Subclinical Hypocalcemia in Dairy Cows: An Integrative Evaluation of Blood Biomarkers, In-Line Milk Composition, and Rumination Behavior
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Housing, Feeding and Management Conditions
2.2. Data Collection
2.3. Grouping
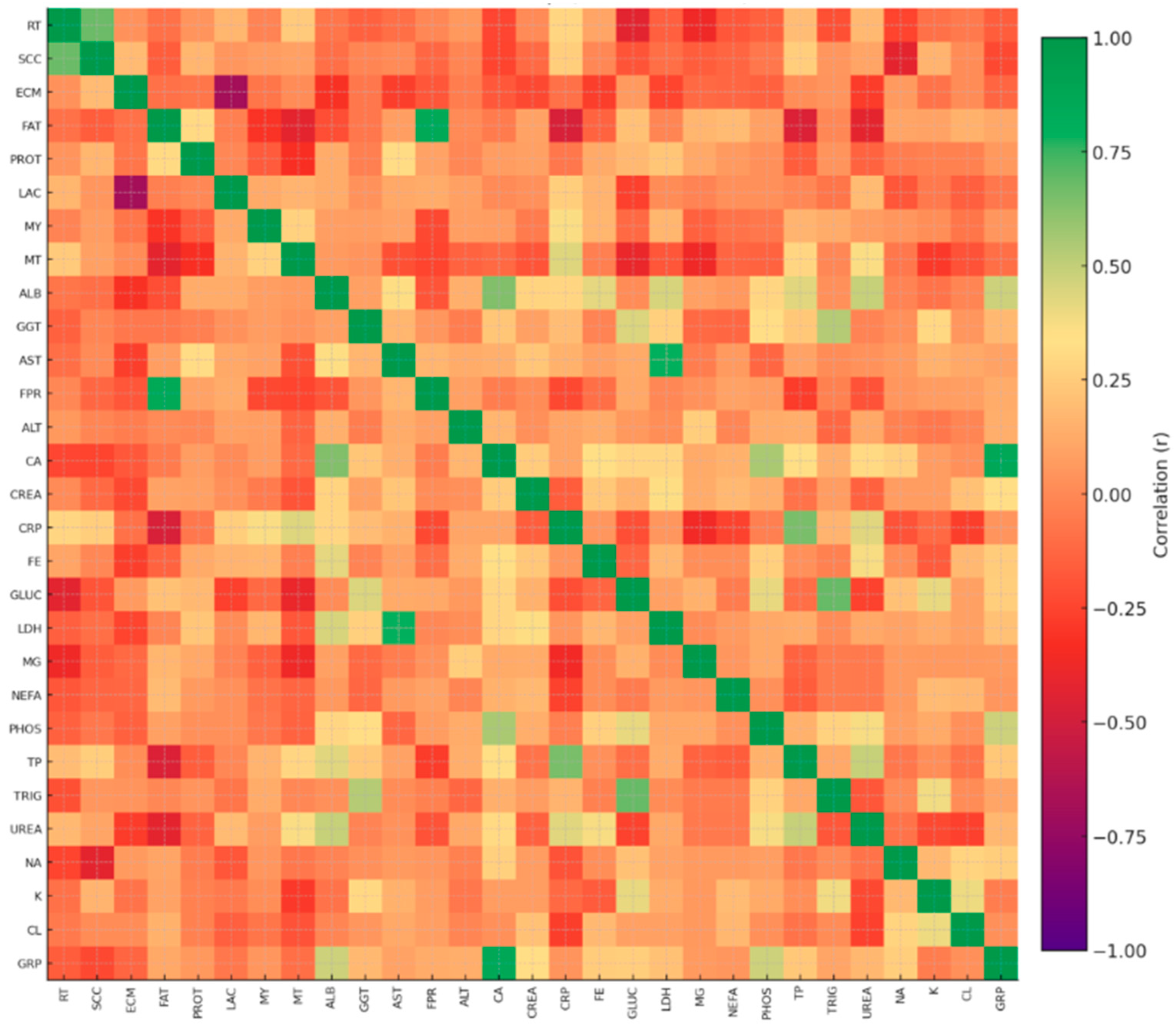
2.4. Statistical Approach
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Horst, R.L.; Goff, J.P.; Reinhardt, T.A. Adapting to the Transition Between Gestation and Lactation: Differences Between Rat, Human and Dairy Cow. J. Mammary Gland. Biol. Neoplasia 2005, 10, 141–156. [Google Scholar] [CrossRef]
- Novo, L.C.; Poindexter, M.B.; Rezende, F.M.; Santos, J.E.P.; Nelson, C.D.; Hernandez, L.L.; Kirkpatrick, B.W.; Peñagaricano, F. Identification of Genetic Variants and Individual Genes Associated with Postpartum Hypocalcemia in Holstein Cows. Sci. Rep. 2023, 13, 21900. [Google Scholar] [CrossRef]
- Chamberlin, W.G.; Middleton, J.R.; Spain, J.N.; Johnson, G.; Ellersieck, M.; Pithua, P. Subclinical Hypocalcemia, Plasma Biochemical Parameters, Lipid Metabolism, Postpartum Disease, and Fertility in Postparturient Dairy Cows. J. Dairy Sci. 2013, 96, 7001–7013. [Google Scholar] [CrossRef] [PubMed]
- Espiritu, H.M.; Al Faruk, S.; Lee, H.-W.; Pioquinto, J.M.; Lee, S.-S.; Cho, Y.-I. Subclinical Hypocalcemia Across Lactation Stages Reflects Potential Metabolic Vulnerability in Korean Holstein Cows. Vet. Sci. 2025, 12, 495. [Google Scholar] [CrossRef] [PubMed]
- Kia, H.A.; Salavati, S. Subclinical Hypocalcemia in Dairy Cows: Pathophysiology, Consequences and Monitoring. Iran. J. Vet. Sci. Technol. 2017, 9, 69198. [Google Scholar]
- Venjakob, S.B.; Borchardt, S. Etiology, Prevalence and Evidence-Based Therapy and Prevention of Periparturient Hypocalcemia. Tierarztl. Prax. Ausg. G 2022, 50, 174–186. [Google Scholar]
- Berean, D.I.; Bogdan, M.; Cimpean, R. Subclinical Hypocalcemia in Dairy Cows: Reproductive and Economic Impacts on Eastern European Farms. Front. Vet. Sci. 2025, 12, 1596239. [Google Scholar] [CrossRef]
- Hernández-Castellano, L.E.; Bruckmaier, R.M. Endocrine Pathways Regulating Calcium Homeostasis around Parturition and Prevention of Hypocalcemia in Periparturient Dairy Cows. Animal 2020, 14, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, S.A.; Alelgn, Z.; Saudik, S.; Molla, W.; Fentie, T.; Jemberu, W.T. Reduced Milk Production, Economic Losses, and Risk Factors Associated with Subclinical Hypocalcemia in Holstein Friesian × Zebu Crossbreed Cows in North-West Ethiopia. Front. Vet. Sci. 2022, 9, 771889. [Google Scholar] [CrossRef]
- Ametaj, B.N. From Parts to Whole: A Systems Biology Approach to Decoding Milk Fever. Vet. Sci. 2025, 12, 347. [Google Scholar] [CrossRef]
- Antanaitis, R.; Džermeikaitė, K.; Krištolaitytė, J.; Girdauskaitė, A.; Arlauskaitė, S.; Tolkačiovaitė, K.; Baumgartner, W. Relation Between Milk Lactose Concentration and Rumination, Feeding and Locomotion Behavior of Early-Lactation Dairy Cows. Animals 2024, 14, 836. [Google Scholar] [CrossRef]
- Džermeikaitė, K.; Bačėninaitė, D.; Antanaitis, R. Innovations in Cattle Farming: Application of Innovative Technologies and Sensors in Disease Diagnosis. Animals 2023, 13, 780. [Google Scholar] [CrossRef]
- Reinhardt, T.A.; Lippolis, J.D.; McCluskey, B.J.; Goff, J.P.; Horst, R.L. Prevalence of Subclinical Hypocalcemia in Dairy Herds. Vet. J. 2011, 188, 122–124. [Google Scholar] [CrossRef]
- Barraclough, R.A.C.; Shaw, D.; Thorup, V.; Haskell, M.; Lee, W.; Macrae, A. Behavior of Dairy Cattle in the Transition Period: Effects of Blood Calcium Status. J. Dairy Sci. 2020, 103, 10604–10613. [Google Scholar] [CrossRef]
- Gladden, N. Postpartum Hypocalcaemia in Dairy Cows. Livestock 2025, 30, 70–76. [Google Scholar] [CrossRef]
- Cardoso, F. Amino Acid Nutrition in Dairy Cattle: Beyond Milk Protein. J. Anim. Sci. 2023, 101, 231–232. [Google Scholar] [CrossRef]
- Campos-Gaona, R.; Correa-Orozco, A.; Salamanca-Carreño, A.; Vélez-Terranova, M. Index Development to Assess Liver Function During the Transition Period in Dairy Cows under Low-Tropic Conditions. Animals 2024, 14, 2056. [Google Scholar] [CrossRef] [PubMed]
- Bach, A. Effects of Nutrition and Genetics on Fertility in Dairy Cows. Reprod. Fertil. Dev. 2018, 31, 40–54. [Google Scholar] [CrossRef]
- Cai, C.; Kong, Y.; Wu, D.; Wang, J. Changes of Macrominerals and Calcitropic Hormones in Periparturient Dairy Cows Subject to Subclinical Hypocalcemia. J. Dairy Res. 2018, 85, 12–15. [Google Scholar] [CrossRef]
- Heilig, M.; Bäuml, D.; Fürll, M. The relevance of Trace Elements Zinc and Iron in Parturient Paresis of Cattle. Tierarztl. Prax. Ausg. G 2014, 43, 199–208. [Google Scholar]
- Weber, J.; Zenker, M.; Köller, G.; Fürll, M.; Freick, M. Clinical Chemistry Investigations in Recumbent and Healthy German Holstein Cows After the Fifth Day in Milk. J. Vet. Res. 2019, 63, 383–390. [Google Scholar] [CrossRef]
- Megahed, A.A.; Hiew, M.; Ragland, D.; Constable, P. Changes in Skeletal Muscle Thickness, Echogenicity, and Plasma Creatinine as Indicators of Protein and Intramuscular Fat Mobilization in Periparturient Dairy Cows. J. Dairy Sci. 2019, 102, 5550–5565. [Google Scholar] [CrossRef]
- Rodrigues, R.; Cooke, R.F.; Ferreira, H.A.O.; Florido, R.R.; Camargo, V.; de Godoy, H.O.; A Bruni, G.; Vasconcelos, J.L.M. Impacts of Subclinical Hypocalcemia on Physiological, Metabolic, and Productive Responses of Holstein × Gir Cows. Transl. Anim. Sci. 2020, 4, 1060–1069. [Google Scholar] [CrossRef]
- van Knegsel, A.T.M.; Hammon, H.M.; Bernabucci, U.; Bertoni, G.; Bruckmaier, R.M.; Goselink, R.M.A.; Gross, J.J.; Kuhla, B.; Metges, C.C.; Parmentier, H.K.; et al. Metabolic Adaptation During Early Lactation: Key to Cow Health and Sustainable Production. CABI Rev. 2014, 1–14. [Google Scholar] [CrossRef]
- Emam, M.H.; Shepley, E.; Mahmoud, M.M.; Ruch, M.; Elmaghawry, S.; Abdelrazik, W.; Abdelaal, A.M.; Crooker, B.A.; Caixeta, L.S. Association Between Prepartum Rumination, Activity and Dry Matter Intake with Subclinical Hypocalcemia and Hypomagnesemia. Animals 2023, 13, 1621. [Google Scholar] [CrossRef] [PubMed]
- Jawor, P.E.; Huzzey, J.M.; LeBlanc, S.J.; Von Keyserlingk, M.A.G. Associations of Subclinical Hypocalcemia at Calving with Milk Yield and Behavioral Parameters Around Parturition. J. Dairy Sci. 2012, 95, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Arlauskaitė, S.; Girdauskaitė, A.; Rutkauskas, A.; Džermeikaitė, K.; Krištolaitytė, J.; Televičius, M.; Malašauskienė, D.; Anskienė, L.; Japertas, S.; Baumgartner, W.; et al. Precision Monitoring of Rumination and Locomotion in Relation to Milk Fat-to-Protein Ratio in Early Lactation Cows. Front. Vet. Sci. 2025, 12, 1632224. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Abbreviation | Measurement Source/System |
|---|---|---|
| Rumination time (min/d) | RT | Lely Astronaut® A3 milking robot/Lely T4C management system |
| Milk yield (kg/d) | MY | |
| Milk fat (%) | F | |
| Milk protein (%) | P | |
| Milk lactose (%) | LAC | |
| Milk temperature °C | MT | |
| Fat-to-protein ratio | FPR | |
| Somatic cell count (103 cells/mL) | SCC | |
| Milk electrical conductivity (Lely Astronaut conductivity score, unitless index) | ECM | |
| Albumin (g/L) | ALB | Hitachi 705 analyzer |
| Aspartate aminotransferase (U/L) | AST | |
| Gamma-glutamyltransferase (U/L) | GGT | |
| Alanine aminotransferase (U/L) | ALT | |
| Calcium (mmol/L) | Ca | |
| Creatinine (umol/L) | CREA | |
| C-reactive protein (mg/L) | CRP | |
| Iron (umol/L) | Fe | |
| Glucose (mmol/L) | GLUC | |
| Lactate dehydrogenase (U/L) | LDH | |
| Magnesium (mmol/L) | Mg | |
| Phosphorus (mmol/L) | PHOS | |
| Total protein (g/L) | TP | |
| Triglycerides (mmol/L) | TRIG | |
| Urea (mmol/L) | UREA | |
| Sodium (mmol/L) | Na | |
| Potassium (mmol/L) | K | |
| Chloride (mmol/L) | Cl | |
| Non-esterified fatty acids (mmol/L) | NEFA | Rx Daytona automated wet chemistry analyzer, Randox Laboratories Ltd. |
| Parameter | Mean Group 1 (n = 20) | SD | Mean Group 2 (n = 55) | SD | p_Value | Cohen_d |
|---|---|---|---|---|---|---|
| RT min/day | 462.65 | 138.31 | 415.45 | 123.62 | 0.189 | 0.370 |
| SCC 103 cells/mL | 382.90 | 405.79 | 215.47 | 281.10 | 0.101 | 0.526 |
| ECM (Lely Astronaut conductivity score. unitless index) | 71.85 | 5.58 | 70.49 | 4.13 | 0.328 | 0.299 |
| F % | 4.06 | 0.87 | 4.28 | 0.90 | 0.332 | −0.253 |
| P % | 3.58 | 0.39 | 3.68 | 0.87 | 0.476 | −0.135 |
| LAC % | 4.55 | 0.28 | 4.53 | 0.11 | 0.681 | 0.156 |
| MY kg/d | 36.05 | 14.37 | 38.48 | 24.26 | 0.320 | −0.157 |
| MT °C | 39.40 | 0.88 | 39.20 | 0.94 | 0.403 | 0.214 |
| ALB g/L *** | 29.13 | 3.77 | 33.50 | 3.65 | 8.71 × 10−5 | −1.186 |
| GGT U/L | 25.39 | 14.33 | 33.67 | 21.51 | 0.061 | −0.417 |
| AST U/L | 80.36 | 37.42 | 88.17 | 33.56 | 0.418 | −0.226 |
| .FPR | 1.13 | 0.21 | 1.20 | 0.22 | 0.270 | −0.285 |
| ALT U/L | 22.71 | 6.66 | 29.34 | 25.09 | 0.077 | −0.304 |
| Ca mmol/L *** | 1.86 | 0.10 | 2.35 | 0.14 | 9.24 × 10−22 | −3.756 |
| CREA umol/L *** | 45.26 | 7.34 | 53.09 | 11.06 | 0.001 | −0.767 |
| CRP mg/L | 9.19 | 4.65 | 9.70 | 5.23 | 0.683 | −0.102 |
| Fe umol/L * | 15.63 | 5.64 | 19.72 | 7.27 | 0.014 | −0.595 |
| GLUC mmol/L ** | 2.86 | 0.65 | 3.46 | 1.09 | 0.005 | −0.608 |
| LDH U/L | 1150.35 | 337.26 | 1321.96 | 362.25 | 0.064 | −0.482 |
| Mg mmol/L | 1.33 | 1.06 | 1.51 | 1.22 | 0.552 | −0.147 |
| NEFA mmol/L | 0.06 | 0.07 | 0.07 | 0.10 | 0.617 | −0.109 |
| PHOS mmol/L *** | 1.63 | 0.38 | 2.09 | 0.39 | 5.45 × 10−5 | −1.192 |
| TP g/L * | 64.64 | 9.80 | 70.04 | 9.91 | 0.043 | −0.547 |
| TRIG mmol/L | 0.11 | 0.03 | 0.12 | 0.06 | 0.206 | −0.238 |
| UREA mmol/L | 4.42 | 1.02 | 4.91 | 1.29 | 0.094 | −0.400 |
| Na mmol/L | 127.05 | 28.16 | 136.49 | 7.57 | 0.154 | −0.599 |
| K mmol/L | 4.33 | 0.49 | 4.29 | 0.36 | 0.771 | 0.089 |
| Cl mmol/L | 92.60 | 7.58 | 93.13 | 8.82 | 0.800 | −0.062 |
| Parameter | p_Value | Cohen_d | q_value |
|---|---|---|---|
| Ca (mmol/L) ** | p > 0.001 | −3.756 | 0.000 |
| PHOS (mmol/L) ** | p > 0.001 | −1.192 | 0.001 |
| ALB (g/L) ** | p > 0.001 | −1.186 | 0.001 |
| CREA (umol/L) ** | 0.001 | −0.767 | 0.006 |
| GLUC (mmol/L) ** | 0.005 | −0.608 | 0.028 |
| Fe (µmol/L) * | 0.014 | −0.595 | 0.065 |
| TP (g/L) * | 0.043 | −0.547 | 0.171 |
| GGT (U/L) | 0.061 | −0.417 | 0.199 |
| LDH (U/L) | 0.064 | −0.482 | 0.199 |
| ALT (U/L) | 0.077 | −0.304 | 0.216 |
| UREA (mmol/L) | 0.094 | −0.400 | 0.235 |
| SCC (×103 cells/mL) | 0.101 | 0.526 | 0.235 |
| Na (mmol/L) | 0.154 | −0.599 | 0.333 |
| RT (min/day) | 0.189 | 0.370 | 0.378 |
| TRIG (mmol/L) | 0.206 | −0.238 | 0.385 |
| FPR (ratio) | 0.270 | −0.285 | 0.472 |
| MY (kg/day) | 0.320 | −0.157 | 0.490 |
| ECM | 0.328 | 0.299 | 0.490 |
| Fat (%) | 0.332 | −0.253 | 0.490 |
| Milk temperature (°C) | 0.403 | 0.214 | 0.557 |
| AST (U/L) | 0.418 | −0.226 | 0.557 |
| Protein (%) | 0.476 | −0.135 | 0.606 |
| Mg (mmol/L) | 0.552 | −0.147 | 0.671 |
| NEFA (mmol/L) | 0.617 | −0.109 | 0.719 |
| LAC (%) | 0.681 | 0.156 | 0.735 |
| CRP (mg/L) | 0.683 | −0.102 | 0.735 |
| K (mmol/L) | 0.771 | 0.089 | 0.799 |
| Cl (mmol/L) | 0.800 | −0.062 | 0.800 |
| var1 | var2 | r |
|---|---|---|
| AST | ALT | 0.175 |
| AST | GGT | 0.318 |
| AST | LDH | 0.790 |
| AST | F % | 0.134 |
| AST | P % | 0.233 |
| AST | LAC % | 0.102 |
| AST | FPR | 0.222 |
| AST | MY | −0.062 |
| AST | RT | 0.016 |
| ALT | GGT | 0.014 |
| ALT | LDH | 0.079 |
| ALT | F | 0.032 |
| ALT | P | −0.011 |
| ALT | LAC | 0.097 |
| ALT | FPR | 0.119 |
| ALT | MY | 0.908 |
| ALT | RT | 0.060 |
| GGT | LDH | 0.268 |
| GGT | F | 0.000 |
| GGT | P | −0.071 |
| GGT | LAC | −0.015 |
| GGT | FPR | 0.132 |
| GGT | MY | −0.075 |
| GGT | RT | 0.047 |
| LDH | F | 0.017 |
| LDH | P | 0.164 |
| LDH | LAC | 0.027 |
| LDH | FPR | 0.026 |
| LDH | MY | −0.096 |
| LDH | RT | −0.025 |
| F | P | 0.287 |
| F | LAC | −0.019 |
| F | FPR | 0.851 |
| F | MY | 0.082 |
| F | RT | −0.145 |
| P | LAC | 0.007 |
| P | FPR | 0.035 |
| P | MY | −0.006 |
| P | RT | −0.027 |
| LAC | FPR | 0.101 |
| LAC | MY | −0.019 |
| LAC | RT | 0.148 |
| FPR | MY | 0.085 |
| FPR | RT | −0.029 |
| MY | RT | −0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigė, S.; Girdauskaitė, A.; Anskienė, L.; Rodaitė, I.; Ginkus, E.; Džermeikaitė, K.; Krištolaitytė, J.; Šertvytytė, G.; Lembovičiūtė, G.; Antanaitis, R. Subclinical Hypocalcemia in Dairy Cows: An Integrative Evaluation of Blood Biomarkers, In-Line Milk Composition, and Rumination Behavior. Life 2025, 15, 1810. https://doi.org/10.3390/life15121810
Grigė S, Girdauskaitė A, Anskienė L, Rodaitė I, Ginkus E, Džermeikaitė K, Krištolaitytė J, Šertvytytė G, Lembovičiūtė G, Antanaitis R. Subclinical Hypocalcemia in Dairy Cows: An Integrative Evaluation of Blood Biomarkers, In-Line Milk Composition, and Rumination Behavior. Life. 2025; 15(12):1810. https://doi.org/10.3390/life15121810
Chicago/Turabian StyleGrigė, Samanta, Akvilė Girdauskaitė, Lina Anskienė, Ieva Rodaitė, Eimantas Ginkus, Karina Džermeikaitė, Justina Krištolaitytė, Greta Šertvytytė, Gabija Lembovičiūtė, and Ramūnas Antanaitis. 2025. "Subclinical Hypocalcemia in Dairy Cows: An Integrative Evaluation of Blood Biomarkers, In-Line Milk Composition, and Rumination Behavior" Life 15, no. 12: 1810. https://doi.org/10.3390/life15121810
APA StyleGrigė, S., Girdauskaitė, A., Anskienė, L., Rodaitė, I., Ginkus, E., Džermeikaitė, K., Krištolaitytė, J., Šertvytytė, G., Lembovičiūtė, G., & Antanaitis, R. (2025). Subclinical Hypocalcemia in Dairy Cows: An Integrative Evaluation of Blood Biomarkers, In-Line Milk Composition, and Rumination Behavior. Life, 15(12), 1810. https://doi.org/10.3390/life15121810










