Sirtuin-1 Regulates Mitochondrial Calcium Uptake Through Mitochondrial Calcium Uptake 1 (MICU1)
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Cells
2.2. Cell Culture
2.3. Mitochondrial Extraction
2.4. Western Blot Analysis
2.5. Cell Immunofluorescence
2.6. RNA Interference
2.7. Bacterial Plasmid Transformation
2.8. Cell Transfection
2.9. Glutathione S-Transferase (GST) Pull-Down Assay
2.10. Co-Immunoprecipitation (Co-IP)
2.11. Mitochondrial Calcium Signal Detection
2.12. Quantification and Statistical Analysis
3. Results
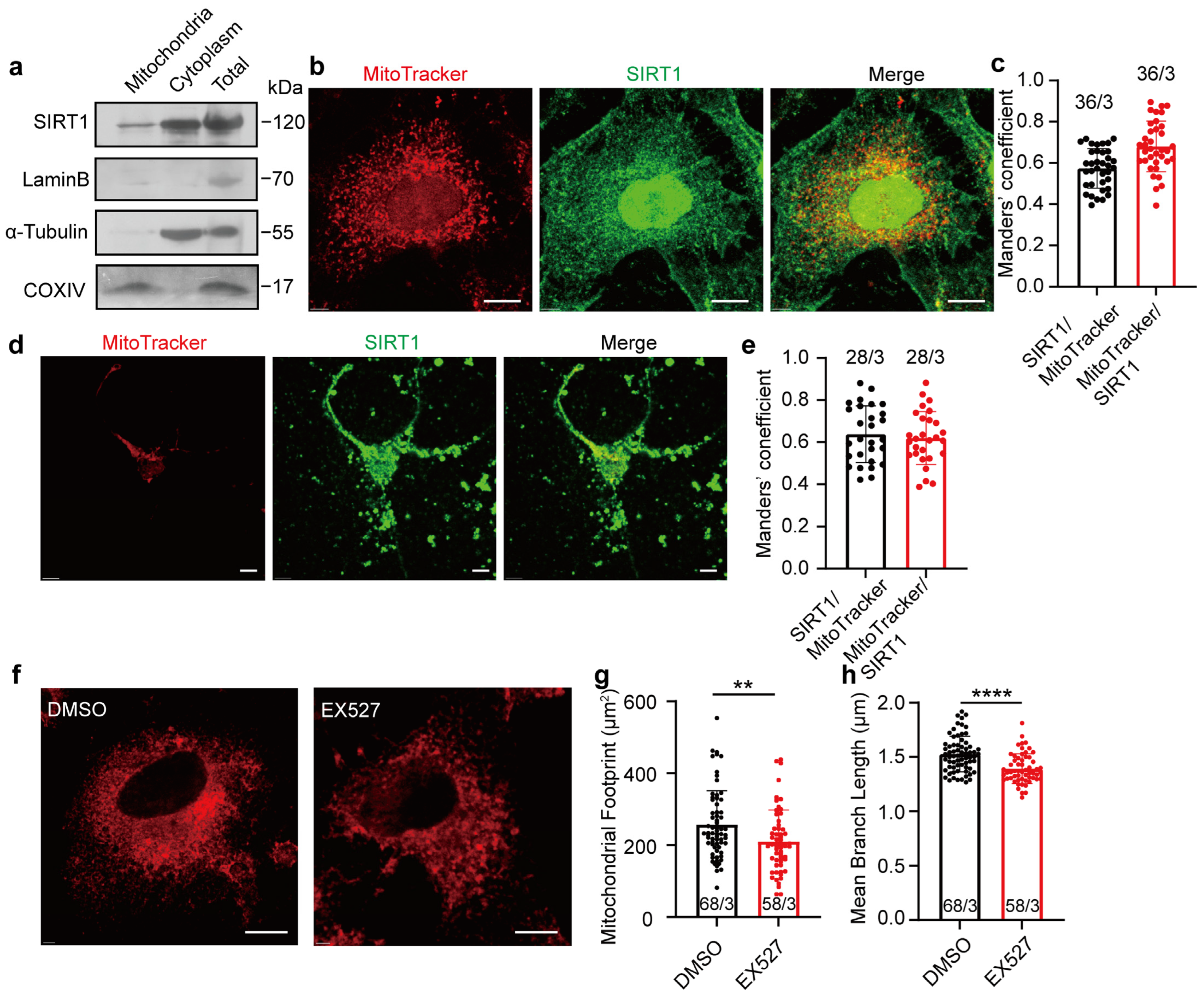
3.1. Long-Term Inhibition of SIRT1 Alters Mitochondrial Morphology in HeLa Cells
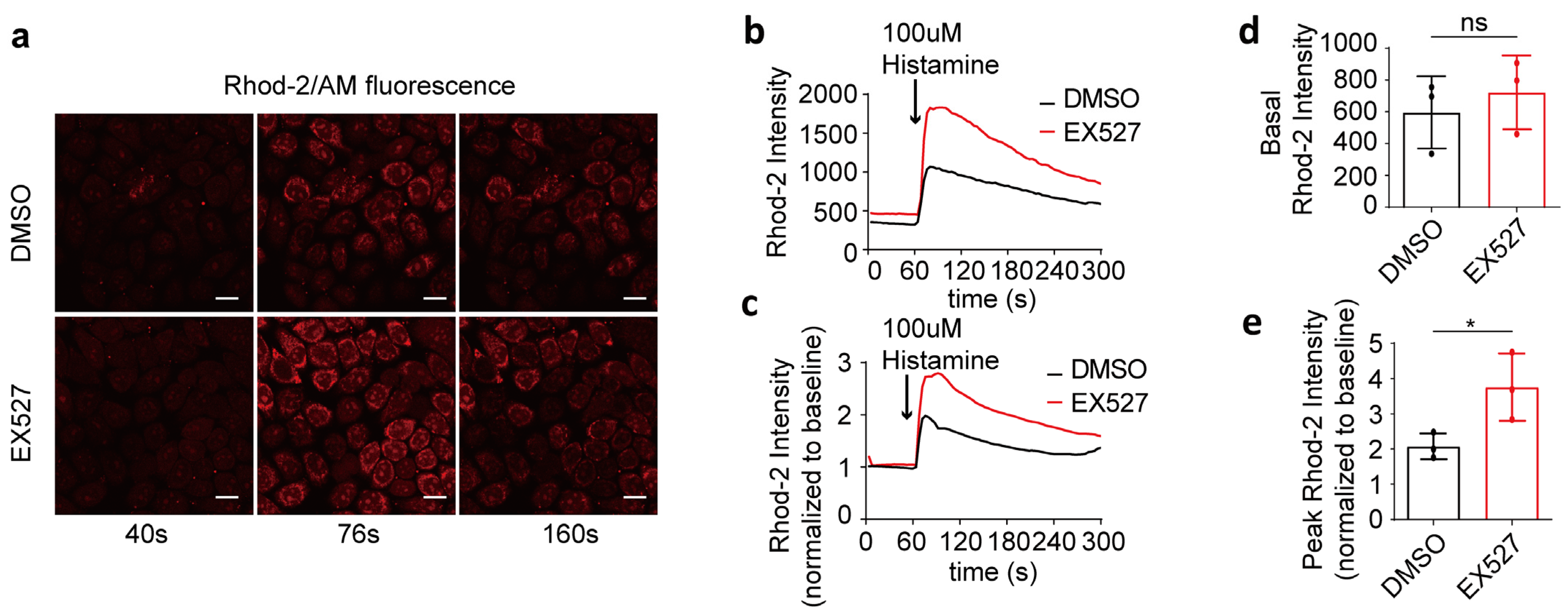
3.2. SIRT1 Inhibitor EX527 Enhanced Histamine-Induced Mitochondrial Calcium Overload in HeLa Cells
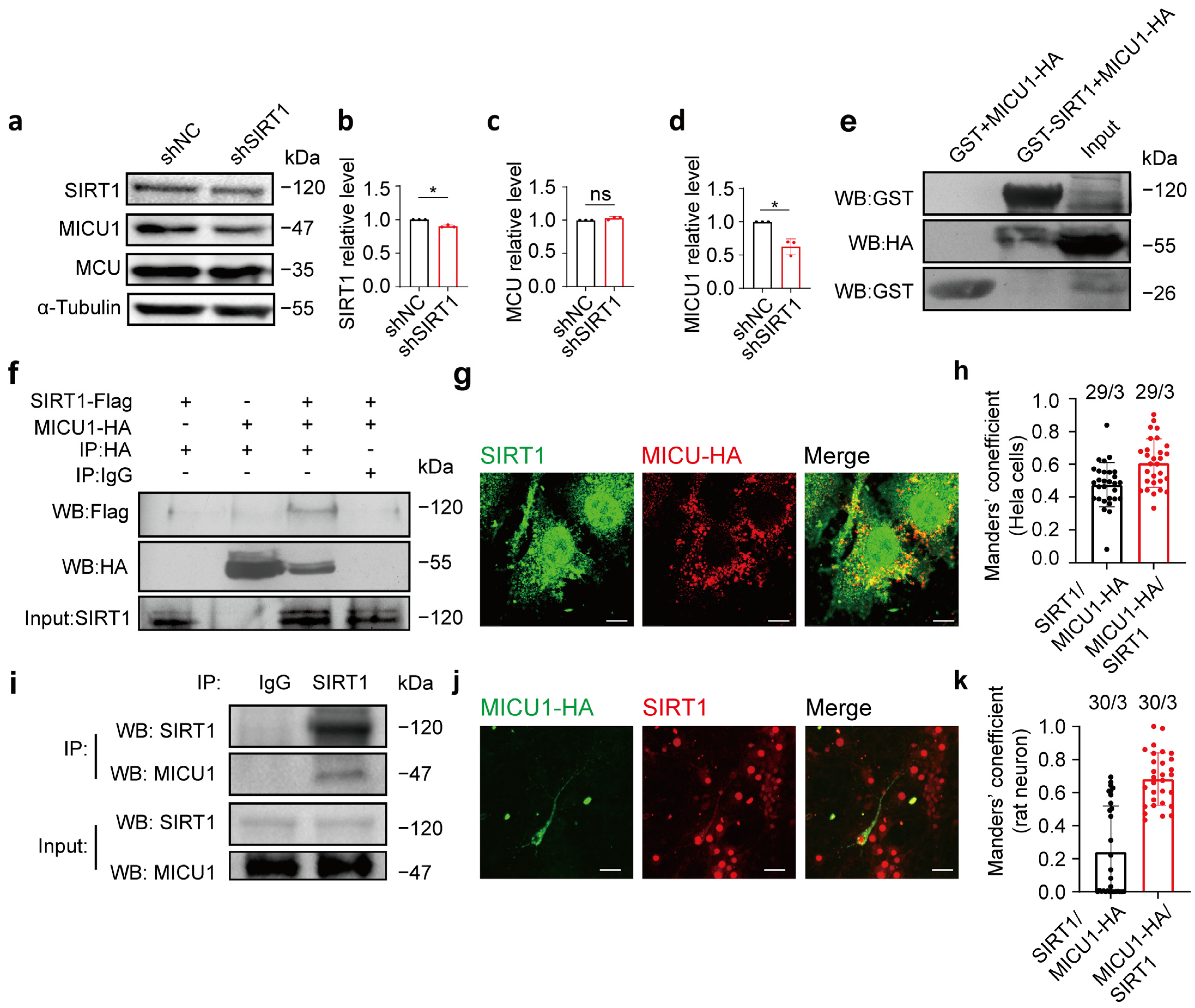
3.3. SIRT1 shRNA Exacerbates Histamine-Induced Mitochondrial Calcium Overload in HeLa Cells
3.4. MICU1 and SIRT1 Interacted with Each Other
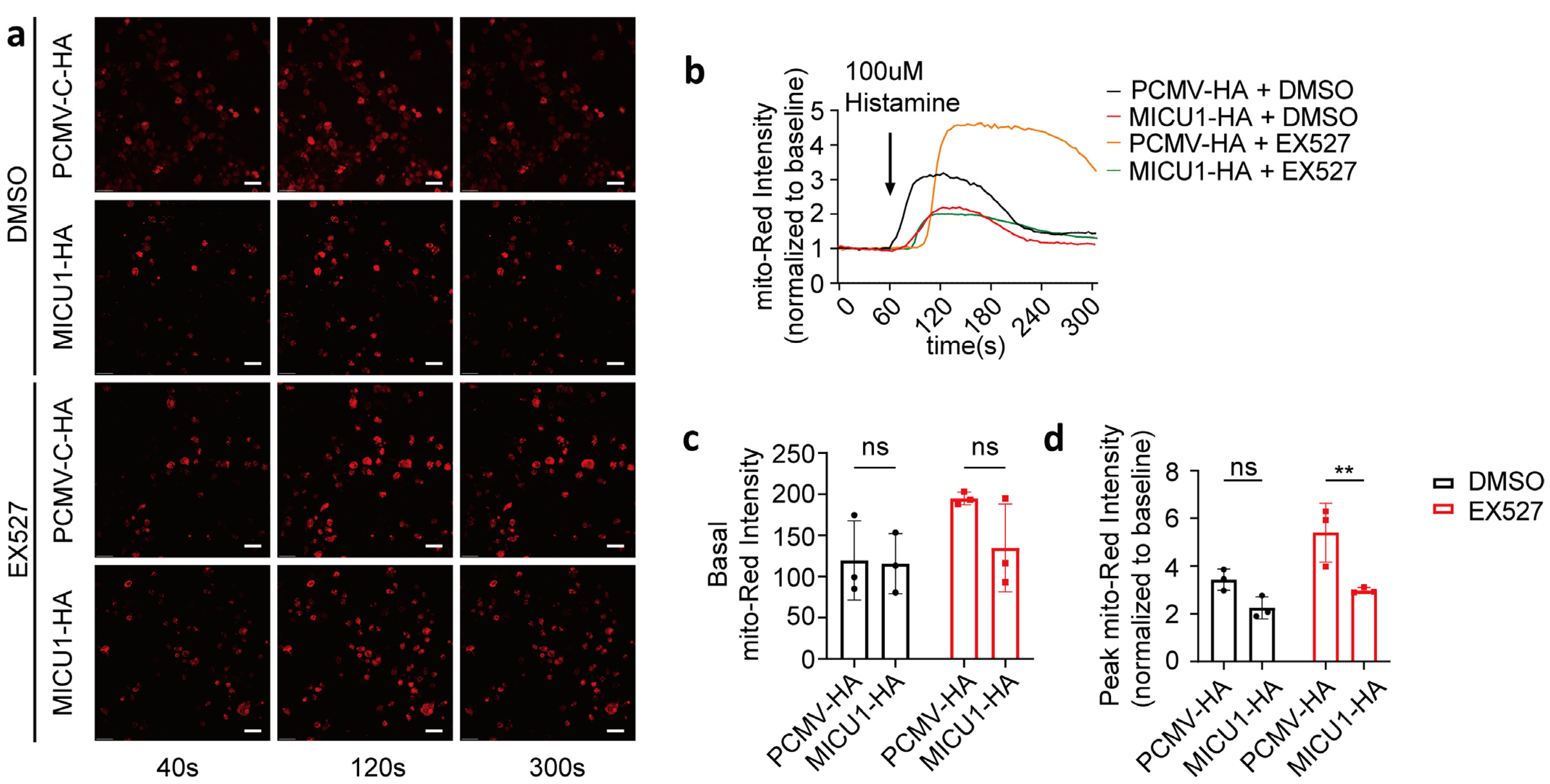
3.5. Overexpression of MICU1 Mitigated the Mitochondrial Calcium Overload Induced by SIRT1 Inhibition
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luongo, T.S.; Lambert, J.P.; Gross, P.; Nwokedi, M.; Lombardi, A.A.; Shanmughapriya, S.; Carpenter, A.C.; Kolmetzky, D.; Gao, E.; van Berlo, J.H.; et al. The mitochondrial Na+/Ca2+ exchanger is essential for Ca2+ homeostasis and viability. Nature 2017, 545, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Shanmughapriya, S.; Tomar, D.; Siddiqui, N.; Lynch, S.; Nemani, N.; Breves, S.L.; Zhang, X.; Tripathi, A.; Palaniappan, P.; et al. Mitochondrial Ca2+ Uniporter Is a Mitochondrial Luminal Redox Sensor that Augments MCU Channel Activity. Mol. Cell 2017, 65, 1014–1028. [Google Scholar] [CrossRef]
- Bick, A.G.; Calvo, S.E.; Mootha, V.K. Evolutionary diversity of the mitochondrial calcium uniporter. Science 2012, 336, 886. [Google Scholar] [CrossRef]
- Pittis, A.A.; Goh, V.; Cebrian-Serrano, A.; Wettmarshausen, J.; Perocchi, F.; Gabaldon, T. Discovery of EMRE in fungi resolves the true evolutionary history of the mitochondrial calcium uniporter. Nat. Commun. 2020, 11, 4031. [Google Scholar] [CrossRef] [PubMed]
- Garbincius, J.F.; Elrod, J.W. Mitochondrial calcium exchange in physiology and disease. Physiol. Rev. 2022, 102, 893–992. [Google Scholar] [CrossRef]
- Fan, M.; Zhang, J.; Tsai, C.W.; Orlando, B.J.; Rodriguez, M.; Xu, Y.; Liao, M.; Tsai, M.F.; Feng, L. Structure and mechanism of the mitochondrial Ca2+ uniporter holocomplex. Nature 2020, 582, 129–133. [Google Scholar] [CrossRef]
- Tsai, C.W.; Rodriguez, M.X.; Van Keuren, A.M.; Phillips, C.B.; Shushunov, H.M.; Lee, J.E.; Garcia, A.M.; Ambardekar, A.V.; Cleveland, J.J.; Reisz, J.A.; et al. Mechanisms and significance of tissue-specific MICU regulation of the mitochondrial calcium uniporter complex. Mol. Cell 2022, 82, 3661–3676. [Google Scholar] [CrossRef]
- Liang, T.; Hang, W.; Chen, J.; Wu, Y.; Wen, B.; Xu, K.; Ding, B.; Chen, J. ApoE4 (Δ272–299) induces mitochondrial-associated membrane formation and mitochondrial impairment by enhancing GRP75-modulated mitochondrial calcium overload in neuron. Cell Biosci. 2021, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- Marinho, D.; Ferreira, I.L.; Lorenzoni, R.; Cardoso, S.M.; Santana, I.; Rego, A.C. Reduction of class I histone deacetylases ameliorates ER-mitochondria cross-talk in Alzheimer’s disease. Aging Cell 2023, 22, e13895. [Google Scholar] [CrossRef]
- Apicco, D.J.; Shlevkov, E.; Nezich, C.L.; Tran, D.T.; Guilmette, E.; Nicholatos, J.W.; Bantle, C.M.; Chen, Y.; Glajch, K.E.; Abraham, N.A.; et al. The Parkinson’s disease-associated gene ITPKB protects against alpha-synuclein aggregation by regulating ER-to-mitochondria calcium release. Proc. Natl. Acad. Sci. USA 2021, 118, e2006476118. [Google Scholar] [CrossRef]
- Beccano-Kelly, D.A.; Cherubini, M.; Mousba, Y.; Cramb, K.; Giussani, S.; Caiazza, M.C.; Rai, P.; Vingill, S.; Bengoa-Vergniory, N.; Ng, B.; et al. Calcium dysregulation combined with mitochondrial failure and electrophysiological maturity converge in Parkinson’s iPSC-dopamine neurons. iScience 2023, 26, 107044. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Y.; Wang, Y.; Chao, Y.; Zhang, J.; Jia, Y.; Tie, J.; Hu, D. Regulation of SIRT1 and Its Roles in Inflammation. Front. Immunol. 2022, 13, 831168. [Google Scholar] [CrossRef]
- Qiu, L.L.; Tan, X.X.; Yang, J.J.; Ji, M.H.; Zhang, H.; Zhao, C.; Xia, J.Y.; Sun, J. Lactate Improves Long-Term Cognitive Impairment Induced by Repeated Neonatal Sevoflurane Exposures Through SIRT1-Mediated Regulation of Adult Hippocampal Neurogenesis and Synaptic Plasticity in Male Mice. Mol. Neurobiol. 2023, 60, 5273–5291. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wu, Z.; Bai, L.; Liu, R.; Ba, Y.; Zhang, H.; Cheng, X.; Zhou, G.; Huang, H. Resveratrol improved hippocampal neurogenesis following lead exposure in rats through activation of SIRT1 signaling. Environ. Toxicol. 2021, 36, 1664–1673. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Tang, S.; Fan, X.; Fang, Y.; Xu, X.; Li, L.; Xu, J.; Li, J.L.; Wang, Z.; Li, X. SIRT1 regulates sphingolipid metabolism and neural differentiation of mouse embryonic stem cells through c-Myc-SMPDL3B. eLife 2021, 10, e67452. [Google Scholar] [CrossRef] [PubMed]
- Surya, K.; Manickam, N.; Jayachandran, K.S.; Kandasamy, M.; Anusuyadevi, M. Resveratrol Mediated Regulation of Hippocampal Neuroregenerative Plasticity via SIRT1 Pathway in Synergy with Wnt Signaling: Neurotherapeutic Implications to Mitigate Memory Loss in Alzheimer’s Disease. J. Alzheimer’s Dis. 2023, 94, S125–S140. [Google Scholar] [CrossRef] [PubMed]
- Marin, C.; Langdon, C.; Alobid, I.; Fuentes, M.; Bonastre, M.; Mullol, J. Recovery of Olfactory Function After Excitotoxic Lesion of the Olfactory Bulbs Is Associated with Increases in Bulbar SIRT1 and SIRT4 Expressions. Mol. Neurobiol. 2019, 56, 5643–5653. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.J.; Zhang, W.Q.; Liu, J.J.; Cui, Y.; Cui, J.Z. Piceatannol protects against cerebral ischemia/reperfusion-induced apoptosis and oxidative stress via the Sirt1/FoxO1 signaling pathway. Mol. Med. Rep. 2020, 22, 5399–5411. [Google Scholar] [CrossRef]
- Li, L.; Zhi, D.; Cheng, R.; Li, J.; Luo, C.; Li, H. The neuroprotective role of SIRT1/PGC-1alpha signaling in limb postconditioning in cerebral ischemia/reperfusion injury. Neurosci. Lett. 2021, 749, 135736. [Google Scholar] [CrossRef] [PubMed]
- Hang, W.; Shu, H.; Wen, Z.; Liu, J.; Jin, Z.; Shi, Z.; Chen, C.; Wang, D.W. N-Acetyl Cysteine Ameliorates High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease and Intracellular Triglyceride Accumulation by Preserving Mitochondrial Function. Front. Pharmacol. 2021, 12, 636204. [Google Scholar] [CrossRef] [PubMed]
- Stoyas, C.A.; Bushart, D.D.; Switonski, P.M.; Ward, J.M.; Alaghatta, A.; Tang, M.B.; Niu, C.; Wadhwa, M.; Huang, H.; Savchenko, A.; et al. Nicotinamide Pathway-Dependent Sirt1 Activation Restores Calcium Homeostasis to Achieve Neuroprotection in Spinocerebellar Ataxia Type 7. Neuron 2020, 105, 630–644. [Google Scholar] [CrossRef]
- Song, S.B.; Park, J.S.; Jang, S.Y.; Hwang, E.S. Nicotinamide Treatment Facilitates Mitochondrial Fission Through Drp1 Activation Mediated by SIRT1-Induced Changes in Cellular Levels of cAMP and Ca2+. Cells 2021, 10, 612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; He, Z.; Fedorova, J.; Logan, C.; Bates, L.; Davitt, K.; Le, V.; Murphy, J.; Li, M.; Wang, M.; et al. Alterations in mitochondrial dynamics with age-related Sirtuin1/Sirtuin3 deficiency impair cardiomyocyte contractility. Aging Cell 2021, 20, e13419. [Google Scholar] [CrossRef] [PubMed]
- Solomon, J.M.; Pasupuleti, R.; Xu, L.; McDonagh, T.; Curtis, R.; DiStefano, P.S.; Huber, L.J. Inhibition of SIRT1 catalytic activity increases p53 acetylation but does not alter cell survival following DNA damage. Mol. Cell. Biol. 2006, 26, 28–38. [Google Scholar] [CrossRef]
- Napper, A.D.; Hixon, J.; McDonagh, T.; Keavey, K.; Pons, J.F.; Barker, J.; Yau, W.T.; Amouzegh, P.; Flegg, A.; Hamelin, E.; et al. Discovery of indoles as potent and selective inhibitors of the deacetylase SIRT1. J. Med. Chem. 2005, 48, 8045–8054. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ding, H.; Tang, X.; Liang, M.; Li, S.; Zhang, J.; Cao, J. Quercetin induces pro-apoptotic autophagy via SIRT1/AMPK signaling pathway in human lung cancer cell lines A549 and H1299 in vitro. Thorac. Cancer 2021, 12, 1415–1422. [Google Scholar] [CrossRef]
- Zinchuk, V.; Zinchuk, O. Quantitative colocalization analysis of confocal fluorescence microscopy images. Curr. Protoc. Cell Biol. 2008, 39, 4.19.1–4.19.16. [Google Scholar] [CrossRef] [PubMed]
- Valente, A.J.; Maddalena, L.A.; Robb, E.L.; Moradi, F.; Stuart, J.A. A simple ImageJ macro tool for analyzing mitochondrial network morphology in mammalian cell culture. Acta Histochem. 2017, 119, 315–326. [Google Scholar] [CrossRef]
- Michishita, E.; Park, J.Y.; Burneskis, J.M.; Barrett, J.C.; Horikawa, I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol. Biol. Cell 2005, 16, 4623–4635. [Google Scholar] [CrossRef]
- Dai, S.H.; Chen, T.; Wang, Y.H.; Zhu, J.; Luo, P.; Rao, W.; Yang, Y.F.; Fei, Z.; Jiang, X.F. Sirt3 protects cortical neurons against oxidative stress via regulating mitochondrial Ca2+ and mitochondrial biogenesis. Int. J. Mol. Sci. 2014, 15, 14591–14609. [Google Scholar] [CrossRef]
- Cheng, A.; Yang, Y.; Zhou, Y.; Maharana, C.; Lu, D.; Peng, W.; Liu, Y.; Wan, R.; Marosi, K.; Misiak, M.; et al. Mitochondrial SIRT3 Mediates Adaptive Responses of Neurons to Exercise and Metabolic and Excitatory Challenges. Cell Metab. 2016, 23, 128–142. [Google Scholar] [CrossRef]
- Price, N.L.; Gomes, A.P.; Ling, A.J.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S.; et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Lee, M.R.; Huang, X.; Messina-Graham, S.; Broxmeyer, H.E. SIRT1 positively regulates autophagy and mitochondria function in embryonic stem cells under oxidative stress. Stem Cells 2014, 32, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Liu, J.; Wu, W.; Guo, T.; Yuan, J.; Wu, Z.; Zheng, Z.; Zhao, Z.; Lin, Q.; Liu, N.; et al. SIRT1 restores mitochondrial structure and function in rats by activating SIRT3 after cerebral ischemia/reperfusion injury. Cell Biol. Toxicol. 2024, 40, 31. [Google Scholar] [CrossRef]
- Marques, P.; Burillo, J.; Gonzalez-Blanco, C.; Jimenez, B.; Garcia, G.; Garcia-Aguilar, A.; Iglesias-Fortes, S.; Lockwood, A.; Guillen, C. Regulation of TSC2 lysosome translocation and mitochondrial turnover by TSC2 acetylation status. Sci. Rep. 2024, 14, 12521. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.P.; Mishra, S.R.; Mahapatra, K.K.; Patil, S.; Efferth, T.; Bhutia, S.K. SIRT1-activating butein inhibits arecoline-induced mitochondrial dysfunction through PGC1alpha and MTP18 in oral cancer. Phytomedicine 2024, 129, 155511. [Google Scholar] [CrossRef] [PubMed]
- Mallilankaraman, K.; Doonan, P.; Cardenas, C.; Chandramoorthy, H.C.; Muller, M.; Miller, R.; Hoffman, N.E.; Gandhirajan, R.K.; Molgo, J.; Birnbaum, M.J.; et al. MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca2+ uptake that regulates cell survival. Cell 2012, 151, 630–644. [Google Scholar] [CrossRef]
- Gottschalk, B.; Malli, R.; Graier, W.F. MICU1 deficiency alters mitochondrial morphology and cytochrome C release. Cell Calcium 2023, 113, 102765. [Google Scholar] [CrossRef] [PubMed]
- Logan, C.V.; Szabadkai, G.; Sharpe, J.A.; Parry, D.A.; Torelli, S.; Childs, A.M.; Kriek, M.; Phadke, R.; Johnson, C.A.; Roberts, N.Y.; et al. Loss-of-function mutations in MICU1 cause a brain and muscle disorder linked to primary alterations in mitochondrial calcium signaling. Nat. Genet. 2014, 46, 188–193. [Google Scholar] [CrossRef]
- Debattisti, V.; Horn, A.; Singh, R.; Seifert, E.L.; Hogarth, M.W.; Mazala, D.A.; Huang, K.T.; Horvath, R.; Jaiswal, J.K.; Hajnoczky, G. Dysregulation of Mitochondrial Ca2+ Uptake and Sarcolemma Repair Underlie Muscle Weakness and Wasting in Patients and Mice Lacking MICU1. Cell Rep. 2019, 29, 1274–1286. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Pei, H.; Liu, Q.; Zhao, M.; Sun, J.; Gao, E.; Ma, X.; Tao, L. MICU1 protects against myocardial ischemia/reperfusion injury and its control by the importer receptor Tom70. Cell Death Dis. 2017, 8, e2923. [Google Scholar] [CrossRef] [PubMed]
- Seegren, P.V.; Harper, L.R.; Downs, T.K.; Zhao, X.Y.; Viswanathan, S.B.; Stremska, M.E.; Olson, R.J.; Kennedy, J.; Ewald, S.E.; Kumar, P.; et al. Reduced mitochondrial calcium uptake in macrophages is a major driver of inflammaging. Nat. Aging 2023, 3, 796–812. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yang, Y.M.; Hu, Y.Y.; Ouyang, L.; Sun, Z.H.; Yin, X.F.; Li, N.; He, Q.Y.; Wang, Y. Inhibition of nuclear deacetylase Sirtuin-1 induces mitochondrial acetylation and calcium overload leading to cell death. Redox Biol. 2022, 53, 102334. [Google Scholar] [CrossRef] [PubMed]
- Jadiya, P.; Kolmetzky, D.W.; Tomar, D.; Di Meco, A.; Lombardi, A.A.; Lambert, J.P.; Luongo, T.S.; Ludtmann, M.H.; Pratico, D.; Elrod, J.W. Impaired mitochondrial calcium efflux contributes to disease progression in models of Alzheimer’s disease. Nat. Commun. 2019, 10, 3885. [Google Scholar] [CrossRef] [PubMed]
- Matteucci, A.; Patron, M.; Vecellio, R.D.; Gastaldello, S.; Amoroso, S.; Rizzuto, R.; Brini, M.; Raffaello, A.; Cali, T. Parkin-dependent regulation of the MCU complex component MICU1. Sci. Rep. 2018, 8, 14199. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.M.; Chen, S.; Almeida, S.; Riley, R.; Goncalves, J.; Oliveira, C.R.; Hayden, M.R.; Nicholls, D.G.; Ellerby, L.M.; Rego, A.C. Mitochondrial-dependent Ca2+ handling in Huntington’s disease striatal cells: Effect of histone deacetylase inhibitors. J. Neurosci. 2006, 26, 11174–11186. [Google Scholar] [CrossRef]
- Singh, R.; Bartok, A.; Paillard, M.; Tyburski, A.; Elliott, M.; Hajnoczky, G. Uncontrolled mitochondrial calcium uptake underlies the pathogenesis of neurodegeneration in MICU1-deficient mice and patients. Sci. Adv. 2022, 8, eabj4716. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Liu, S.; Su, Y.; Zhang, L.; Guo, T.; Wang, X. Sirtuin-1 Regulates Mitochondrial Calcium Uptake Through Mitochondrial Calcium Uptake 1 (MICU1). Life 2025, 15, 174. https://doi.org/10.3390/life15020174
Zhang X, Liu S, Su Y, Zhang L, Guo T, Wang X. Sirtuin-1 Regulates Mitochondrial Calcium Uptake Through Mitochondrial Calcium Uptake 1 (MICU1). Life. 2025; 15(2):174. https://doi.org/10.3390/life15020174
Chicago/Turabian StyleZhang, Xinyi, Shuhu Liu, Yanshan Su, Ling Zhang, Ting Guo, and Xuemin Wang. 2025. "Sirtuin-1 Regulates Mitochondrial Calcium Uptake Through Mitochondrial Calcium Uptake 1 (MICU1)" Life 15, no. 2: 174. https://doi.org/10.3390/life15020174
APA StyleZhang, X., Liu, S., Su, Y., Zhang, L., Guo, T., & Wang, X. (2025). Sirtuin-1 Regulates Mitochondrial Calcium Uptake Through Mitochondrial Calcium Uptake 1 (MICU1). Life, 15(2), 174. https://doi.org/10.3390/life15020174





