Sarin, S.K. Minimal hepatic encephalopathy in patients with extrahepatic portal vein obstruction. Am. J. Gastroenterol. 2008, 3, 1406–1412. [Google Scholar] [CrossRef] [PubMed]
- de Ville, d.G.J.; Clapuyt, P.; Otte, J.B. Extrahilar mesenterico-left portal shunt to relieve extrahepatic portal hypertension after partial liver transplant. Transplantation 1992, 53, 231–232. [Google Scholar]
- Sharif, K.; McKiernan, P.; de Ville, d.G.J. Mesoportal bypass for extrahepatic portal vein obstruction in children: Close to a cure for most! J. Pediatr. Surg. 2010, 45, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Castro Rodríguez, J.; Rodríguez Perálvarez, M.L.; Montero-Álvarez, J.L. Diagnosis and management of Abernethy syndrome. Rev. Esp. Enferm. Dig. 2024, 116, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Franchi-Abella, S.; Gonzales, E.; Ackermann, O.; Branchereau, S.; Pariente, D.; Guérin, F.; International Registry of Congenital Portosystemic Shunt Members. Congenital portosystemic shunts: Diagnosis and treatment. Abdom. Radiol. 2018, 43, 2023–2036. [Google Scholar] [CrossRef] [PubMed]
- Gioia, S.; Nardelli, S.; Riggio, O.; Faccioli, J.; Ridola, L. Cognitive Impairement in Non-Cirrhotic Portal Hypertension: Highlights on Physiopathology, Diagnosis and Management. J. Clin. Med. 2021, 11, 101. [Google Scholar] [CrossRef] [PubMed]
- Meena, B.L.; Narayan SJ, A.; Sarin, S.K. Hepatic encephalopathy in non-cirrhotic portal hypertension. Metab. Brain Dis. 2025, 40, 103. [Google Scholar] [CrossRef]
- Nardone, R.; Taylor, A.C.; Höller, Y.; Brigo, F.; Lochner, P.; Trinka, E. Minimal hepatic encephalopathy: A review. Neurosci. Res. 2016, 111, 1–12. [Google Scholar] [CrossRef]
- D’Antiga, L.; Dacchille, P.; Boniver, C.; Poledri, S.; Schiff, S.; Zancan, L.; Amodio, P. Clues for minimal hepatic encephalopathy in children with noncirrhotic portal hypertension. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 89–94. [Google Scholar] [CrossRef]
- Srivastava, A.; Yadav, S.K.; Lal, R.; Yachha, S.K.; Thomas, M.A.; Saraswat, V.A.; Gupta, R.K. Effect of surgical portosystemic shunt on prevalence of minimal hepatic encephalopathy in children with extrahepatic portal venous obstruction: Assessment by magnetic resonance imaging and psychometry. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Pujol, A.; Graus, F.; Peri, J.; Mercader, J.M.; Rimola, A. Hyperintensity in the globus pallidus on T1-weighted and inversion-recovery MRI: A possible marker of advanced liver disease. Neurology 1991, 41, 1526–1527. [Google Scholar] [CrossRef] [PubMed]
- Montoliu, C.; Urios, A.; Forn, C.; Garcia-Panach, J.; Avila, C.; Gimenez-Garzo, C.; Wassel, A.; Serra, M.A.; Giner-Duran, R.; Gonzalez, O.; et al. Reduced white matter microstructural integrity correlates with cognitive deficits in minimal hepatic encephalopathy. Gut 2014, 63, 1028–1342. [Google Scholar] [CrossRef][Green Version]
- Yadav, S.K.; Srivastava, A.; Srivastava, A.; Thomas, M.A.; Agarwal, J.; Pandey, C.M.; Lal, R.; Yachha, S.K.; Saraswat, V.A.; Gupta, R.K. Encephalopathy assessment in children with extra-hepatic portal vein obstruction with MR, psychometry and critical flicker frequency. J. Hepatol. 2010, 52, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, L.; Shang, Y.; Huang, Y.; Ju, C.; Zheng, H.; Zhao, W.; Liu, J. Identifying Minimal Hepatic Encephalopathy: A New Perspective from Magnetic Resonance Imaging. J. Magn. Reson. Imaging 2025, 61, 11–24. [Google Scholar] [CrossRef]
- Yadav, S.K.; Goel, A.; Saraswat, V.A.; Thomas, M.A.; Wang, E.; Marincola, F.M.; Haris, M.; Gupta, R.K. Evaluation of cognitivity, proinflammatory cytokines, and brain magnetic resonance imaging in minimal hepatic encephalopathy induced by cirrhosis and extrahepatic portal vein obstruction. J. Gastroenterol. Hepatol. 2016, 31, 1986–1994. [Google Scholar] [CrossRef] [PubMed]
- Rudler, M.; Weiss, N.; Perlbarg, V.; Mallet, M.; Tripon, S.; Valabregue, R.; Marjańska, M.; Cluzel, P.; Galanaud, D.; Thabut, D. Combined diffusion tensor imaging and magnetic resonance spectroscopy to predict neurological outcome before transjugular intrahepatic portosystemic shunt. Aliment Pharmacol. Ther. 2018, 48, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Zhang, L.J.; Luo, S.; Ke, J.; Kong, X.; Xu, Q.; Liu, C.; Lu, H.; Lu, G.M. Default mode network functional connectivity: A promising biomarker for diagnosing minimal hepatic encephalopathy: CONSORT-compliant article. Medicine 2014, 93, e227. [Google Scholar] [CrossRef]
- Patel, A.; Kern, M.; Babaei, A.; Samuel, E.A.; Siwiec, R.M.; Chen, G.; Saeian, K.; Li, S.J.; Shaker, R. Objective diagnosis of minimal hepatic encephalopathy (MHE) by analysis of brain resting state functional connectivity. Gastroenterology 2013, 144, S557–S558. [Google Scholar] [CrossRef]
- Elam, J.S.; Glasser, M.F.; Harms, M.P.; Sotiropoulos, S.N.; Andersson, J.L.; Burgess, G.C.; Curtiss, S.W.; Oostenveld, R.; Larson-Prior, L.J.; Schoffelen, J.M.; et al. The Human Connectome Project: A retrospective. Neuroimage 2021, 244, 118543. [Google Scholar] [CrossRef] [PubMed]
- Chiu, B.; Superina, R.A. Encephalopathy caused by a splenorenal shunt can be reversed by performing a mesenteric-to-left portal vein bypass. J. Pediatr. Surg. 2006, 41, 1177–1179. [Google Scholar] [CrossRef] [PubMed]
- Mack, C.L.; Zelko, F.A.; Lokar, J.; Superina, R.; Alonso, E.M.; Blei, A.T.; Whitington, P.F. Surgically restoring portal blood flow to the liver in children with primary extrahepatic portal vein thrombosis improves fluid neurocognitive ability. Pediatrics 2006, 117, e405-12. [Google Scholar] [CrossRef]
- Nieto-Castanon, A.; Whitfield-Gabrieli, S. CONN Functional Connectivity Toolbox: RRID SCR_009550, Release 22; Boston, MA, USA, 2022; Available online: https://web.conn-toolbox.org/ (accessed on 12 February 2022).
- Behzadi, Y.; Restom, K.; Liau, J.; Liu, T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 2007, 37, 90–101. [Google Scholar] [CrossRef]
- Sparacia, G.; Parla, G.; Mamone, G.; Caruso, M.; Torregrossa, F.; Grasso, G. Resting-State Functional Magnetic Resonance Imaging for Surgical Neuro-Oncology Planning: Towards a Standardization in Clinical Settings. Brain Sci. 2021, 11, 1613. [Google Scholar] [CrossRef]
- Nichols, T.E.; Holmes, A.P. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum. Brain Mapp. 2002, 15, 1–25. [Google Scholar] [CrossRef]
- Smith, S.M.; Nichols, T.E. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 2009, 44, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Salimi-Khorshidi, G.; Smith, S.M.; Nichols, T.E. Adjusting the effect of non stationarity in cluster-based and TFCE inference. Neuroimage 2011, 54, 2006–2019. [Google Scholar] [CrossRef] [PubMed]
- Sparacino, L.; Faes, L.; Mijatović, G.; Parla, G.; Lo Re, V.; Miraglia, R.; de Ville de Goyet, J.; Sparacia, G. Statistical Approaches to Identify Pairwise and High-Order Brain Functional Connectivity Signatures on a Single-Subject Basis. Life 2023, 13, 2075. [Google Scholar] [CrossRef]
- Glickman, M.E.; Rao, S.R.; Schultz, M.R. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J. Clin. Epidemiol. 2014, 67, 850–857. [Google Scholar] [CrossRef]
- Llansola, M.; Izquierdo-Altarejos, P.; Montoliu, C.; Mincheva, G.; Palomares-Rodriguez, A.; Pedrosa, M.A.; Arenas, Y.M.; Felipo, V. Role of peripheral inflammation in minimal hepatic encephalopathy. Metab. Brain Dis. 2024, 39, 1667–1677. [Google Scholar] [CrossRef] [PubMed]
- Kibble, H.; Shawcross, D.L. The microbiome in portal hypertension. Clin. Liver Dis. 2023, 22, 70–74. [Google Scholar] [CrossRef]
- Lombardi, M.; Troisi, J.; Motta, B.M.; Torre, P.; Masarone, M.; Persico, M. Gut-Liver Axis Dysregulation in Portal Hypertension: Emerging Frontiers. Nutrients 2024, 16, 1025. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Wang, J.; Ji, F.; Qin, B.; Geng, J.; Kong, G.; Li, Z. Improvement of gut microbiome and intestinal permeability following splenectomy plus pericardial devascularization in hepatitis B virus-related cirrhotic portal hypertension. Front. Immunol. 2022, 13, 941830. [Google Scholar] [CrossRef]
- Gitto, S.; Vizzutti, F.; Baldi, S.; Campani, C.; Navari, N.; Falcini, M.; Venturi, G.; Montanari, S.; Roccarina, D.; Arena, U.; et al. Transjugular intrahepatic Porto-systemic shunt positively influences the composition and metabolic functions of the gut microbiota in cirrhotic patients. Dig. Liver Dis. 2023, 55, 622–628. [Google Scholar] [CrossRef]
- Jiang, W.; Zhou, Z.; Li, G.; Yin, W.; Wu, Z.; Wang, L.; Ghanbari, M.; Li, G.; Yap, P.T.; Howell, B.R.; et al. Mapping the evolution of regional brain network efficiency and its association with cognitive abilities during the first twenty-eight months of life. Dev. Cogn. Neurosci. 2023, 63, 101284. [Google Scholar] [CrossRef] [PubMed]
- Gozdas, E.; Holland, S.K.; Altaye, M.; CMIND Authorship Consortium. Developmental changes in functional brain networks from birth through adolescence. Hum. Brain Mapp. 2019, 40, 1434–1444. [Google Scholar] [CrossRef] [PubMed]
- Lillegard, J.B.; Hanna, A.M.; McKenzie, T.J.; Moir, C.R.; Ishitani, M.B.; Nagorney, D.M. A single-institution review of portosystemic shunts in children: An ongoing discussion. HPB Surg. 2010, 2010, 964597. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sparacia, G.; Parla, G.; Miraglia, R.; de Ville de Goyet, J. Brain Functional Connectivity Significantly Improves After Surgical Eradication of Porto-Systemic Shunting in Pediatric Patients. Life 2025, 15, 290. https://doi.org/10.3390/life15020290
Sparacia G, Parla G, Miraglia R, de Ville de Goyet J. Brain Functional Connectivity Significantly Improves After Surgical Eradication of Porto-Systemic Shunting in Pediatric Patients. Life. 2025; 15(2):290. https://doi.org/10.3390/life15020290
Chicago/Turabian StyleSparacia, Gianvincenzo, Giuseppe Parla, Roberto Miraglia, and Jean de Ville de Goyet. 2025. "Brain Functional Connectivity Significantly Improves After Surgical Eradication of Porto-Systemic Shunting in Pediatric Patients" Life 15, no. 2: 290. https://doi.org/10.3390/life15020290
APA StyleSparacia, G., Parla, G., Miraglia, R., & de Ville de Goyet, J. (2025). Brain Functional Connectivity Significantly Improves After Surgical Eradication of Porto-Systemic Shunting in Pediatric Patients. Life, 15(2), 290. https://doi.org/10.3390/life15020290






