Mechanisms Involved in Cell Wall Remodeling in Etiolated Rice Shoots Grown Under Osmotic Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Measurement of the Mechanical Properties of the Cell Wall
2.3. Determination of Cell Wall Constituents
2.4. Determination of CW-PRX and PAL Activities
2.5. Gene Expression Analysis
2.6. Statistical Analysis
3. Results
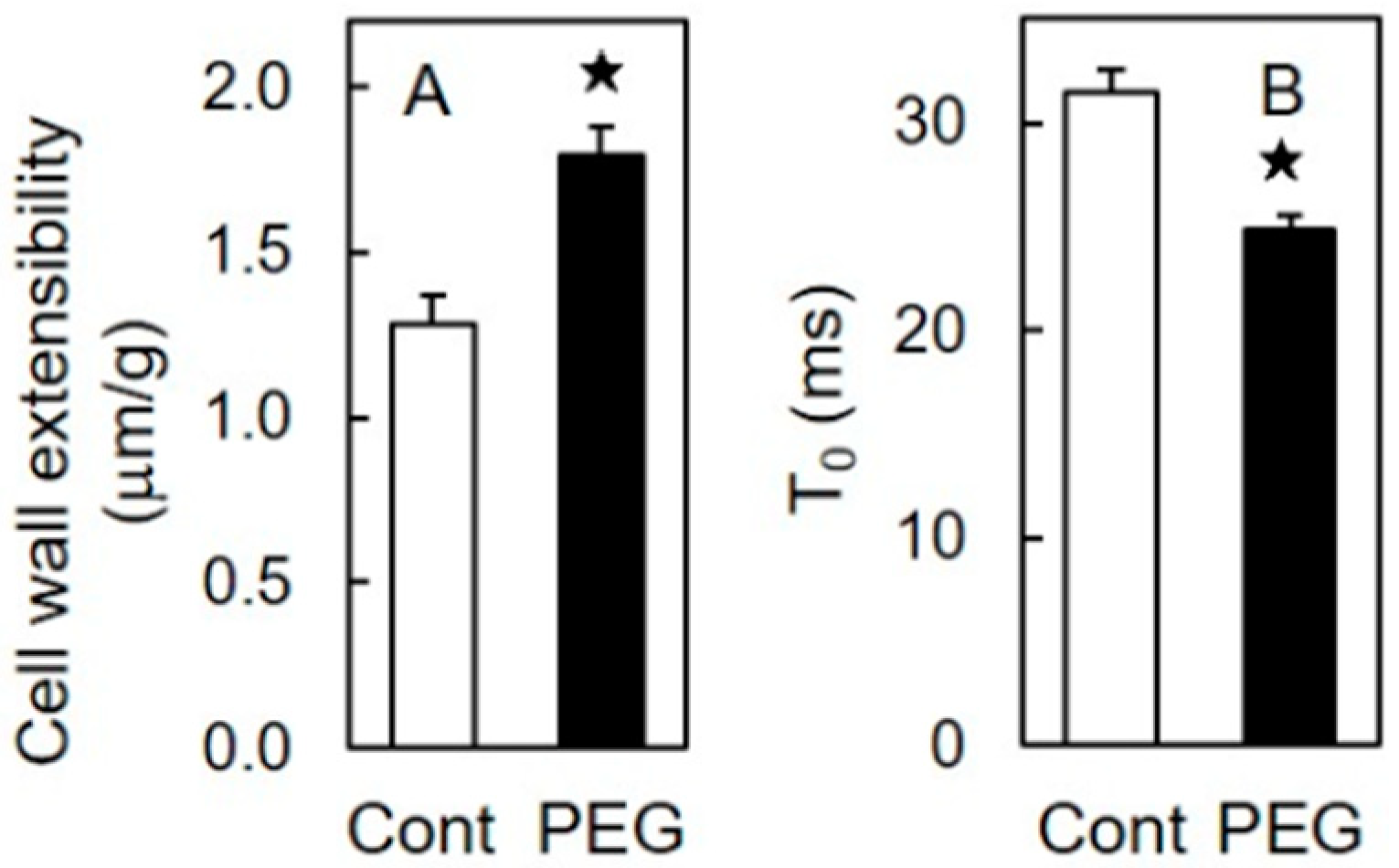
3.1. Effects of Osmotic Stress on Shoot Growth and Mechanical Properties of the Cell Wall
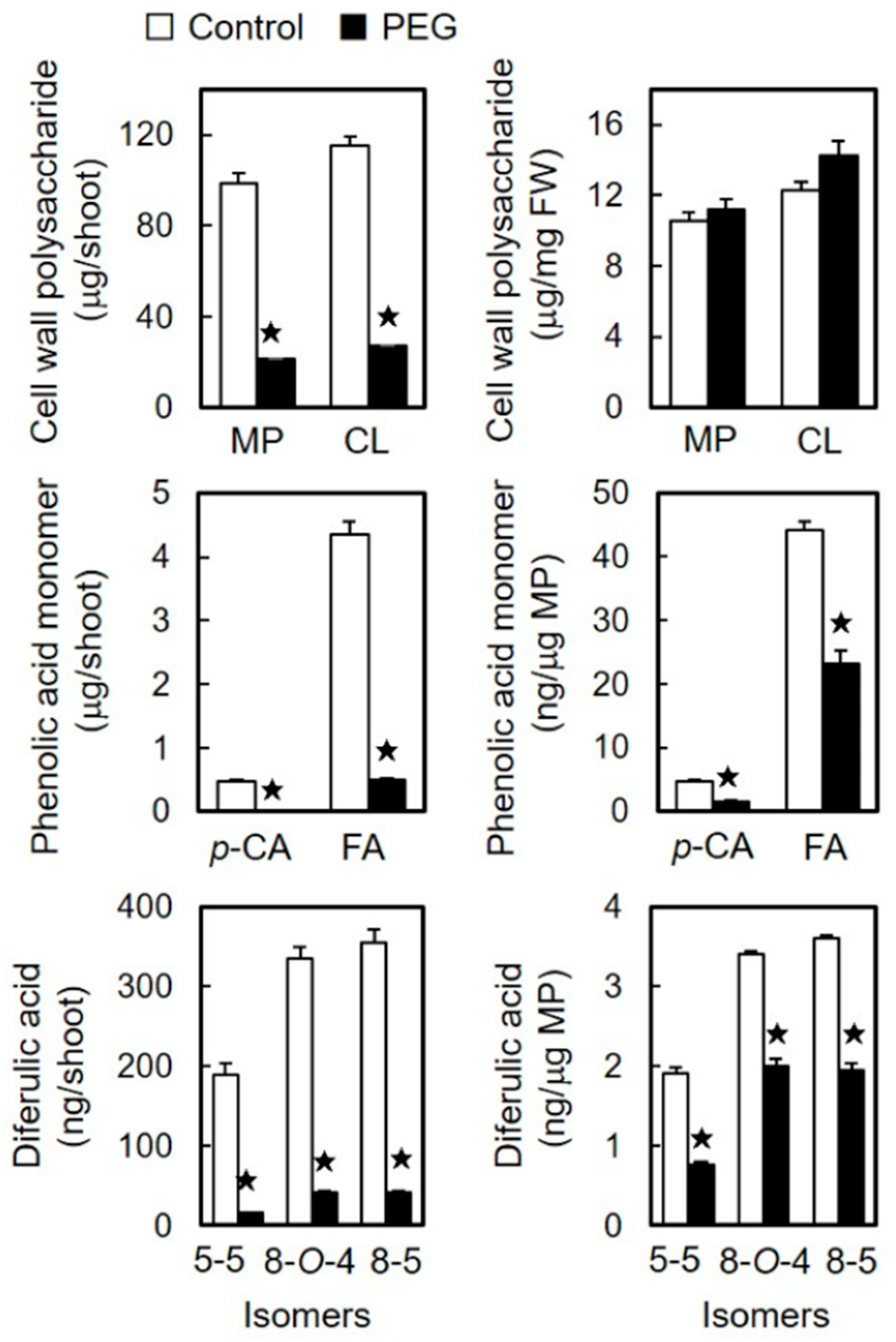
3.2. Effects of Osmotic Stress on the Amounts of Cell Wall Constituents
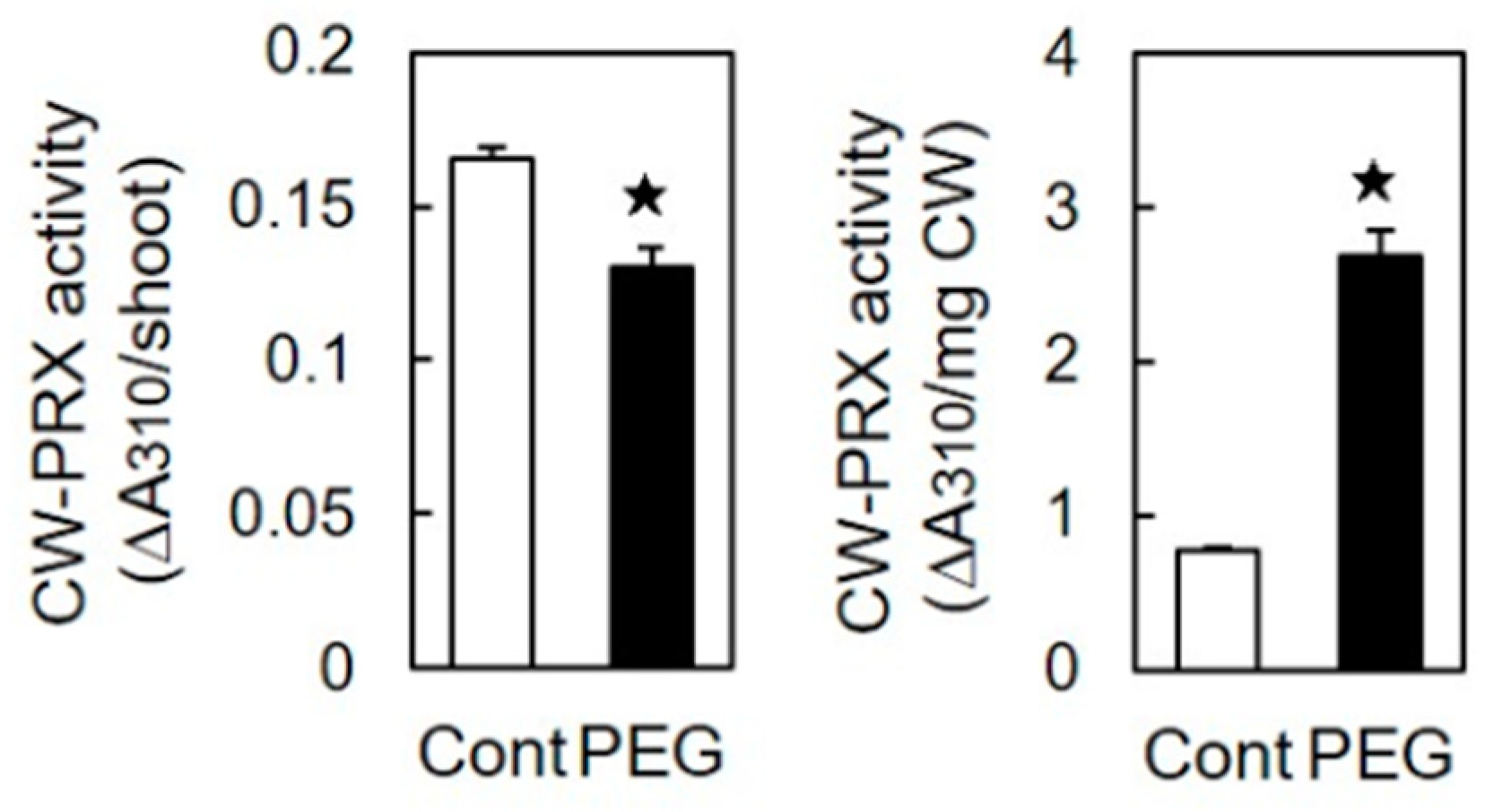
3.3. Effects of Osmotic Stress on Activity and Gene Expression of CW-PRX
3.4. Effects of Osmotic Stress on Activity and Gene Expression of PAL
3.5. Effects of Osmotic Stress on Gene Expression of BADH Acyltransferases
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cosgrove, D.J. Building an extensible cell wall. Plant Physiol. 2022, 189, 1246–1277. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J. Structure and growth of plant cell walls. Nature Rev. Mol. Cell Biol. 2024, 25, 340–358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Gao, Y.; Zhang, L.; Zhou, Y. The plant cell wall: Biosynthesis, construction, and functions. J. Integr. Plant Biol. 2021, 63, 251–272. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.T.; Kieber, J.J. Dynamic construction, perception, and remodeling of plant cell walls. Annu. Rev. Plant Biol. 2020, 71, 39–69. [Google Scholar] [CrossRef]
- Le Gall, H.; Philippe, F.; Domon, J.-M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell wall metabolism in response to abiotic stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef]
- Tenhaken, R. Cell wall remodeling under abiotic stress. Front. Plant Sci. 2015, 5, 771. [Google Scholar] [CrossRef]
- Novaković, L.; Guo, T.; Bacic, A.; Sampathkumar, A.; Johnson, K. Hitting the wall-Sensing and signaling pathways involved in plant cell wall remodeling in response to abiotic stress. Plants 2018, 7, 89. [Google Scholar] [CrossRef]
- Takahashi, D.; Soga, K.; Kikuchi, T.; Kutsuno, T.; Hao, P.; Sasaki, K.; Nishiyama, Y.; Kidokoro, S.; Sampathkumar, A.; Bacic, A.; et al. Structural changes in cell wall pectic polymers contribute to freezing tolerance induced by cold acclimation in plants. Curr. Biol. 2024, 34, 958–968. [Google Scholar] [CrossRef]
- Hoson, T. Physiological functions of plant cell coverings. J. Plant Res. 2002, 115, 277–282. [Google Scholar] [CrossRef]
- Mabuchi, A.; Soga, K.; Wakabayashi, K.; Hoson, T. Phenotypic screening of Arabidopsis T-DNA insertion lines for cell wall mechanical properties revealed ANTHOCYANINLESS2, a cell wall-related gene. J. Plant Physiol. 2016, 191, 29–35. [Google Scholar] [CrossRef]
- Kikukawa, K.; Takigawa-Imamura, H.; Soga, K.; Kotake, T.; Higaki, T. Smooth elongation of pavement cells induced by RIC1 overexpression leads to marginal protrusions of the cotyledon in Arabidopsis thaliana. Plant Cell Physiol. 2023, 64, 1356–1371. [Google Scholar] [CrossRef] [PubMed]
- Yamaguch, A.; Soga, K.; Wakabayashi, K.; Hoson, T. Modification of xyloglucan metabolism during a decrease in cell wall extensibility in 1-aminocyclopropane-1-carboxylic acid-treated azuki bean epicotyls. Plants 2023, 12, 367. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, K.; Soga, K.; Hoson, T.; Masuda, H. The modification of cell wall properties is involved in the growth inhibition of rice coleoptiles induced by lead stress. Life 2023, 13, 471. [Google Scholar] [CrossRef] [PubMed]
- Carpita, N.C. Structural and biogenesis of the cell walls of grasses. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 445–476. [Google Scholar] [CrossRef]
- Hatfield, R.D.; Rancour, D.M.; Marita, J.M. Grass cell walls: A story of cross-linking. Front. Plant Sci. 2017, 7, 2056. [Google Scholar] [CrossRef]
- Grabber, J.H.; Hatfield, R.D.; Ralph, J.; Zon, J.; Amrhein, N. Ferulate cross-linking in cell walls isolated from maize cell suspensions. Phytochemistry 1995, 40, 1077–1082. [Google Scholar] [CrossRef]
- Saulnier, L.; Crepeau, M.-J.; Lahaye, M.; Thibault, J.-F.; Garcia-Conesa, M.T.; Kroon, P.A.; Williamson, G. Isolation and structural determination of two 5,5′-diferuloyl oligosaccharides indicate that maize heteroxylans are covalently cross-linked by oxidatively coupled ferulates. Carbohydr. Res. 1999, 320, 82–92. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Soga, K.; Kamisaka, S.; Hoson, T. Increase in the level of arabinoxylan-hydroxycinnamate network in cell walls of wheat coleoptiles grown under continuous hypergravity conditions. Physiol. Plant. 2005, 125, 127–134. [Google Scholar] [CrossRef]
- Hossain, M.T.; Soga, K.; Wakabayashi, K.; Kamisaka, S.; Fujii, S.; Yamamoto, R.; Hoson, T. Modification of chemical properties of cell walls by silicon and its role in regulation of the cell wall extensibility in oat leaves. J. Plant Physiol. 2007, 164, 385–393. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Soga, K.; Hoson, T.; Kotake, T.; Yamazaki, T.; Higashibata, A.; Ishioka, N.; Shimazu, T.; Fukui, K.; Osada, I.; et al. Suppression of hydroxycinnamate network formation in cell walls of rice shoots grown under microgravity conditions in space. PLoS ONE 2015, 10, e0137992. [Google Scholar] [CrossRef]
- Barros, J.; Dixon, R.A. Plant phenylalanine/tyrosine ammonia-lyases. Trends. Plant Sci. 2020, 25, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Rohde, A.; Morreel, K.; Ralph, J.; Goeminne, G.; Hostyn, V.; De Rycke, R.; Kushnir, S.; Van Doorsselaere, J.; Joseleau, J.P.; Vuylsteke, M.; et al. Molecular phenotyping of the pal1 and pal2 mutants of Arabidopsis thaliana reveals far-reaching consequences on phenylpropanoid, amino acid, and carbohydrate metabolism. Plant Cell 2004, 16, 2749–2771. [Google Scholar] [CrossRef]
- Huang, J.; Gu, M.; Lai, Z.; Fan, B.; Shi, K.; Zhou, Y.-H.; Yu, J.-Q.; Chen, Z. Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiol. 2010, 153, 1526–1538. [Google Scholar] [CrossRef] [PubMed]
- Buanafina, M.M.d.O.; Morris, P. The impact of cell wall feruloylation on plant growth, responses to environmental stress, plant pathogens and cell wall degradability. Agronomy 2022, 12, 1874. [Google Scholar] [CrossRef]
- Chandrakanth, N.N.; Zhang, C.; Freeman, J.; de Souza, W.R.; Bartley, L.E.; Mitchell, R.A.C. Modification of plant cell walls with hydroxycinnamic acids by BAHD acyltransferases. Front. Plant Sci. 2023, 13, 1088879. [Google Scholar] [CrossRef]
- Xu, D.; Wang, Z.; Zhuang, W.; Wang, T.; Xie, Y. Family characteristics, phylogenetic reconstruction, and potential applications of the plant BAHD acyltransferase family. Front. Plant Sci. 2023, 14, 1218914. [Google Scholar] [CrossRef]
- Wallace, G.; Fry, S.C. In vitro peroxidase-catalysed oxidation of ferulic acid esters. Phytochemistry 1995, 39, 1291–1299. [Google Scholar] [CrossRef]
- González, L.F.; Rojas, M.C.; Perez, F.J. Diferulate and lignin formation is related to biochemical differences of wall-bound peroxidases. Phytochemistry 1999, 50, 711–717. [Google Scholar] [CrossRef]
- Kumar, S.; Sachdeva, S.; Bhat, K.V.; Vats, S. Plant responses to drought stress: Physiological, biochemical and molecular basis. In Biotic and Abiotic Stress Tolerance in Plants; Springer Nature: Singapore, 2018; pp. 1–25. [Google Scholar]
- Sato, H.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Complex plant responses to drought and heat stress under climate change. Plant J. 2024, 117, 1873–1892. [Google Scholar] [CrossRef]
- Oguz, M.C.; Aycan, M.; Oguz, E.; Poyraz, I.; Yildiz, M. Drought stress tolerance in plants: Interplay of molecular, biochemical and physiological responses in important development stages. Physiologia 2022, 2, 180–197. [Google Scholar] [CrossRef]
- Hura, T.; Hura, K.; Grzesiak, S. Possible contribution of cell-wall-bound ferulic acid in drought resistance and recovery in triticale seedlings. J. Plant Physiol. 2009, 166, 1720–1733. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.N.; Hanstein, S.; Faust, F.; Eitenmüller, P.T.; Pitann, B.; Schubert, S. Diferulic acid in the cell wall may contribute to the suppression of shoot growth in the first phase of salt stress in maize. Phytochemistry 2014, 102, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Calderone, S.; Mauri, N.; Manga-Robles, A.; Fornale, S.; Garcia-Mir, L.; Centeno, M.-L.; Sanchez-Retuerta, C.; Ursache, R.; Acebes, J.-L.; Campos, N.; et al. Diverging cell wall strategies for drought adaptation in two maize inbreds with contrasting lodging resistance. Plant Cell Environ. 2024, 47, 1747–1768. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, K.; Hoson, T.; Kamisaka, S. Osmotic stress suppresses the cell wall stiffening and the increase in cell wall-bound ferulic and diferulic acids in wheat coleoptiles. Plant Physiol. 1997, 113, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Shimamoto, K.; Kyozuka, J. Rice as a model for comparative genomics of plants. Annu. Rev. Plant Biol. 2002, 53, 399–419. [Google Scholar] [CrossRef]
- Yamamoto, R.; Shinozaki, K.; Masuda, Y. Stress-relaxation properties of plant cell walls with special reference to auxin action. Plant Cell Physiol. 1970, 11, 947–956. [Google Scholar] [CrossRef]
- Sakurai, N. Cell wall functions in growth and development. A physical and chemical point of view. Bot. Mag. Tokyo 1991, 104, 235–251. [Google Scholar] [CrossRef]
- Passardi, F.; Longet, D.; Penel, C.; Dunand, C. The class III peroxidase multigenic family in rice and its evolution in land plants. Phytochemistry 2004, 65, 1879–1893. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Soga, K.; Hoson, T. Phenylalanine ammonia-lyase and cell wall peroxidase are cooperatively involved in the extensive formation of ferulate network in cell walls of developing rice shoots. J. Plant Physiol. 2012, 169, 262–267. [Google Scholar] [CrossRef]
- Chazen, O.; Neumann, P.M. Hydraulic signals from the roots and rapid cell wall-hardening in growing maize (Zea mays L.) leaves are primary responses to polyethylene glycol-induced water deficits. Plant Physiol. 1994, 104, 1385–1392. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Soga, K.; Hoson, T. Cell wall oxalate oxidase modifies the ferulate metabolism in cell walls of wheat shoots. J. Plant Physiol. 2011, 168, 1997–2000. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Kao, C.H. Osmotic stress-induced changes in cell wall peroxidase activity and hydrogen peroxide level in roots of rice seedlings. Plant Growth Regul. 2002, 37, 177–184. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and biological functions in plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-J.; Yang, M.-F.; Chen, H.; Qu, L.-Q.; Chen, F.; Shen, S.-H. Abscisic acid pretreatment enhances salt tolerance of rice seedlings: Proteomic evidence. Biochim. Biophys. Acta 2010, 1804, 929–940. [Google Scholar] [CrossRef]
- D’Auria, J.C. Acyltransferases in plants: A good time to be BAHD. Curr. Opin. Plant Biol. 2006, 9, 331–340. [Google Scholar] [CrossRef]
- Piston, F.; Uauy, C.; Fu, L.; Langston, J.; Labavitch, J.; Dubcovsky, J. Down-regulation of four putative arabinoxylan feruloyl transferase genes from family PF02458 reduces ester-linked ferulate content in rice cell walls. Planta 2010, 231, 677–691. [Google Scholar] [CrossRef]
- Bartley, L.E.; Peck, M.L.; Kim, S.R.; Ebert, B.; Manisseri, C.; Chiniquy, D.M.; Sykes, R.; Gao, L.; Rautengarten, C.; Vega-Sanchez, M.E.; et al. Overexpression of a BAHD acyltransferase, OsAT10, alters rice cell wall hydroxycinnamic acid content and saccharification. Plant Physiol. 2013, 161, 1615–1633. [Google Scholar] [CrossRef]
- Withers, S.; Lu, F.; Kim, H.; Zhu, Y.; Ralph, J.; Wilkerson, C.G. Identification of grass-specific enzyme that acylates monolignols with p-coumarate. J. Biol. Chem. 2012, 287, 8347–8355. [Google Scholar] [CrossRef]
- Karlen, S.; Zhang, C.; Peck, M.L.; Smith, R.A.; Padmakshan, D.; Helmich, K.E.; Free, H.C.A.; Lee, S.; Smith, B.G.; Lu, F.; et al. Monolignol ferulate conjugates are naturally incorporated into plant lignins. Sci. Adv. 2016, 2, e1600393. [Google Scholar] [CrossRef]
- Choudhury, S.; Moulic, D.; Ghosh, D.; Soliman, M.; Alkhedaide, A.; Gaber, A.; Hossain, A. Drought-induced oxidative stress in pearl millet (Cenchrus americanus L.) at seedling stage: Survival mechanisms through alteration of morphophysiological and antioxidants activity. Life 2022, 12, 1171. [Google Scholar] [CrossRef]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic acid and abiotic stress tolerance in crop plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Hoson, T.; Kamisaka, S. Abscisic acid suppresses the increase in cell wall-bound ferulic and diferulic acid levels in dark-grown wheat (Triticum aestivum L.) coleoptiles. Plant Cell Physiol. 1997, 38, 811–817. [Google Scholar] [CrossRef][Green Version]


| Treatment | Shoot Growth Rate (mm/day) | ||
|---|---|---|---|
| 2 d~3 d | 3 d~4 d | 4 d~5 d | |
| Control | 5.13 ± 0.28 a | 5.05 ± 0.40 a | 4.62 ± 0.42 a |
| PEG | 0.63 ± 0.23 b | 2.13 ± 0.34 b | 2.11 ± 0.39 b |
| PEG → Water | 4.72 ± 0.49 a | ||
| Class III PRX Genes RAP ID | Signal Intensity | Ratio (PEG/Control) | |
|---|---|---|---|
| Control | PEG | ||
| Os01g0205900 (2) | 193 | 135 | 0.70 |
| Os01g0263300 (3) | 315 | 374 | 1.19 |
| Os01g0270300 (4) | 160 | 166 | 1.04 |
| Os07g0157000 (7) | 355 | 368 | 1.04 |
| Os01g0294700 (11) | 873 | 796 | 0.91 |
| Os01g0326000 (12) | 56 | 45 | 0.80 |
| Os01g0962700 (20) | 95 | 101 | 1.06 |
| Os02g0236800 (26) | 207 | 209 | 1.01 |
| Os02g0236600 (27) | 116 | 109 | 0.94 |
| Os02g0833900 (32) | 364 | 291 | 0.80 |
| Os03g0121200 (33) | 216 | 177 | 0.82 |
| Os03g0234900 (39) | 69 | 63 | 0.91 |
| Os03g0339300 (41) | 301 | 335 | 1.11 |
| Os04g0465100 (55) | 88 | 86 | 0.98 |
| Os04g0656800 (58) | 129 | 85 | 0.66 |
| Os04g0688100 (59) | 106 | 133 | 1.25 |
| Os05g0134400 (65) | 1829 | 1692 | 0.93 |
| Os05g0135200 (69) | 43 | 76 | 1.77 |
| Os05g0135500 (71) | 556 | 375 | 0.67 |
| Os05g0499300 (74) | 162 | 168 | 1.04 |
| Os06g0547400 (86) | 171 | 122 | 0.71 |
| Os06g0681600 (89) | 63 | 57 | 0.90 |
| Os06g0695500 (90) | 60 | 42 | 0.70 |
| Os07g0104100 (97) | 198 | 143 | 0.72 |
| Os07g0677100 (110) | 68 | 9 | 0.13 |
| Os07g0677200 (111) | 103 | 10 | 0.10 |
| Os07g0677300 (112) | 148 | 7 | 0.05 |
| Os07g0694300 (116) | 40 | 96 | 2.40 |
| Os10g0109300 (125) | 107 | 112 | 1.05 |
| Os10g0536700 (128) | 114 | 107 | 0.94 |
| Os11g0661600 (134) | 60 | 71 | 1.18 |
| Os12g0191500 (137) | 185 | 75 | 0.41 |
| Os12g0530100 (138) | 59 | 77 | 1.31 |
| Os01g0378100 | 303 | 335 | 1.11 |
| Os03g0434800 | 197 | 311 | 1.58 |
| PAL Genes RAP ID | Signal Intensity | Ratio (PEG/Control) | |
|---|---|---|---|
| Control | PEG | ||
| Os02g0626100 | 487 | 368 | 0.76 |
| Os02g0626400 | 1153 | 702 | 0.61 |
| Os02g0626600 | 10 | 12 | 1.20 |
| Os02g0627100 | 168 | 91 | 0.54 |
| Os04g0518100 | 294 | 183 | 0.62 |
| Os04g0518400 | 12 | 15 | 1.25 |
| Os04g0518200 | 210 | 181 | 0.86 |
| Os05g0427400 | 60 | 38 | 0.63 |
| Os05g0558900 | 264 | 202 | 0.77 |
| Os11g0708900 | 5 | 6 | 1.20 |
| Os12g0520200 | 19 | 16 | 0.84 |
| BAHD Acyltransferase Genes RAP ID | Signal Intensity | Ratio (PEG/Control) | |
|---|---|---|---|
| Control | PEG | ||
| Os01g0615300 (OsAT1) | 203 | 202 | 1.00 |
| Os01g0615200 (OsAT2) | 52 | 52 | 1.00 |
| Os05g0136900 (OsAT3) | 15 | 13 | 0.87 |
| Os01g0291500 (OsAT4) | 162 | 119 | 0.73 |
| Os05g0278500 (OsAT5) | 46 | 32 | 0.70 |
| Os01g0179000 (OsAT6) | 8 | 7 | 0.88 |
| Os05g0179300 (OsAT7) | 128 | 106 | 0.83 |
| Os06g0595800 (OsAT8) | 213 | 180 | 0.85 |
| Os01g0185300 (OsAT9) | 31 | 24 | 0.77 |
| Os06g0594600 (OsAT10) | 18 | 12 | 0.67 |
| Os04g0175500 (OsAT12) | 53 | 67 | 1.26 |
| Os10g0108700 (OsAT15) | 176 | 139 | 0.79 |
| Os10g0122500 (OsAT18) | 5 | 4 | 0.80 |
| Os04g0172400 (OsAT19) | 4 | 4 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wakabayashi, K.; Shibatsugu, M.; Hattori, T.; Soga, K.; Hoson, T. Mechanisms Involved in Cell Wall Remodeling in Etiolated Rice Shoots Grown Under Osmotic Stress. Life 2025, 15, 196. https://doi.org/10.3390/life15020196
Wakabayashi K, Shibatsugu M, Hattori T, Soga K, Hoson T. Mechanisms Involved in Cell Wall Remodeling in Etiolated Rice Shoots Grown Under Osmotic Stress. Life. 2025; 15(2):196. https://doi.org/10.3390/life15020196
Chicago/Turabian StyleWakabayashi, Kazuyuki, Motomi Shibatsugu, Takayuki Hattori, Kouichi Soga, and Takayuki Hoson. 2025. "Mechanisms Involved in Cell Wall Remodeling in Etiolated Rice Shoots Grown Under Osmotic Stress" Life 15, no. 2: 196. https://doi.org/10.3390/life15020196
APA StyleWakabayashi, K., Shibatsugu, M., Hattori, T., Soga, K., & Hoson, T. (2025). Mechanisms Involved in Cell Wall Remodeling in Etiolated Rice Shoots Grown Under Osmotic Stress. Life, 15(2), 196. https://doi.org/10.3390/life15020196







