Influence of Nutrient Medium Components on In Vitro Tuberization of Solanum tuberosum L. and Subsequent Minituber Production in Aeroponic and Greenhouse Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Location
2.2. Plant Material and Culture Conditions
2.3. Microtuber Induction
2.4. Assessment of Microtuber Production
2.5. Use of Microtubers as Planting Material for the Aeroponic and Greenhouse Systems
- Macronutrients (in mM): nitrogen (as NO3−): 15 mM; phosphorus (as H2PO4−): 1 mM; potassium (as K⁺): 6 mM; calcium (as Ca2⁺): 5 mM; magnesium (as Mg2⁺): 2 mM; sulfur (as SO42−): 2 mM.
- Micronutrients (in µM): iron (as Fe-EDTA): 100 µM; boron (as H3BO3): 50 µM; manganese (as MnSO4): 10 µM; zinc (as ZnSO4): 1 µM; copper (as CuSO4): 0.5 µM; molybdenum (as Na2MoO4): 0.05 µM.
- 1.
- First Growth Stage (from mass germination to the beginning of budding)
- 2.
- Second Growth Stage (from the start of budding to the beginning of flowering)
- 3.
- Third Growth Stage (from the beginning of flowering to the start of wilting of the foliage)
- 2021: Planting occurred on 15 April, utilizing microtubers in both greenhouse and aeroponic systems. Harvest was completed on 14 July.
- 2022: Planting commenced on 18 April, again employing microtubers in both greenhouse and aeroponic systems. Harvest concluded on 17 July.
- 2023: Planting began on 10 April, with microtubers planted in both greenhouse and aeroponic systems. Harvest was finalized on 9 July.
2.6. Nutrient Composition of Potato Tubers
2.7. Biochemical Analysis of Minitubers
- Dry matter content was measured using the thermogravimetric method, according to which samples were dried to a constant weight at 105 °C (ISO 1026:1982) [41].
- Sugar content was assessed using Bertrand’s method, which involves the reduction of copper (II) ions to copper (I) oxide under alkaline conditions (AOAC Official Method 923.09) [42].
- Vitamin C content was determined using UV spectrophotometry, following the titration of ascorbic acid with a dye (Negi, 2022) [43].
- Nitrate content was analyzed using the Griess reagent method, which involves the diazotization of nitrite with sulfanilamide and subsequent coupling with N-(1-naphthyl) ethylenediamine dihydrochloride to form a colored azo dye (METTLER TOLEDO, n.d.) [44].
- Starch content was evaluated using the enzymatic colorimetric method, whereby starch is enzymatically hydrolyzed to glucose and the glucose is quantified (AOAC Official Method 996.11) [45].
2.8. Statistical Analysis
3. Results
3.1. Effect of Sucrose Concentration on Microtuber Induction and Characteristics
3.2. Percentage of Plants Induced to Form Microtubers
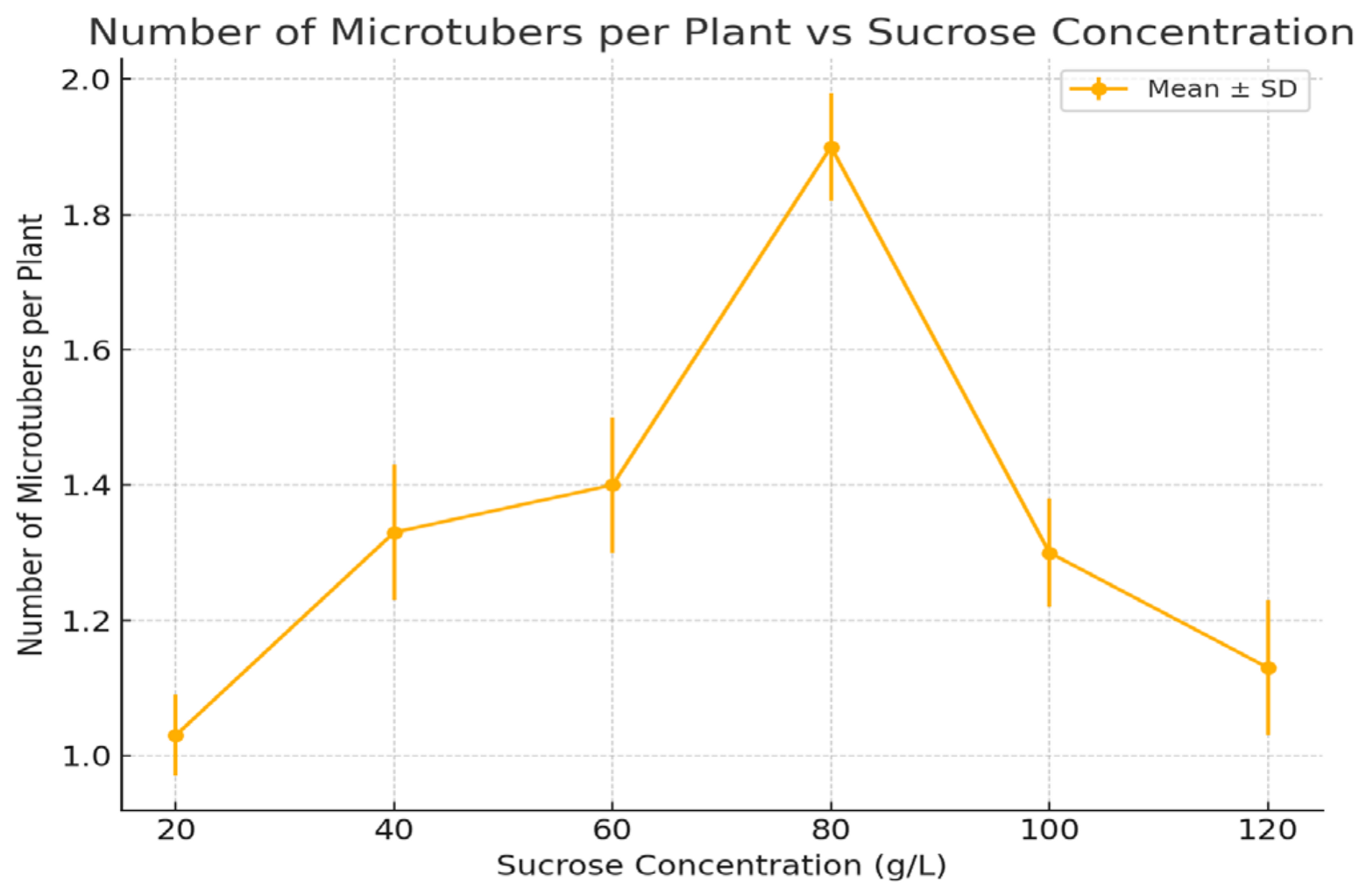
3.3. Number of Microtubers per Plant
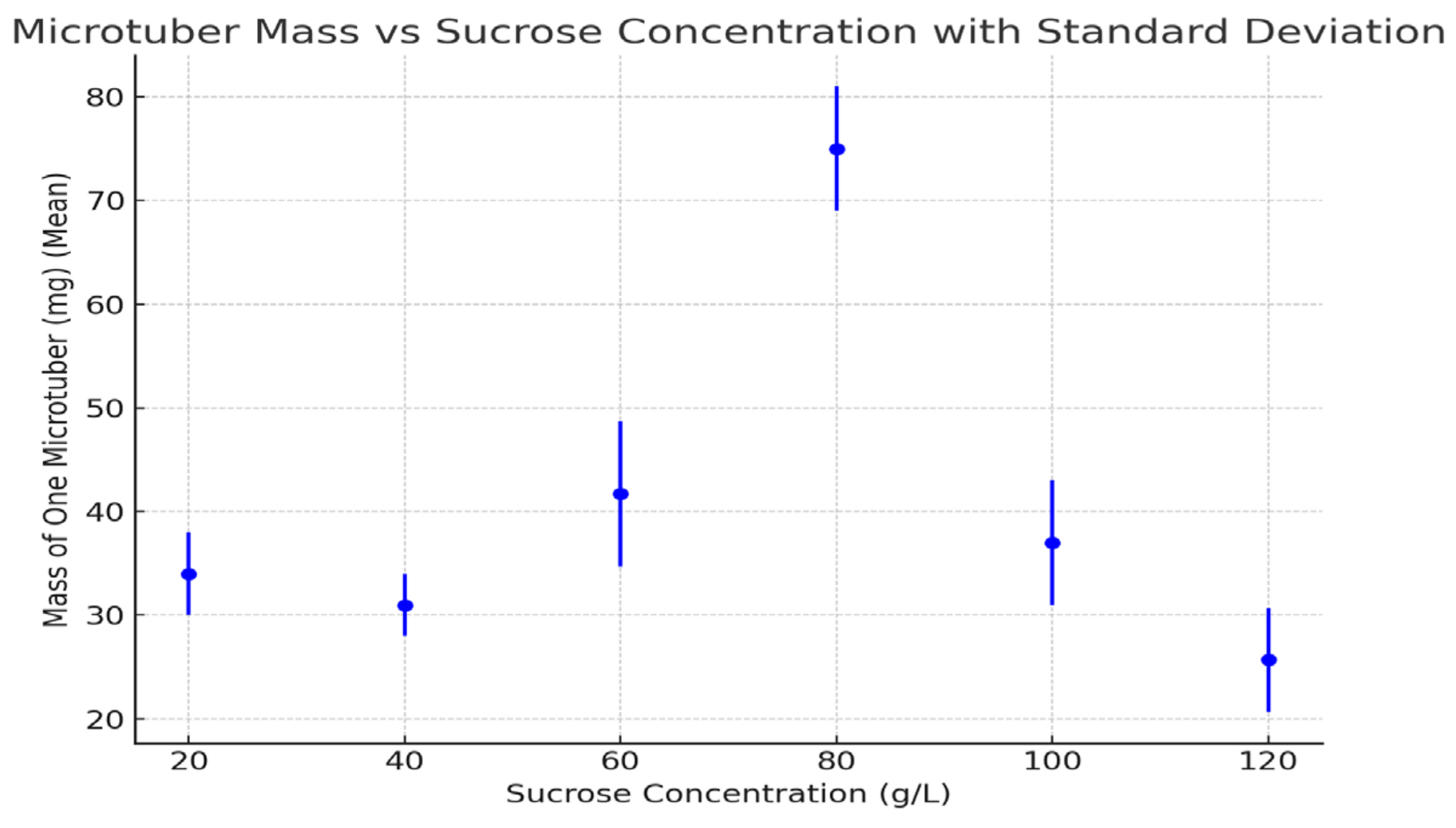
3.4. Mass of One Microtuber
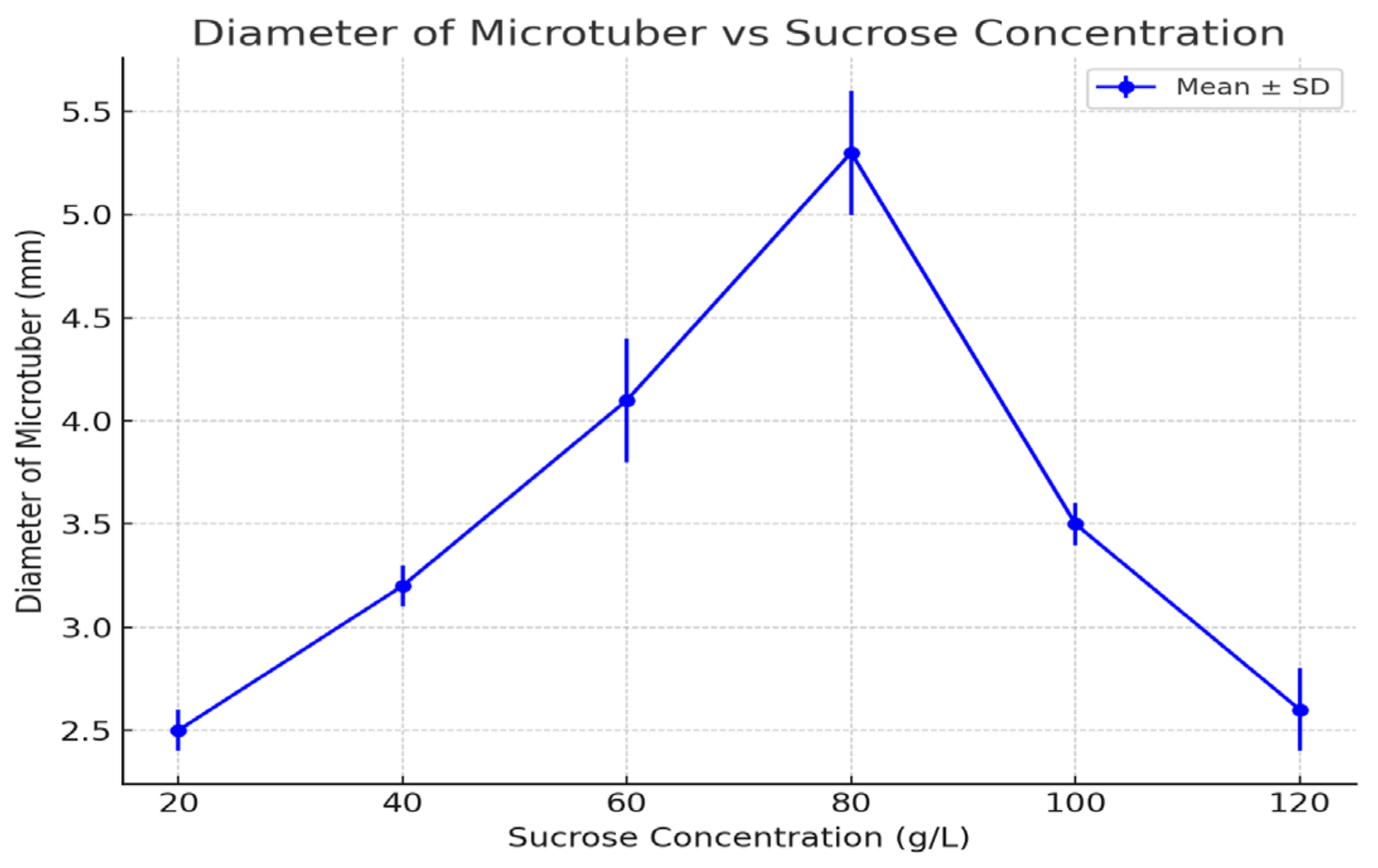
3.5. Diameter of Microtubers
3.6. Effect of Cytokinins on Tuber Formation
- BAP: A steady increase in the number of microtubers per shoot was observed as BAP concentrations increased. Starting with the control (1.60 microtubers per shoot), the number nearly tripled at the peak concentration of 3.0 mg/L, reaching 4.60 microtubers per shoot. After this peak, a decline in performance was noted at higher concentrations.
- Kin: While Kin also promoted microtuber formation, the increase was more modest compared to BAP. At 3.0 mg/L Kin, the number of microtubers peaked at 4.00 ± 0.10 per shoot. As concentrations increased further, a noticeable decline in the number of microtubers was observed. The microtuber weight in Kin treatments did not reach the same levels as those observed with BAP. The highest weight (140.0 ± 10.0 mg) was observed at 4.0 mg/L Kin. However, as with BAP, higher concentrations led to a decrease in weight.
3.7. Evaluation of Potato Growth Metrics and Survival Rates in Greenhouse and Aeroponic Systems
3.8. Comparative Analysis of Potato Minituber Composition in Aeroponic and Greenhouse Systems
4. Discussion
4.1. Incorporating Recent Studies on Controlled Environment Agriculture (CEA)
4.2. Economic Efficiency and Environmental Sustainability
4.3. Directions for Future Research
4.4. Practical Recommendations
- For producers, we recommend the use of sucrose concentrations of approximately 80 g/L and 3.0 mg/L BAP to optimize minituber production. Aeroponic systems are ideal for high-density minituber production, while greenhouse systems are better suited for producing larger tubers intended for field planting.
- We recommend that researchers focus on optimizing sucrose concentrations and hormonal treatments for different potato varieties and investigate the effects of additional phytohormones, such as gibberellins and other less studied hormones, to enhance in vitro microtuber formation, followed by the propagation of these microtubers to improve minituber quality in greenhouse and aeroponic systems
- We recommend that policymakers invest in aeroponic and greenhouse technologies, as well as training and energy-saving systems, to improve minituber production. Standardized guidelines for sucrose and growth regulator use would simplify seed potato production protocols.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sawicka, B.; Umachandran, K.; Pszczółkowski, P. Innovative Potato Production Technology and Its Influence on Quality of Tubers. In Proceedings of the International Conference on Emerging Technology and Interdisciplinary Sciences, Online, 4 December 2021; pp. 174–195. [Google Scholar]
- Gong, H.; Igiraneza, C.; Dusengemungu, L. Major In Vitro Techniques for Potato Virus Elimination and Post Eradication Detection Methods. A Review. Am. J. Potato Res. 2019, 96, 379–389. [Google Scholar] [CrossRef]
- Anikina, I.; Kamarova, A.; Issayeva, K.; Issakhanova, S.; Mustafayeva, N.; Insebayeva, M.; Mukhamedzhanova, A.; Khan, S.M.; Ahmad, Z.; Lho, L.H.; et al. Plant Protection from Virus: A Review of Different Approaches. Front. Plant Sci. 2023, 14, 1163270. [Google Scholar] [CrossRef] [PubMed]
- Diener, T.O. Potato Spindle Tuber “Virus”: IV. A Replicating, Low Molecular Weight RNA. Virology 1971, 45, 411–428. [Google Scholar] [CrossRef]
- Navarro, B.; Turina, M. Viroid and Viroid-Like Elements in Plants and Plant-Associated Microbiota: A New Layer of Biodiversity for Plant Holobionts. New Phytol. 2024, 244, 1216–1222. [Google Scholar] [CrossRef]
- Herrera-Isidron, L.; Valencia-Lozano, E.; Uribe-Lopez, B.; Délano-Frier, J.P.; Barraza, A.; Cabrera-Ponce, J.L. Molecular Insights into the Role of Sterols in Microtuber Development of Potato Solanum tuberosum L. Plants 2024, 13, 2391. [Google Scholar] [CrossRef]
- Rahman, M.H.; Islam, M.J.; Mumu, U.H.; Ryu, B.-R.; Lim, J.-D.; Azad, M.O.K.; Cheong, E.J.; Lim, Y.-S. Effect of Light Quality on Seed Potato (Solanum tuberosum L.) Tuberization When Aeroponically Grown in a Controlled Greenhouse. Plants 2024, 13, 737. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.H.; Islam, M.J.; Mumu, U.H.; Ryu, B.-R.; Lim, J.-D.; Azad, M.O.K.; Cheong, E.J.; Lim, Y.-S. The Growth and Tuber Yield of Potatoes (Solanum tuberosum L.) under Varying LED Light Spectrums in Controlled Greenhouse Conditions. Horticulturae 2024, 10, 254. [Google Scholar] [CrossRef]
- Buckseth, T.; Singh, R.K.; Tiwari, J.K.; Sharma, A.K.; Singh, S.; Chakrabarti, S.K. A novel sustainable aeroponic system for healthy seed potato production in India – An update. Indian J. Agric. Sci. 2020, 90, 243–248. [Google Scholar] [CrossRef]
- Broćić, Z.; Oljača, J.; Pantelić, D.; Rudić, J.; Momčilović, I. Potato Aeroponics: Effects of Cultivar and Plant Origin on Minituber Production. Horticulturae 2022, 8, 915. [Google Scholar] [CrossRef]
- Barbaś, P.; Sawicka, B.; Pszczółkowski, P.; Hameed, T.S.; Farhan, A.K. Optimization of Potato Cultivation Through the Use of Biostimulator Supporter. Agronomy 2024, 14, 2430. [Google Scholar] [CrossRef]
- Martirosyan, Y.T.; Kharchenko, P.N. Plant Tray and Aerohydroponic Plant with Its Use. Patent RF No RU88246U1; Bulletin No 31, 10 November 2009. [Google Scholar]
- Martirosyan, Y.T.; Kosobryukhov, A.A.; Martirosyan, V.V. Aeroponic Technologies in Virus-Free Seed Production: Advantages and Perspectives. Dostizheniya Nauk. I Tekhniki APK 2016, 30, 47–51. (In Russian) [Google Scholar]
- Tunio, M.H.; Qureshi, S.A.; Naeem, F.; Lakhiar, I.A.; Memon, N.A.; Chandio, F.A.; Bhatti, A.A. Potato Production in Aeroponics: An Emerging Food Growing System in Sustainable Agriculture for Food Security. Chil. J. Agric. Res. 2020, 80, 118–132. [Google Scholar] [CrossRef]
- Wheeler, R.; Herridge, L. NASA Plant Researchers Explore Question of Deep-Space Food Crops. NASA. 2016. Available online: https://api.semanticscholar.org/CorpusID:111818786 (accessed on 10 November 2024).
- Kargapolova, K.Y.; Burygin, G.L.; Tkachenko, O.V.; Evseeva, N.V.; Pukhalskiy, Y.V.; Belimov, A.A. Effectiveness of Inoculation of In Vitro-Grown Potato Microplants with Rhizosphere Bacteria of the Genus Azospirillum. Plant Cell Tissue Organ Cult. 2020, 141, 351–359. [Google Scholar] [CrossRef]
- Mamiya, K.; Tanabe, K.; Onishi, N. Production of Potato (Solanum tuberosum L.) Microtubers Using Plastic Culture Bags. Plant Biotechnol. 2020, 37, 233–238. [Google Scholar] [CrossRef]
- Boubaker, H.; Saadaoui, W.; Dasgan, H.Y.; Tarchoun, N.; Gruda, N.S. Enhancing Seed Potato Production from In Vitro Plantlets and Microtubers Through Biofertilizer Application: Investigating Effects on Plant Growth, Tuber Yield, Size, and Quality. Agronomy 2023, 13, 2541. [Google Scholar] [CrossRef]
- Sadawarti, M.; Pandey, K.K.; Singh, B.P.; Samadiya, R.K. A Review on Potato Microtuber Storability and Dormancy. J. Appl. Nat. Sci. 2016, 8, 2319–2324. [Google Scholar] [CrossRef]
- Yagiz, A.K.; Yavuz, C.; Tarim, C.; Demirel, U.; Caliskan, M.E. Effects of Growth Regulators, Media, and Explant Types on Microtuberization of Potato. Am. J. Potato Res. 2020, 97, 523–530. [Google Scholar] [CrossRef]
- Daurov, D.; Daurova, A.; Sapakhova, Z.; Kanat, R.; Akhmetzhanova, D.; Abilda, Z.; Toishimanov, M.; Raissova, N.; Otynshiyev, M.; Zhambakin, K.; et al. The Impact of Growth Regulators and Cultivation Conditions of Temporary Immersion Systems (TISs) on the Morphological Characteristics of Potato Explants and Microtubers. Agronomy 2024, 14, 1782. [Google Scholar] [CrossRef]
- Reisi, A.; Askari, N.; Sadat Hosseini, M.; Motlagh, B.P.; Ghahremani, R. Far-Red Spectrum Leads to Enhanced In Vitro Microtuberization in Potato (Solanum tuberosum cv. Sante). Plant Cell Tissue Organ Cult. 2024, 156, 45. [Google Scholar] [CrossRef]
- Dutt, S.; Manjul, A.S.; Raigond, P.; Singh, B.; Siddappa, S.; Bhardwaj, V.; Kawar, P.G.; Patil, V.U.; Kardile, H.B. Key Players Associated with Tuberization in Potato: Potential Candidates for Genetic Engineering. Crit. Rev. Biotechnol. 2017, 37, 942–957. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Arif, Y.; Siddiqui, H.; Upadhyaya, C.P.; Pichtel, J.; Hayat, S. Critical Factors Responsible for Potato Tuberization. Bot. Rev. 2023, 89, 421–437. [Google Scholar] [CrossRef]
- Abeytilakarathna, P.D. Factors Affect to Stolon Formation and Tuberization in Potato: A Review. Agric. Rev. 2022, 43, 91–97. [Google Scholar] [CrossRef]
- Dutta, M.; Mali, S.; Raturi, V.; Zinta, G. Transcriptional and Post-transcriptional Regulation of Tuberization in Potato (Solanum tuberosum L.). J. Plant Growth Regul. 2024, 43, 1–24. [Google Scholar] [CrossRef]
- Plantenga, F.D.M.; Bergonzi, S.; Abelenda, J.A.; Bachem, C.W.B.; Visser, R.G.F.; Heuvelink, E.; Marcelis, L.F.M. The Tuberization Signal StSP6A Represses Flower Bud Development in Potato. J. Exp. Bot. 2019, 70, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Hannapel, D.J.; Sharma, P.; Lin, T.; Banerjee, A.K. The Multiple Signals That Control Tuber Formation. Plant Physiol. 2017, 174, 845–856. [Google Scholar] [CrossRef]
- Kumar, P.; Jaisuriyan, K.; Gopika, B.; Subhash, B. Aeroponics: A Modern Agriculture Technology Under Controlled Environment. In Hydroponics; Kumar, N., Ed.; Encyclopedia of Sustainability Science and Technology Series; Springer: New York, NY, USA, 2024. [Google Scholar]
- Garzón, J.; Montes, L.; Garzón, J.; Lampropoulos, G. Systematic Review of Technology in Aeroponics: Introducing the Technology Adoption and Integration in Sustainable Agriculture Model. Agronomy 2023, 13, 2517. [Google Scholar] [CrossRef]
- Bag, T.K.; Srivastava, A.K.; Yadav, S.K.; Gurjar, M.S.; Diengdoh, L.C.; Rai, R.; Singh, S. Potato (Solanum tuberosum) Aeroponics for Quality Seed Production in North Eastern Himalayan Region of India. Indian J. Agric. Sci. 2015, 85, 1360–1364. [Google Scholar] [CrossRef]
- Sahakyan, A.D.; Barseghyan, A.A.; Melyan, G.G.; Martirosyan, Y.T. Comparative Assessment of Potato Minitubers Yield Produced from In Vitro Plants in Greenhouse and Aeroponics. Subtrop. Ornam. Hortic. 2018, 66, 179–184. [Google Scholar] [CrossRef]
- Sahakyan, A.J.; Melyan, G.H.; Melikyan, A.S.; Barseghyan, A.H. Production of Healthy Potato Minitubers In Vitro and Their Efficiency Assessment. AgriScience Technol. 2022, 3, 268–274. [Google Scholar] [CrossRef]
- Dyikanova, M.E.; Deniskina, N.F.; Levshin, A.G.; Gasparyan, S.V.; Ivashova, O.N.; Gasparyan, I.N. The Influence of Agrotechnical Practices on the Early Production of Potatoes of the Red Scarlett Variety. IOP Conf. Ser. Earth Environ. Sci. 2022, 1112, 012097. [Google Scholar] [CrossRef]
- Salem, J.; Hassanein, A.M. In Vitro Propagation, Microtuberization, and Molecular Characterization of Three Potato Cultivars. Biol. Plant. 2017, 61, 427–437. [Google Scholar] [CrossRef]
- Sivakumar, P.; Sasikala, K.; Prabha, T.; Vijai Selvaraj, K.S.; Karunakaran, V.; Selvamurugan, M.; Shree, G.G.K.; Kiruba, M. Microtuber Induction and Plant Regeneration of Potato (Solanum tuberosum L.): A Review. Int. J. Plant Soil Sci. 2024, 36, 166–176. [Google Scholar] [CrossRef]
- Khorsandi, S.; Motallebi Azar, A.; Zaare-Nahandi, F.; Mokhtarzadeh, S. Effect of Different Concentrations of BAP and Putrescine on Potato Microtuberization (cv. Agria). J. Inst. Sci. Technol. 2020, 10, 723–731. [Google Scholar] [CrossRef]
- Gudeva, L.K.; Trajkova, F.; Stojkova, I. Effect of Plant Growth Regulators and Sucrose on Microtuberization of Potato (Solanum tuberosum L.). Rom. Agric. Res. 2016, 33, 1–7. [Google Scholar]
- Tsama Subrahmanyeswari, T.; Bandyopadhyay, S.; Gantait, S. Direct Regeneration-Mediated In Vitro Propagation and Microtuberization of Potato: An Appraisal of the Past Ten-Year Period. S. Afr. J. Bot. 2024, 172, 768–778. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants Without Soil. Calif. Agric. Exp. Stn. Circ. 1950, 347, 32–35. [Google Scholar]
- ISO 1026:1982; Fruit and Vegetable Products—Determination of Dry Matter Content—Method by Drying Under Reduced Pressure. International Organization for Standardization: Geneva, Switzerland, 1982. Available online: https://www.iso.org/standard/5498.html (accessed on 21 November 2024).
- AOAC Official Method 923.09. Association of Official Analytical Chemists. Sugars (Reducing) in Plants: Bertrand Method. 2023. Available online: https://www.aoac.org/official-methods-of-analysis/ (accessed on 21 November 2024).
- Negi, S. Analysis of Total Vitamin C Contents in Various Fruits and Vegetables by UV-Spectrophotometry. Sch. Res. J. Interdiscip. Stud. 2022, 42, 17723–17730. [Google Scholar] [CrossRef]
- METTLER TOLEDO. (n.d.). Nitrate Content Analysis Using the Griess Reagent Method. Available online: https://www.mt.com/dam/Analytical/anachemapplications/titration/M720/M720_2015.pdf (accessed on 21 November 2024).
- AOAC Official Method 996.11. Starch (Total) in Cereal Products. Association of Official Analytical Chemists. 1996. Available online: https://img.21food.cn/img/biaozhun/20100108/177/11285185.pdf (accessed on 21 November 2024).
- Wazir, A.; Gul, Z.; Hussain, M.; Khan, Z.U.; Saleem, M.; Khurshid, I. Effect of Biochar on Crop Growth and Yield. Int. J. Agron. Agric. Res. 2015, 7, 118–124. [Google Scholar]
- Fufa, M.; Diro, M. Microtuber Induction of Two Potato (Solanum tuberosum L.) Varieties. Adv. Crop Sci. Technol. 2014, 2, 122. [Google Scholar] [CrossRef]
- Cui, X.H.; Murthy, H.N.; Wu, C.H.; Paek, K.Y. Sucrose-Induced Osmotic Stress Affects Biomass, Metabolite, and Antioxidant Levels in Root Suspension Cultures of Hypericum perforatum L. Plant Cell Tissue Organ Cult. 2010, 103, 7–14. [Google Scholar] [CrossRef]
- Askari, N. Evaluation of Sucrose Benefits to Tuberization in ‘Sante’ Potato Cultivar In Vitro. Int. J. Hortic. Sci. Technol. 2023, 11, 437–444. [Google Scholar]
- Mohamed, A.E.S.; Girgis, N.D. Factors Affecting In Vitro Tuberization of Potato. Bull. Natl. Res. Cent. 2023, 47, 80. [Google Scholar] [CrossRef]
- AL-Hussaini, Z.A.; Yousif, S.H.A.; AL-Ajeely, S.A. The Role of Sucrose and Light Duration on In Vitro Tuberization for Two Cultivars of Potato (Solanum tuberosum L.). Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 277–283. [Google Scholar]
- Homsuwan, N.; Mapiyaphun, K.; Ngampanya, B. Effect of Sucrose on Microtuber Induction and Inulin Accumulation in Jerusalem Artichoke (Helianthus tuberosus L.). CMUJ Nat. Sci. 2021, 20, e2021063. [Google Scholar] [CrossRef]
- Hossain, M.S.; Hossain, M.M.; Hossain, T.; Haque, M.M.; Zakaria, M.; Sarkar, M.D. Varietal Performance of Potato on Induction and Development of Microtuber in Response to Sucrose. Ann. Agric. Sci. 2017, 62, 75–81. [Google Scholar] [CrossRef]
- Emara, H.A.; Hamza, E.M.; Fekry, W.A. In Vitro Propagation and Microtuber Formation of Potato in Relation to Different Concentrations of Some Growth Regulators and Sucrose. Middle East J. Agric. Res. 2017, 6, 1029–1037. [Google Scholar]
- Islam, M.S.; Roni, M.Z.K.; Jamal Uddin, A.F.M.; Shimasaki, K. Tracing the Role of Sucrose in Potato Microtuber Formation In Vitro. Plant Omics J. 2017, 10, 15–19. [Google Scholar] [CrossRef]
- Lastdrager, J.; Hanson, J.; Smeekens, S. Sugar Signals and the Control of Plant Growth and Development. J. Exp. Bot. 2014, 65, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Sosnowski, J.; Truba, M.; Vasileva, V. The Impact of Auxin and Cytokinin on the Growth and Development of Selected Crops. Agriculture 2023, 13, 724. [Google Scholar] [CrossRef]
- Hasrak, S.; Zarghami, R. Comparison of Minituber Production in Designed Aeroponic System and Soil Cultivation. Acta Physiol. Plant. 2023, 45, 57. [Google Scholar] [CrossRef]
- Çalışkan, M.E.; Yavuz, C.; Yağız, A.K.; Demirel, U.; Çalışkan, S. Comparison of Aeroponics and Conventional Potato Mini Tuber Production Systems at Different Plant Densities. Potato Res. 2021, 64, 41–53. [Google Scholar] [CrossRef]
- Čížek, M.; Komárková, Z. Comparison of Aeroponics Technology with a Conventional System of Production of Potato Minitubers in the Conditions of the Czech Republic. Plant Soil Environ. 2022, 68, 366–374. [Google Scholar] [CrossRef]
- Serderov, V.K.; Chanbabaev, T.G.; Serderova, D.V. Change of Maintenance of Dry Substances and Starch in Tubers of Potato in Dependence on Terms of Till. Veg. Crops Russ. 2019, 2, 80–83. [Google Scholar] [CrossRef]
- Lyskova, I.V.; Sintsova, N.F.; Kratyuk, E.I. Accumulation and Morphological Properties of Starch in Tubers of New Potato Hybrids. Agrar. Nauka Evro-Sev.-Vostoka (Agric. Sci. Euro-North-East) 2023, 24, 367–376. [Google Scholar] [CrossRef]
- Golovko, T.K.; Tabalenkova, G.N. Source–Sink Relationships in Potato Plants. Russ. J. Plant Physiol. 2019, 66, 664–671. [Google Scholar] [CrossRef]
- Min, A. Aeroponic Systems Design: Considerations and Challenges. J. Agric. Eng. 2022, 54, 19. [Google Scholar] [CrossRef]
- Faisal, A.; Rajashekar, V.; Biswal, P.; Mukherjee, A.; Bhowmick, G.D. Advancing Agriculture with Aeroponics: A Critical Review of Methods, Benefits, and Limitations. In Hydroponics; Kumar, N., Ed.; Encyclopedia of Sustainability Science and Technology Series; Springer: New York, NY, USA, 2024; pp. 1–16. [Google Scholar] [CrossRef]
- Ojo, M.O.; Zahid, A. Deep Learning in Controlled Environment Agriculture: A Review of Recent Advancements, Challenges and Prospects. Sensors 2022, 22, 7965. [Google Scholar] [CrossRef] [PubMed]
- Heuvelink, E.; Hemming, S.; Marcelis, L.F.M. Some Recent Developments in Controlled-Environment Agriculture: On Plant Physiology, Sustainability, and Autonomous Control. J. Hortic. Sci. Biotechnol. 2024, 100, 1–11. [Google Scholar] [CrossRef]
- Kim, J.; Park, H.; Seo, C.; Kim, H.; Choi, G.; Kim, M.; Kim, B.; Lee, W. Sustainable and Inflatable Aeroponics Smart Farm System for Water Efficiency and High-Value Crop Production. Appl. Sci. 2024, 14, 4931. [Google Scholar] [CrossRef]
- Barbosa, G.L.; Gadelha, F.D.A.; Kublik, N.; Proctor, A.; Reichelm, L.; Weissinger, E.; Wohlleb, G.M.; Halden, R.U. Comparison of Land, Water, and Energy Requirements of Lettuce Grown Using Hydroponic vs. Conventional Agricultural Methods. Int. J. Environ. Res. Public Health 2015, 12, 6879–6891. [Google Scholar] [CrossRef]
- Lakhiar, I.A.; Gao, J.; Syed, T.N.; Chandio, F.A.; Buttar, N.A. Modern Plant Cultivation Technologies in Agriculture under Controlled Environment: A Review on Aeroponics. J. Plant Interact. 2018, 13, 338–352. [Google Scholar] [CrossRef]
- Buckseth, T.; Sharma, A.K.; Pandey, K.K.; Singh, B.P.; Muthuraj, R. Methods of Pre-Basic Seed Potato Production with Special Reference to Aeroponics—A Review. Scientia Hortic. 2016, 204, 79–87. [Google Scholar] [CrossRef]


| Sucrose Concentration (g/L) | Percentage of Plants Induced to form Microtubers (%) | Number of Microtubers per Plant (Mean ± SD) | Mass of One Microtuber (mg) (Mean ± SD) | Diameter of One Microtuber (mm) (Mean ± SD) |
|---|---|---|---|---|
| 20 | 10.0 ± 2.1 d | 1.03 ± 0.06 c | 34.0 ± 4.0 b | 2.5 ± 0.1 e |
| 40 | 38.0 ± 3.5 c | 1.33 ± 0.1 b | 31.0 ± 3.0 b | 3.2 ± 0.1 d |
| 60 | 46.5 ± 3.0 b | 1.40 ± 0.1 b | 41.7 ± 7.0 b | 4.1 ± 0.3 b |
| 80 | 63.3 ± 4.2 a | 1.90 ± 0.08 a | 75.0 ± 6.0 a | 5.3 ± 0.3 a |
| 100 | 50.4 ± 2.8 b | 1.30 ± 0.08 b | 37.0 ± 6.0 b | 3.5 ± 0.1 c |
| 120 | 46.6 ± 3.1 b | 1.13 ± 0.1 c | 25.7 ± 5.0 c | 2.6 ± 0.2 e |
| Treatment | Cytokinin Concentration (mg/L) | In Vitro Tuberization (%) |
|---|---|---|
| BAP | 0 (control) | 55.5 e |
| 0.25 | 56.7 e | |
| 0.5 | 60.0 de | |
| 1.0 | 66.6 de | |
| 2.0 | 70.0 c | |
| 2.5 | 75.0 c | |
| 3.0 | 90.0 a | |
| 4.0 | 55.0 e | |
| 5.0 | 36.5 f | |
| Kin | 0.25 | 56.6 e |
| 0.5 | 60.0 e | |
| 1.0 | 66.7 d | |
| 2.0 | 70.0 cd | |
| 2.5 | 71.7 c | |
| 3.0 | 76.5 c | |
| 4.0 | 80.5 b | |
| 5.0 | 46.6 f |
| Treatment | Phytohormone Concentration (mg/L) | Microtubers per Shoot (Mean ± SD) | Weight of One Microtuber (mg, Mean ± SD) | Diameter of one Microtuber (mm, Mean ± SD) |
|---|---|---|---|---|
| Control | 0.00 | 1.60 ± 0.10 h | 60.0 ± 10.0 ef | 5.30 ± 0.10 e |
| BAP | 0.25 | 1.95 ± 0.08 g | 90.0 ± 10.0 e | 6.25 ± 0.05 d |
| 0.50 | 2.30 ± 0.08 f | 130.0 ± 10.0 d | 6.67 ± 0.15 c | |
| 1.0 | 2.95 ± 0.10 e | 150.0 ± 20.0 d | 6.80 ± 0.10 c | |
| 2.0 | 3.48 ± 0.15 d | 260.0 ± 20.0 c | 7.17 ± 0.15 b | |
| 2.5 | 4.10 ± 0.15 b | 350.0 ± 20.0 b | 7.45 ± 0.05 b | |
| 3.0 | 4.60 ± 0.10 a | 410.0 ± 20.0 a | 8.43 ± 0.20 a | |
| 4.0 | 3.70 ± 0.14 c | 370.0 ± 10.0 b | 6.50 ± 0.38 c | |
| 5.0 | 1.40 ± 0.15 h | 50.0 ± 20.0 f | 4.50 ± 0.20 f | |
| Kin | 0.25 | 2.50 ± 0.14 f | 70.0 ± 10.0 ef | 4.15 ± 0.10 f |
| 0.50 | 2.75 ± 0.16 e | 80.0 ± 20.0 ef | 4.25 ± 0.12 f | |
| 1.0 | 3.40 ± 0.15 d | 90.0 ± 10.0 e | 4.43 ± 0.15 f | |
| 2.0 | 3.85 ± 0.10 c | 100.0 ± 20.0 d | 4.10 ± 0.26 f | |
| 2.5 | 3.65 ± 0.16 cd | 120.0 ± 20.0 d | 4.20 ± 0.26 f | |
| 3.0 | 4.00 ± 0.10 b | 110.0 ± 20.0 d | 4.50 ± 0.20 f | |
| 4.0 | 3.40 ± 0.08 d | 140.0 ± 10.0 d | 4.00 ± 0.10 g | |
| 5.0 | 2.10 ± 0.16 g | 90.0 ± 10.0 e | 3.13 ± 0.20 h |
| Potato Variety | Origin of Mini Tubers | Survival Rate (%) (Mean ± SD) | Number of Stems per Microtuber (Mean ± SD) | Number of Mini Tubers per Microtuber (Mean ± SD) | Number of Tubers (%) <25 mm (Mean ± SD) |
|---|---|---|---|---|---|
| ‘Red Scarlet’ | Aeroponic | 95.0 ± 4.08 a | 2.1 ± 0.14 b | 32.4 ± 2.6 a | 82 ± 4.0 a |
| Greenhouse | 80.0 ± 4.08 b | 3.2 ± 0.18 a | 7.5 ± 1.9 b | 26 ± 5.4 b |
| Biochemical Parameter | Aeroponic System (Mean ± SD) | Greenhouse System (Mean ± SD) | Statistical Significance |
|---|---|---|---|
| Dry Matter Content (%) | 16.9 ± 1.5 | 19.8 ± 1.2 | p < 0.05 |
| Reducing Sugars Content (%) | 1.02 ± 0.03 | 0.36 ± 0.02 | p < 0.05 |
| Vitamin C Content (mg/100 g) | 18.5 ± 1.0 | 16.3 ± 0.8 | p < 0.05 |
| Nitrate Content (mg/kg) | 45.3 ± 3.5 | 49.7 ± 2.8 | p < 0.05 |
| Starch Content (%) | 10.3 ± 0.9 | 17.8 ± 1.0 | p < 0.05 |
| Amylose Content (%) | 27.9 ± 1.5 | 28.1 ± 1.1 | p > 0.05 |
| Crude Protein Content (%) | 1.93 ± 0.24 | 2.43 ± 0.19 | p < 0.05 |
| Total Protein Content (%) | 0.98 ± 0.03 | 1.05 ± 0.04 | p < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melyan, G.H.; Martirosyan, Y.T.; Sahakyan, A.J.; Sayadyan, H.Y.; Melikyan, A.S.; Barseghyan, A.H.; Vardanyan, A.S.; Martirosyan, H.S.; Harutyunyan, M.G.; Mkrtchyan, A.L.; et al. Influence of Nutrient Medium Components on In Vitro Tuberization of Solanum tuberosum L. and Subsequent Minituber Production in Aeroponic and Greenhouse Conditions. Life 2025, 15, 241. https://doi.org/10.3390/life15020241
Melyan GH, Martirosyan YT, Sahakyan AJ, Sayadyan HY, Melikyan AS, Barseghyan AH, Vardanyan AS, Martirosyan HS, Harutyunyan MG, Mkrtchyan AL, et al. Influence of Nutrient Medium Components on In Vitro Tuberization of Solanum tuberosum L. and Subsequent Minituber Production in Aeroponic and Greenhouse Conditions. Life. 2025; 15(2):241. https://doi.org/10.3390/life15020241
Chicago/Turabian StyleMelyan, Gayane Hrant, Yuri Tsatur Martirosyan, Aghvan Jumshud Sahakyan, Hovik Yakshibek Sayadyan, Andreas Shmavon Melikyan, Andranik Hakob Barseghyan, Arayik Sajan Vardanyan, Hamlet Sargis Martirosyan, Margarita Gurgen Harutyunyan, Anzhela Liparit Mkrtchyan, and et al. 2025. "Influence of Nutrient Medium Components on In Vitro Tuberization of Solanum tuberosum L. and Subsequent Minituber Production in Aeroponic and Greenhouse Conditions" Life 15, no. 2: 241. https://doi.org/10.3390/life15020241
APA StyleMelyan, G. H., Martirosyan, Y. T., Sahakyan, A. J., Sayadyan, H. Y., Melikyan, A. S., Barseghyan, A. H., Vardanyan, A. S., Martirosyan, H. S., Harutyunyan, M. G., Mkrtchyan, A. L., Hakobjanyan, I. L., Dangyan, K. S., Terteryan, K. H., Khazaryan, K. A., & Galstyan, M. H. (2025). Influence of Nutrient Medium Components on In Vitro Tuberization of Solanum tuberosum L. and Subsequent Minituber Production in Aeroponic and Greenhouse Conditions. Life, 15(2), 241. https://doi.org/10.3390/life15020241







