The Role of Renalase in Cardiovascular Disease: A Comprehensive Review of Its Molecular Biology, Genetic Associations, and Clinical Significance
Abstract
1. Introduction
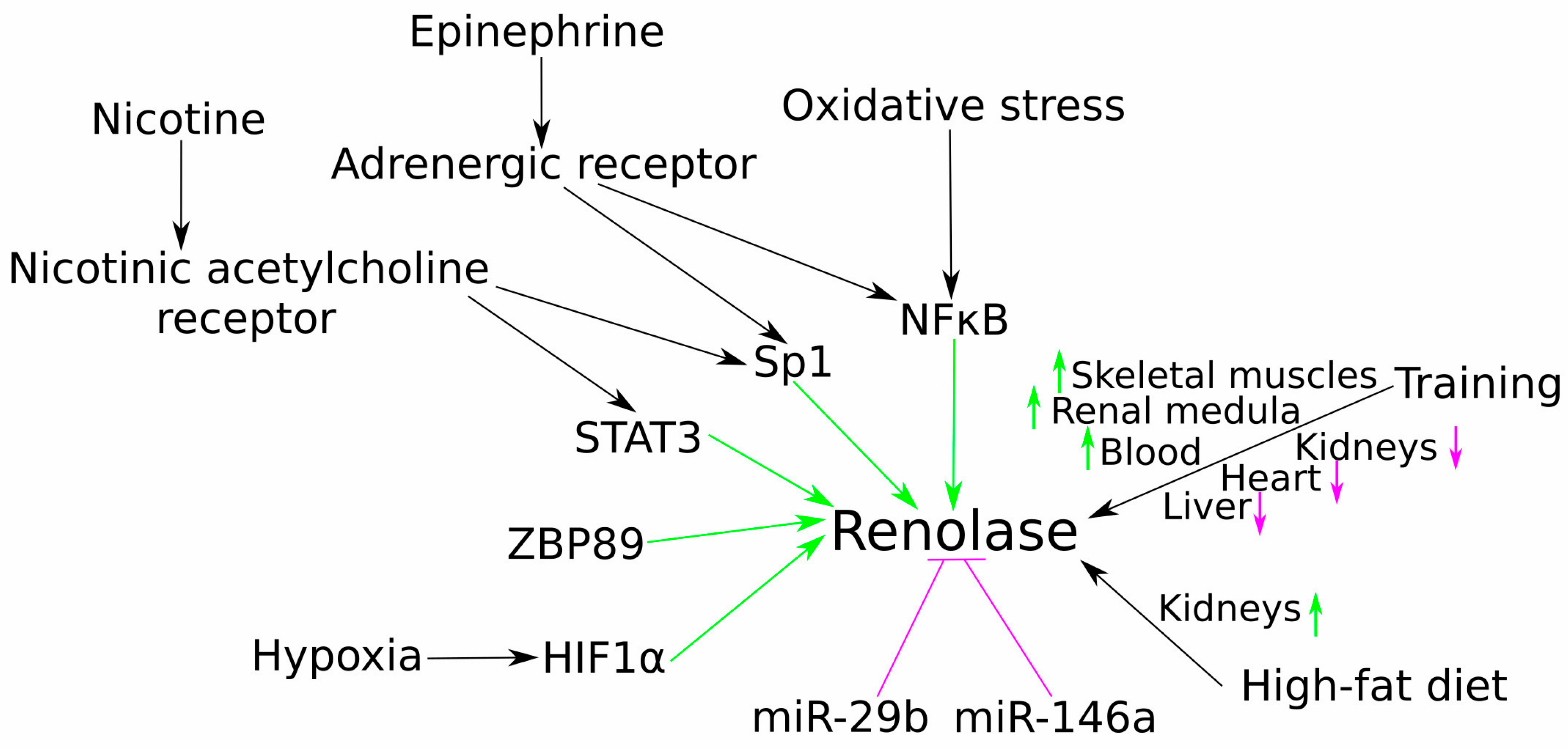
2. Molecular and Physiological Regulation of Renalase Gene Expression
3. Renalase and Its Association with Blood Pressure Regulation
Mechanistic Insights from Preclinical Models
4. Renalase in Preclinical Cardiovascular Disease Models
5. Renalase Gene Polymorphisms and Their Association with Cardiovascular Diseases and Related Phenotypes
6. Circulating Renalase as a Potential Biomarker in Cardiovascular and Related Diseases
6.1. Hypertension and Related Conditions
6.2. Coronary Artery Disease (CAD)
6.3. Atrial Fibrillation (AF)
6.4. Heart Failure (HF)
6.5. Broader Cardiovascular Risk Factors and Major Adverse Cardiovascular Events (MACEs)
7. Discussion
7.1. Renalase in Specific Cardiovascular Contexts: Synthesis and Critical Appraisal
- •
- Hypertension and Related Syndromes:
- •
- CAD and Atherosclerosis:
- •
- Heart Failure (HF) and Cardiac Remodelling:
- •
- Atrial Fibrillation (AF):
- •
- Chronic Kidney Disease (CKD) and Broader Cardiovascular Risk:
7.2. Key Challenges and Limitations in Current Renalase Research
- •
- Methodological Heterogeneity in Renalase Quantification: A primary obstacle is the profound lack of standardisation in measuring renalase. Studies employ different ELISA kits, report values in widely disparate units (µg/mL and ng/mL), assess different biofluids (serum, plasma, urine), and may not distinguish between total and potentially active renalase. This makes direct comparisons of absolute values across studies nearly impossible and contributes significantly to perceived inconsistencies in findings. Specific limitations of ELISA-based methods include potential variability in antibody specificity and affinity for different renalase isoforms or post-translationally modified forms, a lack of universally accepted calibrators, and significant inter-laboratory variation. Without a harmonised approach, the field cannot establish reliable reference ranges or validated prognostic cut-offs.
- •
- Inconsistency and Context-Dependency of Findings: As highlighted above, the direction and strength of association between renalase (levels or SNPs) and clinical parameters often vary considerably depending on the specific disease, its stage, patient demographics (age, sex, ethnicity), comorbidities, and even medication use. This suggests that renalase is not a “one-size-fits-all” biomarker and its interpretation requires careful contextualisation.
- •
- Discerning Causation from Correlation: Many human studies are cross-sectional or observational, establishing associations rather than causal relationships. It is often unclear whether altered renalase levels are a pathogenic driver, a compensatory mechanism, or simply an epiphenomenon of the underlying disease state.
- •
- Functional Impact of Genetic Variants: While numerous RNLS SNPs have been linked to CVD risk, the functional consequences of most of these variants on renalase expression, protein stability, or enzymatic/signalling activity remain largely uncharacterised.
- •
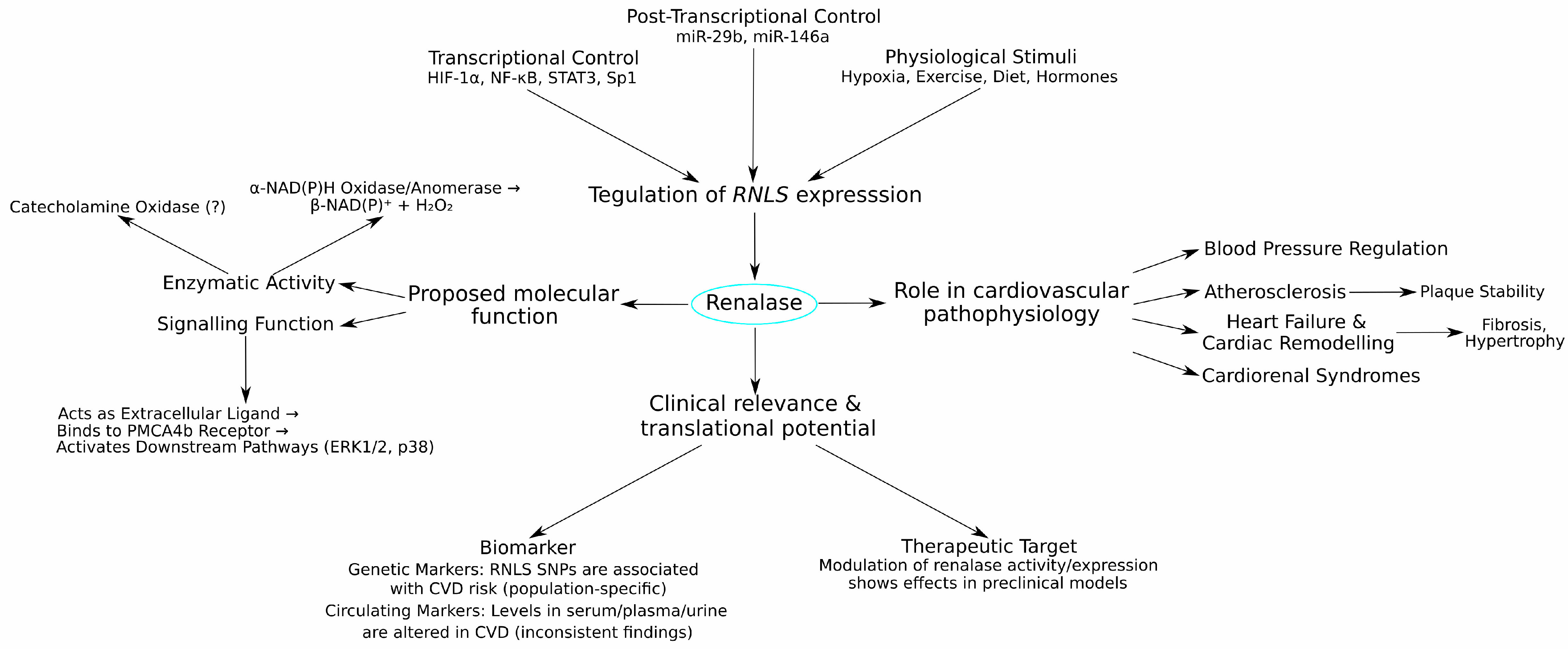
- Uncertainty Regarding Primary Enzymatic Functions: A critical challenge remains the unresolved debate over renalase’s primary enzymatic function in vivo. While the α-NAD(P)H oxidase activity provides a plausible biochemical role with clear implications for cellular redox balance, the identification of a specific cell surface receptor, PMCA4b, lends strong, independent support to its function as an extracellular signalling molecule, acting akin to a cytokine (as illustrated in Figure 4). These roles are not mutually exclusive; renalase may function differently inside the cell versus in circulation. However, the lack of definitive in vivo evidence for either catecholamine degradation or significant NAD(P)H isomer oxidation in circulation complicates the interpretation of its physiological effects.
7.3. Future Directions and Opportunities
- •
- Standardisation and Methodological Advancements:
- •
- Develop and validate standardised, robust, and widely accessible assays for quantifying renalase levels and, if possible, its specific enzymatic activity in various biological samples. Establishing reference materials and agreed-upon reporting units is critical.
- •
- Further refine techniques to differentiate between various renalase isoforms and post-translationally modified forms that may have distinct biological activities.
- •
- Elucidating Fundamental Biology and Mechanisms:
- •
- Conduct definitive studies to identify the primary physiological substrates and products of renalase in vivo under varying conditions.
- •
- Deepen the understanding of renalase signalling pathways, including receptor interactions beyond PMCA4b and downstream intracellular events (see Figure 4 for the current model).
- •
- Perform comprehensive functional characterisation of disease-associated RNLS SNPs to determine their impact on gene expression, protein function, and ultimately, cellular and organismal phenotypes.
- •
- Preclinical and Translational Research:
- •
- Utilise advanced preclinical models (e.g., tissue-specific conditional knockouts/ins, humanised mice) to dissect the context-specific roles of renalase in different organs and cell types during CVD development and progression.
- •
- Investigate the therapeutic potential of modulating renalase (e.g., via recombinant protein, gene therapy, or pharmacological agents) in well-defined disease models, with clear mechanistic readouts.
- •
- Robust Human Clinical Studies:
- •
- Design and execute large-scale, prospective, multi-ethnic cohort studies to rigorously assess the diagnostic, prognostic, and predictive value of renalase (levels and SNPs) for specific cardiovascular outcomes, using the standardised assays developed above.
- •
- Explore the utility of renalase in combination with other biomarkers to enhance predictive accuracy for complex cardiovascular phenotypes.
- •
- Crucially, for any proposed clinical application, studies must address practical considerations such as assay cost-effectiveness, turnaround time, and potential integration into existing clinical guidelines.
8. Summary and Conclusions: Renalase in the Cardiovascular Nexus—Current Standing and Future Horizons
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chong, B.; Jayabaskaran, J.; Jauhari, S.M.; Chan, S.P.; Goh, R.; Kueh, M.T.W.; Li, H.; Chin, Y.H.; Kong, G.; Anand, V.V.; et al. Global Burden of Cardiovascular Diseases: Projections from 2025 to 2050. Eur. J. Prev. Cardiol. 2025, 32, 1001–1015. [Google Scholar] [CrossRef]
- Sánchez-Viñas, A.; Corral-Partearroyo, C.; Gil-Girbau, M.; Peñarrubia-María, M.T.; Gallardo-González, C.; Olmos-Palenzuela, M.-C.; Aznar-Lou, I.; Serrano-Blanco, A.; Rubio-Valera, M. Effectiveness and Cost-Effectiveness of an Intervention to Improve Initial Medication Adherence to Treatments for Cardiovascular Diseases and Diabetes in Primary Care: Study Protocol for a Pragmatic Cluster Randomised Controlled Trial and Economic Model (the IMA-cRCT Study). BMC Prim. Care 2022, 23, 170. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Mocumbi, A.O. Cardiovascular Health Care in Low- and Middle-Income Countries. Circulation 2024, 149, 557–559. [Google Scholar] [CrossRef]
- Luengo-Fernandez, R.; Walli-Attaei, M.; Gray, A.; Torbica, A.; Maggioni, A.P.; Huculeci, R.; Bairami, F.; Aboyans, V.; Timmis, A.D.; Vardas, P.; et al. Economic Burden of Cardiovascular Diseases in the European Union: A Population-Based Cost Study. Eur. Heart J. 2023, 44, 4752–4767. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.J.; Ordóñez-Mena, J.M.; Jones, N.R.; Roalfe, A.K.; Lay-Flurrie, S.; Marshall, T.; Hobbs, F.D.R. National Trends in Heart Failure Mortality in Men and Women, United Kingdom, 2000–2017. Eur. J. Heart Fail. 2021, 23, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Netala, V.R.; Teertam, S.K.; Li, H.; Zhang, Z. A Comprehensive Review of Cardiovascular Disease Management: Cardiac Biomarkers, Imaging Modalities, Pharmacotherapy, Surgical Interventions, and Herbal Remedies. Cells 2024, 13, 1471. [Google Scholar] [CrossRef]
- Xu, J.; Li, G.; Wang, P.; Velazquez, H.; Yao, X.; Li, Y.; Wu, Y.; Peixoto, A.; Crowley, S.; Desir, G.V. Renalase Is a Novel, Soluble Monoamine Oxidase That Regulates Cardiac Function and Blood Pressure. J. Clin. Investig. 2005, 115, 1275–1280. [Google Scholar] [CrossRef]
- Strausberg, R.L.; Feingold, E.A.; Klausner, R.D.; Collins, F.S. The Mammalian Gene Collection. Science 1999, 286, 455–457. [Google Scholar] [CrossRef]
- Guo, X.; Wang, L.; Velazquez, H.; Safirstein, R.; Desir, G.V. Renalase: Its Role as a Cytokine, and an Update on Its Association with Type 1 Diabetes and Ischemic Stroke. Curr. Opin. Nephrol. Hypertens. 2014, 23, 513–518. [Google Scholar] [CrossRef]
- Wang, L.; Velazquez, H.; Chang, J.; Safirstein, R.; Desir, G.V. Identification of a Receptor for Extracellular Renalase. PLoS ONE 2015, 10, e0122932. [Google Scholar] [CrossRef] [PubMed]
- Baraka, A.; El Ghotny, S. Cardioprotective Effect of Renalase in 5/6 Nephrectomized Rats. J. Cardiovasc. Pharmacol. Ther. 2012, 17, 412–416. [Google Scholar] [CrossRef]
- Hennebry, S.C.; Eikelis, N.; Socratous, F.; Desir, G.; Lambert, G.; Schlaich, M. Renalase, a Novel Soluble FAD-Dependent Protein, Is Synthesized in the Brain and Peripheral Nerves. Mol. Psychiatry 2010, 15, 234–236. [Google Scholar] [CrossRef]
- Desir, G.V. Regulation of Blood Pressure and Cardiovascular Function by Renalase. Kidney Int. 2009, 76, 366–370. [Google Scholar] [CrossRef]
- Wang, L.; Velazquez, H.; Moeckel, G.; Chang, J.; Ham, A.; Lee, H.T.; Safirstein, R.; Desir, G.V. Renalase Prevents AKI Independent of Amine Oxidase Activity. J. Am. Soc. Nephrol. 2014, 25, 1226–1235. [Google Scholar] [CrossRef]
- Milani, M.; Ciriello, F.; Baroni, S.; Pandini, V.; Canevari, G.; Bolognesi, M.; Aliverti, A. FAD-Binding Site and NADP Reactivity in Human Renalase: A New Enzyme Involved in Blood Pressure Regulation. J. Mol. Biol. 2011, 411, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Moran, G.R. The Catalytic Function of Renalase: A Decade of Phantoms. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2016, 1864, 177–186. [Google Scholar] [CrossRef]
- Beaupre, B.A.; Hoag, M.R.; Roman, J.; Försterling, F.H.; Moran, G.R. Metabolic Function for Human Renalase: Oxidation of Isomeric Forms of β-NAD(P)H That Are Inhibitory to Primary Metabolism. Biochemistry 2015, 54, 795–806. [Google Scholar] [CrossRef]
- Czubilińska-Łada, J.; Gliwińska, A.; Badeński, A.; Szczepańska, M. Associations between Renalase Concentration and the Occurrence of Selected Diseases. Endokrynol. Pol. 2020, 71, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Beaupre, B.A.; Hoag, M.R.; Moran, G.R. Renalase Does Not Catalyze the Oxidation of Catecholamines. Arch. Biochem. Biophys. 2015, 579, 62–66. [Google Scholar] [CrossRef]
- Pandini, V.; Ciriello, F.; Tedeschi, G.; Rossoni, G.; Zanetti, G.; Aliverti, A. Synthesis of Human Renalase1 in Escherichia Coli and Its Purification as a FAD-Containing Holoprotein. Protein Expr. Purif. 2010, 72, 244–253. [Google Scholar] [CrossRef]
- Beaupre, B.A.; Carmichael, B.R.; Hoag, M.R.; Shah, D.D.; Moran, G.R. Renalase Is an α-NAD(P)H Oxidase/Anomerase. J. Am. Chem. Soc. 2013, 135, 13980–13987. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Han, P.; Wang, J.; Sun, H.; Shao, M. Renalase Overexpression in ER-Positive Breast Cancer. Int. J. Clin. Exp. Pathol. 2018, 11, 1297–1307. [Google Scholar]
- Guo, X.; Hollander, L.; MacPherson, D.; Wang, L.; Velazquez, H.; Chang, J.; Safirstein, R.; Cha, C.; Gorelick, F.; Desir, G.V. Inhibition of Renalase Expression and Signaling Has Antitumor Activity in Pancreatic Cancer. Sci. Rep. 2016, 6, 22996. [Google Scholar] [CrossRef]
- Guo, X.; Jessel, S.; Qu, R.; Kluger, Y.; Chen, T.-M.; Hollander, L.; Safirstein, R.; Nelson, B.; Cha, C.; Bosenberg, M.; et al. Inhibition of Renalase Drives Tumour Rejection by Promoting T Cell Activation. Eur. J. Cancer 2022, 165, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xu, J.; Wang, P.; Velazquez, H.; Li, Y.; Wu, Y.; Desir, G.V. Catecholamines Regulate the Activity, Secretion, and Synthesis of Renalase. Circulation 2008, 117, 1277–1282. [Google Scholar] [CrossRef]
- Desir, G.V.; Tang, L.; Wang, P.; Li, G.; Sampaio-Maia, B.; Quelhas-Santos, J.; Pestana, M.; Velazquez, H. Renalase Lowers Ambulatory Blood Pressure by Metabolizing Circulating Adrenaline. J. Am. Heart Assoc. 2012, 1, e002634. [Google Scholar] [CrossRef]
- Sonawane, P.J.; Gupta, V.; Sasi, B.K.; Kalyani, A.; Natarajan, B.; Khan, A.A.; Sahu, B.S.; Mahapatra, N.R. Transcriptional Regulation of the Novel Monoamine Oxidase Renalase: Crucial Roles of Transcription Factors Sp1, STAT3, and ZBP89. Biochemistry 2014, 53, 6878–6892. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Huang, K.; Huang, D.; Yang, L.; Gao, L.; Wang, X.; Huang, D.; Li, X.; Wang, C.; Zhang, F.; et al. Renalase Is a Novel Target Gene of Hypoxia-Inducible Factor-1 in Protection against Cardiac Ischaemia–Reperfusion Injury. Cardiovasc. Res. 2015, 105, 182–191. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, G.; Xing, T.; Lu, Z.; Li, J.; Peng, C.; Liu, G.; Wang, N. Renalase Contributes to the Renal Protection of Delayed Ischaemic Preconditioning via the Regulation of Hypoxia-inducible Factor-1α. J. Cell. Mol. Med. 2015, 19, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Gu, J.; Guo, J.; Chen, K.; Li, H.; Wang, J. Renalase Attenuates Mouse Fatty Liver Ischemia/Reperfusion Injury through Mitigating Oxidative Stress and Mitochondrial Damage via Activating SIRT1. Oxidative Med. Cell. Longev. 2019, 2019, 7534285. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Cai, H.; Zhao, Q.; Xing, T.; Li, J.; Wang, N. Epinephrine Evokes Renalase Secretion via A-Adrenoceptor/NF-κB Pathways in Renal Proximal Tubular Epithelial Cells. Kidney Blood Press. Res. 2014, 39, 252–259. [Google Scholar] [CrossRef]
- Aoki, K.; Yanazawa, K.; Tokinoya, K.; Sugasawa, T.; Suzuki, T.; Yoshida, Y.; Nakano, T.; Omi, N.; Kawakami, Y.; Takekoshi, K. Renalase Is Localized to the Small Intestine Crypt and Expressed upon the Activation of NF-κB P65 in Mice Model of Fasting-Induced Oxidative Stress. Life Sci. 2021, 267, 118904. [Google Scholar] [CrossRef] [PubMed]
- Kalyani, A.; Sonawane, P.J.; Khan, A.A.; Subramanian, L.; Ehret, G.B.; Mullasari, A.S.; Mahapatra, N.R. Post-Transcriptional Regulation of Renalase Gene by miR-29 and miR-146 MicroRNAs: Implications for Cardiometabolic Disorders. J. Mol. Biol. 2015, 427, 2629–2646. [Google Scholar] [CrossRef]
- Dziedzic, M.; Powrózek, T.; Orłowska, E.; Koch, W.; Kukula-Koch, W.; Gaweł, K.; Bednarek-Skublewska, A.; Małecka-Massalska, T.; Milanowski, J.; Petkowicz, B.; et al. Relationship between microRNA-146a Expression and Plasma Renalase Levels in Hemodialyzed Patients. PLoS ONE 2017, 12, e0179218. [Google Scholar] [CrossRef]
- Özkan, G.; Ulusoy, Ş.; Geyik, E.; Erdem, Y. Down-regulation of miRNA 145 and Up-regulation of miRNA 4516 May Be Associated with Primary Hypertension. J Clin. Hypertens. 2019, 21, 1724–1731. [Google Scholar] [CrossRef]
- Luo, M.; Cao, S.; Lv, D.; He, L.; He, Z.; Li, L.; Li, Y.; Luo, S.; Chang, Q. Aerobic Exercise Training Improves Renal Injury in Spontaneously Hypertensive Rats by Increasing Renalase Expression in Medulla. Front. Cardiovasc. Med. 2022, 9, 922705. [Google Scholar] [CrossRef]
- Tokinoya, K.; Shiromoto, J.; Sugasawa, T.; Yoshida, Y.; Aoki, K.; Nakagawa, Y.; Ohmori, H.; Takekoshi, K. Influence of Acute Exercise on Renalase and Its Regulatory Mechanism. Life Sci. 2018, 210, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Tokinoya, K.; Yoshida, Y.; Sugasawa, T.; Takekoshi, K. Moderate-intensity Exercise Increases Renalase Levels in the Blood and Skeletal Muscle of Rats. FEBS Open Bio 2020, 10, 1005–1012. [Google Scholar] [CrossRef]
- Tokinoya, K.; Ono, S.; Aoki, K.; Yanazawa, K.; Shishikura, Y.; Sugasawa, T.; Takekoshi, K. Gene Expression Level of Renalase in the Skeletal Muscles Is Increased with High-Intensity Exercise Training in Mice on a High-Fat Diet. Physiol. Int. 2021, 108, 274–284. [Google Scholar] [CrossRef]
- Desir, G.V.; Wang, L.; Peixoto, A.J. Human Renalase: A Review of Its Biology, Function, and Implications for Hypertension. J. Am. Soc. Hypertens. 2012, 6, 417–426. [Google Scholar] [CrossRef]
- Farzaneh-Far, R.; Desir, G.V.; Na, B.; Schiller, N.B.; Whooley, M.A. A Functional Polymorphism in Renalase (Glu37Asp) Is Associated with Cardiac Hypertrophy, Dysfunction, and Ischemia: Data from the Heart and Soul Study. PLoS ONE 2010, 5, e13496. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, J.; Velazquez, H.; Wang, P.; Li, G.; Liu, D.; Sampaio-Maia, B.; Quelhas-Santos, J.; Russell, K.; Russell, R.; et al. Renalase Deficiency Aggravates Ischemic Myocardial Damage. Kidney Int. 2011, 79, 853–860. [Google Scholar] [CrossRef]
- Desir, G.V. Renalase Deficiency in Chronic Kidney Disease, and Its Contribution to Hypertension and Cardiovascular Disease. Curr. Opin. Nephrol. Hypertens. 2008, 17, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Crockett, S.L.; Harris, M.; Boatwright, N.; Su, R.L.; Yarboro, M.T.; Berger, C.D.; Shelton, E.L.; Reese, J.; Segar, J.L. Role of Dopamine and Selective Dopamine Receptor Agonists on Mouse Ductus Arteriosus Tone and Responsiveness. Pediatr. Res. 2020, 87, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Sizova, D.; Velazquez, H.; Sampaio-Maia, B.; Quelhas-Santos, J.; Pestana, M.; Desir, G.V. Renalase Regulates Renal Dopamine and Phosphate Metabolism. Am. J. Physiol.—Ren. Physiol. 2013, 305, F839–F844. [Google Scholar] [CrossRef]
- Quelhas-Santos, J.; Serrão, M.P.; Soares-Silva, I.; Fernandes-Cerqueira, C.; Simões-Silva, L.; Pinho, M.J.; Remião, F.; Sampaio-Maia, B.; Desir, G.V.; Pestana, M. Renalase Regulates Peripheral and Central Dopaminergic Activities. Am. J. Physiol.—Ren. Physiol. 2015, 308, F84–F91. [Google Scholar] [CrossRef]
- Wang, S.; Lu, X.; Yang, J.; Wang, H.; Chen, C.; Han, Y.; Ren, H.; Zheng, S.; He, D.; Zhou, L.; et al. Regulation of Renalase Expression by D5 Dopamine Receptors in Rat Renal Proximal Tubule Cells. Am. J. Physiol.—Ren. Physiol. 2014, 306, F588–F596. [Google Scholar] [CrossRef]
- Ferrari, M.; Cosentino, M.; Marino, F.; Bombelli, R.; Rasini, E.; Lecchini, S.; Frigo, G. Dopaminergic D1-like Receptor-Dependent Inhibition of Tyrosine Hydroxylase mRNA Expression and Catecholamine Production in Human Lymphocytes. Biochem. Pharmacol. 2004, 67, 865–873. [Google Scholar] [CrossRef]
- Singh, R.R.; Denton, K.M. Renal Denervation: A Treatment for Hypertension and Chronic Kidney Disease. Hypertension 2018, 72, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Guo, Y.; Tan, L.; Tang, X.; Yang, Q.; Yang, K. Impact of Renal Denervation on Renalase Expression in Adult Rats with Spontaneous Hypertension. Exp. Ther. Med. 2012, 4, 493–496. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vijayakumar, A.; Mahapatra, N.R. Renalase: A Novel Regulator of Cardiometabolic and Renal Diseases. Hypertens. Res. 2022, 45, 1582–1598. [Google Scholar] [CrossRef]
- Severina, I.S.; Fedchenko, V.I.; Veselovsky, A.V.; Medvedev, A.E. The history of renalase from amine oxidase to alpha-NAD(P)H-oxidase/anomerase. Biomeditsinskaya Khimiya 2015, 61, 667–679. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, M.; Ma, C.; Liu, W.; Liu, H.; Wang, N.; Kang, Q.; Li, P. Valsartan Promoting Atherosclerotic Plaque Stabilization by Upregulating Renalase: A Potential-Related Gene of Atherosclerosis. J. Cardiovasc. Pharmacol. Ther. 2015, 20, 509–519. [Google Scholar] [CrossRef]
- Wu, Y.; Quan, C.; Yang, Y.; Liang, Z.; Jiang, W.; Li, X. Renalase Improves Pressure Overload-Induced Heart Failure in Rats by Regulating Extracellular Signal-Regulated Protein Kinase 1/2 Signaling. Hypertens. Res. 2021, 44, 481–488. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, L.; Wen, J.; Zhang, F.; Gu, S.; Wang, F.; Yin, J.; Wang, N. Cardiac-Specific Renalase Overexpression Alleviates CKD-Induced Pathological Cardiac Remodeling in Mice. Front. Cardiovasc. Med. 2022, 9, 1061146. [Google Scholar] [CrossRef]
- Orlowska-Baranowska, E.; Gadomska Vel Betka, L.; Gora, J.; Baranowski, R.; Pedzich-Placha, E.; Zakrzewski, D.; Dlugosz, A.; Kossowska, H.; Zebrowska, A.; Zakoscielna, E.; et al. Functional Polymorphism of the Renalase Gene Is Associated with Cardiac Hypertrophy in Female Patients with Aortic Stenosis. PLoS ONE 2017, 12, e0186729. [Google Scholar] [CrossRef]
- Shen, H.; Yang, J.; Xue, W.; Wei, Z.; Li, L.; Guan, J.; Li, X.; Wu, X. Renalase Rs2296545 Variant Improve Hypertension Susceptibility by Modifying Binding Affinity to Catecholamines in Obstructive Sleep Apnea. Hypertens. Res. 2024, 47, 3200–3213. [Google Scholar] [CrossRef]
- Khater, M.H.; Abd EL-Hassib, D.M.; Sabry, J.H.; Elkilany, R.M.; Ameen, S.G. Association Between Renalase Gene Polymorphism (Rs2296545) and Hypertension in Egyptian Chronic Kidney Disease Patients. Cureus 2023, 15, e47903. [Google Scholar] [CrossRef] [PubMed]
- Akbari, H.; Asadikaram, G.; Vakili, S.; Masoumi, M. Atorvastatin and Losartan May Upregulate Renalase Activity in Hypertension but Not Coronary Artery Diseases: The Role of Gene Polymorphism. J. Cell. Biochem. 2019, 120, 9159–9171. [Google Scholar] [CrossRef]
- Izadpanah, P.; Asadian, F.; Jangjou, A. Association of Serum Renalase Levels and Renalase Rs10887800 Polymorphism with Unstable Angina Pectoris Patients Having Metabolic Syndrome. Diabetes Metab. Syndr. Obes. 2020, 13, 3249–3259. [Google Scholar] [CrossRef] [PubMed]
- Ramroodi, N.; Khorrami, A.; Hashemi, S.M.; Rezaei, M.; Shahraki-ghadim, H.; Salimi, S. The Effect of Renalase Rs2576178 and Rs10887800 Polymorphisms on Ischemic Stroke Susceptibility in Young Patients (<50 Years): A Case-Control Study and In Silico Analysis. Dis. Markers 2021, 2021, 5542292. [Google Scholar] [CrossRef]
- Hu, N.; Wang, J.; Hu, P.; Li, Z. Investigation of Renalase Gene Rs2576178 Polymorphism in Patients with Coronary Artery Disease. Biosci. Rep. 2018, 38, BSR20180839. [Google Scholar] [CrossRef]
- Heydarpour, M.; Parksook, W.W.; Hopkins, P.N.; Pojoga, L.H.; Williams, G.H.; Williams, J.S. A Candidate Locus in the Renalase Gene and Susceptibility to Blood Pressure Responses to the Dietary Salt. J. Hypertens. 2023, 41, 723–732. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.; Hu, G.-L.; Chu, C.; Zhang, X.-Y.; Du, M.-F.; Zou, T.; Zhou, Q.; Liao, Y.-Y.; Ma, Q.; et al. Associations of Renalase With Blood Pressure and Hypertension in Chinese Adults. Front. Cardiovasc. Med. 2022, 9, 800427. [Google Scholar] [CrossRef]
- Lemiesz, M.; Tenderenda-Banasiuk, E.; Sosnowska, D.; Taranta-Janusz, K.; Wasilewska, A. Serum Renalase Levels in Adolescents with Primary Hypertension. Pediatr. Cardiol. 2018, 39, 1258–1264. [Google Scholar] [CrossRef]
- Martynowicz, H.; Wieckiewicz, M.; Poreba, R.; Wojakowska, A.; Smardz, J.; Januszewska, L.; Markiewicz-Gorka, I.; Mazur, G.; Pawlas, K.; Gac, P. The Relationship between Sleep Bruxism Intensity and Renalase Concentration—An Enzyme Involved in Hypertension Development. J. Clin. Med. 2019, 9, 16. [Google Scholar] [CrossRef]
- Martynowicz, H.; Czerwińska, K.; Wojakowska, A.; Januszewska, L.; Markiewicz-Górka, I.; Więckiewicz, M.; Mazur, G.; Pawlas, K.; Poręba, R.; Gać, P. Renalase and Hypertension—Demographic and Clinical Correlates in Obstructive Sleep Apnea. Sleep Breath. 2021, 25, 669–675. [Google Scholar] [CrossRef]
- Czerwińska, K.; Januszewska, L.; Markiewicz-Górka, I.; Jaremków, A.; Martynowicz, H.; Pawlas, K.; Mazur, G.; Poręba, R.; Gać, P. Selenoprotein P, Peroxiredoxin-5, Renalase and Selected Cardiovascular Consequences Tested in Ambulatory Blood Pressure Monitoring and Echocardiography. Antioxidants 2023, 12, 1187. [Google Scholar] [CrossRef] [PubMed]
- Wybraniec, M.T.; Bożentowicz-Wikarek, M.; Chudek, J.; Mizia-Stec, K. Urinary Renalase Concentration in Patients with Preserved Kidney Function Undergoing Coronary Angiography. Nephrology 2018, 23, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-H.; Sheu, W.H.-H.; Lee, W.-J.; Wang, J.-S.; Fu, C.-P.; Liang, K.-W.; Lee, I.-T. Synergistic Effect of Renalase and Chronic Kidney Disease on Endothelin-1 in Patients with Coronary Artery Disease—A Cross-Sectional Study. Sci. Rep. 2018, 8, 7378. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-T.; Sheu, W.H.-H. Serum Renalase Levels Are Predicted by Brain-Derived Neurotrophic Factor and Associated with Cardiovascular Events and Mortality after Percutaneous Coronary Intervention. J. Clin. Med. 2018, 7, 437. [Google Scholar] [CrossRef] [PubMed]
- Safdar, B.; Guo, X.; Johnson, C.; D’Onofrio, G.; Dziura, J.; Sinusas, A.J.; Testani, J.; Rao, V.; Desir, G. Elevated Renalase Levels in Patients with Acute Coronary Microvascular Dysfunction—A Possible Biomarker for Ischemia. Int. J. Cardiol. 2019, 279, 155–161. [Google Scholar] [CrossRef]
- Wybraniec, M.T.; Wieczorek, J.; Woźniak-Skowerska, I.; Hoffmann, A.; Nowak, S.; Cichoń, M.; Szydło, K.; Wnuk-Wojnar, A.; Chudek, J.; Więcek, A.; et al. Renalase Is Associated with Adverse Left Atrial Remodelling and Disease Burden in Patients with Atrial Fibrillation Undergoing Pulmonary Vein Isolation. Kardiol. Pol. 2018, 76, 1232–1241. [Google Scholar] [CrossRef]
- Stojanovic, D.; Mitic, V.; Petrovic, D.; Stojanovic, M.; Ignjatovic, A.; Stefanovic, N.; Cvetkovic, T.; Bojanic, V.; Kocic, G.; Ilic, M.D. Association of Plasma Renalase and Left Ventricle Mass Index in Heart Failure Patients Stratified to the Category of the Ejection Fraction: A Pilot Study. Dis. Markers 2019, 2019, 7265160. [Google Scholar] [CrossRef]
- Stojanovic, D.; Mitic, V.; Stojanovic, M.; Petrovic, D.; Ignjatovic, A.; Stefanovic, N.; Cvetkovic, T.; Kocic, G.; Bojanic, V.; Deljanin Ilic, M. The Partnership between Renalase and Ejection Fraction as a Risk Factor for Increased Cardiac Remodeling Biomarkers in Chronic Heart Failure Patients. Curr. Med. Res. Opin. 2020, 36, 909–919. [Google Scholar] [CrossRef]
- Stojanovic, D.; Mitic, V.; Stojanovic, M.; Petrovic, D.; Ignjatovic, A.; Milojkovic, M.; Dunjic, O.; Milenkovic, J.; Bojanic, V.; Deljanin Ilic, M. The Discriminatory Ability of Renalase and Biomarkers of Cardiac Remodeling for the Prediction of Ischemia in Chronic Heart Failure Patients With the Regard to the Ejection Fraction. Front. Cardiovasc. Med. 2021, 8, 691513. [Google Scholar] [CrossRef]
- Gok Oguz, E.; Akoglu, H.; Ulusal Okyay, G.; Karaveli Gursoy, G.; Yildirim, T.; Merhametsiz, O.; Cimen, T.; Canbakan, B.; Yeter, E.; Ayli, M.D. Increased Serum Renalase in Peritoneal Dialysis Patients: Is It Related to Cardiovascular Disease Risk? Nefrología 2017, 37, 189–194. [Google Scholar] [CrossRef]
- Knop, W.; Serwin, N.M.; Cecerska-Heryć, E.; Grygorcewicz, B.; Dołęgowska, B.; Gomółka, A.; Wiśniewska, M.; Ciechanowski, K. Elevated Levels of Renalase, the β-NAD(P)H Isomerase, Can Be Used as Risk Factors of Major Adverse Cardiovascular Events and All-Cause Death in Patients with Chronic Kidney Disease. Biomolecules 2021, 11, 1514. [Google Scholar] [CrossRef]
- Cerqueira, A.; Quelhas-Santos, J.; Ferreira, I.; Sampaio, S.; Relvas, M.; Marques, N.; Dias, C.C.; Pestana, M. Circulating Renalase as Predictor of Renal and Cardiovascular Outcomes in Pre-Dialysis CKD Patients: A 5-Year Prospective Cohort Study. Life 2021, 11, 210. [Google Scholar] [CrossRef] [PubMed]
- Żórawik, A.; Hajdusianek, W.; Markiewicz-Górka, I.; Jaremków, A.; Pawlas, K.; Martynowicz, H.; Mazur, G.; Poręba, R.; Gać, P. Coexistence of Cardiovascular Risk Factors and Blood Renalase Concentration. Int. J. Mol. Sci. 2023, 24, 16666. [Google Scholar] [CrossRef] [PubMed]

| SNP(s) | Patient Cohort Characteristics | Key Findings | References |
|---|---|---|---|
| rs2296545 (Glu37Asp) | 657 elderly Polish patients with aortic stenosis | Asp37 variant associated with higher left ventricular mass, intraventricular septal thickness, posterior wall thickness, and relative wall thickness in female patients only; no association in male patients. | [57] |
| 126 middle-aged Chinese Han patients with OSA | Serum renalase levels higher in severe OSA group; positive correlation with BP in non-OSA group, negative correlation in severe OSA group. CG genotype associated with increased hypertension risk in the severe OSA group. | [58] | |
| 60 middle-aged Egyptian patients with CKD (hypertensive and normotensive) | CC genotype and C allele associated with increased risk of CKD and higher risk of developing hypertensive CKD. | [59] | |
| rs10887800 | 260 elderly Iranian patients with CAD and hypertension | Renalase activity higher in hypertension patients. No gender differences in renalase activity. No association of SNP with risk/protection against hypertension and/or CAD. Atorvastatin/losartan increased renalase activity (hypertension); losartan reduced renalase activity (CAD) vs. controls. | [60] |
| 134 elderly Iranian patients with MetS and USAP | Renalase levels higher in USAP + MetS patients (23.28 ± 4.09 µg/dL) vs. healthy controls (20.81 ± 2.73 µg/dL). AG and GG genotypes more common in USAP + MetS patients. | [61] | |
| rs2576178 rs10887800 | 154 middle-aged Iranian patients with IS | rs10887800 AG genotype associated with a 1.6-fold higher risk of IS. No significant association for rs2576178. | [62] |
| rs2576178 | 449 elderly Chinese CAD patients | GG genotype or G allele associated with higher CAD risk in females, smokers, and individuals who consume alcohol. No association with general clinical parameters. | [63] |
| rs1932531, rs77324767, rs10749565, JHU_10.89888367, rs77287889, rs868872, rs10509536; (TCTTAGTT haplotype) rs10887801 | 720 middle-aged American patients with hypertension | On high-salt diet, 7 SNPs (TCTTAGTT haplotype) associated with SBP, pulse pressure, and mean arterial pressure. rs10887801 TT genotype carriers had higher SSBP and lower plasma renin activity. | [64] |
| rs7922058, rs1935582, rs2576178, rs10887800, rs796945, rs2296545 | 488 middle-aged and elderly Chinese hypertensive patients | rs7922058: associated with 14-year change in systolic BP. rs1935582 & rs2576178: associated with 14-year change in mean arterial pressure. rs2576178, rs1935582, rs10887800, rs796945, & rs2296545: associated with 14-year change in diastolic BP. rs1935582, rs2576178, & rs796945: associated with hypertension incidence. Renalase gene expression decreased in renal biopsies of hypertensive vs. normotensive controls. | [65] |
| Condition Studied/Main Grouping | Patient/Cohort Description | n | Renalase Levels (Mean ± SD or Median [IQR/Range]) & Units | Key Associations and Findings | References |
|---|---|---|---|---|---|
| Hypertension & RelatedConditions | |||||
| Primary Hypertension | Polish adolescent patients | 88 | Serum: Patients: Med 29.8 [26.1–35.8] µg/mL Controls: Med 26.8 [22.96–29.4] µg/mL | Higher in patients. Correlated with serum uric acid. Correlated with systolic & diastolic BP in hypertensive patients. | [66] |
| Sleep Bruxism (Hypertensive vs. Normotensive) | Polish middle-aged patients | 87 | Serum: Hypertensive: 133.33 ± 160.71 ng/mL Normotensive Controls: 219.23 ± 220.58 ng/mL | Lower in hypertensive group. Negatively correlated with BMI (bruxism group). Cut-off > 212.5 ng/mL associated higher bruxism episodes & BMI with lower renalase. | [67] |
| Suspected Obstructive Sleep Apnoea (OSA) | Polish middle-aged patients | 113 | Serum: Mod/Sev OSA: 139.56 ± 175.72 ng/mL No OSA (Controls): 230.97 ± 240.50 ng/mL | Lower in moderate/severe OSA. Negatively correlated with AHI (hypertensive group, males, <60 yrs). Lower renalase, hypertension, higher BMI, male gender independently associated with higher AHI. | [68] |
| Polish middle-aged patients | 101 | Serum: Hypertensive: 159.16 ± 207.19 ng/mL No OSA (Controls): 212.56 ± 238.79 ng/mL OSA group (general): 167.37 ± 197.29 ng/mL | Negatively correlated with pulse pressure, IVSEDD, & LA diameter. Lower in hypertensive group vs. no OSA; no significant difference in general OSA group vs. no OSA. | [69] | |
| Hypertension | Chinese middle-aged & elderly patients | 488 | Serum: Hypertensive: 27.2 ± 0.4 µg/mL Normotensive: 25.1 ± 0.2 µg/mL | Higher in hypertensive patients. Correlated with BP and associated with hypertension risk. | [65] |
| Coronary Artery Disease (CAD) | |||||
| CI-AKI post-CA/PCI | Polish elderly patients with CAD | 95 | Urinary (Renalase/Creatinine ratio): Pre-CA/PCI: 2843.6 ng/mg 6 h Post-CA/PCI: 1540.7 ng/mg (Decreased in both CI-AKI & non-CI-AKI subgroups) | Urinary ratio decreased 6 h post-CA/PCI. Decrease below 25th percentile suggested as CI-AKI predictor. | [70] |
| CAD with/without CKD | Non-diabetic elderly Taiwanese patients | 342 | Serum: CAD + CKD: 46.8 ± 17.1 ng/mL CAD (no CKD): 33.9 ± 9.9 ng/mL | Higher renalase (and ET-1) in CAD + CKD. High renalase with CKD associated with increased ET-1. | [71] |
| Post-PCI Outcomes | Taiwanese elderly CAD patients | 152 | Serum: Pre-PCI: 47.5 ± 17.3 ng/mL Post-PCI: 35.9 ± 11.3 ng/mL | Reduced post-PCI. Pre-PCI BDNF predicted renalase reduction. Post-PCI renalase ≥ 35 ng/mL associated with higher risk of MI, stroke, death. | [72] |
| Coronary Microvascular Dysfunction (CMD) | American middle-aged patients with chest pain (PET/CT) | 80 | Serum: CMD: Med 5503 [IQR 3070] ng/mL CAD: Med 4069 [IQR 1850] ng/mL Controls: Med 4266 [IQR 1503] ng/mL | Higher in CMD patients. Independent predictor of CMD. | [73] |
| Atrial Fibrillation (AF) | |||||
| AF undergoing PVI | Polish elderly patients | 69 | Serum: AF (PVI): Mean 27.99 µg/mL Controls: Mean 21.48 µg/mL Persistent AF: 19.05 µg/mL (vs. 28.77 µg/mL non-persistent) | Higher in AF (PVI) vs. controls. Lower in persistent AF & with pre-PVI AF episodes. Lower levels associated with higher heart rate, AF burden, LA diameter, less negative LA strain. No prediction of AF recurrence (6 mo). | [74] |
| Heart Failure (HF) | |||||
| HF (HFrEF, HFmrEF, HFpEF) | Serbian elderly patients | 75 | Plasma: HFrEF: 147.33 ± 29.07 ng/mL HFmrEF: 118.58 ± 19.61 ng/mL HFpEF: 117.31 ± 32.83 ng/mL | Higher in HFrEF. Correlated with LV mass index & BNP in HFrEF. | [75] |
| HF (HFrEF vs. HFpEF) | Serbian elderly patients | 76 | Plasma: HFrEF: 126.26 ± 8.45 ng/mL HFpEF: 106.68 ± 13.54 ng/mL | Higher in HFrEF. Correlated with remodelling biomarkers (galectin-3, sST2, GDF-15, syndecan-1, BNP, cystatin); negatively with LVEF. | [76] |
| Chronic HF (CHF) | Serbian elderly patients | 77 | Plasma: HFrEF: 147.52 ± 29.39 ng/mL HFpEF: 122.63 ± 38.61 ng/mL | Increased renalase (and other biomarkers) in HFrEF were independent predictors of ischaemia. | [77] |
| CVD Risk Factors/MACE/CKD | |||||
| Peritoneal Dialysis (PD) | Turkish middle-aged patients | 40 | Serum: PD: Med 176.5 [100–278.3] ng/mL Controls: Med 122 [53.3–170.0] ng/mL | Higher in PD patients. Correlated with CRP, negatively with RRF. No correlation with EAT or LVMI. | [78] |
| Chronic Kidney Disease (CKD) | Polish elderly patients (incl. haemodialysed) | 90 | Serum: Haemodialysed CKD: 35.6 ± 13.5 µg/mL Controls: 21.8 ± 9.2 µg/mL | Correlated with all-cause death (haemodialysed subgroup). Correlated with MACE & all-cause death (entire CKD pop.). Cut-offs: >25 µg/mL (MACE risk); >30 µg/mL (death risk in haemodialysed). | [79] |
| Pre-dialysis CKD | Portuguese elderly patients | 40 | Serum: CKD Stage 4–5: Med 83.53 [78.6–95.2] µg/mL CKD Stage 1–3: Med 42.03 [37.45–60.07] µg/mL | Higher in advanced CKD stages. Associated with various biochemical parameters, eGFR, CKD progression, hospitalisation, all-cause mortality. Negatively correlated with Hb, HDL. No MACE association reported. | [80] |
| Multiple CVD Risk Factors | Polish middle-aged patients with diverse risk factors | 96 | Serum: Lower RF group: 284.33 ± 232.20 ng/mL Higher RF group (≥4 RFs): 90.33 ± 127.50 ng/mL | Higher renalase with fewer CVD risk factors. Lower renalase associated with obesity, smoking, lack of physical activity. Cut-off < 38.98 ng/mL associated with ≥5 CVD risk factors. | [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dabravolski, S.A.; Omelchenko, A.V.; Korchagina, E.R.; Maltseva, O.N.; Pavshintsev, V.V.; Kovyanova, T.I. The Role of Renalase in Cardiovascular Disease: A Comprehensive Review of Its Molecular Biology, Genetic Associations, and Clinical Significance. Life 2025, 15, 1581. https://doi.org/10.3390/life15101581
Dabravolski SA, Omelchenko AV, Korchagina ER, Maltseva ON, Pavshintsev VV, Kovyanova TI. The Role of Renalase in Cardiovascular Disease: A Comprehensive Review of Its Molecular Biology, Genetic Associations, and Clinical Significance. Life. 2025; 15(10):1581. https://doi.org/10.3390/life15101581
Chicago/Turabian StyleDabravolski, Siarhei A., Andrey V. Omelchenko, Elizaveta R. Korchagina, Olga N. Maltseva, Vsevolod V. Pavshintsev, and Tatiana I. Kovyanova. 2025. "The Role of Renalase in Cardiovascular Disease: A Comprehensive Review of Its Molecular Biology, Genetic Associations, and Clinical Significance" Life 15, no. 10: 1581. https://doi.org/10.3390/life15101581
APA StyleDabravolski, S. A., Omelchenko, A. V., Korchagina, E. R., Maltseva, O. N., Pavshintsev, V. V., & Kovyanova, T. I. (2025). The Role of Renalase in Cardiovascular Disease: A Comprehensive Review of Its Molecular Biology, Genetic Associations, and Clinical Significance. Life, 15(10), 1581. https://doi.org/10.3390/life15101581





