Advancing the Diagnosis and Treatment of Early Chronic Pancreatitis Through Innovation in Imaging and Biomarker Profiling—A Narrative Review
Abstract
1. Introduction
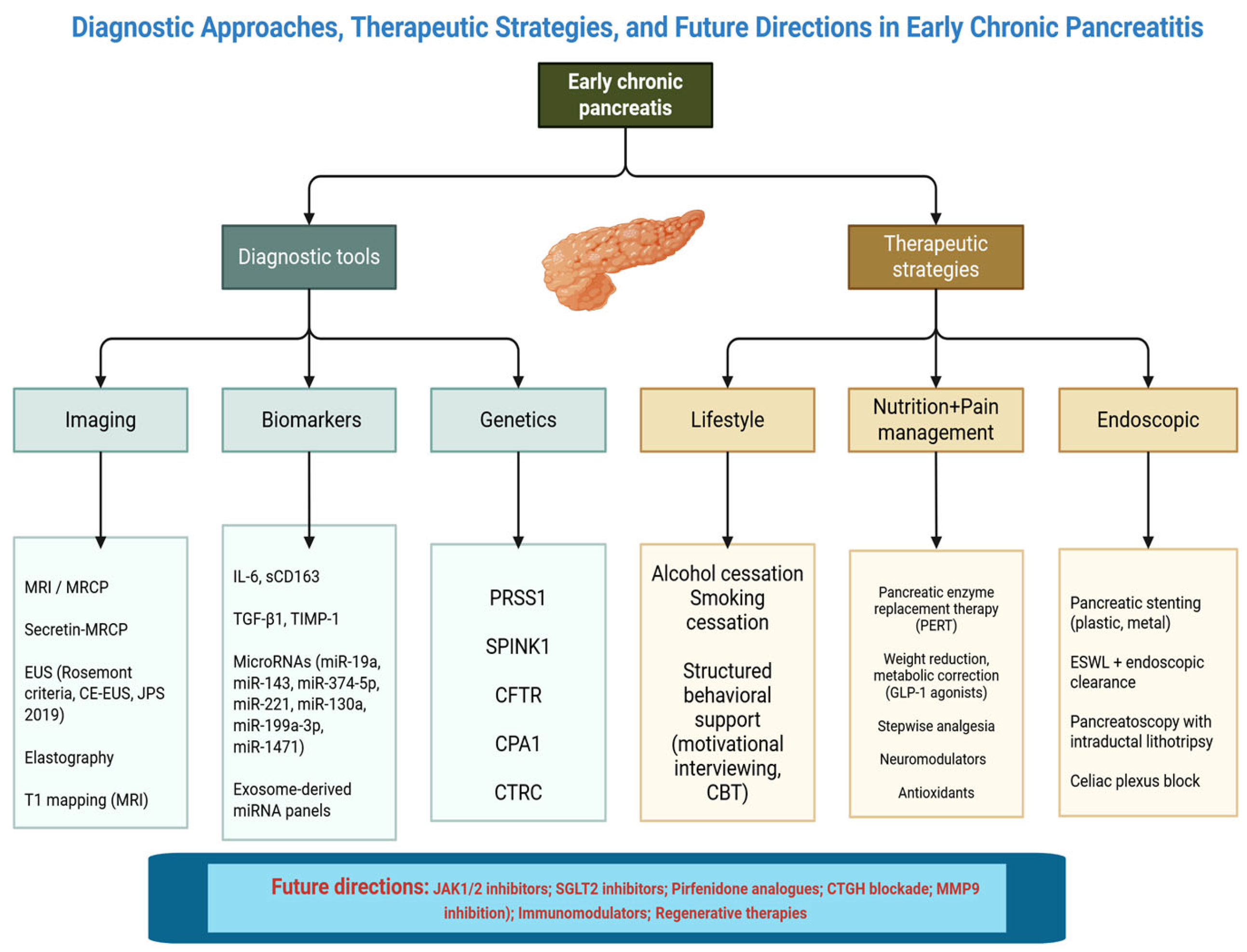
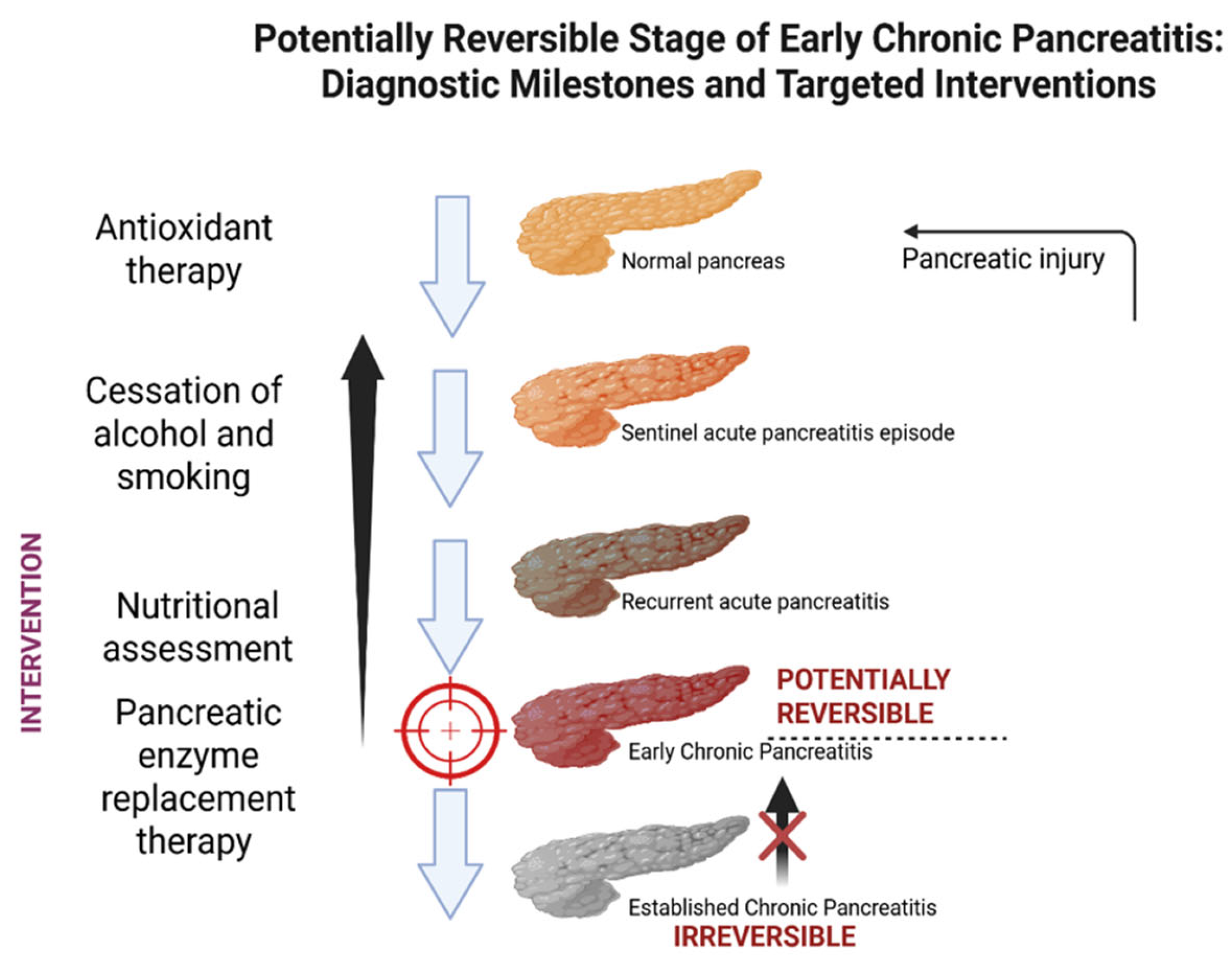
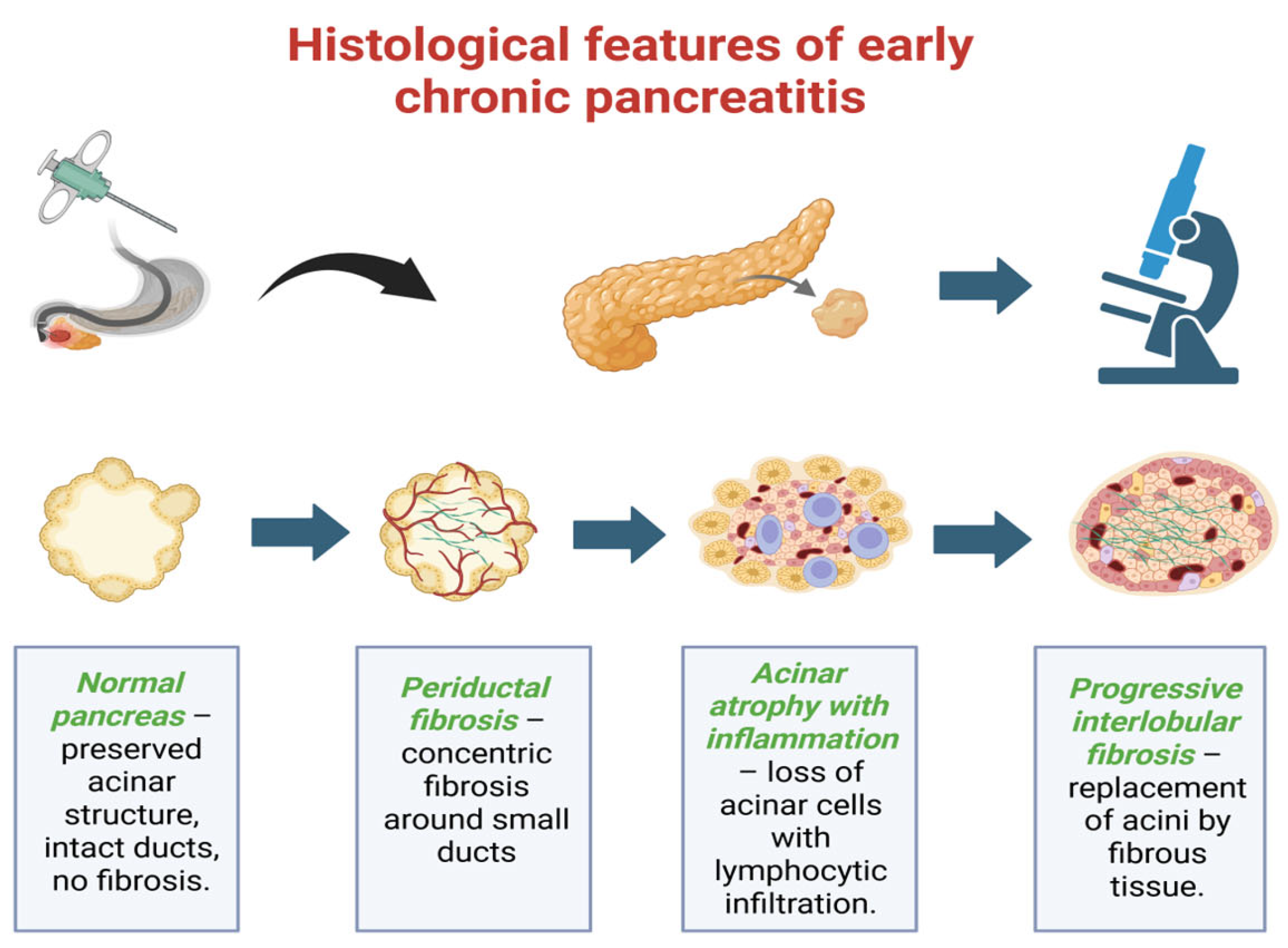
2. Early Chronic Pancreatitis: Definition and Diagnostic Criteria
3. Diagnostic Modalities
3.1. Imaging Techniques
3.2. Biomarkers
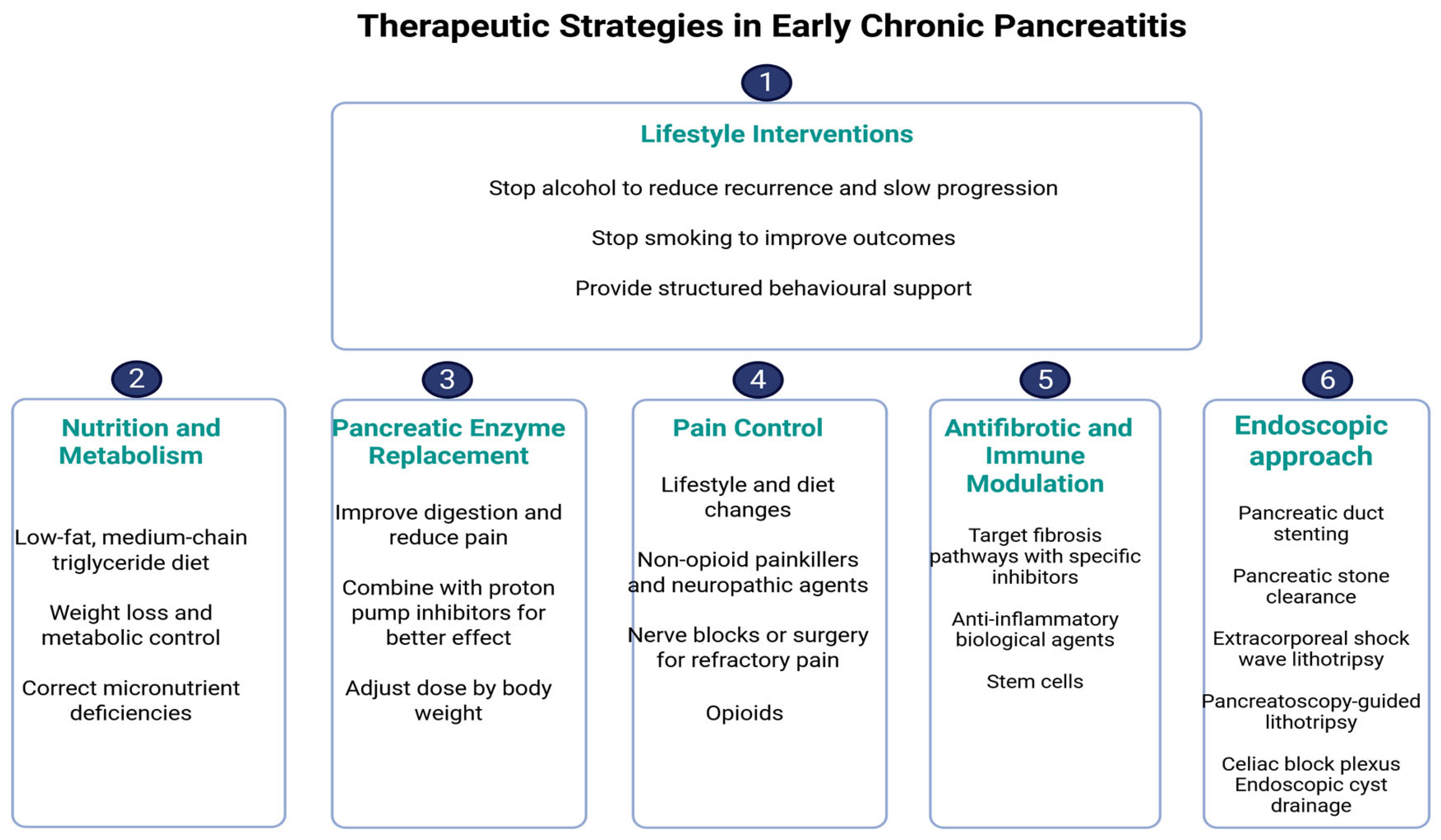
4. Therapeutic Strategies in Early Chronic Pancreatitis
4.1. Lifestyle Interventions
4.2. Nutritional Optimization and Metabolic Correction
4.3. Enzyme Supplementation: Timing and Rationale
4.4. Pain Control: Step-Up Approach
4.5. Antifibrotics and Immune Modulators
4.6. Role of Endoscopy
5. Limitations
6. Future Perspectives and Research Gaps
6.1. Biomarker Validation
6.2. Imaging Standardization
6.3. Precision Medicine and Registries
6.4. Therapeutic Innovation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| AIP | Autoimmune Pancreatitis |
| CFTR | Cystic Fibrosis Transmembrane Conductance Regulator |
| CP | Chronic Pancreatitis |
| CT | Computed Tomography |
| CTSI | Computed Tomography Severity Index |
| ECP | Early Chronic Pancreatitis |
| EUS | Endoscopic Ultrasound |
| ERCP | Endoscopic Retrograde Cholangiopancreatography |
| GWAS | Genome-Wide Association Studies |
| IL-6 | Interleukin-6 |
| JPS | Japanese Pancreas Society |
| MRI | Magnetic Resonance Imaging |
| MRCP | Magnetic Resonance Cholangiopancreatography |
| NGS | Next-Generation Sequencing |
| PERT | Pancreatic Enzyme Replacement Therapy |
| PRSS1 | Protease, Serine, 1 (cationic trypsinogen gene) |
| RAP | Recurrent Acute Pancreatitis |
| sCD163 | Soluble CD163 (macrophage activation marker) |
| SPINK1 | Serine Protease Inhibitor Kazal Type 1 |
| TNF-α | Tumor Necrosis Factor-alpha |
References
- Khurmatullina, A.R.; Andreev, D.N.; Maev, I.V.; Kucheryavyy, Y.A.; Beliy, P.A.; Dzhafarova, A.R.; Cherenkova, V.V.; Sokolov, F.S. Prevalence and Risk of Sarcopenia in Patients with Chronic Pancreatitis: Systematic Review and Meta-Analysis. Nutrients 2025, 17, 870. [Google Scholar] [CrossRef]
- Lankisch, P.G.; Apte, M.; Banks, P.A. Acute Pancreatitis. Lancet 2015, 386, 85–96. [Google Scholar] [CrossRef]
- Yamamiya, A.; Tomonaga, K.; Hoshi, K.; Nagashima, K.; Minaguchi, T.; Haruyama, Y.; Irisawa, A. The Risk Factors for Progression to Chronic Pancreatitis in Patients with Past-History of Acute Pancreatitis: A Retrospective Analysis Based on Mechanistic Definition. J. Clin. Med. 2022, 11, 2209. [Google Scholar] [CrossRef]
- Strum, W.B.; Boland, C.R. Advances in Acute and Chronic Pancreatitis. World J. Gastroenterol. 2023, 29, 1194–1201. [Google Scholar] [CrossRef]
- Takasaki, Y.; Ishii, S.; Fujisawa, T.; Ushio, M.; Takahashi, S.; Yamagata, W.; Ito, K.; Suzuki, A.; Ochiai, K.; Tomishima, K.; et al. Endoscopic Ultrasonography Findings of Early and Suspected Early Chronic Pancreatitis. Diagnostics 2020, 10, 1018. [Google Scholar] [CrossRef] [PubMed]
- Sekine, M.; Tanaka, A.; Akimoto, M.; Miura, T.; Fujiwara, J.; Noda, H.; Rikiyama, T.; Ohnishi, H.; Mashima, H. A Comparative Study of Endoscopic Ultrasonography and Histopathology Images for the Diagnosis of Early Chronic Pancreatitis. Pancreas 2021, 50, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Brizi, M.G.; Perillo, F.; Cannone, F.; Tuzza, L.; Manfredi, R. The Role of Imaging in Acute Pancreatitis. Radiol. Med. 2021, 126, 1017–1029. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, S.J.; Xiao, A.Y.; Wu, L.M.; Windsor, J.A.; Forsmark, C.E.; Petrov, M.S. Frequency of Progression From Acute to Chronic Pancreatitis and Risk Factors: A Meta-analysis. Gastroenterology 2015, 149, 1490–1500.e1. [Google Scholar] [CrossRef]
- García, J.S.; Delgado Cordón, F. Role of imaging in the diagnosis of chronic pancreatitis. Radiol. Engl. Ed. 2019, 61, 247–258. [Google Scholar] [CrossRef]
- Metelli, F.; Manfredi, G.; Pagano, N.; Buscarini, E.; Crino, S.F.; Armellini, E. The Role of Endoscopic Ultrasound and Ancillary Modalities in Pancreatic Imaging in Chronic Pancreatitis. Diagnostics 2024, 14, 1233. [Google Scholar] [CrossRef]
- Thierens, N.; Verdonk, R.C.; Löhr, J.M.; van Santvoort, H.C.; Bouwense, S.A.; van Hooft, J.E. Chronic Pancreatitis. Lancet 2025, 404, 2605–2618. [Google Scholar] [CrossRef] [PubMed]
- Whitcomb, D.C.; Shimosegawa, T.; Chari, S.T.; Forsmark, C.E.; Frulloni, L.; Garg, P.; Hegyi, P.; Hirooka, Y.; Irisawa, A.; Ishikawa, T.; et al. International Consensus Statements on Early Chronic Pancreatitis. Pancreatology 2018, 18, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Takasaki, Y.; Fujisawa, T.; Ishii, S.; Tomishima, K.; Takahashi, S.; Ikoma, I.; Jimbo, Y.; Ota, H.; Kabemura, D.; et al. Current Situation and Problems in Diagnosis of Early Chronic Pancreatitis. Pancreas 2023, 52, e275–e281. [Google Scholar] [CrossRef] [PubMed]
- Uc, A.; Perito, E.R.; Pohl, J.F.; Shah, U.; Abu-El-Haija, M.; Barth, B.; Bellin, M.D.; Ellery, K.M.; Fishman, D.S.; Gariepy, C.E.; et al. International Study Group of Pediatric Pancreatitis: In Search for a CuRE Cohort Study: Design and Rationale for INSPPIRE 2 from the Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer. Pancreas 2018, 47, 1222–1228. [Google Scholar] [CrossRef]
- Abu-El-Haija, M.; Nathan, J.D. Pediatric Chronic Pancreatitis: Updates in the 21st Century. Pancreatology 2018, 18, 354–359. [Google Scholar] [CrossRef]
- Wang, M.; Gao, F.; Wang, X.; Liu, Y.; Ji, R.; Cang, L.; Shi, Y. Magnetic Resonance Elastography and T1 Mapping for Early Diagnosis and Classification of Chronic Pancreatitis. J. Magn. Reson. Imaging 2018, 48, 837–845. [Google Scholar] [CrossRef]
- Khair, A.M.; McIlvain, G.; McGarry, M.D.J.; Kandula, V.; Yue, X.; Kaur, G.; Averill, L.W.; Choudhary, A.K.; Johnson, C.L.; Nikam, R.M. Clinical Application of Magnetic Resonance Elastography in Pediatric Neurological Disorders. Pediatr. Radiol. 2023, 53, 2712–2722. [Google Scholar] [CrossRef]
- Meher, S.; Mishra, T.S.; Sasmal, P.K.; Rath, S.; Sharma, R.; Rout, B.; Sahu, M.K. Role of Biomarkers in Diagnosis and Prognostic Evaluation of Acute Pancreatitis. J. Biomark. 2015, 2015, 519534. [Google Scholar] [CrossRef]
- Frossard, J.-L.; Hadengue, A.; Pastor, C.M. New Serum Markers for the Detection of Severe Acute Pancreatitis in Humans. Am. J. Respir. Crit. Care Med. 2001, 164, 162–170. [Google Scholar] [CrossRef]
- Yadav, D.; Conwell, D.L.; Pandol, S.J.; Steen, H.; Feng, Z.; Li, L.; Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer. Diagnostic and Prognostic Biomarkers of Chronic Pancreatitis: A Conceptual Framework Based on the PRoBE Design. Gastroenterology 2024, 166, 957–962.e3. [Google Scholar] [CrossRef]
- Poulsen, C.L.; Brock, C.; Olesen, S.S.; Nøjgaard, C.; Frøkjær, J.B.; Drewes, A.M. IL-6 and Pancreatic Injury in ECP. Pancreas 2023, 52, 341–347. [Google Scholar] [CrossRef]
- Poulsen, V.V.; Hadi, A.; Werge, M.P.; Karstensen, J.G.; Novovic, S. Circulating Biomarkers Involved in the Development of and Progression to Chronic Pancreatitis—A Literature Review. Biomolecules 2024, 14, 239. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Monserrate, Z.; Gumpper, K.; Pita, V.; Hart, P.A.; Forsmark, C.; Whitcomb, D.C.; Yadav, D.; Waldron, R.T.; Pandol, S.; Steen, H.; et al. Biomarkers of Chronic Pancreatitis: A systematic literature review. Pancreatology 2021, 21, 323–333. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Löhr, J.M.; Dominguez-Munoz, E.; Rosendahl, J.; Besselink, M.; Mayerle, J.; Lerch, M.M.; Haas, S.; Akisik, F.; Kartalis, N.; Iglesias-Garcia, J.; et al. United European Gastroenterology Evidence-Based Guidelines for the Diagnosis and Therapy of Chronic Pancreatitis (HaPanEU). United Eur. Gastroenterol. J. 2017, 5, 153–199. [Google Scholar] [CrossRef]
- Ammann, R.W. A Clinically Based Classification System for Alcoholic Chronic Pancreatitis: Summary of an International Workshop on Chronic Pancreatitis. Pancreas 1997, 14, 215–221. [Google Scholar] [CrossRef]
- LaRusch, J.; Whitcomb, D.C. Genetics of pancreatitis. Curr. Opin. Gastroenterol. 2011, 27, 467–474. [Google Scholar] [CrossRef]
- Shimosegawa, T. Japanese Clinical Diagnostic Criteria for Chronic Pancreatitis 2009: The Summary and Process for Proposals. Pancreas 2010, 39, 700–701. [Google Scholar] [CrossRef]
- Stevens, T.; Dumot, J.A.; Zuccaro, G.; Vargo, J.J.; Parsi, M.A.; Lopez, R.; Kirchner, H.L.; Purich, E.; Conwell, D.L. Evaluation of duct-cell and acinar-cell function and endosonographic abnormalities in patients with suspected chronic pancreatitis. Clin. Gastroenterol. Hepatol. 2009, 7, 114–119. [Google Scholar] [CrossRef]
- Tirkes, T.; Lin, C.; Fogel, E.L.; Sherman, S.S.; Wang, Q.; Sandrasegaran, K. T1 Mapping for Diagnosis of Mild Chronic Pancreatitis. J. Magn. Reson. Imaging 2017, 45, 1171–1176. [Google Scholar] [CrossRef]
- Masamune, A.; Nabeshima, T.; Kikuta, K.; Hamada, S.; Nakano, E.; Kume, K.; Kanno, A.; Sato, A.; Tachibana, Y.; Inatomi, O.; et al. Prospective Study of Early Chronic Pancreatitis Diagnosed Based on the Japanese Diagnostic Criteria. J. Gastroenterol. 2019, 54, 928–935. [Google Scholar] [CrossRef]
- Hegyi, P.J.; Soós, A.; Tóth, E.; Ébert, A.; Venglovecz, V.; Márta, K.; Mátrai, P.; Mikó, A.; Bajor, J.; Sarlós, P.; et al. Evidence for Diagnosis of Early Chronic Pancreatitis after Three Episodes of Acute Pancreatitis: A Cross-Sectional Multicentre International Study with Experimental Animal Model. Sci. Rep. 2021, 11, 1367. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Iovanna, J.; Santofimia-Castaño, P. Targeting Fibrosis: The Bridge That Connects Pancreatitis and Pancreatic Cancer. Int. J. Mol. Sci. 2021, 22, 4970. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petrone, M.C.; Arcidiacono, P.G.; Perri, F.; Carrara, S.; Boemo, C.; Testoni, P.A. Chronic pancreatitis-like changes detected by endoscopic ultrasound in subjects without signs of pancreatic disease: Do these indicate age-related changes, effects of xenobiotics, or early chronic pancreatitis? Pancreatology 2010, 10, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Irisawa, A.; Bhutani, M.S.; Shibukawa, G.; Yamabe, A.; Fujisawa, M.; Igarashi, R.; Arakawa, N.; Yoshida, Y.; Abe, Y.; et al. Significance of normal appearance on endoscopic ultrasonography in the diagnosis of early chronic pancreatitis. Endosc. Ultrasound 2018, 7, 110–118. [Google Scholar] [CrossRef]
- Minaguchi, T.; Yamamiya, A.; Tominaga, K.; Kashima, K.; Kunogi, Y.; Sakuma, F.; Fukushi, K.; Nagashima, K.; Izawa, N.; Yamabe, A.; et al. Measuring optimal ultrasound speed using endoscopic ultrasound in patients with chronic pancreatitis, including early stage. Dig. Endosc. 2022, 34, 1214–1221. [Google Scholar] [CrossRef]
- Subramanian, S.K.; Brahmbhatt, B.; Bailey-Lundberg, J.M.; Thosani, N.C.; Mutha, P. Lifestyle Medicine for the Prevention and Treatment of Pancreatitis and Pancreatic Cancer. Diagnostics 2024, 14, 614. [Google Scholar] [CrossRef]
- Xu, D.; Liu, C.; Zhou, J. Long-term Quality of Life after Acute Pancreatitis: A Systematic Review and Meta-Analysis. BMC Gastroenterol. 2025, 25, 502. [Google Scholar] [CrossRef]
- Sheel, A.R.G.; Baron, R.D.; Sarantitis, I.; Ramesh, J.; Ghaneh, P.; Raraty, M.G.T.; Yip, V.; Sutton, R.; Goulden, M.R.; Campbell, F.; et al. The diagnostic value of Rosemont and Japanese diagnostic criteria for ‘indeterminate’, ‘suggestive’, ‘possible’ and ‘early’ chronic pancreatitis. Pancreatology 2018, 18, 774–784. [Google Scholar] [CrossRef]
- Beyer, G.; Habtezion, A.; Werner, J.; Lerch, M.M.; Mayerle, J. Chronic pancreatitis. Lancet 2020, 396, 499–512. [Google Scholar] [CrossRef]
- Schneider, A.; Löhr, J.M.; Singer, M.V. The M-ANNHEIM classification of chronic pancreatitis: Introduction of a unifying classification system based on a review of previous classifications of the disease. J. Gastroenterol. 2007, 42, 101–119. [Google Scholar] [CrossRef]
- Nagahama, M.; Takano, Y.; Niiya, F.; Tamai, N.; Noda, J.; Yamawaki, M.; Azami, T. Early Chronic Pancreatitis Findings by Endoscopic Ultrasonography (EUS) in Asymptomatic Patients with Pancreas Divisum. Diagnostics 2025, 15, 253. [Google Scholar] [CrossRef]
- de Rijk, F.E.M.; van Veldhuisen, C.L.; Besselink, M.G.; van Santvoort, H.C.; Bruno, M.J. Diagnosis and treatment of exocrine pancreatic insufficiency in chronic pancreatitis: An international expert survey and case vignette study. Pancreatology 2022, 22. [Google Scholar] [CrossRef]
- Sendler, M.; Weiss, F.U.; Golchert, J.; Homuth, G.; van den Brandt, C.; Mahajan, U.M.; Partecke, L.I.; Döring, P.; Gukovsky, I.; Gukovskaya, A.S.; et al. Cathepsin B-Mediated Activation of Trypsinogen in Endocytosing Macrophages Increases Severity of Pancreatitis in Mice. Gastroenterology 2018, 154, 704–718.e10. [Google Scholar] [CrossRef]
- Farr, K.P.; Moses, D.; Haghighi, K.S.; Phillips, P.A.; Hillenbrand, C.M.; Chua, B.H. Imaging Modalities for Early Detection of Pancreatic Cancer: Current State and Future Research Opportunities. Cancers 2022, 14, 2539. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Ha, H.I.; Jang, J.Y.; Lee, J.W.; Choi, J.Y.; Bang, S.; Lee, C.H.; Kim, W.B.; Lee, S.S.; Kim, S.C.; et al. High-resolution pancreatic computed tomography for assessing pancreatic ductal adenocarcinoma resectability: A multicenter prospective study. Eur. Radiol. 2023, 33, 5965–5975. [Google Scholar] [CrossRef] [PubMed]
- IAP/APA/EPC/IPC/JPS Working Group. International Association of Pancreatology Revised Guidelines on Acute Pancreatitis 2025: Supported and Endorsed by the American Pancreatic Association, European Pancreatic Club, Indian Pancreas Club, and Japan Pancreas Society. Pancreatology 2025, 25, 770–814. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.; Dasari, V.; Jain, D. Management of Pancreatic Calculi in Chronic Pancreatitis: A Review Article. Cureus 2023, 15, e35788. [Google Scholar] [CrossRef]
- Inomata, N.; Masuda, A.; Yamakawa, K.; Takenaka, M.; Tsujimae, M.; Toyama, H.; Sofue, K.; Sakai, A.; Kobayashi, T.; Tanaka, T.; et al. Lobularity Rather Than Hyperechoic Foci/Stranding on Endoscopic Ultrasonography Is Associated with More Severe Histological Features in Chronic Pancreatitis. J. Gastroenterol. Hepatol. 2023, 38, 103–111. [Google Scholar] [CrossRef]
- Koh, C.J.; Lakhtakia, S.; Kida, M.; Lesmana, C.R.A.; Ang, T.L.; Vu, C.K.F.; Aye, T.T.; Park, S.H.; Almadi, M.A.; Chong, C.C.N.; et al. Defining the endoscopic ultrasound features of chronic pancreatitis in Asians: A multicenter validation study. Endoscopy 2021, 53, 595–602. [Google Scholar] [CrossRef]
- LeBlanc, J.K.; Chen, J.-H.; Al-Haddad, M.; Juan, M.; Okumu, W.; McHenry, L.; Cote, G.; Sherman, S.; DeWitt, J.M. Endoscopic Ultrasound and Histology in Chronic Pancreatitis. Pancreas 2014, 43, 440–444. [Google Scholar] [CrossRef]
- Wang, D.B.; Yu, J.; Fulcher, A.S.; Turner, M.A. Pancreatitis in patients with pancreas divisum: Imaging features at MRI and MRCP. World J. Gastroenterol. 2013, 19, 4907–4916. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-García, J.; de la Iglesia-García, D.; Lariño-Noia, J.; Domínguez-Muñoz, J.E. Endoscopic Ultrasound (EUS) Guided Elastography. Diagnostics 2023, 13, 1686. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Crinò, S.F.; Ramai, D.; Madhu, D.; Fugazza, A.; Carrara, S.; Spadaccini, M.; Mangiavillano, B.; Gkolfakis, P.; Mohan, B.P.; et al. Comparative diagnostic performance of different techniques for EUS-guided fine-needle biopsy sampling of solid pancreatic masses: A network meta-analysis. Gastrointest. Endosc. 2023, 97, 839–848.e5. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Tanioka, K.; Kawaji, Y.; Tamura, T.; Nuta, J.; Hatamaru, K.; Itonaga, M.; Yoshida, T.; Ida, Y.; Maekita, T.; et al. Utility of elastography with endoscopic ultrasonography shear-wave measurement for diagnosing chronic pancreatitis. Gut Liver 2020, 14, 659–664. [Google Scholar] [CrossRef]
- Iglesias-García, J.; Domínguez-Muñoz, J.E.; Castiñeira-Alvariño, M.; Luaces-Regueira, M.; Lariño-Noia, J. Quantitative Elastography Associated with Endoscopic Ultrasound for the Diagnosis of Chronic Pancreatitis. Endoscopy 2013, 45, 781–788. [Google Scholar] [CrossRef]
- Domínguez-Muñoz, J.E.; Lariño-Noia, J.; Alvarez-Castro, A.; Nieto, L.; Lojo, S.; Leal, S.; de la Iglesia-García, D.; Iglesias-García, J. Endoscopic ultrasound-based multimodal evaluation of the pancreas in patients with suspected early chronic pancreatitis. United Eur. Gastroenterol. J. 2020, 8, 790–797. [Google Scholar] [CrossRef]
- Kuwahara, T.; Hirooka, Y.; Kawashima, H.; Ohno, E.; Ishikawa, T.; Kawai, M.; Suhara, H.; Takeyama, T.; Hashizume, K.; Koya, T.; et al. Quantitative diagnosis of chronic pancreatitis using EUS elastography. J. Gastroenterol. 2017, 52, 868–874. [Google Scholar] [CrossRef]
- Wallace, M.B.; Hawes, R.H.; Durkalski, V.; Chak, A.; Mallery, S.; Catalano, M.F.; Wiersema, M.J.; Bhutani, M.S.; Ciaccia, D.; Kochman, M.L.; et al. The reliability of EUS for the diagnosis of chronic pancreatitis: Interobserver agreement among experienced endosonographers. Gastrointest. Endosc. 2001, 53, 294–299. [Google Scholar] [CrossRef]
- Ge, Q.C.; Zhang, Y.D. Surveillance imaging in early CP: Complementary EUS and MRCP. World J. Gastroenterol. 2021, 27, 4342–4357. [Google Scholar] [CrossRef]
- Schreyer, A.G.; Jung, M.; Riemann, J.F.; Niessen, C.; Pregler, B.; Grenacher, L.; Hoffmeister, A. S3 guideline for chronic pancreatitis—Diagnosis, classification and therapy for the radiologist. RoFo Fortschritte Geb. Röntgenstrahlen Bildgeb. Verfahr. 2014, 186, 1002–1008. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Huang, J.; Chen, X.; Xu, Z. Multiparametric mapping magnetic resonance imaging of pancreatic disease. Front. Physiol. 2020, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Manikkavasakar, S.; AlObaidy, M.; Busireddy, K.K.; Ramalho, M.; Nilmini, V.; Alagiyawanna, M.; Semelka, R.C. Magnetic resonance imaging of pancreatitis: An update. World J. Gastroenterol. 2014, 20, 14760–14777. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.C.; Dietrich, C.F.; Bhutani, M.S.; Zhang, B.Z.; Zhang, Y.; Wang, Y.D.; Zhang, J.J.; Wu, Y.F.; Sun, S.Y.; Guo, J.T. Comprehensive Review of Diagnostic Modalities for Early Chronic Pancreatitis. World J. Gastroenterol. 2021, 27, 4342–4357. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Bhatti, K.M.; Ahmed, M.; Malik, K.A.; Rehman, S.; Abdulgader, A.; Kausar, A.; Canelo, R. C-Reactive Protein as a Predictor of Complicated Acute Pancreatitis: Reality or a Myth? Cureus 2021, 13, e19265. [Google Scholar] [CrossRef]
- Bai, Y.; Qin, X.; Ao, X.; Ran, T.; Zhou, C.; Zou, D. The Role of EUS in the Diagnosis of Early Chronic Pancreatitis. Endosc. Ultrasound 2024, 13, 232–238. [Google Scholar] [CrossRef]
- Dogra, V.; Peer, J.A.; Gilkar, I.A.; Mushtaq, U.; Ahmed, I. Role of C-Reactive Protein in Acute Pancreatitis: An Observational Study in a Tertiary Care Centre. Int. Surg. J. 2022, 9, 559–562. [Google Scholar] [CrossRef]
- Hagn-Meincke, R.; Novovic, S.; Hadi, A.; Jensen, A.B.; Drewes, A.M.; Krarup, H.; Frøkjær, J.B.; Park, W.G.; Jørgensen, P.L.; Møller, H.J.; et al. Circulating Biomarkers of Macrophage Activation in Different Stages of Chronic Pancreatitis: A Pilot Study. Pancreas 2025, 54, e331–e339. [Google Scholar] [CrossRef]
- Chuliá-Peris, L.; Carreres-Rey, C.; Gabasa, M.; Alcaraz, J.; Carretero, J.; Pereda, J. Matrix Metalloproteinases and Their Inhibitors in Pulmonary Fibrosis: EMMPRIN/CD147 Comes into Play. Int. J. Mol. Sci. 2022, 23, 6894. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stojek, M.; Adrych, K.; Rojek, L.; Smoczynski, M.; Sledzinski, T.; Szrok, S.; Swierczynski, J. Decreased serum platelet derived growth factor BB levels in acute and increased in chronic pancreatitis. World J. Gastroenterol. 2014, 20, 13127–13132. [Google Scholar] [CrossRef]
- Miron, N.; Miron, M.-M.; Milea, V.G.I.; Cristea, V. Proinflammatory Cytokines: An Insight into Pancreatic Oncogenesis. Roum. Arch. Microbiol. Immunol. 2010, 69, 183–189. Available online: https://pubmed.ncbi.nlm.nih.gov/21462832/ (accessed on 16 September 2025).
- Lee, B.; Jones, E.K.; Manohar, M.; Li, L.; Yadav, D.; Conwell, D.L.; Hart, P.A.; Vege, S.S.; Fogel, E.L.; Serrano, J.; et al. Distinct Serum Immune Profiles Define the Spectrum of Acute and Chronic Pancreatitis from the Multicenter Prospective Evaluation of Chronic Pancreatitis for Epidemiologic and Translational Studies (PROCEED) Study. Gastroenterology 2023, 165, 173–186. [Google Scholar] [CrossRef]
- Rao, S.A.; Kunte, A.R. Interleukin-6: An Early Predictive Marker for Severity of Acute Pancreatitis. Indian J. Crit. Care Med. 2017, 21, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Tornel Avelar, A.I.; Gerrard, C.N.; Priego Parra, B.A. Phospholipase D2: A Biomarker Implicated in Various Pancreatic Diseases beyond Acute Pancreatitis. World J. Gastroenterol. 2025, 31, 108271. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Dong, S.; Chen, Z.; Li, X.; Jiang, W. New Challenges for microRNAs in Acute Pancreatitis: Progress and Treatment. J. Transl. Med. 2022, 20, 192. [Google Scholar] [CrossRef] [PubMed]
- Seidel, T.; Ohri, N.; Glaß, M.; Sunami, Y.; Müller, L.P.; Kleeff, J. Stromal Cells in Early Inflammation-Related Pancreatic Carcinogenesis—Biology and Its Potential Role in Therapeutic Targeting. Cancers 2025, 17, 1541. [Google Scholar] [CrossRef]
- Xiang, H.; Tao, X.; Xia, S.; Qu, J.; Song, H.; Liu, J.; Shang, D. Targeting MicroRNA Function in Acute Pancreatitis. Front. Physiol. 2017, 8, 726. [Google Scholar] [CrossRef]
- Azadinejad, H.; Farhadi Rad, M.; Babaeizad, A.; Samadi, A. MicroRNA Profiling in Pancreatic Cancer and Chronic Pancreatitis: Novel Insights and Pathway Analysis. Hum. Gene 2025, 44, 201410. [Google Scholar] [CrossRef]
- Nakamura, K.; Zhu, Z.; Roy, S.; Jun, E.; Han, H.; Munoz, R.M.; Nishiwada, S.; Sharma, G.; Cridebring, D.; Zenhausern, F.; et al. An Exosome-Based Transcriptomic Signature for Noninvasive, Early Detection of Patients with Pancreatic Ductal Adenocarcinoma: A Multicenter Cohort Study. Gastroenterology 2022, 163, 1252–1266.e2. [Google Scholar] [CrossRef]
- Khan, E.; Chakrabarty, S.; Shariff, S.; Bardhan, M. Genetics and Genomics of Chronic Pancreatitis with a Focus on Disease Biology and Molecular Pathogenesis. Glob. Med. Genet. 2023, 10, 324–334. [Google Scholar] [CrossRef]
- Resell, M.; Qvigstad, G.; Wang, T.C.; Quante, A.S.; González-Fernández, Á.; Waldum, H.; Chen, D.; Zhao, C.-M. Translational Studies on Pancreatic Cancer and Gastric Cancer: A Methodology in PhD Thesis. Front. Pharmacol. 2025, 16, 1604017. [Google Scholar] [CrossRef]
- Sahin-Tóth, M. Genetic Risk in Chronic Pancreatitis: The Misfolding-Dependent Pathway. Curr. Opin. Gastroenterol. 2017, 33, 390–395. [Google Scholar] [CrossRef]
- Previdi, M.C.; Carotenuto, P.; Zito, D.; Pandolfo, R.; Braconi, C. Noncoding RNAs as Novel Biomarkers in Pancreatic Cancer: What Do We Know? Future Oncol. 2017, 13, 443–453. [Google Scholar] [CrossRef]
- Ionescu, V.A.; Gheorghe, G.; Bleotu, C.; Puiu, L.; Mambet, C.; Diaconu, C.C.; Diaconu, C.C. Circulating miRNAs as Non-Invasive Biomarkers in Pancreatic Cancer: A Two-Phase Plasma-Based Study. J. Clin. Med. 2025, 14, 6430. [Google Scholar] [CrossRef] [PubMed]
- Dixit, A.K.; Sarver, A.E.; Yuan, Z.; George, J.; Barlass, U.; Cheema, H.; Sareen, A.; Banerjee, S.; Dudeja, V.; Dawra, R.; et al. Comprehensive Analysis of MicroRNA Signature of Mouse Pancreatic Acini: Overexpression of miR-21-3p in Acute Pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G974–G980. [Google Scholar] [CrossRef] [PubMed]
- Orkin, S.; Holovach, P.; Thompson, T.; Farrell, P.; Nasr, A.; Vitale, D.; Ibrahim, S.; Kotha, N.; Estes, J.; Hornung, L.; et al. Nutritional Parameters Following First Episode of Pediatric Acute Pancreatitis. Clin. Nutr. ESPEN 2024, 49, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Bang, S.; Jeon, T.J.; Cho, J.H.; Lee, K.J. Risk of and Factors Influencing the Progression from Acute to Recurrent Acute to Chronic Pancreatitis. Pancreatology 2025, 25, 624–630. [Google Scholar] [CrossRef]
- Yadav, D.; Slivka, A.; Sherman, S.; Hawes, R.H.; Anderson, M.A.; Burton, F.R.; Brand, R.E.; Lewis, M.D.; Gardner, T.B.; Gelrud, A.; et al. Smoking Is Underrecognized as a Risk Factor for Chronic Pancreatitis. Pancreatology 2010, 10, 713–719. [Google Scholar] [CrossRef]
- Pezzilli, R.; Bini, L.; Fantini, L.; Baroni, E.; Campana, D.; Tomassetti, P.; Corinaldesi, R. Quality of Life in Chronic Pancreatitis. World J. Gastroenterol. 2006, 12, 6249–6251. [Google Scholar] [CrossRef]
- Han, E.F.; Koea, J.; Hammill, C.; Srinivasa, S. The importance of smoking cessation in pancreatitis. ANZ J. Surg. 2022, 92, 2780–2781. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ocskay, K.; Juhász, M.F.; Farkas, N.; Zádori, N.; Szakó, L.; Szakács, Z.; Szentesi, A.; Erőss, B.; Miklós, E.; Zemplényi, A.; et al. Recurrent Acute Pancreatitis Prevention by the Elimination of Alcohol and Cigarette Smoking (REAPPEAR): Protocol of a Randomised Controlled Trial and a Cohort Study. BMJ Open 2022, 12, e050821. [Google Scholar] [CrossRef]
- Sanchez, R.J.; Ge, W.; Wei, W.; Ponda, M.P.; Rosenson, R.S. The Association of Triglyceride Levels with the Incidence of Initial and Recurrent Acute Pancreatitis. Lipids Health Dis. 2021, 20, 72. [Google Scholar] [CrossRef] [PubMed]
- Trang, T.; Chan, J.; Graham, D.Y. Pancreatic enzyme replacement therapy for pancreatic exocrine insufficiency in the 21th century. World J. Gastroenterol. 2014, 20, 11467–11485. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Li, Z.; Su, Y.; Sun, J.Y.; Xu, C.H.; Kong, X.Q.; Sun, W. Obesity, Visceral Adipose Tissue, and Essential Hypertension: Evidence from a Mendelian Randomization Study and Mediation Analysis. J. Clin. Hypertens. 2025, 27, e70045. [Google Scholar] [CrossRef] [PubMed]
- Bruni, A.; Rossi, G.; Mancini, L.; De Luca, F.; Romano, M.; Conti, C.; Esposito, G.; Bianchi, P.; Ferrara, L.; Greco, M.; et al. Nutritional Management in Chronic Pancreatitis. Nutrients 2025, 17, 2720. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Y.; Li, H.; Zhang, J.; Chen, F.; Liu, Q.; Sun, M.; Zhao, L.; Yang, X.; Wu, D.; et al. Adherence to the Mediterranean Diet Is Associated with Reduced Chronic Pancreatitis Risk: A Longitudinal Cohort Study. Food Funct. 2024, 15, 10452–10463. [Google Scholar] [CrossRef]
- Li, B.; Guo, S.; Zong, W.; Chu, Y.; Zhang, Q.; Yin, X.; Mao, T.; Li, X. Association between Dietary Mineral Intake and New Onset Diabetes/Pre-Diabetes after Chronic Pancreatitis. Front. Nutr. 2025, 11, 1461468. [Google Scholar] [CrossRef]
- Bélanger, V.; Delorme, J.; Napartuk, M.; Bouchard, I.; Meloche, C.; Curnier, D.; Sultan, S.; Laverdière, C.; Sinnett, D.; Marcil, V. Early Nutritional Intervention to Promote Healthy Eating Habits in Pediatric Oncology: A Feasibility Study. Nutrients 2022, 14, 1024. [Google Scholar] [CrossRef]
- Bagheri, A.; Asoudeh, F.; Rezaei, S.; Babaei, M.; Esmaillzadeh, A. The Effect of Mediterranean Diet on Body Composition, Inflammatory Factors, and Nutritional Status in Patients with Cachexia Induced by Colorectal Cancer: A Randomized Clinical Trial. Integr. Cancer Ther. 2023, 22, 15347354231195322. [Google Scholar] [CrossRef]
- Lewis, D.M.; Shahid, A. Survey of Pancreatic Enzyme Replacement Therapy Dosing Experiences in Adults with Exocrine Pancreatic Insufficiency. Healthcare 2023, 11, 2316. [Google Scholar] [CrossRef]
- Fujisaki, T.; Shirahama, Y.; Sheng, F.; Ikeda, K.; Osada, N.; Tanaka, S.; Zhang, Y.; Tsujita, K. The Effect of Lipid-Lowering Therapy on Coronary Artery Plaque in East Asia Population. JACC Asia 2025, 5, 1032–1047. [Google Scholar] [CrossRef]
- Pawelec, N.; Durko, Ł.; Małecka-Wojciesko, E. Changes Connected to Early Chronic Pancreatitis and Early Pancreatic Cancer in Endoscopic Ultrasonography (EUS): Clinical Implications. Cancers 2025, 17, 1891. [Google Scholar] [CrossRef]
- Capasso, M.; Venezia, L.; Antonini, F.; Barresi, L.; de Nucci, G.; Valvano, M. Pancreatic Enzyme Replacement Therapy: Not Only in Chronic Pancreatitis. J. Gastrointest. Liver Dis. 2025, 34, 390–399. [Google Scholar] [CrossRef]
- Graham, D.Y. An Enteric-Coated Pancreatic Enzyme Preparation That Works. Dig. Dis. Sci. 1979, 24, 906–909. [Google Scholar] [CrossRef]
- Ko, K.H.; An, J.M.; Son, M.S.; Chung, J.B.; Hahm, K.B. Antioxidant Therapy in Chronic Pancreatitis—Promises and Pitfalls. Ann. Transl. Med. 2019, 7 (Suppl. 3), S115. [Google Scholar] [CrossRef]
- Swentek, L.; Chung, D.; Ichii, H. Antioxidant Therapy in Pancreatitis. Antioxidants 2021, 10, 657. [Google Scholar] [CrossRef]
- Brennan, G.T.; Saif, M.W. Pancreatic Enzyme Replacement Therapy: A Concise Review. JOP 2019, 20, 121–125. [Google Scholar] [PubMed]
- Ketwaroo, G.A.; Graham, D.Y. Rational Use of Pancreatic Enzymes for Pancreatic Insufficiency and Pancreatic Pain. Adv. Exp. Med. Biol. 2019, 1148, 323–343. [Google Scholar] [CrossRef] [PubMed]
- Cañamares-Orbís, P.; García-Rayado, G.; Alfaro-Almajano, E. Nutritional Support in Pancreatic Diseases. Nutrients 2022, 14, 4570. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.E.; Tang, G.; Wang, X.; Forsmark, C.E.; Yadav, D. Pancreatic Enzyme Replacement Therapy in Patients with Non-Pancreatic Digestive Conditions: A Nationwide Claims Analysis. Dig. Dis. Sci. 2023, 68, 1754–1761. [Google Scholar] [CrossRef]
- Enweluzo, C.; Tlhabano, L. Pain Management in Chronic Pancreatitis: Taming the Beast. Clin. Exp. Gastroenterol. 2013, 6, 167–171. [Google Scholar] [CrossRef]
- Nicoletti, A.; Vitale, F.; Paratore, M.; Quero, G.; Negri, M.; Nista, E.C.; Alfieri, S.; Gasbarrini, A.; Zileri Dal Verme, L. Neuropancreatology: The Nervous System and Pain Management in Pancreatic Diseases. Life 2024, 14, 299. [Google Scholar] [CrossRef]
- Tłustochowicz, K.; Gajewska, D.; Kolodziejczyk, M.; Adamczyk, J.; Jurek, T.; Dabrowski, M.; Piotrowski, M.; Cichoz-Lach, H.; Majka, J.; Dziki, A.; et al. Treatment Strategies for Chronic Pancreatitis (CP). Pharmaceuticals 2025, 18, 311. [Google Scholar] [CrossRef]
- Ma, K.W.; Kwan, C.; She, W.H.; Wong, N.W.Y.; Chan, A.C.Y.; Tsang, S.H.Y.; Cheung, T.T.; Dai, W.C.; Chok, K.S.H.; Fung, A.K.Y.; et al. Endoscopic versus Surgical Intervention for Painful Chronic Pancreatitis. J. Clin. Med. 2021, 10, 2636. [Google Scholar] [CrossRef] [PubMed]
- Chow, R.P.; Nguyen, K.; Gou, W.; Green, E.; Morgan, K.; Lancaster, W.; Helke, K.; Strange, C.; Wang, H.; Davis, D.; et al. A Novel Cellular Therapy to Treat Pancreatic Pain in Experimental Chronic Pancreatitis Using Human Alpha-1 Antitrypsin Overexpressing Mesenchymal Stromal Cells. Biomedicines 2021, 9, 1695. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Chen, Y.; Zhang, L.; Wu, J.; Li, H.; Huang, X.; Zhao, Y.; Sun, K.; Yang, F.; Xu, Z.; et al. Navigating Chronic Pancreatitis Pain: A Pathophysiological Review. Front. Physiol. 2025, 16, 1622845. [Google Scholar] [CrossRef]
- Hofer, D.M.; Harnik, M.; Lehmann, T.; Stüber, F.; Baumbach, P.; Dreiling, J.; Meissner, W.; Stamer, U.M. Trajectories of Pain and Opioid Use up to One Year after Surgery: Analysis of a European Registry. Br. J. Anaesth. 2024, 132, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, G.; Dupoiron, D.; Seegers, V.; Lebrec, N.; Boré, F.; Dubois, P.Y.; Leblanc, D.; Delorme, T.; Jubier-Hamon, S. Intrathecal Drug Delivery Systems for Refractory Pancreatic Cancer Pain: Observational Follow-Up Study over an 11-Year Period in a Comprehensive Cancer Center. Anesth. Analg. 2018, 126, 2038–2046. [Google Scholar] [CrossRef]
- Nag, D.S.; Swain, B.P.; Anand, R.; Barman, T.K.; Vatsala. Pain Management in Chronic Pancreatitis. World J. Clin. Cases 2024, 12, 2016–2022. [Google Scholar] [CrossRef]
- Mondal, S.; Adhikari, N.; Banerjee, S.; Amin, S.A.; Jha, T. Matrix Metalloproteinase-9 (MMP-9) and Its Inhibitors in Cancer: A Minireview. Eur. J. Med. Chem. 2020, 194, 112260. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Li, J.; Jia, Y.; Xie, X.; Ding, Y.; Cao, F.; Li, F. Inhibition of MMP9 Ameliorates Neutrophil Extracellular Traps-Mediated Necroptosis through Regulation of Impaired Autophagy in Severe Acute Pancreatitis. Int. Immunopharmacol. 2025, 162, 115109. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Morsi, M.; Ghoneim, N.I.; Abdel-Daim, M.M.; El-Badri, N. Mesenchymal Stromal Cell Therapy for Pancreatitis: A Systematic Review. Oxid. Med. Cell Longev. 2018, 2018, 3250864. [Google Scholar] [CrossRef]
- Gerges, C.; Albers, D.; Schmitz, L.; Goni, E.; Cappello, A.; Schirra, J.; Casper, M.; Dormann, A.J.; Hartmann, D.; Hollenbach, M.; et al. Digital Single-Operator Pancreatoscopy for the Treatment of Symptomatic Pancreatic Duct Stones: A Prospective Multicenter Cohort Trial. Endoscopy 2023, 55, 150–157. [Google Scholar] [CrossRef]
- Keith, S.W.; Maio, V.; Arafat, H.A.; Alcusky, M.; Karagiannis, T.; Rabinowitz, C.; Lavu, H.; Louis, D.Z. Angiotensin Blockade Therapy and Survival in Pancreatic Cancer: A Population Study. BMC Cancer 2022, 22, 150. [Google Scholar] [CrossRef]
- Wei, X.; Yao, W.; Li, H.; Qian, J.; Xie, Y.; Zhang, Z.; Lu, H.; Shi, L.; Lin, X. B and NK Cells Closely Correlate with the Condition of Patients with Acute Pancreatitis. Gastroenterol. Res. Pract. 2019, 2019, 7568410. [Google Scholar] [CrossRef]
- Chen, C.; Demirkhanyan, L.; Gondi, C.S. The Multifaceted Role of miR-21 in Pancreatic Cancers. Cells 2024, 13, 948. [Google Scholar] [CrossRef]
- Sherman, S.; Kozarek, R.A.; Costamagna, G.; Reddy, D.N.; Tarnasky, P.; Shah, R.J.; Slivka, A.; Fogel, E.; Watkins, J.; Delhaye, M.; et al. Soft Self-Expandable Metal Stent to Treat Painful Pancreatic Duct Strictures Secondary to Chronic Pancreatitis: A Prospective Multicenter Trial. Gastrointest. Endosc. 2023, 97, 472–481.e3. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, A.A.; Krige, J.E.; Bornman, P.C. Biliary Tract Obstruction in Chronic Pancreatitis. HPB 2007, 9, 421–428. [Google Scholar] [CrossRef] [PubMed]
- van Veldhuisen, C.L.; Kempeneers, M.A.; de Rijk, F.E.M.; Bouwense, S.A.; Bruno, M.J.; Fockens, P.; Poley, J.W.; Ali, U.A.; Bollen, T.L.; Busch, O.R.; et al. Long-Term Outcomes of Early Surgery vs. Endoscopy First in Chronic Pancreatitis: Follow-Up Analysis of the ESCAPE Randomized Clinical Trial. JAMA Surg. 2025, 160, 126–133. [Google Scholar] [CrossRef]
- Ito, K.; Iwashita, T.; Uemura, S.; Kogure, H.; Hamada, T.; Okuno, M.; Sasaki, T.; Ishigami, M.; Maeda, S.; Matsuda, K.; et al. Current Status and Future Perspectives for Endoscopic Therapies for Complications of Chronic Pancreatitis. Dig. Endosc. 2025, 37, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Komar, H.M.; Serpa, G.; Kerscher, C.; Schwoegl, E.; Mace, T.A.; Jin, M.; Yang, M.C.; Chen, C.S.; Bloomston, M.; Ostrowski, M.C.; et al. Inhibition of Jak/STAT Signaling Reduces the Activation of Pancreatic Stellate Cells In Vitro and Limits Caerulein-Induced Chronic Pancreatitis In Vivo. Sci. Rep. 2017, 7, 1787. [Google Scholar] [CrossRef]
- Khakhar, Z.; Manji, S.; Patel, R.K.; Ali, S.K. Sodium-Glucose Transport Protein 2 (SGLT2) Inhibitors and the Risk of Pancreatitis: A Case Report. Cureus 2024, 16, e62957. [Google Scholar] [CrossRef]
- Guo, H.L.; Liang, X.S.; Zeng, X.P.; Liu, Y.; Li, Z.S.; Wang, L.J.; Hu, L.H. Pirfenidone Alleviates Chronic Pancreatitis via Suppressing the Activation of Pancreatic Stellate Cells and the M1 Polarization of Macrophages. Int. Immunopharmacol. 2024, 130, 111691. [Google Scholar] [CrossRef] [PubMed]
- Li, B.Q.; Liu, X.Y.; Mao, T.; Zheng, T.H.; Zhang, P.; Zhang, Q.; Zhang, Y.; Li, X.Y. The Research Progress of Anti-Inflammatory and Anti-Fibrosis Treatment of Chronic Pancreatitis. Front. Oncol. 2022, 12, 1050274. [Google Scholar] [CrossRef] [PubMed]
- Mititelu, A.; Grama, A.; Colceriu, M.C.; Benţa, G.; Popoviciu, M.S.; Pop, T.L. Role of Interleukin 6 in Acute Pancreatitis: A Possible Marker for Disease Prognosis. Int. J. Mol. Sci. 2024, 25, 8283. [Google Scholar] [CrossRef] [PubMed]
- Dhali, A.; Kipkorir, V.; Srichawla, B.S.; Kumar, H.; Rathna, R.B.; Ongidi, I.; Chaudhry, T.; Morara, G.; Nurani, K.; Cheruto, D.; et al. Artificial Intelligence Assisted Endoscopic Ultrasound for Detection of Pancreatic Space-Occupying Lesion: A Systematic Review and Meta-Analysis. Int. J. Surg. 2023, 109, 4298–4308. [Google Scholar] [CrossRef]
- Nikpanah, M.; Morgan, D.E. Magnetic Resonance Imaging in the Evaluation and Management of Acute Pancreatitis: A Review of Current Practices and Future Directions. Clin. Imaging 2024, 107, 110086. [Google Scholar] [CrossRef]
- Wu, D.; Cai, W.; Wu, Z.; Huang, Y.; Mukherjee, R.; Peng, J.; Huang, W.; Li, Q.; Xia, Q.; Jiang, K. Multi-omics Profiles Reveal Immune Microenvironment Alterations Associated with PD-L1 Checkpoint in Acute Pancreatitis in the Early Phase. Biochem. Biophys. Res. Commun. 2025, 751, 151451. [Google Scholar] [CrossRef]
- Han, X.; Liao, R.; Li, X.; Zhang, C.; Huo, S.; Qin, L.; Xiong, Y.; He, T.; Xiao, G.; Zhang, T. Mesenchymal stem cells in treating human diseases: Molecular mechanisms and clinical studies. Signal Transduct. Target. Ther. 2025, 10, 262. [Google Scholar] [CrossRef]
| Domain | Consensus Statement/Conclusion | Clinical Implication |
|---|---|---|
| Definition | ECP is a stage of disease characterized by ongoing pathogenic mechanisms without end-stage damage. | Emphasizes a dynamic process; shifts focus from imaging to mechanism-based definition. |
| Pathophysiology | Chronic pancreatitis arises from repeated injury, inadequate repair, and progressive fibrosis. | Highlights the need for early identification of risk factors and repeated inflammatory events. |
| Diagnostic Focus | Traditional imaging findings are not required to define ECP. | Diagnosis should be based on mechanistic understanding, not just structural changes. |
| Mechanistic Model | CP should be defined by underlying biological processes (e.g., inflammation, fibrosis, genetics). | Supports use of biomarkers and risk stratification tools to guide diagnosis and therapy. |
| Role of Genetics | Genetic variants (e.g., PRSS1, SPINK1, CFTR) modify susceptibility and progression risk. | Genetic testing is useful in early or idiopathic cases, especially in young patients. |
| EUS and Imaging | Imaging may appear normal or nonspecific in ECP. | Reliance solely on CT/MRI may delay diagnosis; EUS and secretin-MRCP offer more sensitivity. |
| Biomarkers | Need for validated biomarkers of early-stage disease and progression. | Research should prioritize non-invasive diagnostic and prognostic molecular tools. |
| Clinical Presentation | Symptoms in early CP may mimic functional GI disorders. | Requires low threshold of clinical suspicion; overlap with irritable bowel syndrome/dyspepsia is frequent. |
| Goal of Diagnosis | To identify at-risk individuals before irreversible injury occurs. | Enables potential disease modification through early lifestyle and therapeutic intervention. |
| Prognostic Stratification | Disease evolution is heterogeneous; not all patients with RAP will progress to CP. | Risk prediction tools should integrate clinical, genetic, and biomarker data. |
| No. | First Author (Year) | Country | Study Type | No. Patients/Data | Main Objective/Imaging | Key Findings |
|---|---|---|---|---|---|---|
| 1 | Japanese Pancreas Society (2009) [27] | Japan | Expert consensus | N/A | EUS | Proposed first diagnostic criteria for ECP based on EUS and clinical features. |
| 2 | Whitcomb et al. (2018) [12] | International | Expert consensus | N/A | Multimodal | Defined the concept of early CP and proposed mechanistic classification and diagnostic model. |
| 3 | Stevens T. et al. (2009) [28] | USA | Narrative review | N/A | EUS | Highlighted EUS as sensitive but operator-dependent; advocated for integrated imaging criteria. |
| 4 | Tirkes et al. (2019) [29] | USA (Indiana University) | Prospective | 69 | MRI T1 mapping | T1 mapping identified significant differences between controls, ECP, and definite CP. |
| 5 | Masamune et al. (2019) [30] | Japan | Multicentre cohort study | 83 | MRI + EUS | Found that 4.8% progressed to CP while 36.1% regressed; lifestyle changes were protective. |
| 7 | Hegyi et al. (2021) [31] | Hungary | Cross-species study | Human + mouse model | Histology + Imaging | ≥3 AP episodes were associated with irreversible fibrotic changes in pancreas tissue. |
| 8 | Yamamiya et al. (2022) [3] | Japan | Retrospective cohort | 100 | Clinical criteria + EUS | High AUDIT-C scores predicted CP progression in alcohol-related ECP. |
| 9 | Poulsen et al. (2024) [22] | Romania | Narrative review | N/A | Biomarkers | Reviewed IL-6, sCD163, MMP-9 as potential markers of ECP progression. |
| Modality | Purpose/Description | Advantages | Limitations | Clinical Utility |
|---|---|---|---|---|
| Transabdominal Ultrasound (US) | Initial assessment tool for pancreas (parenchyma and duct). | Non-invasive, inexpensive, widely available. | Limited by bowel gas; sensitivity reduced in early/mild disease. | First-line screen; follow-up with advanced imaging if suspicion remains high. |
| CE-US | Assesses pancreatic perfusion and microvascular changes. | Can detect inflammation, vascular alterations. | Operator- and equipment-dependent; less standardized for CP. | Adjunctive tool in experienced centres, notably for vascular findings. |
| EUS | High-resolution imaging of pancreatic parenchyma and ducts. | Excellent spatial resolution; detects subtle parenchymal/duct abnormalities. | Interobserver variability; invasive, operator-dependent; sedation required. | Gold standard for early structural detection; ideal for borderline cases. |
| EUS Elastography | Measures tissue stiffness to detect early fibrosis. | Quantitative assessment; identifies preclinical fibrotic changes. | Lacks standardized cut-offs; technique-dependent; evolving evidence. | Promising tool for fibrosis staging alongside conventional EUS. |
| EUS-guided nCLE | In vivo microscopic imaging via confocal laser endomicroscopy. | Cellular-level resolution; distinguishes fibrosis from other lesions. | Invasive (requires FNA), risk of bleeding/infection, expensive. | May aid differentiation from malignancy in select cases. |
| CT | Cross-sectional imaging for parenchymal/duct morphology, excludes complications. | Good for advanced disease; detects calcifications/complications. | Poor sensitivity in early stage; radiation exposure; low soft tissue contrast. | Useful for suspicion of complications or when other modalities unavailable. |
| MRI/MRCP | Visualizes ductal anatomy, parenchyma, and fluid collections; secretin-enhanced MRCP (sMRCP) increases functional assessment. | High soft tissue contrast; secretin reveals ductal function. | Availability/cost issues; contraindications in metal implants; interpretation variability. | Excellent non-invasive option for both structure and function assessment. |
| MRI Elastography | Quantifies tissue stiffness to indicate early fibrosis. | Non-invasive, quantitative fibrosis measurement. | Emerging technique; needs standardization, limited access. | May become adjunct to MRI in fibrosis quantification. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coseru, A.-I.; Floria, D.E.; Simiras, C.; Vulpoi, R.A.; Rosca, V.; Nemteanu, R.; Petrea, O.; Ciortescu, I.; Barboi, O.-B.; Balan, G.G.; et al. Advancing the Diagnosis and Treatment of Early Chronic Pancreatitis Through Innovation in Imaging and Biomarker Profiling—A Narrative Review. Life 2025, 15, 1574. https://doi.org/10.3390/life15101574
Coseru A-I, Floria DE, Simiras C, Vulpoi RA, Rosca V, Nemteanu R, Petrea O, Ciortescu I, Barboi O-B, Balan GG, et al. Advancing the Diagnosis and Treatment of Early Chronic Pancreatitis Through Innovation in Imaging and Biomarker Profiling—A Narrative Review. Life. 2025; 15(10):1574. https://doi.org/10.3390/life15101574
Chicago/Turabian StyleCoseru, Alexandru-Ionut, Diana Elena Floria, Constantin Simiras, Radu Alexandru Vulpoi, Vadim Rosca, Roxana Nemteanu, Oana Petrea, Irina Ciortescu, Oana-Bogdana Barboi, Gheorghe G. Balan, and et al. 2025. "Advancing the Diagnosis and Treatment of Early Chronic Pancreatitis Through Innovation in Imaging and Biomarker Profiling—A Narrative Review" Life 15, no. 10: 1574. https://doi.org/10.3390/life15101574
APA StyleCoseru, A.-I., Floria, D. E., Simiras, C., Vulpoi, R. A., Rosca, V., Nemteanu, R., Petrea, O., Ciortescu, I., Barboi, O.-B., Balan, G. G., Sfarti, C., Gîlca-Blanariu, G.-E., Mihai, C., Gheorghe, L., Plesa, A., & Drug, V.-L. (2025). Advancing the Diagnosis and Treatment of Early Chronic Pancreatitis Through Innovation in Imaging and Biomarker Profiling—A Narrative Review. Life, 15(10), 1574. https://doi.org/10.3390/life15101574






