Virosomes: Beyond Vaccines
Abstract
1. Introduction
1.1. Development of Vaccines
1.2. Overview of Virosomes
2. Materials and Methods
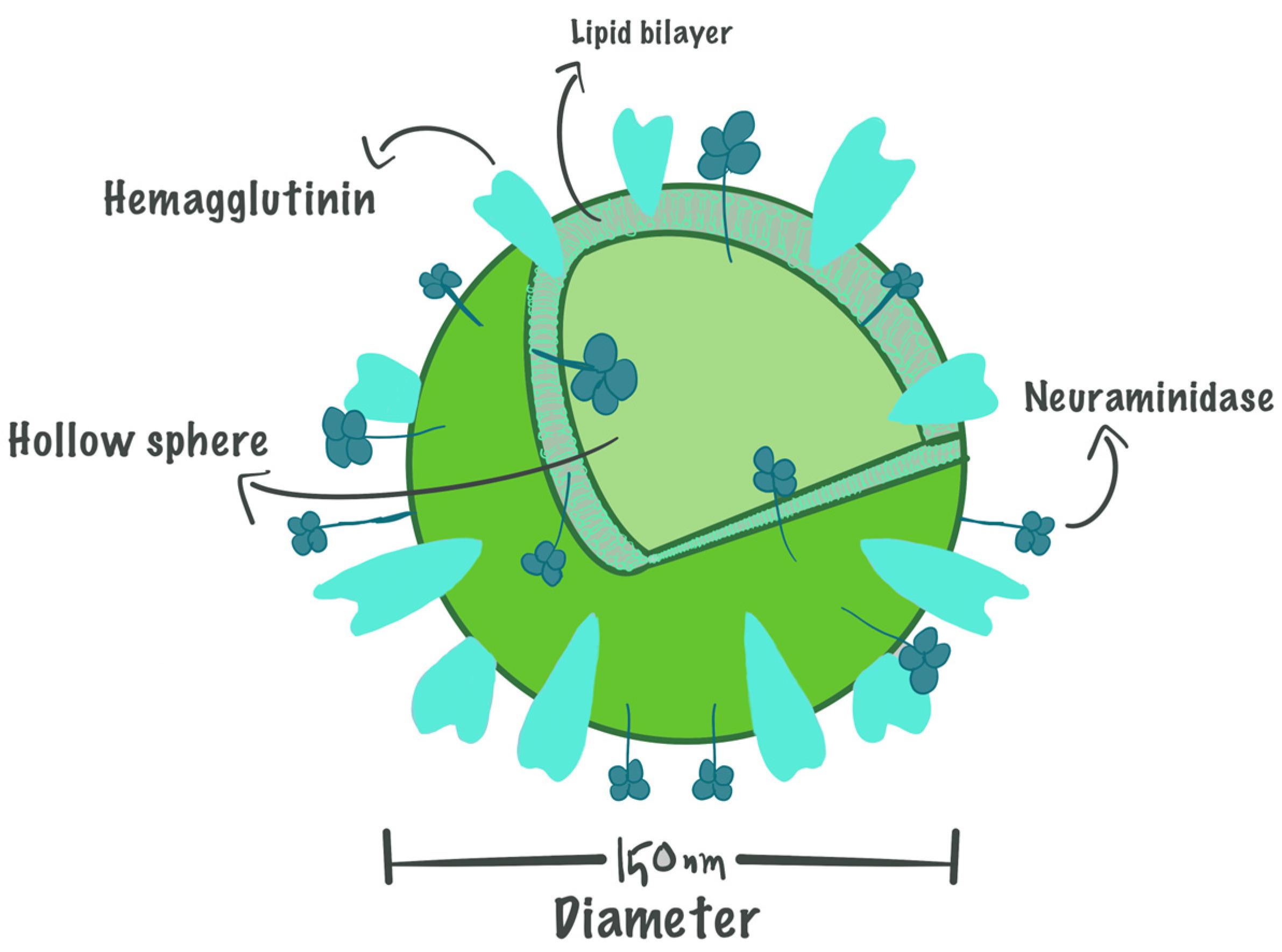
3. Structure of Virosomes
3.1. The Basic Structure of Virosomes
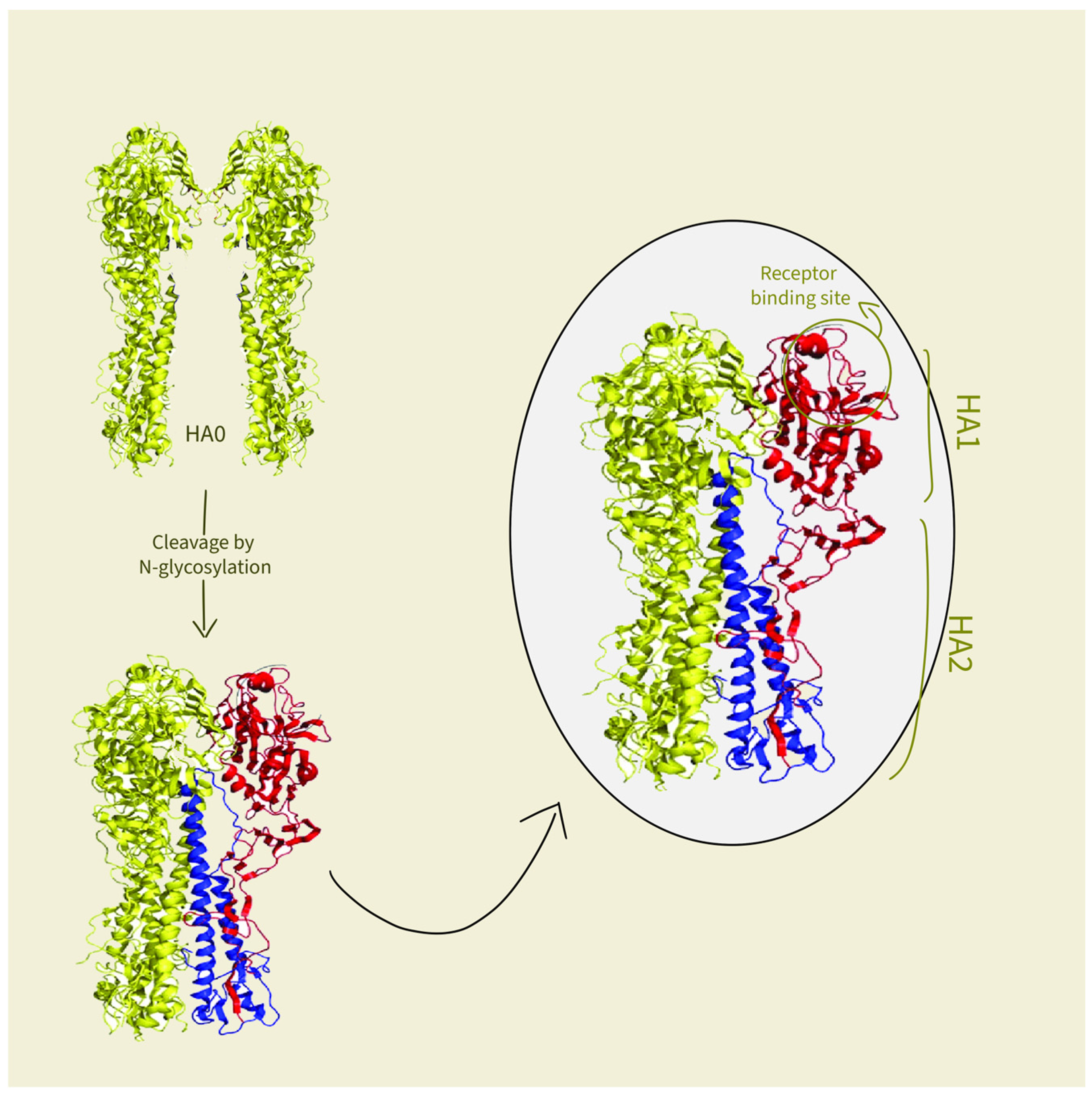
3.2. Roles of Components in Virosomes Activity
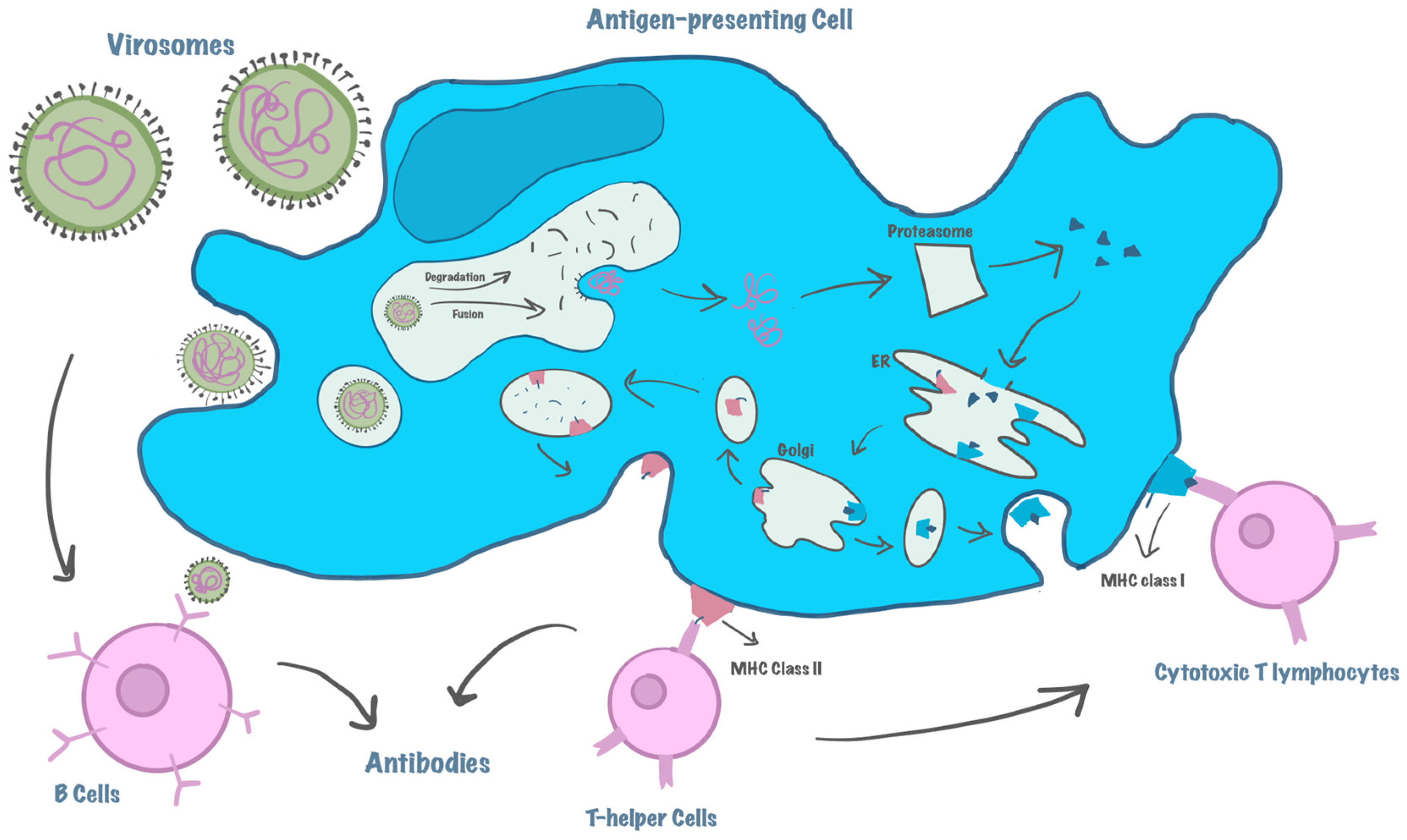
4. Interaction of Virosomes with the Immune System
4.1. Mechanism of Action
4.2. Stimulation of the Immune System—MHC Activation
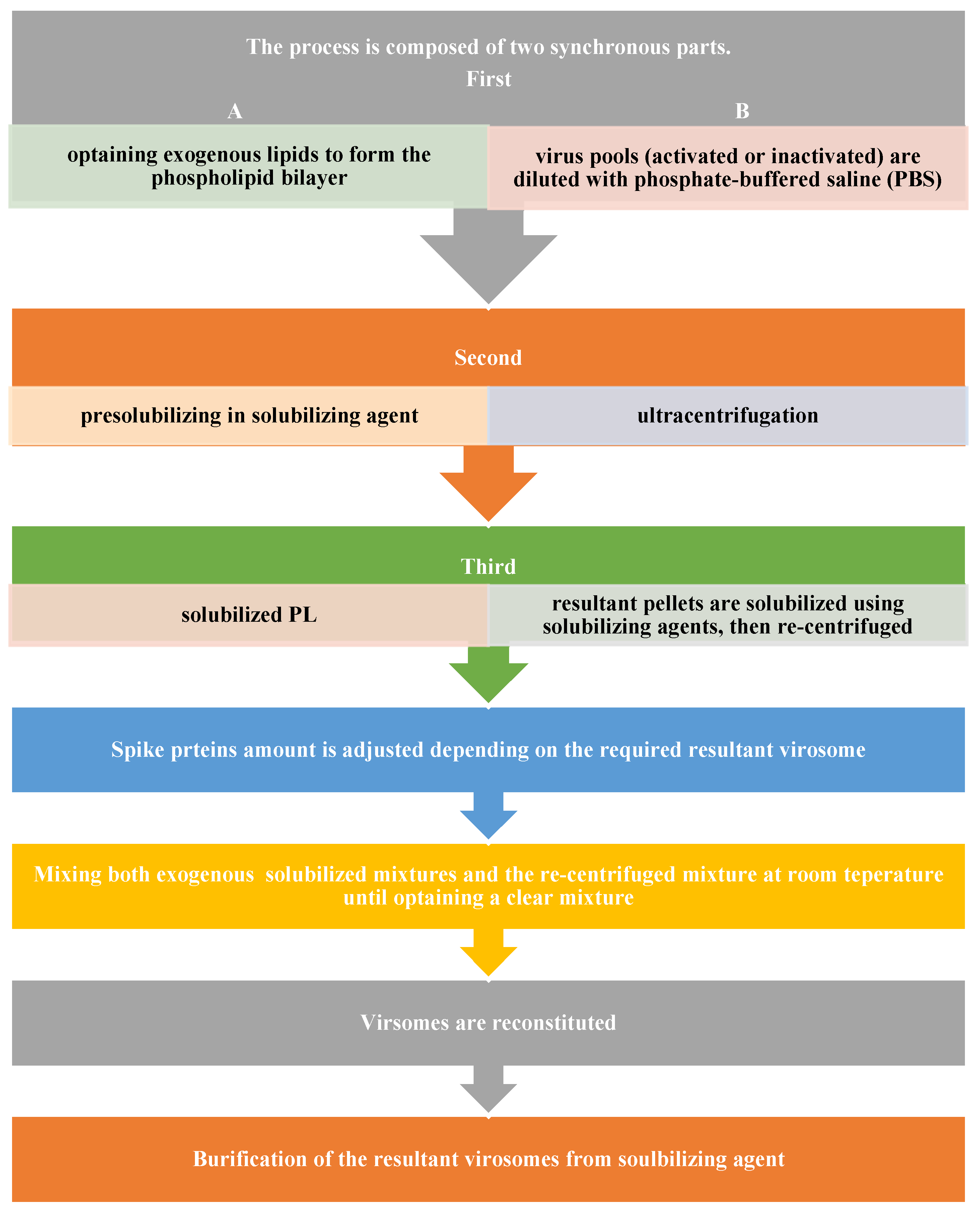
5. Manufacturing of Virosomes
5.1. Preparation of Virosomes
5.2. Preparation of Influenza Virosome
5.3. Advances in Manufacturing Methods—Cell-Free Protein Synthesis
6. Applications of Virosomes
6.1. Viral Vaccines
6.1.1. Influenza Vaccines
6.1.2. Hepatitis A Vaccines
6.1.3. COVID-19
6.2. Vaccines in Clinical Development
6.2.1. Hepatitis C
6.2.2. Immunodeficiency Virus Type 1 (HIV)
6.2.3. Mumps Vaccine—DNA Virosome
6.2.4. Virosomal Malaria Vaccine
6.3. Virosomes as a Delivery System of Drugs
6.3.1. Virosomes as a Delivery System for Anti-Cancer
6.3.2. Anti-Alzheimer Virosomes
6.3.3. Candida albicans Virosomal Vaccine
7. Advantages of Virosomes
7.1. Industrial Advantages
7.2. Potency and Efficacy
8. Patents
8.1. Preparation Techniques
8.2. Lyophilization of Virosomes
8.3. Virosomal Vaccines
8.4. Delivery of Genetic Material
9. Future Prospects
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CTLs | Cytotoxic T-lymphocytes |
| CpG | Cytosine phosphoguanine |
| GMP | Good manufacturing processes |
| NA | Neuraminidase |
| HA | Hemagglutinin |
| PL | Phospholipids |
| PK | Pharmacokinetic properties |
| PA | Pharmaceutical agent |
| APCs | Antigen-presenting cells |
| MHC | Major histocompatibility complex |
| TAP | Transporters of antigenic peptides |
| TCR | T-cell receptor |
| Ig | Immunoglobulin |
| DTA | Diphtheria toxin A |
| OVA | Ovalbumin |
| DCs | Dendritic cells |
| DPPC | 1,2-dipalmitoyl-sn-glycero-3-phosphocholine |
| MPLA | Monophosphoryl lipid A |
| DOTAP | 1,2-Dioleoyl-3-trimethylammonium-propane |
| DODAP | 1,2-Dioleoyl-3-dimethylammonium-propane |
| PC | Phosphatidylcholine |
| PBS | Phosphate-buffered saline |
| OEG | Oligoethylene glycol |
| MDP | Muramyl dipeptide |
| CFPS | Cell-free protein synthesis |
| VLPs | Virus-like particles |
| PCR | Polymerase chain reaction |
| CECF | Continuous exchange cell-free synthesis platform |
| IRIV | Immunopotentiating reconstituted influenza virosome |
| NHP | Nonhuman primates |
| HIV | Human immunodeficiency virus |
| MACIVIVA | Manufacturing Process for Cold Chain Independent Virosome-based Vaccines |
| MuV | Mumps virus |
| F | Fusion gene |
| TAA | Tumor-associated antigens |
| PTH-rP | Parathyroid hormone-related protein |
| AMA-1 | Apical membrane antigen-1 |
| Cs | Circumsporozoite |
| APP | Amyloid precursor protein |
| AD | Alzheimer disease |
| Aβ | Amyloid beta |
| Sap | Secretory aspartyl proteinases |
| rtSap2 | N-terminally truncated Sap2 protein |
| RVVC | Recurrent vulvovaginal infection by Candida albicans |
| IM | Intramuscular |
| SC | Subcutaneous |
| ID | Intradermal |
| IN | Intranasal |
| TEAE | Treatment-emergent adverse event |
| EPO | European Patent Office |
| HAV | Hepatitis A virus |
| HBsAG | Hepatitis B envelope protein |
| RSV | Respiratory syncytial virus |
| ACPs | Anti-cancer peptides |
| CPPs | Cell penetration peptides |
| CYP | Cytochrome P |
References
- Guimarães, L.E.; Baker, B.; Perricone, C.; Shoenfeld, Y. Vaccines, adjuvants and autoimmunity. Pharmacol. Res. 2015, 100, 190–209. [Google Scholar] [CrossRef]
- Vetter, V.; Denizer, G.; Friedland, L.R.; Krishnan, J.; Shapiro, M. Understanding modern-day vaccines: What you need to know. Ann. Med. 2018, 50, 110–120. [Google Scholar] [CrossRef]
- Subramanian, R.; Graham, A.L.; Grenfell, B.T.; Arinaminpathy, N. Universal or Specific? A Modeling-Based Comparison of Broad-Spectrum Influenza Vaccines against Conventional, Strain-Matched Vaccines. PLoS Comput. Biol. 2016, 12, e1005204. [Google Scholar] [CrossRef]
- Doherty, P.C.; Turner, S.J.; Webby, R.G.; Thomas, P.G. Influenza and the challenge for immunology. Nat. Immunol. 2006, 7, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Cytotoxic T Cell Overview—PS. Available online: https://www.thermofisher.com/sa/en/home/life-science/cell-analysis/cell-analysis-learning-center/immunology-at-work/cytotoxic-t-cell-overview.html (accessed on 19 January 2025).
- Levine, M.M.; Dougan, G.; Good, M.F.; Nabel, G.J.; Nataro, J.P.; Rappuoli, R. (Eds.) New Generation Vaccines; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Liu, M.A. DNA vaccines: An historical perspective and view to the future. Immunol. Rev. 2011, 239, 62–84. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Akbar, M.; Iqbal, B.; Ali, F.; Sharma, N.K.; Kumar, N.; Najmi, A.; Albratty, M.; Alhazmi, H.A.; Madkhali, O.A.; et al. Virosome: An engineered virus for vaccine delivery. Saudi Pharm. J. SPJ 2023, 31, 752–764. [Google Scholar] [CrossRef] [PubMed]
- CDC. Adjuvants and Vaccines. Vaccine Safety. 20 December 2024. Available online: https://www.cdc.gov/vaccine-safety/about/adjuvants.html (accessed on 19 January 2025).
- Das, A.; Ali, N. Nanovaccine: An emerging strategy. Expert Rev. Vaccines 2021, 20, 1273–1290. [Google Scholar] [CrossRef]
- Gheibi Hayat, S.M.; Darroudi, M. Nanovaccine: A novel approach in immunization. J. Cell Physiol. 2019, 234, 12530–12536. [Google Scholar] [CrossRef]
- Stegmann, T.; Wiekmeijer, A.S.; Kwappenberg, K.; van Duikeren, S.; Bhoelan, F.; Bemelman, D.; Beenakker, T.J.M.; Krebber, W.-J.; Arens, R.; Melief, C.J.M. Enhanced HPV16 E6/E7+ tumor eradication via induction of tumor-specific T cells by therapeutic vaccination with virosomes presenting synthetic long peptides. Cancer Immunol. Immunother. 2023, 72, 2851–2864. [Google Scholar] [CrossRef]
- Asadi, K.; Gholami, A. Virosome-based nanovaccines; a promising bioinspiration and biomimetic approach for preventing viral diseases: A review. Int. J. Biol. Macromol. 2021, 182, 648–658. [Google Scholar] [CrossRef]
- Karpe, S.; Gupta, K.; Vyas, G.; Rana, P.; Khan, F.; Kumar, R. Virosome: A vector in vaccine delivery. BIO Web Conf. 2024, 86, 01020. [Google Scholar] [CrossRef]
- Moser, C.; Amacker, M.; Kammer, A.R.; Rasi, S.; Westerfeld, N.; Zurbriggen, R. Influenza virosomes as a combined vaccine carrier and adjuvant system for prophylactic and therapeutic immunizations. Expert Rev. Vaccines 2007, 6, 711–721. [Google Scholar] [CrossRef] [PubMed]
- De Bernardis, F.; Amacker, M.; Arancia, S.; Sandini, S.; Gremion, C.; Zurbriggen, R.; Moser, C.; Cassone, A. A virosomal vaccine against candidal vaginitis: Immunogenicity, efficacy and safety profile in animal models. Vaccine 2012, 30, 4490–4498. [Google Scholar] [CrossRef] [PubMed]
- Zurbriggen, R.; Amacker, M.; Kammer, A.R.; Westerfeld, N.; Borghgraef, P.; Van Leuven, F.; Van der Auwera, I.; Wera, S. Virosome-based active immunization targets soluble amyloid species rather than plaques in a transgenic mouse model of Alzheimer’s disease. J. Mol. Neurosci. 2005, 27, 157–166. [Google Scholar] [CrossRef]
- Correale, P.; Cusi, M.G.; Sabatino, M.; Micheli, L.; Pozzessere, D.; Nencini, C.; Valensin, P.; Petrioli, R.; Giorgi, G.; Zurbriggen, R.; et al. Tumour-associated antigen (TAA)-specific cytotoxic T cell (CTL) response in vitro and in a mouse model, induced by TAA-plasmids delivered by influenza virosomes. Eur. J. Cancer 2001, 37, 2097–2103. [Google Scholar] [CrossRef]
- De Bruijn, I.A.; Nauta, J.; Gerez, L.; Palache, A.M. The virosomal influenza vaccine Invivac®: Immunogenicity and tolerability compared to an adjuvanted influenza vaccine (Fluad®) in elderly subjects. Vaccine 2006, 24, 6629–6631. [Google Scholar] [CrossRef]
- Mischler, R.; Metcalfe, I.C. Inflexal®V a trivalent virosome subunit influenza vaccine: Production. Vaccine 2002, 20, B17–B23. [Google Scholar] [CrossRef]
- Bovier, P.A. Epaxal®: A virosomal vaccine to prevent hepatitis A infection. Expert Rev. Vaccines 2008, 7, 1141–1150. [Google Scholar] [CrossRef]
- Object. Monophosphoryl Lipid A-Adjuvanted Virosomes with Ni-Chelating Lipids for Attachment of Conserved Viral Proteins as Cross-Protective Influenza Vaccine. Available online: https://core.ac.uk/reader/159515978?utm_source=linkout (accessed on 31 December 2024).
- Cusi, M.G.; Zurbriggen, R.; Valassina, M.; Bianchi, S.; Durrer, P.; Valensin, P.E.; Donati, M.; Glück, R. Intranasal Immunization with Mumps Virus DNA Vaccine Delivered by Influenza Virosomes Elicits Mucosal and Systemic Immunity. Virology 2000, 277, 111–118. [Google Scholar] [CrossRef]
- Thompson, F.M.; Porter, D.W.; Okitsu, S.L.; Westerfeld, N.; Vogel, D.; Todryk, S.; Poulton, I.; Correa, S.; Hutchings, C.; Berthoud, T.; et al. Evidence of blood stage efficacy with a virosomal malaria vaccine in a phase IIa clinical trial. PLoS ONE 2008, 3, e1493. [Google Scholar] [CrossRef]
- Firdaus, F.Z.; Skwarczynski, M.; Toth, I. Developments in Vaccine Adjuvants. In Vaccine Design: Methods and Protocols, Volume 3. Resources for Vaccine Development; Thomas, S., Ed.; Springer: New York, NY, USA, 2022; pp. 145–178. [Google Scholar] [CrossRef]
- Kalra, N.; Dhanya, V.; Saini, V.; Jeyabalan, D.G. Virosomes: As a Drug Delivery Carrier. Am. J. Adv. Drug Deliv. 2013, 1, 29–35. Available online: https://www.primescholars.com/articles/virosomes-as-a-drug-delivery-carrier.pdf (accessed on 31 December 2024).
- He, H.; Yuan, D.; Wu, Y.; Cao, Y. Pharmacokinetics and Pharmacodynamics Modeling and Simulation Systems to Support the Development and Regulation of Liposomal Drugs. Pharmaceutics 2019, 11, 110. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, R.; Jain, V.K.; Nagpal, S. Comparison of Virosome vs. Liposome as drug delivery vehicle using HepG2 and CaCo2 cell lines. J. Microencapsul. 2021, 38, 263–275. [Google Scholar] [CrossRef]
- Gamblin, S.J.; Vachieri, S.G.; Xiong, X.; Zhang, J.; Martin, S.R.; Skehel, J.J. Hemagglutinin Structure and Activities. Cold Spring Harb. Perspect. Med. 2021, 11, a038638. [Google Scholar] [CrossRef] [PubMed]
- Böttcher-Friebertshäuser, E.; Klenk, H.; Garten, W. Activation of influenza viruses by proteases from host cells and bacteria in the human airway epithelium. Pathog. Dis. 2013, 69, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Bungener, L.; Serre, K.; Bijl, L.; Leserman, L.; Wilschut, J.; Daemen, T.; Machy, P. Virosome-mediated delivery of protein antigens to dendritic cells. Vaccine 2002, 20, 2287–2295. [Google Scholar] [CrossRef] [PubMed]
- Gamblin, S.J.; Skehel, J.J. Influenza Hemagglutinin and Neuraminidase Membrane Glycoproteins. J. Biol. Chem. 2010, 285, 28403–28409. [Google Scholar] [CrossRef]
- Bungener, L.; Idema, J.; Ter Veer, W.; Huckriede, A.; Daemen, T.; Wilschut, J. Virosomes in vaccine development: Induction of Cytotoxic T lymphocyte activity with virosome-encapsulated protein antigens. J. Liposome Res. 2002, 12, 155–163. [Google Scholar] [CrossRef]
- Armitage, R.J.; Alderson, M.R. B-cell stimulation. Curr. Opin. Immunol. 1995, 7, 243–247. [Google Scholar] [CrossRef]
- Huckriede, A.; Bungener, L.; Stegmann, T.; Daemen, T.; Medema, J.; Palache, A.M.; Wilschut, J. The virosome concept for influenza vaccines. Vaccine 2005, 23, S26–S38. [Google Scholar] [CrossRef]
- Herzog, C.; Hartmann, K.; Künzi, V.; Kürsteiner, O.; Mischler, R.; Lazar, H.; Glück, R. Eleven years of Inflexal® V—A virosomal adjuvanted influenza vaccine. Vaccine 2009, 27, 4381–4387. [Google Scholar] [CrossRef]
- Bron, R.; Ortiz, A.; Wilschut, J. Cellular Cytoplasmic Delivery of a Polypeptide Toxin by Reconstituted Influenza Virus Envelopes (Virosomes). Biochemistry 1994, 33, 9110–9117. [Google Scholar] [CrossRef]
- Yewdell, J.W.; Bennink, J.R.; Hosaka, Y. Cells process exogenous proteins for recognition by cytotoxic T lymphocytes. Science 1988, 239, 637–640. [Google Scholar] [CrossRef]
- Hosaka, Y.; Sasao, F.; Ohara, R. Cell-mediated lysis of heat-inactivated influenza virus-coated murine targets. Vaccine 1985, 3 (Suppl. S3), 245–251. [Google Scholar] [CrossRef] [PubMed]
- Bungener, L.; Huckriede, A.; De Mare, A.; De Vries-Idema, J.; Wilschut, J.; Daemen, T. Virosome-mediated delivery of protein antigens in vivo: Efficient induction of class I MHC-restricted cytotoxic T lymphocyte activity. Vaccine 2005, 23, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, F.N.; Haach, V.; Bellaver, F.V.; Bombassaro, G.; Gava, D.; da Silva, L.P.; Baron, L.F.; Simonelly, M.; Carvalho, W.A.; Schaefer, R.; et al. Immunological profile of mice immunized with a polyvalent virosome-based influenza vaccine. Virol. J. 2023, 20, 187. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Rijken, P.; Ugwoke, M. Method for Preparing Virosomes. US20160199480A1, 20 October 2020. Available online: https://patents.google.com/patent/US20160199480A1/en (accessed on 29 December 2024).
- Kusumoto, S.; Inage, M.; Shiba, T.; Azuma, I.; Yamamura, Y. Synthesis of long chain fatty acid esters of N-acetylmuramyl-L-alanyl-D-isoglutamine in relation to antitumor activity. Tetrahedron. Lett. 1978, 19, 4899–4902. [Google Scholar] [CrossRef]
- Ando, S.; Tsuge, H.; Mayumi, T. Effect of cholesterol or phospholipids incorporation on vesicle formation of muramyldipeptide derivative B30-MDP. Colloid Polym. Sci. 1996, 274, 178–185. [Google Scholar] [CrossRef]
- Ando, S.; Tsuge, H.; Mayumi, T. Preparation of influenza virosome vaccine with muramyldipeptide derivative B30-MDP. J. Microencapsul. 1997, 14, 79–90. [Google Scholar] [CrossRef]
- Liu, H.; Tu, Z.; Feng, F.; Shi, H.; Chen, K.; Xu, X. Virosome, a hybrid vehicle for efficient and safe drug delivery and its emerging application in cancer treatment. Acta Pharm. 2015, 65, 105–116. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, B.; Li, B.; Luo, Y.; Ma, M.; Chen, Y.; Liu, H.; Xie, H.; Yang, T.; Zhao, X.; et al. Cell-free protein synthesis of influenza virus hemagglutinin HA2-integrated virosomes for siRNA delivery. Int. J. Pharm. 2022, 623, 121890. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Luo, Y.; Yang, T.; Zhao, X.; Ding, P. Virosome, a promising delivery vehicle for siRNA delivery and its novel preparation method. J. Drug Deliv. Sci. Technol. 2022, 74, 103490. [Google Scholar] [CrossRef]
- Dondapati, S.K.; Stech, M.; Zemella, A.; Kubick, S. Cell-Free Protein Synthesis: A Promising Option for Future Drug Development. Biodrugs 2020, 34, 327–348. [Google Scholar] [CrossRef]
- Gregorio, N.E.; Levine, M.Z.; Oza, J.P. A User’s Guide to Cell-Free Protein Synthesis. Methods Protoc. 2019, 2, 24. [Google Scholar] [CrossRef] [PubMed]
- Bundy, B.C.; Swartz, J.R. Efficient disulfide bond formation in virus-like particles. J. Biotechnol. 2011, 154, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Bundy, B.C.; Franciszkowicz, M.J.; Swartz, J.R. Escherichia coli-based cell-free synthesis of virus-like particles. Biotechnol. Bioeng. 2008, 100, 28–37. [Google Scholar] [CrossRef]
- Thoring, L.; Wüstenhagen, D.A.; Borowiak, M.; Stech, M.; Sonnabend, A.; Kubick, S. Cell-Free Systems Based on CHO Cell Lysates: Optimization Strategies, Synthesis of “Difficult-to-Express” Proteins and Future Perspectives. PLoS ONE 2016, 11, e0163670. [Google Scholar] [CrossRef] [PubMed]
- Quast, R.B.; Sonnabend, A.; Stech, M.; Wüstenhagen, D.A.; Kubick, S. High-yield cell-free synthesis of human EGFR by IRES-mediated protein translation in a continuous exchange cell-free reaction format. Sci. Rep. 2016, 6, 30399. [Google Scholar] [CrossRef]
- Gurramkonda, C.; Rao, A.; Borhani, S.; Pilli, M.; Deldari, S.; Ge, X.; Pezeshk, N.; Han, T.; Tolosa, M.; Kostov, Y.; et al. Improving the recombinant human erythropoietin glycosylation using microsome supplementation in CHO cell-free system. Biotechnol. Bioeng. 2018, 115, 1253–1264. [Google Scholar] [CrossRef]
- Mineev, K.S.; Lyukmanova, E.N.; Krabben, L.; Serebryakova, M.V.; Shulepko, M.A.; Arseniev, A.S.; Kordyukova, L.V.; Veit, M. Structural investigation of influenza virus hemagglutinin membrane-anchoring peptide. Protein Eng. Des. Sel. 2013, 26, 547–552. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, Y.; Jia, T.; Zhang, K.; Li, J.; Wang, L. Development of a microRNA delivery system based on bacteriophage MS2 virus-like particles. FEBS J. 2012, 279, 1198–1208. [Google Scholar] [CrossRef]
- Jahangir, M.A.; Mohanty, D.; Choudhury, A.; Imam, S.S. Theranostic Applications of Functionalized Vesicular Carriers. In Multifunctional and Targeted Theranostic Nanomedicines: Formulation, Design and Applications; Jain, K., Jain, N.K., Eds.; Springer Nature: Singapore, 2023; pp. 49–76. [Google Scholar] [CrossRef]
- Mellid-Carballal, R.; Gutierrez-Gutierrez, S.; Rivas, C.; Garcia-Fuentes, M. Viral protein-based nanoparticles (part 2): Pharmaceutical applications. Eur. J. Pharm. Sci. 2023, 189, 106558. [Google Scholar] [CrossRef]
- Doud, M.B.; Lee, J.M.; Bloom, J.D. How single mutations affect viral escape from broad and narrow antibodies to H1 influenza hemagglutinin. Nat. Commun. 2018, 9, 1386. [Google Scholar] [CrossRef] [PubMed]
- Prachanronarong, K.L.; Canale, A.S.; Liu, P.; Somasundaran, M.; Hou, S.; Poh, Y.-P.; Han, T.; Zhu, Q.; Renzette, N.; Zeldovich, K.B.; et al. Mutations in Influenza A Virus Neuraminidase and Hemagglutinin Confer Resistance against a Broadly Neutralizing Hemagglutinin Stem Antibody. J. Virol. 2019, 93, e01639-18. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.; Amsen, D.; Blander, J.M. Co-ordination of Incoming and Outgoing Traffic in Antigen-Presenting Cells by Pattern Recognition Receptors and T Cells. Traffic 2011, 12, 1669–1676. [Google Scholar] [CrossRef] [PubMed]
- Nair-Gupta, P.; Baccarini, A.; Tung, N.; Seyffer, F.; Florey, O.; Huang, Y.; Banerjee, M.; Overholtzer, M.; Roche, P.A.; Tampé, R.; et al. TLR Signals Induce Phagosomal MHC-I Delivery from the Endosomal Recycling Compartment to Allow Cross-Presentation. Cell 2014, 158, 506–521. [Google Scholar] [CrossRef]
- Raghunath, G.; Dyer, R.B. Kinetics of Histidine-Tagged Protein Association to Nickel-Decorated Liposome Surfaces. Langmuir 2019, 35, 12550–12561. [Google Scholar] [CrossRef]
- Watson, D.S.; Platt, V.M.; Cao, L.; Venditto, V.J.; Szoka, F.C. Antibody Response to Polyhistidine-Tagged Peptide and Protein Antigens Attached to Liposomes via Lipid-Linked Nitrilotriacetic Acid in Mice. Clin. Vaccine Immunol. 2011, 18, 289–297. [Google Scholar] [CrossRef]
- Glück, R. Intranasal immunization against influenza. J. Aerosol Med. 2002, 15, 221–228. [Google Scholar] [CrossRef]
- Mutsch, M.; Zhou, W.; Rhodes, P.; Bopp, M.; Chen, R.T.; Linder, T.; Spyr, C.; Steffen, R. Use of the inactivated intranasal influenza vaccine and the risk of Bell’s palsy in Switzerland. N. Engl. J. Med. 2004, 350, 896–903. [Google Scholar] [CrossRef]
- Moser, C.; Amacker, M.; Zurbriggen, R. Influenza virosomes as a vaccine adjuvant and carrier system. Expert Rev. Vaccines 2011, 10, 437–446. [Google Scholar] [CrossRef]
- Wiedermann, G.; Ambrosch, F.; Kollaritsch, H.; Hofmann, H.; Kunz, C.; D’HOndt, E.; Delem, A.; André, F.; Safary, A.; Stéphenne, J. Safety and immunogenicity of an inactivated hepatitis A candidate vaccine in healthy adult volunteers. Vaccine 1990, 8, 581–584. [Google Scholar] [CrossRef]
- Bovier, P.A. Recent advances with a virosomal hepatitis A vaccine. Expert Opin. Biol. Ther. 2008, 8, 1177–1185. [Google Scholar] [CrossRef]
- Bovier, P.A.; Bock, J.; Loutan, L.; Farinelli, T.; Glueck, R.; Herzog, C. Long-term immunogenicity of an inactivated virosome hepatitis A vaccine. J. Med. Virol. 2002, 68, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadeh-Goudarzi, K.; Nematollahi, M.H.; Khanbabaei, H.; Nave, H.H.; Mirzaei, H.R.; Pourghadamyari, H.; Sahebkar, A. Targeted Delivery of CRISPR/Cas13 as a Promising Therapeutic Approach to Treat SARS-CoV-2. Curr. Pharm. Biotechnol. 2021, 22, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Golmai, P.; Larsen, C.P.; DeVita, M.V.; Wahl, S.J.; Weins, A.; Rennke, H.G.; Bijol, V.; Rosenstock, J.L. AKI in the Setting of COVID-19: Histopathologic and Ultrastructural Findings in Postmortem Kidney Biopsy: PO0830. J. Am. Soc. Nephrol. 2020, 31, 295. [Google Scholar] [CrossRef]
- Fernandes, B.; Castro, R.; Bhoelan, F.; Bemelman, D.; Gomes, R.A.; Costa, J.; Gomes-Alves, P.; Stegmann, T.; Amacker, M.; Alves, P.M.; et al. Insect Cells for High-Yield Production of SARS-CoV-2 Spike Protein: Building a Virosome-Based COVID-19 Vaccine Candidate. Pharmaceutics 2022, 14, 854. [Google Scholar] [CrossRef]
- Pevion Biotech Ltd. A Phase I Single-Blind Randomised Placebo Controlled Dose Escalating Study of One Virosome Formulated CD4 and Two Virosomes Formulated CD8 Hepatitis C Virus (HCV) Vaccine Components (PEV2A and PEV2B) Administered to Healthy Adult Volunteers. clinicaltrials.gov. 2010. Available online: https://clinicaltrials.gov/study/NCT00445419 (accessed on 31 December 2024).
- Torresi, J.; Johnson, D.; Wedemeyer, H. Progress in the development of preventive and therapeutic vaccines for hepatitis C virus. J. Hepatol. 2011, 54, 1273–1285. [Google Scholar] [CrossRef]
- Montero, M.; van Houten, N.E.; Wang, X.; Scott, J.K. The Membrane-Proximal External Region of the Human Immunodeficiency Virus Type 1 Envelope: Dominant Site of Antibody Neutralization and Target for Vaccine Design. Microbiol. Mol. Biol. Rev. 2008, 72, 54–84. [Google Scholar] [CrossRef]
- Bomsel, M.; Tudor, D.; Drillet, A.-S.; Alfsen, A.; Ganor, Y.; Roger, M.-G.; Mouz, N.; Amacker, M.; Chalifour, A.; Diomede, L.; et al. Immunization with HIV-1 gp41 Subunit Virosomes Induces Mucosal Antibodies Protecting Nonhuman Primates against Vaginal SHIV Challenges. Immunity 2011, 34, 269–280. [Google Scholar] [CrossRef]
- Leroux-Roels, G.; Maes, C.; Clement, F.; Van Engelenburg, F.; Dobbelsteen, M.V.D.; Adler, M.; Amacker, M.; Lopalco, L.; Bomsel, M.; Chalifour, A.; et al. Randomized Phase I: Safety, Immunogenicity and Mucosal Antiviral Activity in Young Healthy Women Vaccinated with HIV-1 Gp41 P1 Peptide on Virosomes. PLoS ONE 2013, 8, e55438. [Google Scholar] [CrossRef]
- Amacker, M.; Smardon, C.; Mason, L.; Sorrell, J.; Jeffery, K.; Adler, M.; Bhoelan, F.; Belova, O.; Spengler, M.; Punnamoottil, B.; et al. New GMP manufacturing processes to obtain thermostable HIV-1 gp41 virosomes under solid forms for various mucosal vaccination routes. npj Vaccines 2020, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Periodic Reporting for Period 3—MACIVIVA (MAnufacturing Process for Cold-Chain Independent VIrosome-Based VAccines)|H2020. CORDIS|European Commission. Available online: https://cordis.europa.eu/project/id/646122/reporting (accessed on 12 January 2025).
- Bosch, S.; de Beaurepaire, L.; Allard, M.; Mosser, M.; Heichette, C.; Chrétien, D.; Jegou, D.; Bach, J.-M. Trehalose prevents aggregation of exosomes and cryodamage. Sci. Rep. 2016, 6, 36162. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.S.; Renard, A.; Boato, F.; Vogel, D.; Naegeli, M.; Zurbriggen, R.; Robinson, J.A.; Pluschke, G. Induction of Parasite Growth-Inhibitory Antibodies by a Virosomal Formulation of a Peptidomimetic of Loop I from Domain III of Plasmodium falciparum Apical Membrane Antigen 1. Infect. Immun. 2003, 71, 4749–4758. [Google Scholar] [CrossRef] [PubMed]
- Okitsu, S.L.; Kienzl, U.; Moehle, K.; Silvie, O.; Peduzzi, E.; Mueller, M.S.; Sauerwein, R.W.; Matile, H.; Zurbriggen, R.; Mazier, D.; et al. Structure-activity-based design of a synthetic malaria peptide eliciting sporozoite inhibitory antibodies in a virosomal formulation. Chem. Biol. 2007, 14, 577–587. [Google Scholar] [CrossRef]
- Cusi, M.G. Applications of Influenza Virosomes as a Delivery System. Hum. Vaccines 2006, 2, 1–7. [Google Scholar] [CrossRef]
- Daemen, T.; Demare, A.; Bungener, L.; Dejonge, J.; Huckriede, A.; Wilschut, J. Virosomes for antigen and DNA delivery. Adv. Drug Deliv. Rev. 2005, 57, 451–463. [Google Scholar] [CrossRef]
- Karunagaran, D.; Tzahar, E.; Beerli, R.R.; Chen, X.; Graus-Porta, D.; Ratzkin, B.J.; Seger, R.; Hynes, N.E.; Yarden, Y. ErbB-2 is a common auxiliary subunit of NDF and EGF receptors: Implications for breast cancer. EMBO J. 1996, 15, 254–264. [Google Scholar] [CrossRef]
- Niehans, G.A.; Singleton, T.P.; Dykoski, D.; Kiang, D.T. Stability of HER-2/neu expression over time and at multiple metastatic sites. J. Natl. Cancer Inst. 1993, 85, 1230–1235. [Google Scholar] [CrossRef]
- Waelti, E.; Wegmann, N.; Schwaninger, R.; Wetterwald, A.; Wingenfeld, C.; Rothen-Rutishauser, B.; Gimmi, C.D. Targeting HER-2/neu with Antirat Neu Virosomes for Cancer Therapy1. Cancer Res. 2002, 62, 437–444. [Google Scholar]
- Cragg, G.M.; Newman, D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, R.; Jain, V.K.; Nagpal, S. Preparation and characterization of nanocurcumin based hybrid virosomes as a drug delivery vehicle with enhanced anticancerous activity and reduced toxicity. Sci. Rep. 2021, 11, 368. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sidhu, J.; Lui, F.; Tsao, J.W. Alzheimer Disease. In StatPearls; StatPearls Publishing: Petersburg, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK499922/ (accessed on 1 January 2025).
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Thawabteh, A.M.; Ghanem, A.W.; AbuMadi, S.; Thaher, D.; Jaghama, W.; Karaman, D.; Karaman, R. Promising Natural Remedies for Alzheimer’s Disease Therapy. Molecules 2025, 30, 922. [Google Scholar] [CrossRef] [PubMed]
- De Bernardis, F.; Sullivan, P.A.; Cassone, A. Aspartyl proteinases of Candida albicans and their role in pathogenicity. Med. Mycol. 2001, 39, 303–313. [Google Scholar] [CrossRef]
- Naglik, J.R.; Challacombe, S.J.; Hube, B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol. Mol. Biol. Rev. 2003, 67, 400–428. [Google Scholar] [CrossRef]
- De Bernardis, F.; Boccanera, M.; Adriani, D.; Spreghini, E.; Santoni, G.; Cassone, A. Protective role of antimannan and anti-aspartyl proteinase antibodies in an experimental model of Candida albicans vaginitis in rats. Infect. Immun. 1997, 65, 3399–3405. [Google Scholar] [CrossRef]
- Sandini, S.; La Valle, R.; Deaglio, S.; Malavasi, F.; Cassone, A.; De Bernardis, F. A highly immunogenic recombinant and truncated protein of the secreted aspartic proteases family (rSap2t) of Candida albicans as a mucosal anticandidal vaccine. FEMS Immunol. Med. Microbiol. 2011, 62, 215–224. [Google Scholar] [CrossRef]
- Ramphal, R. IND15—Berna Products: Virosome vaccines: Advantages in safety. Clin. Microbiol. Infect. 2000, 6, 249–250. [Google Scholar] [CrossRef]
- Loutan, L.; Bovier, P.; Althaus, B.; Glück, R. Inactivated virosome hepatitis A vaccine. Lancet 1994, 343, 322–324. [Google Scholar] [CrossRef]
- Manolova, V.; Flace, A.; Bauer, M.; Schwarz, K.; Saudan, P.; Bachmann, M.F. Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 2008, 38, 1404–1413. [Google Scholar] [CrossRef]
- Sekiya, T.; Ohno, M.; Nomura, N.; Handabile, C.; Shingai, M.; Jackson, D.C.; Brown, L.E.; Kida, H. Selecting and Using the Appropriate Influenza Vaccine for Each Individual. Viruses 2021, 13, 971. [Google Scholar] [CrossRef] [PubMed]
- Osterholm, M.T.; Kelley, N.S.; Sommer, A.; Belongia, E.A. Efficacy and effectiveness of influenza vaccines: A systematic review and meta-analysis. Lancet Infect. Dis. 2012, 12, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Scheifele, D.W.; McNeil, S.A.; Ward, B.J.; Dionne, M.; Cooper, C.; Coleman, B.; Loeb, M.; Rubinstein, E.; McElhaney, J.; Hatchette, T.; et al. Safety, immunogenicity, and tolerability of three influenza vaccines in older adults. Hum. Vaccines Immunother. 2013, 9, 2460–2473. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Singh, J.; Gautam, R.K.; Farooqui, N.; Manocha, N.; Malhotra, H. Adjuvant and Vaccine Safety. In Emerging Pathways of Vaccine Adjuvants; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2025; pp. 235–253. [Google Scholar] [CrossRef]
- Gasparini, R.; Amicizia, D.; Lai, P.L.; Rossi, S.; Panatto, D. Effectiveness of adjuvanted seasonal influenza vaccines (Inflexal V® and Fluad®) in preventing hospitalization for influenza and pneumonia in the elderly. Hum. Vaccines Immunother. 2013, 9, 144–152. [Google Scholar] [CrossRef]
- Naro, M.D. Generation of Virosome Particles. US20130259928A1, 3 October 2013. Available online: https://patents.google.com/patent/US20130259928A1/en (accessed on 13 January 2025).
- Adriaansen, J.; Doro, F. Improved Formulations for Virosomes. EP3082769B1, 31 January 2018. Available online: https://patents.google.com/patent/EP3082769B1/en (accessed on 13 January 2025).
- Zurbriggen, R.; Amacker, M.; Rasi, S. Lyophilization of Virosomes. US20080268028A1, 30 October 2008. Available online: https://patents.google.com/patent/US20080268028A1/en (accessed on 14 January 2025).
- Affairs O of R. Lyophilization of Parenteral (7/93). FDA. 2022. Available online: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/inspection-guides/lyophilization-parenteral-793 (accessed on 14 January 2025).
- Alphavirus—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/medicine-and-dentistry/alphavirus (accessed on 14 January 2025).
- Olmsted, R.; Keith, P.; Dryga, S.; Caley, I.; Maughan, M.; Johnston, R.; Davis, N.; Swanstrom, R. Alphavirus Vectors and Virosomes with Modified HIV Genes for Use as Vaccines. US20050123555A1, 9 June 2005. Available online: https://patents.google.com/patent/US20050123555A1/en (accessed on 14 January 2025).
- Fleury, S.; Bomsel, M.; Zurbriggen, R. Virosome-Like Vesicles Comprising gp41-Derived Antigens. US9216156B2, 22 December 2015. Available online: https://patents.google.com/patent/US9216156B2/en?q=(virosome)&q=(antigen)&q=(vaccine)&q=(influenza)&q=(lipid+bilayer)&before=priority:20100930&scholar (accessed on 14 January 2025).
- Gluck, R.; Mischler, R. Hepatitis A Virus in a Reconstituted Influenza Virosome and Use as a Vaccine. US5565203A, 15 October 1996. Available online: https://patents.google.com/patent/US5565203A/en (accessed on 14 January 2025).
- Moser, C.; Assero, G.; Fichera, E.; Ventura, D.; Lempereur, L.; Felnerova, D. Virosome Particles Comprising Antigens from Influenza Virus and Hepatitis B Virus. EP1652914A1, 3 May 2006. Available online: https://patents.google.com/patent/EP1652914A1/no (accessed on 14 January 2025).
- Coller, B.A.; Henderickx, V.; Garcon, N.M.J. Vaccine Compositions Comprising Virosomes and a Saponin Adjuvant. EP2269638A2, 5 January 2011. Available online: https://patents.google.com/patent/EP2269638A2/en (accessed on 14 January 2025).
- Walti, E.R.; Gluck, R.; Klein, P. Cationic Virosomes as Transfer System for Genetic Material. US6210708B1, 3 April 2001. Available online: https://patents.google.com/patent/US6210708B1/en (accessed on 16 January 2025).
- Asadikaram, G.; Poustforoosh, A.; Pardakhty, A.; Torkzadeh-Mahani, M.; Nematollahi, M.H. Niosomal virosome derived by vesicular stomatitis virus glycoprotein as a new gene carrier. Biochem. Biophys. Res. Commun. 2021, 534, 980–987. [Google Scholar] [CrossRef]
- Caley, I.; Davis, N.; Dryga, S.; Johnston, R.; Keith, P.; Maughan, M.; Olmsted, R.; Swanstrom, R. Alphavirus Vectors and Virosomes with Modified HIV Genes for Use as Vaccines. AU2001273313A1, 21 January 2002. Available online: https://patents.google.com/patent/AU2001273313A1/en (accessed on 16 January 2025).
- Rajalakshmi, M.; Suveena, S.; Vijayalakshmia, P.; Indu, S.; Roy, A.; Ludas, A. DaiCee: A database for anti-cancer compounds with targets and side effect profiles. Bioinformation 2020, 16, 843–848. [Google Scholar] [CrossRef]
- Shimizu, C. Side effects of anticancer treatment and the needs for translational research on toxicity: A clinician’s perspective. Nihon Yakurigaku Zasshi Folia Pharmacol. Jpn. 2015, 146, 72–75. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Zhang, W.; Li, X. Bioactive peptides for anticancer therapies. Biomater. Transl. 2023, 4, 5–17. [Google Scholar] [CrossRef]
- Soon, T.N.; Chia, A.Y.Y.; Yap, W.H.; Tang, Y.Q. Anticancer Mechanisms of Bioactive Peptides. Protein Pept. Lett. 2020, 27, 823–830. [Google Scholar] [CrossRef]
- Marqus, S.; Pirogova, E.; Piva, T.J. Evaluation of the use of therapeutic peptides for cancer treatment. J. Biomed. Sci. 2017, 24, 21. [Google Scholar] [CrossRef]
- Bidwell, G.L.; Raucher, D. Therapeutic peptides for cancer therapy. Part I—Peptide inhibitors of signal transduction cascades. Expert Opin. Drug Deliv. 2009, 6, 1033–1047. [Google Scholar] [CrossRef]
- Schellens, J.H.M.; Malingré, M.M.; Kruijtzer, C.M.F.; Bardelmeijer, H.A.; van Tellingen, O.; Schinkel, A.H.; Beijnen, J.H. Modulation of oral bioavailability of anticancer drugs: From mouse to man. Eur. J. Pharm. Sci. 2000, 12, 103–110. [Google Scholar] [CrossRef]
| Mumps Virosomes | Mucosal Immunity Response | Cellular Immunity Response |
|---|---|---|
| GC9-Virosome | Instigated a humoral response with a significant increase in IgA production. | Triggered T-helper cell 2 response, characterized by increased levels of IL-10 and IgG1 |
| GC23-Virosome | No response | Triggered T-helper cell 1 response, characterized by increased levels of IL-2 and IgG |
| Virosome Patent | Novel Composition | Current Assignee | Patent Office | Legal Status |
|---|---|---|---|---|
| Patent on the preparation Technique | ||||
| Generation of a virosome particle | Deriving HA expressed on plants and mixing them with phospholipids to generate the plant-IRIV89. | FRANVAX Srl | United States | Abandoned |
| Method for preparing virosome | Virosome reconstitution using a solubilizing agent, followed by purification by BIOBEADS | Janssen Vaccines and Prevention BV | European Patent Office (EPO), and the United States | Active |
| Improved formulation of virosomes | Adding KH2PO4/Na2HPO4 buffer at a pH ranging between 6.5 and 8, and trehalose during manufacturing, so that the final product could withstand freezing | Janssen Vaccines and Prevention BV | EPO | Active |
| Lyophilization | Lyophilization gives the advantage of adding adjuvants that can enhance the immune response | HELVETIC AIRWAYS AG | United States | Abandoned |
| Virosomal vaccines | ||||
| Alphavirus | RNA replicon systems, which contain nucleic acid sequences encoding antigens for eliciting an immune response to HIV | University of North Carolina at Chapel Hill, Alphavax Inc. | Australia [119], then the United States | Abandoned |
| HIV | Virosome-like vesicles comprising gp41-derived antigens | Pevion Biotech Ltd., Institut National de la Sante et de la Recherche Medicale INSERM, Mymetics Corp. | United States | Active |
| Hepatitis A | Hepatitis A virus (HAV) antigen was adsorbed on the surface of the virosomes so it could elucidate immunogenicity potentiation | Cilag GmbH International | United States | Expired |
| Hepatitis B | HBsAG was incorporated in the liposomal surface of the influenza virosome, which is used to link the HBc to the virosome structure | Cilag GmbH International | EPO | Withdrawn |
| Influenza and RSV | Vaccine compositions comprising virosomes and a saponin adjuvant | GlaxoSmithKline Biologicals SA | EPO | Withdrawn |
| Delivery of genetic materials | ||||
| Anti-cancer, leukemia | Cationic virosomes as a transfer system for active pharmaceutical ingredients | Nika Health Products Ltd. | United States | Expired |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salameh, H.K.; Safi, M.M.; Karaman, R. Virosomes: Beyond Vaccines. Life 2025, 15, 1567. https://doi.org/10.3390/life15101567
Salameh HK, Safi MM, Karaman R. Virosomes: Beyond Vaccines. Life. 2025; 15(10):1567. https://doi.org/10.3390/life15101567
Chicago/Turabian StyleSalameh, Hadeel K., Mohammed M. Safi, and Rafik Karaman. 2025. "Virosomes: Beyond Vaccines" Life 15, no. 10: 1567. https://doi.org/10.3390/life15101567
APA StyleSalameh, H. K., Safi, M. M., & Karaman, R. (2025). Virosomes: Beyond Vaccines. Life, 15(10), 1567. https://doi.org/10.3390/life15101567








