DYRK1A Up-Regulation Specifically Impairs a Presynaptic Form of Long-Term Potentiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Genotyping
2.2. Quantitative In Situ Hybridization (ISH)
2.3. Protein Extraction and Western Blot Analysis
2.4. Laser-Assisted Microdissection, Total RNA Preparation and Quantitative Real-Time PCR (Q-RT-PCR) Analysis
2.5. Proximity Ligation Assay (PLA) Analysis
2.6. Electrophysiological Analysis on 152F7 Juvenile Mice
2.7. Electrophysiological Analysis of 189N3 Adult Mice
2.8. Bioinformatics Analysis
2.9. Reagents
2.10. Ethics Statement
3. Results
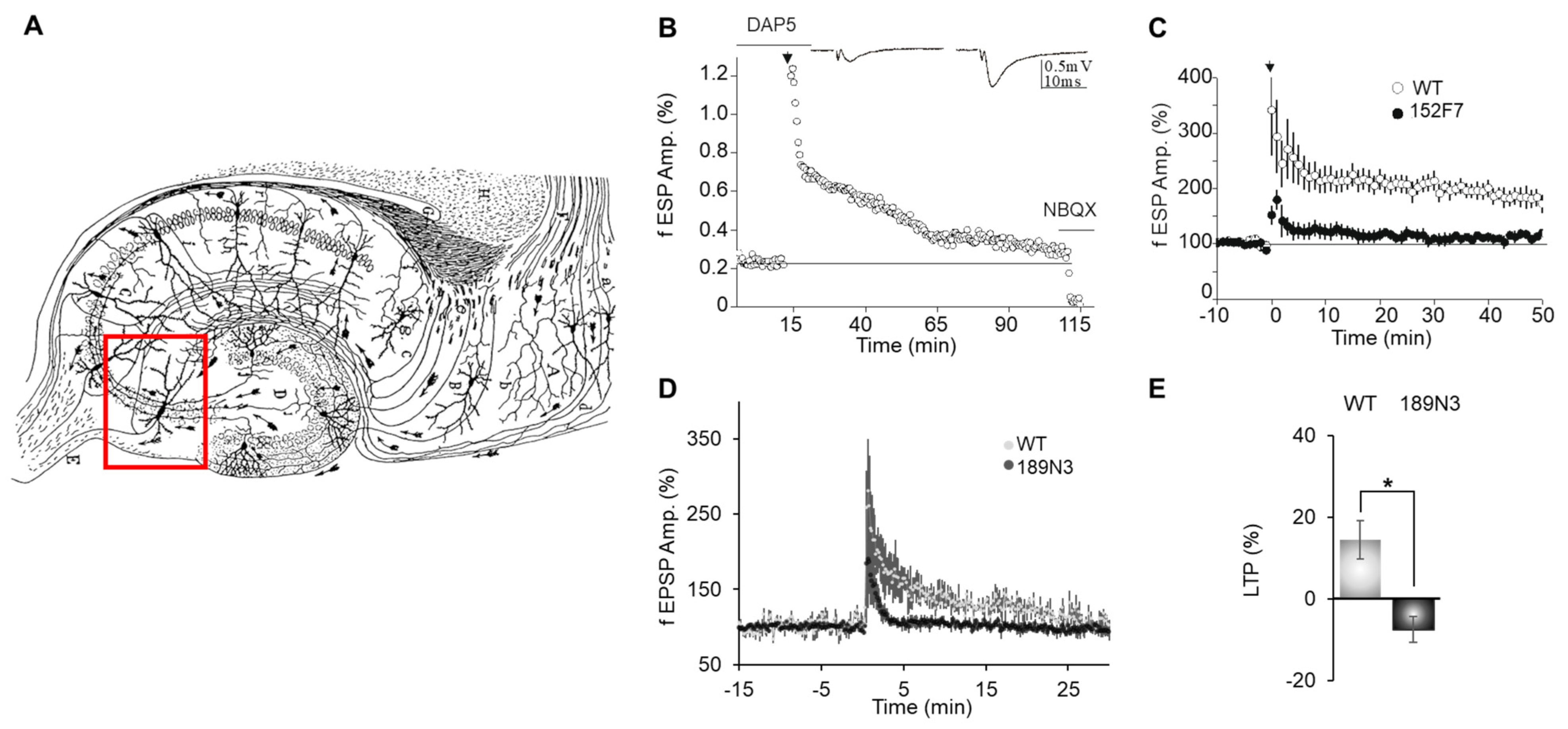
3.1. Dyrk1A Up-Regulation Specifically Impairs a Presynaptic Form of LTP at the DG-CA3 Hippocampal Synapse
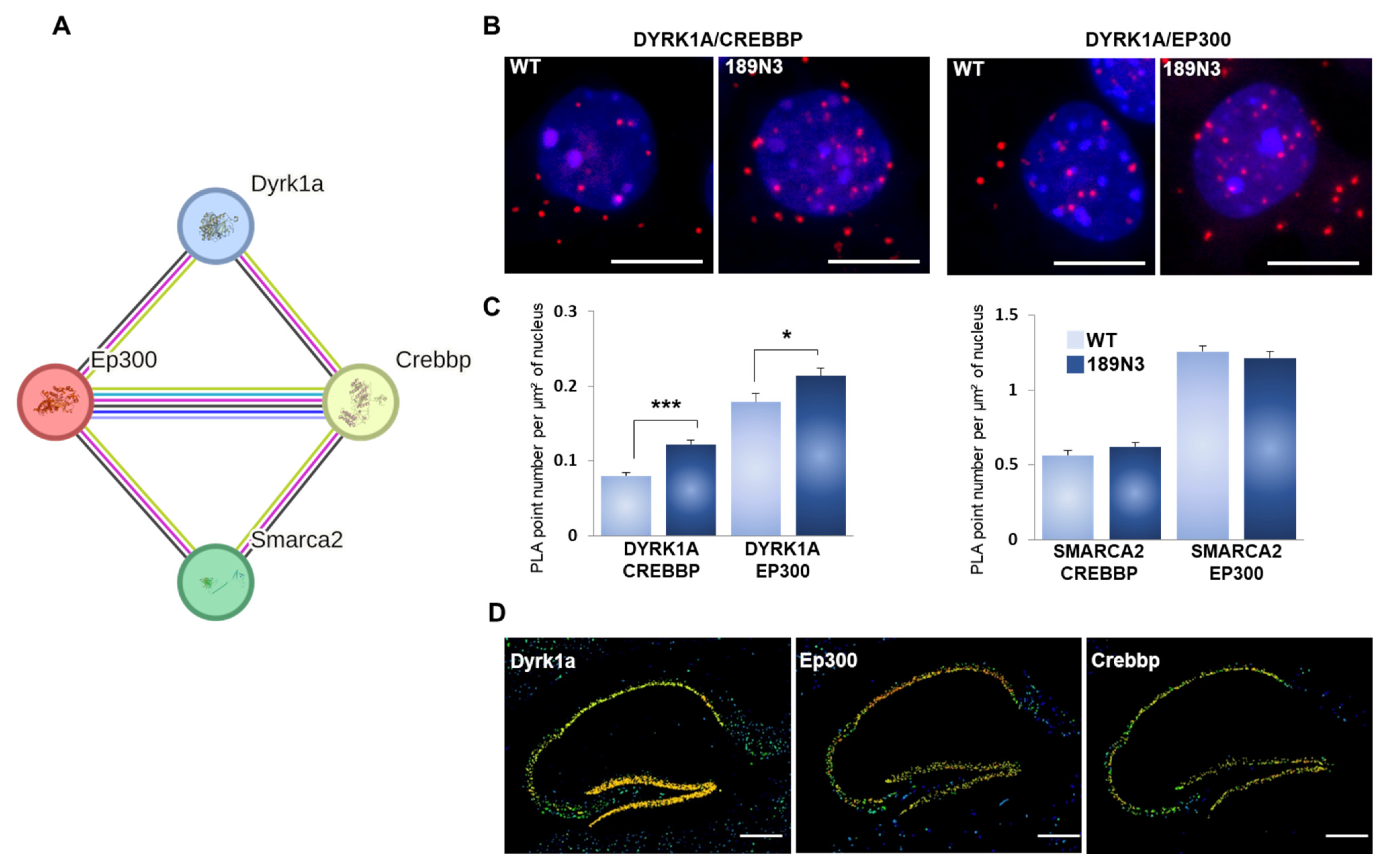
3.2. Mouse DYRK1A Interacts with the Chromatin Remodelers EP300 and CREBBP in the Brain
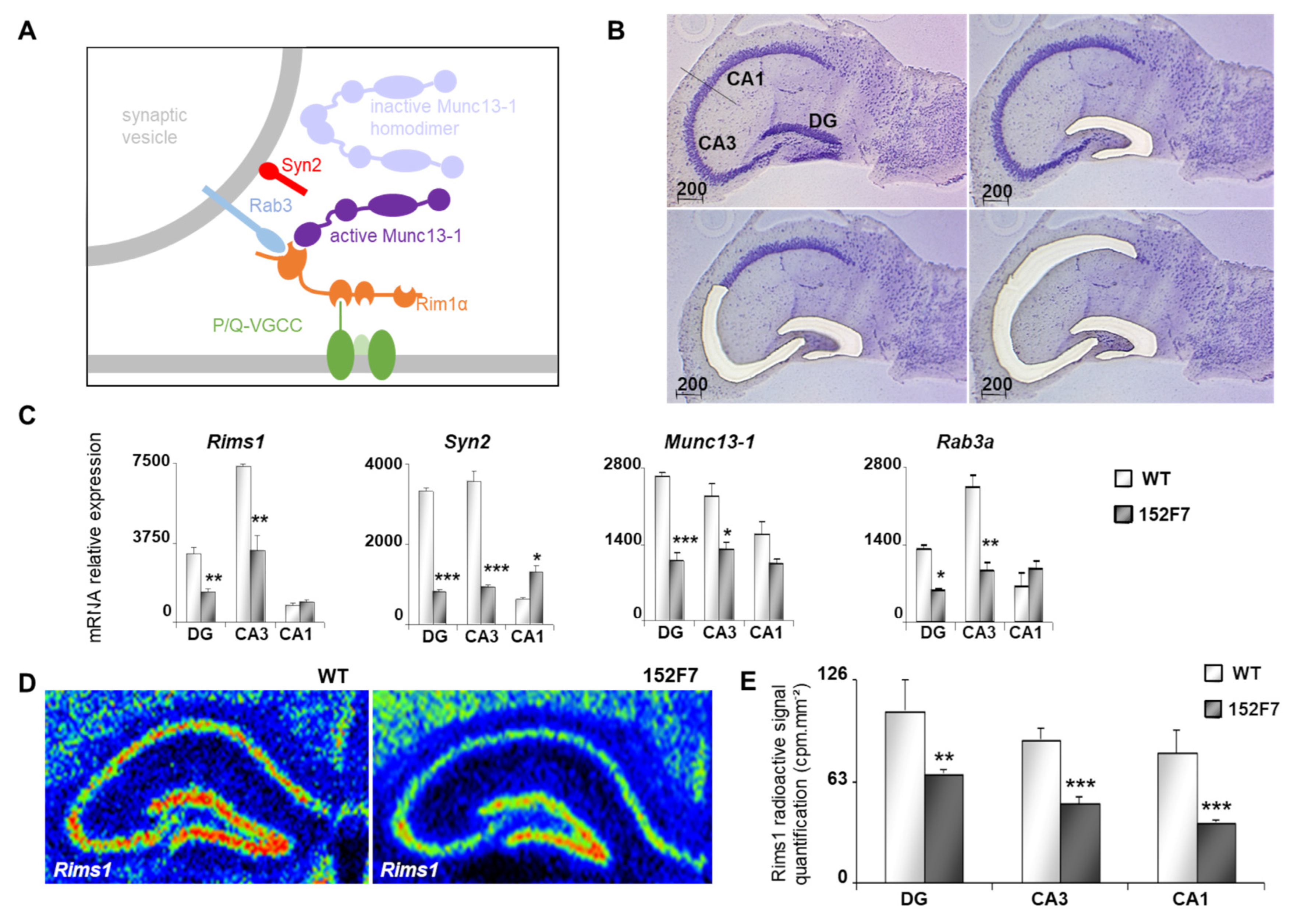
3.3. Dyrk1A Up-Regulation Affects the Transcription of Genes Encoding Presynaptic Proteins
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antonarakis, S.E. Down syndrome and the complexity of genome dosage imbalance. Nat. Rev. Genet. 2017, 18, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Lott, I.T.; Head, E. Dementia in Down syndrome: Unique insights for Alzheimer disease research. Nat. Rev. Neurol. 2019, 15, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Dierssen, M. Down syndrome: The brain in trisomic mode. Nat. Rev. Neurosci. 2012, 13, 844–858. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, F.K.; Al-Janabi, T.; Hardy, J.; Karmiloff-Smith, A.; Nizetic, D.; Tybulewicz, V.L.J.; Fisher, E.M.C.; Strydom, A. A genetic cause of Alzheimer disease: Mechanistic insights from Down syndrome. Nat. Rev. Neurosci. 2015, 16, 564–574. [Google Scholar] [CrossRef]
- Ahn, K.J.; Jeong, H.K.; Choi, H.S.; Ryoo, S.R.; Kim, Y.J.; Goo, J.S.; Choi, S.-Y.; Han, J.-S.; Ha, I.; Song, W.-J. DYRK1A BAC transgenic mice show altered synaptic plasticity with learning and memory defects. Neurobiol. Dis. 2006, 22, 463–472. [Google Scholar] [CrossRef]
- Guedj, F.; Pereira, P.L.; Najas, S.; Barallobre, M.J.; Chabert, C.; Souchet, B.; Sebrie, C.; Verney, C.; Herault, Y.; Arbones, M.; et al. DYRK1A: A master regulatory protein controlling brain growth. Neurobiol. Dis. 2012, 46, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Thomazeau, A.; Lassalle, O.; Iafrati, J.; Souchet, B.; Guedj, F.; Janel, N.; Chavis, P.; Delabar, J.; Manzoni, O.J. Prefrontal Deficits in a Murine Model Overexpressing the Down Syndrome Candidate Gene Dyrk1a. J. Neurosci. 2014, 34, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Liu, C.; Yu, T.; Zhang, L.; Meng, K.; Xing, Z.; Belichenko, P.V.; Kleschevnikov, A.M.; Pao, A.; Peresie, J.; et al. Genetic dissection of the Down syndrome critical region. Hum. Mol. Genet. 2015, 24, 6540–6551. [Google Scholar] [CrossRef] [PubMed]
- Arbones, M.L.; Thomazeau, A.; Nakano-Kobayashi, A.; Hagiwara, M.; Delabar, J.M. DYRK1A and cognition: A lifelong relationship. Pharmacol. Ther. 2019, 194, 199–221. [Google Scholar] [CrossRef] [PubMed]
- Kandel, E.R. The molecular biology of memory storage: A dialogue between genes and synapses. Science 2001, 294, 1030–1038. [Google Scholar] [CrossRef]
- Nicoll, R.A. A Brief History of Long-Term Potentiation. Neuron 2017, 93, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Castillo, P.E. Presynaptic LTP and LTD of Excitatory and Inhibitory Synapses. Cold Spring Harb. Perspect. Biol. 2012, 4, a005728. [Google Scholar] [CrossRef]
- Monday, H.R.; Younts, T.J.; Castillo, P.E. Long-Term Plasticity of Neurotransmitter Release: Emerging Mechanisms and Contributions to Brain Function and Disease. Annu. Rev. Neurosci. 2018, 41, 299–322. [Google Scholar] [CrossRef]
- Smith, D.J.; Stevens, M.E.; Sudanagunta, S.P.; Bronson, R.T.; Makhinson, M.; Watabe, A.M.; O’Dell, T.J.; Fung, J.; Weier, H.-U.G.; Cheng, J.-F.; et al. Functional screening of 2 Mb of human chromosome 21q22.2 in transgenic mice implicates minibrain in learning defects associated with Down syndrome. Nat. Genet. 1997, 16, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, K. Identification of Two Novel Primate-Specific Genes in DSCR. DNA Res. 2002, 9, 89–97. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, M.; Wang, J.; Luo, Y.; Huang, K.; Shi, X.; Liu, Y.; Li, J.; Lai, Z.; Xue, S.; Gao, H.; et al. Identify Down syndrome transcriptome associations using integrative analysis of microarray database and correlation-interaction network. Hum. Genom. 2018, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Souchet, B.; Guedj, F.; Sahún, I.; Duchon, A.; Daubigney, F.; Badel, A.; Yanagawa, Y.; Barallobre, M.J.; Dierssen, M.; Yu, E.; et al. Excitation/inhibition balance and learning are modified by Dyrk1a gene dosage. Neurobiol. Dis. 2014, 69, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.; Duchon, A.; Manousopoulou, A.; Loaëc, N.; Villiers, B.; Pani, G.; Karatas, M.; Mechling, A.E.; Harsan, L.-A.; Limanton, E.; et al. Correction of cognitive deficits in mouse models of Down syndrome by a pharmacological inhibitor of DYRK1A. Dis. Models Mech. 2018, 11, dmm035634. [Google Scholar] [CrossRef]
- Knierim, J.J. The hippocampus. Curr. Biol. 2015, 25, R1116–R1121. [Google Scholar] [CrossRef]
- Lepagnol-Bestel, A.M.; Zvara, A.; Maussion, G.; Quignon, F.; Ngimbous, B.; Ramoz, N.; Imbeaud, S.; Loe-Mie, Y.; Benihoud, K.; Agier, N.; et al. DYRK1A interacts with the REST/NRSF-SWI/SNF chromatin remodelling complex to deregulate gene clusters involved in the neuronal phenotypic traits of Down syndrome. Hum. Mol. Genet. 2009, 18, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Viard, J.; Loe-Mie, Y.; Daudin, R.; Khelfaoui, M.; Plancon, C.; Boland, A.; Tejedor, F.; Huganir, R.L.; Kim, E.; Kinoshita, M.; et al. Chr21 protein-protein interactions: Enrichment in proteins involved in intellectual disability, autism, and late-onset Alzheimer’s disease. Life Sci. Alliance 2022, 5, e202101205. [Google Scholar] [CrossRef] [PubMed]
- Söderberg, O.; Gullberg, M.; Jarvius, M.; Ridderstråle, K.; Leuchowius, K.J.; Jarvius, J.; Wester, K.; Hydbring, P.; Bahram, F.; Larsson, L.-G.; et al. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat. Methods 2006, 3, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.E.; Madison, D.V. Presynaptic FMR1 genotype influences the degree of synaptic connectivity in a mosaic mouse model of fragile X syndrome. J. Neurosci. 2007, 27, 4014–4018. [Google Scholar] [CrossRef]
- García-Cerro, S.; Martínez, P.; Vidal, V.; Corrales, A.; Flórez, J.; Vidal, R.; Rueda, N.; Arbonés, M.L.; Martínez-Cué, C. Overexpression of Dyrk1A is implicated in several cognitive, electrophysiological and neuromorphological alterations found in a mouse model of Down syndrome. PLoS ONE 2014, 9, e106572. [Google Scholar] [CrossRef] [PubMed]
- Nicoll, R.A.; Schmitz, D. Synaptic plasticity at hippocampal mossy fibre synapses. Nat. Rev. Neurosci. 2005, 6, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Varjosalo, M.; Keskitalo, S.; Van Drogen, A.; Nurkkala, H.; Vichalkovski, A.; Aebersold, R.; Gstaiger, M. The protein interaction landscape of the human CMGC kinase group. Cell Rep. 2013, 3, 1306–1320. [Google Scholar] [CrossRef]
- Vélot, L.; Lessard, F.; Bérubé-Simard, F.A.; Tav, C.; Neveu, B.; Teyssier, V.; Boudaoud, I.; Dionne, U.; Lavoie, N.; Bilodeau, S.; et al. Proximity-dependent Mapping of the Androgen Receptor Identifies Kruppel-like Factor 4 as a Functional Partner. Mol. Cell. Proteom. 2021, 20, 100064. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, M.B.; Zhang, Y.J.; Zhong, X.; Dai, H.; Yan, L.; Wu, N.-H.; Zhang, Y.; Shen, Y.-F. A switch from hBrm to Brg1 at IFNγ-activated sequences mediates the activation of human genes. Cell Res. 2010, 20, 1345–1360. [Google Scholar] [CrossRef] [PubMed]
- Südhof, T.C. Neurotransmitter release: The last millisecond in the life of a synaptic vesicle. Neuron 2013, 80, 675–690. [Google Scholar] [CrossRef]
- Acuna, C.; Guo, Q.; Burré, J.; Sharma, M.; Sun, J.; Südhof, T.C. Microsecond dissection of neurotransmitter release: SNARE-complex assembly dictates speed and Ca2+ sensitivity. Neuron 2014, 82, 1088–1100. [Google Scholar] [CrossRef]
- Castillo, P.E.; Janz, R.; Südhof, T.C.; Tzounopoulos, T.; Malenka, R.C.; Nicoll, R.A. Rab3A is essential for mossy fibre long-term potentiation in the hippocampus. Nature 1997, 388, 590–593. [Google Scholar] [CrossRef]
- Lonart, G.; Janz, R.; Johnson, K.M.; Südhof, T.C. Mechanism of action of rab3A in mossy fiber LTP. Neuron 1998, 21, 1141–1150. [Google Scholar] [CrossRef]
- Castillo, P.E.; Schoch, S.; Schmitz, F.; Südhof, T.C.; Malenka, R.C. RIM1alpha is required for presynaptic long-term potentiation. Nature 2002, 415, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Calakos, N. Munc13-1 is required for presynaptic long-term potentiation. J. Neurosci. 2011, 31, 12053–12057. [Google Scholar] [CrossRef]
- Spillane, D.M.; Rosahl, T.W.; Südhof, T.C.; Malenka, R.C. Long-term potentiation in mice lacking synapsins. Neuropharmacology 1995, 34, 1573–1579. [Google Scholar] [CrossRef]
- Milacic, M.; Beavers, D.; Conley, P.; Gong, C.; Gillespie, M.; Griss, J.; Haw, R.; Jassal, B.; Matthews, L.; May, B.; et al. The Reactome Pathway Knowledgebase 2024. Nucleic Acids Res. 2024, 52, D672–D678. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Dhanasekaran, A.R.; Gardiner, K.J. Mouse models of Down syndrome: Gene content and consequences. Mamm. Genome 2016, 27, 538–555. [Google Scholar] [CrossRef] [PubMed]
- Herault, Y.; Delabar, J.M.; Fisher, E.M.C.; Tybulewicz, V.L.J.; Yu, E.; Brault, V. Rodent models in Down syndrome research: Impact and future opportunities. Dis. Models Mech. 2017, 10, 1165–1186. [Google Scholar] [CrossRef]
- Shaw, P.R.; Klein, J.A.; Aziz, N.M.; Haydar, T.F. Longitudinal neuroanatomical and behavioral analyses show phenotypic drift and variability in the Ts65Dn mouse model of Down syndrome. Dis. Model Mech. 2020, 13, dmm046243. [Google Scholar] [CrossRef]
- Tomás Ahumada Saavedra, J.; Chevalier, C.; Zupan, A.B.; Herault, Y. Ripply3 overdosage induces mid-face shortening through Tbx1 downregulation in Down syndrome models. bioRxiv 2024. bioRxiv:2024.09.13.612914. Available online: https://www.biorxiv.org/content/10.1101/2024.09.13.612914v1 (accessed on 18 January 2025).
- Fulton, S.L.; Wenderski, W.; Lepack, A.E.; Eagle, A.L.; Fanutza, T.; Bastle, R.M.; Ramakrishnan, A.; Hays, E.C.; Neal, A.; Bendl, J.; et al. Rescue of deficits by Brwd1 copy number restoration in the Ts65Dn mouse model of Down syndrome. Nat. Commun. 2022, 13, 6384. [Google Scholar] [CrossRef] [PubMed]
- De Toma, I.; Ortega, M.; Aloy, P.; Sabidó, E.; Dierssen, M. DYRK1A Overexpression Alters Cognition and Neural-Related Proteomic Pathways in the Hippocampus That Are Rescued by Green Tea Extract and/or Environmental Enrichment. Front. Mol. Neurosci. 2019, 12, 272. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.; De Toma, I.; Fernández-Blanco, Á.; Calderón, A.; Barahona, L.; Trullàs, R.; Sabidó, E.; Dierssen, M. Proteomic profiling reveals mitochondrial dysfunction in the cerebellum of transgenic mice overexpressing DYRK1A, a Down syndrome candidate gene. Front. Mol. Neurosci. 2022, 15, 1015220. [Google Scholar] [CrossRef]
- Manubens-Gil, L.; Pons-Espinal, M.; Gener, T.; Ballesteros-Yañez, I.; de Lagrán, M.M.; Dierssen, M. Deficits in neuronal architecture but not over-inhibition are main determinants of reduced neuronal network activity in a mouse model of overexpression of Dyrk1A. Cereb. Cortex 2024, 34, bhad431. [Google Scholar] [CrossRef] [PubMed]
- Vona, C.D.; Bezdan, D.; Islam, A.B.; Salichs, E.; López-Bigas, N.; Ossowski, S.; de la Luna, S. Chromatin-wide profiling of DYRK1A reveals a role as a gene-specific RNA polymerase II CTD kinase. Mol. Cell 2015, 57, 506–520. [Google Scholar] [PubMed]
- Li, S.; Xu, C.; Fu, Y.; Lei, P.J.; Yao, Y.; Yang, W.; Zhang, Y.; Washburn, M.P.; Florens, L.; Jaiswal, M.; et al. DYRK1A interacts with histone acetyl transferase p300 and CBP and localizes to enhancers. Nucleic Acids Res. 2018, 46, 11202–11213. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, J.M.; Malleret, G.; Touzani, K.; Vronskaya, S.; Ishii, S.; Kandel, E.R.; Barco, A. Chromatin Acetylation, Memory, and LTP Are Impaired in CBP+/− Mice. Neuron 2004, 42, 947–959. [Google Scholar] [CrossRef]
- Guan, Z.; Giustetto, M.; Lomvardas, S.; Kim, J.H.; Miniaci, M.C.; Schwartz, J.H.; Thanos, D.; Kandel, E.R. Integration of Long-Term-Memory-Related Synaptic Plasticity Involves Bidirectional Regulation of Gene Expression and Chromatin Structure. Cell 2002, 111, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.A.; Guan, Z.; Barco, A.; Xu, S.; Kandel, E.R.; Schwartz, J.H. CREB-binding protein controls response to cocaine by acetylating histones at the fosB promoter in the mouse striatum. Proc. Natl. Acad. Sci. USA 2005, 102, 19186–19191. [Google Scholar] [CrossRef]
- Lacombe, D.; Bloch-Zupan, A.; Bredrup, C.; Cooper, E.B.; Houge, S.D.; García-Miñaúr, S.; Kayserili, H.; Larizza, L.; Gonzalez, V.L.; A Menke, L.; et al. Diagnosis and management in Rubinstein-Taybi syndrome: First international consensus statement. J. Med. Genet. 2024, 61, 503–519. [Google Scholar] [CrossRef]
- Shi, H.; He, Y.; Zhou, Y.; Huang, J.; Maher, K.; Wang, B.; Tang, Z.; Luo, S.; Tan, P.; Wu, M.; et al. Spatial atlas of the mouse central nervous system at molecular resolution. Nature 2023, 622, 552–561. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lepagnol-Bestel, A.-M.; Haziza, S.; Viard, J.; Salin, P.A.; Duchon, A.; Herault, Y.; Simonneau, M. DYRK1A Up-Regulation Specifically Impairs a Presynaptic Form of Long-Term Potentiation. Life 2025, 15, 149. https://doi.org/10.3390/life15020149
Lepagnol-Bestel A-M, Haziza S, Viard J, Salin PA, Duchon A, Herault Y, Simonneau M. DYRK1A Up-Regulation Specifically Impairs a Presynaptic Form of Long-Term Potentiation. Life. 2025; 15(2):149. https://doi.org/10.3390/life15020149
Chicago/Turabian StyleLepagnol-Bestel, Aude-Marie, Simon Haziza, Julia Viard, Paul A. Salin, Arnaud Duchon, Yann Herault, and Michel Simonneau. 2025. "DYRK1A Up-Regulation Specifically Impairs a Presynaptic Form of Long-Term Potentiation" Life 15, no. 2: 149. https://doi.org/10.3390/life15020149
APA StyleLepagnol-Bestel, A.-M., Haziza, S., Viard, J., Salin, P. A., Duchon, A., Herault, Y., & Simonneau, M. (2025). DYRK1A Up-Regulation Specifically Impairs a Presynaptic Form of Long-Term Potentiation. Life, 15(2), 149. https://doi.org/10.3390/life15020149





