Oral Treatment with the Vimentin-Targeting Compound ALD-R491 Mitigates Hyperinflammation, Multi-Organ Injury, and Mortality in CLP-Induced Septic Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Compound and Purity Quotient
2.2. Animals
2.3. Drug Treatment and Groups
2.4. Cecal Ligation and Punction-Induced Mice Sepsis
2.5. Disease Severity Evaluation
2.6. Evaluation of Lung Edema
2.7. Systemic Inflammation Analysis
2.8. Measurement of Common Biomarkers for Sepsis and Tissue Damage
2.9. Histological Examination
2.10. Real-Time PCR Gene Expression Quantification
2.11. Statistical Analysis
3. Results
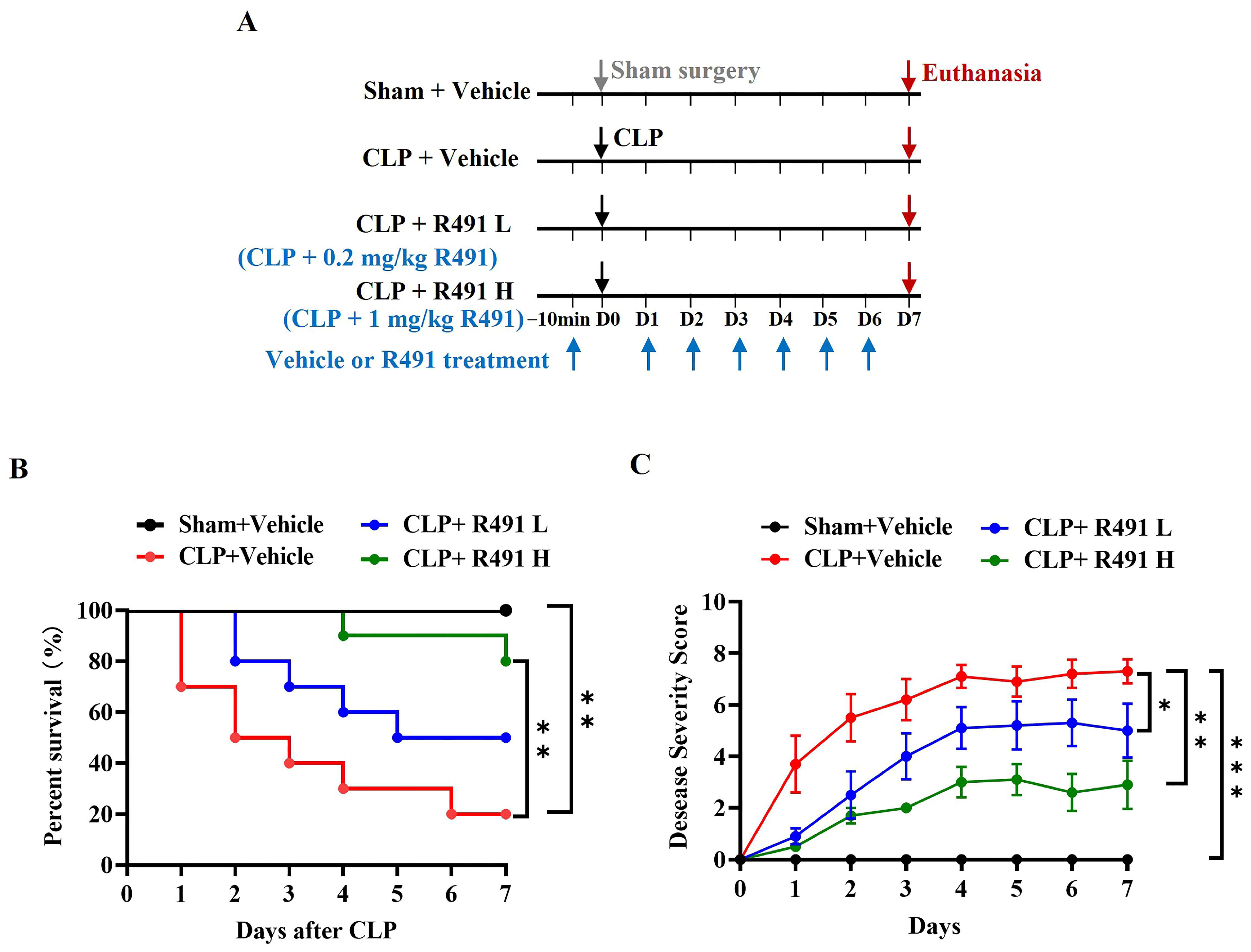
3.1. ALD-R491 Significantly Reduced Disease Severity and Mortality in Mice with CLP-Induced Sepsis
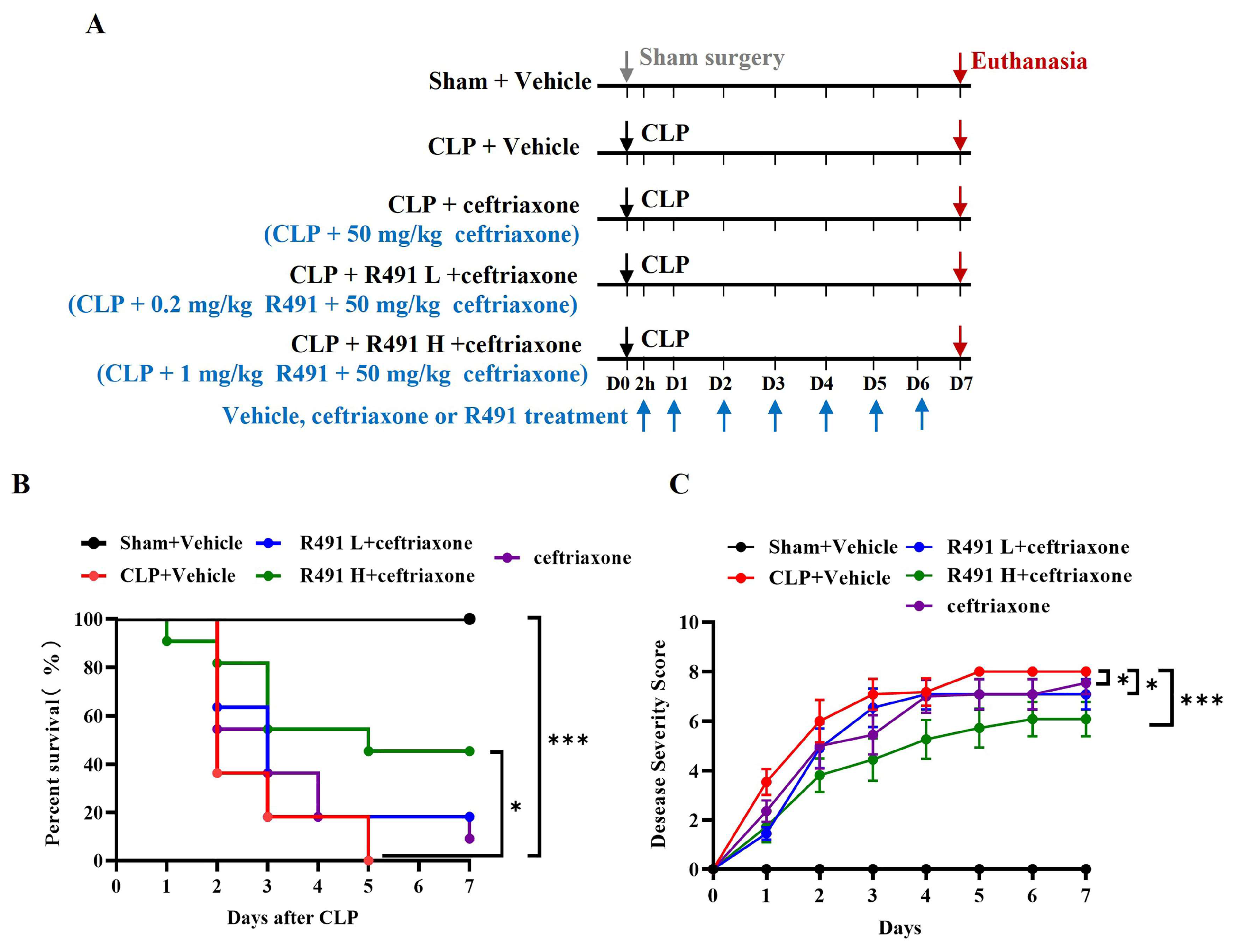
3.2. ALD-R491 Combined with Ceftriaxone Significantly Reduced Disease Severity and Mortality in Mice with CLP-Induced Sepsis
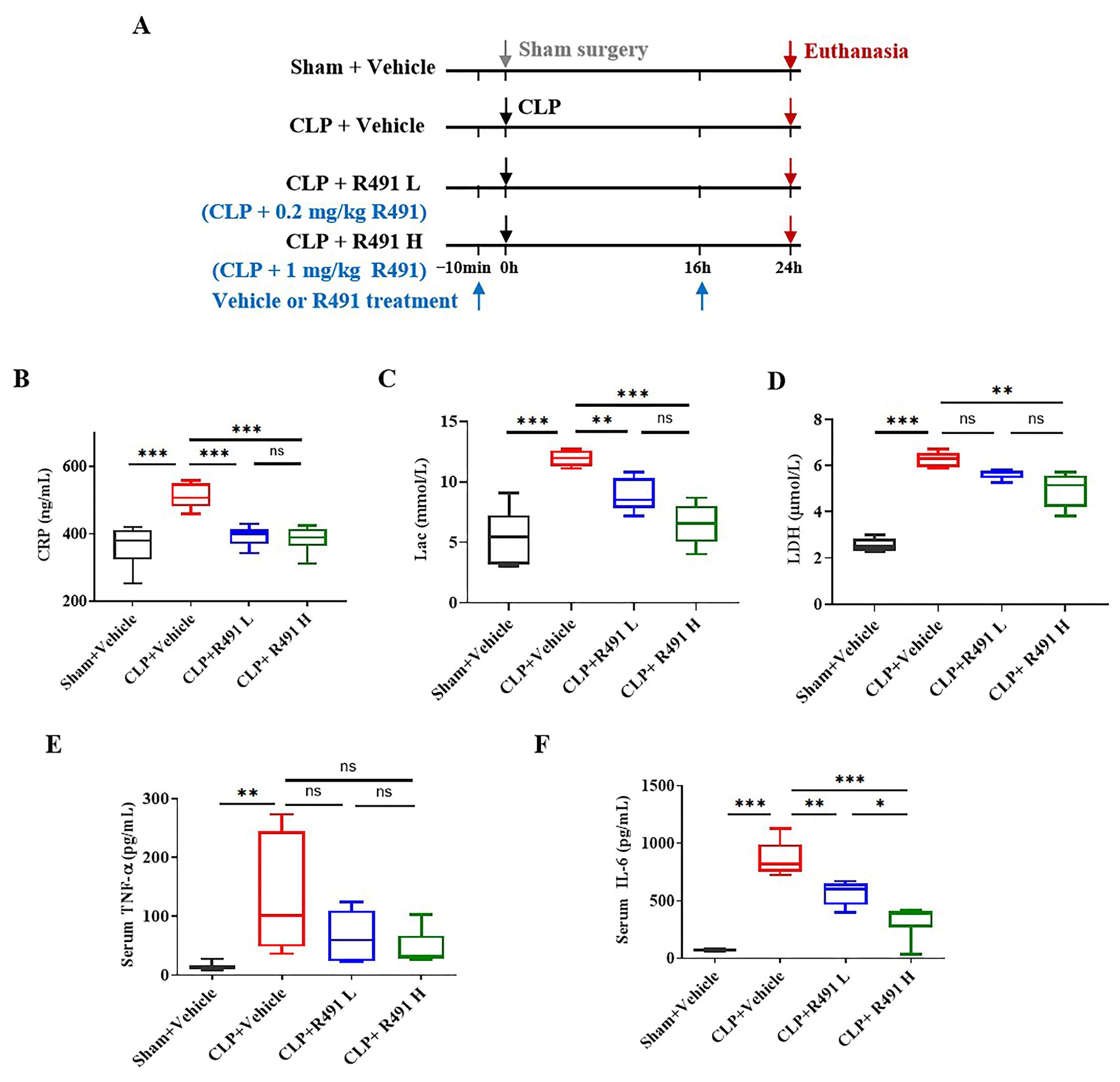
3.3. ALD-R491 Significantly Reduced Systemic Inflammation and Cellular Injury in Septic Mice
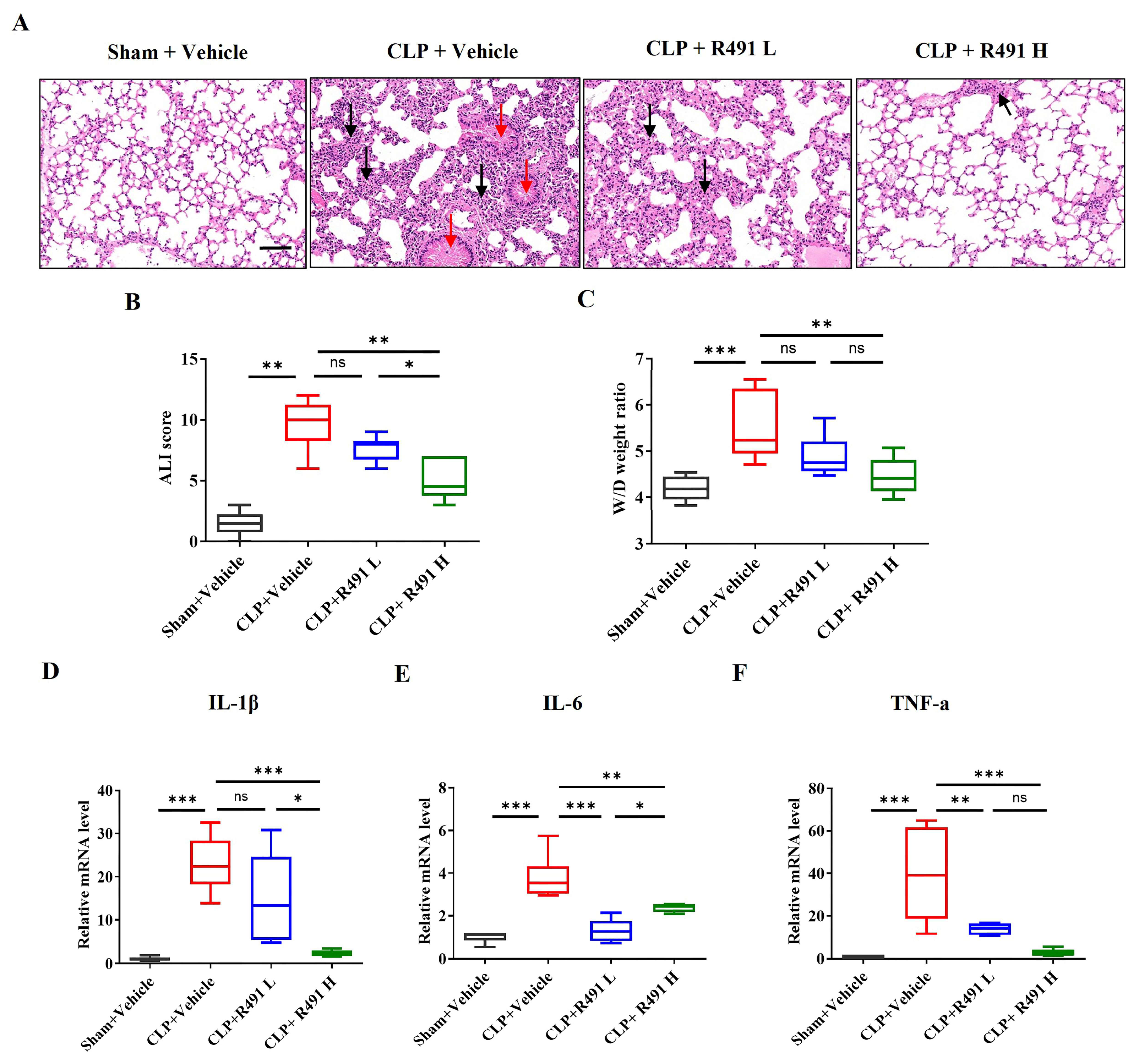
3.4. ALD-R491 Significantly Alleviated Acute Lung Injury and Inflammation
3.5. ALD-R491 Significantly Alleviated Acute Liver Injury and Inflammation
3.6. ALD-R491 Significantly Alleviates Acute Kidney Injury and Restores Renal Function
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. What Is Sepsis? Available online: https://www.cdc.gov/sepsis/about/index.html (accessed on 28 March 2025).
- Buchman, T.G.; Simpson, S.Q.; Sciarretta, K.L.; Finne, K.P.; Sowers, N.; Collier, M.; Chavan, S.; Oke, I.; Pennini, M.E.; Santhosh, A.; et al. Sepsis Among Medicare Beneficiaries: 2. The Trajectories of Sepsis, 2012–2018. Crit. Care Med. 2020, 48, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef]
- World Health Organization. Global Report on the Epidemiology and Burden of Sepsis: Current Evidence, Identifying Gaps and Future Directions; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Cavaillon, J.M.; Singer, M.; Skirecki, T. Sepsis therapies: Learning from 30 years of failure of translational research to propose new leads. EMBO Mol. Med. 2020, 12, e10128. [Google Scholar] [CrossRef]
- Jarczak, D.; Kluge, S.; Nierhaus, A. Sepsis—Pathophysiology and Therapeutic Concepts. Front. Med. 2021, 8, 628302. [Google Scholar] [CrossRef]
- Colucci-Guyon, E.; Portier, M.M.; Dunia, I.; Paulin, D.; Pournin, S.; Babinet, C. Mice lacking vimentin develop and reproduce without an obvious phenotype. Cell 1994, 79, 679–694. [Google Scholar] [CrossRef]
- Ridge, K.M.; Eriksson, J.E.; Pekny, M.; Goldman, R.D. Roles of vimentin in health and disease. Genes Dev. 2022, 36, 391–407. [Google Scholar] [CrossRef]
- Miao, C.; Zhao, S.; Etienne-Manneville, S.; Jiu, Y. The diverse actions of cytoskeletal vimentin in bacterial infection and host defense. J. Cell Sci. 2023, 136, jcs260509. [Google Scholar] [CrossRef]
- Mak, T.N.; Brüggemann, H. Vimentin in Bacterial Infections. Cells 2016, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Ramos, I.; Stamatakis, K.; Oeste, C.L.; Pérez-Sala, D. Vimentin as a Multifaceted Player and Potential Therapeutic Target in Viral Infections. Int. J. Mol. Sci. 2020, 21, 4675. [Google Scholar] [CrossRef]
- Mor-Vaknin, N.; Legendre, M.; Yu, Y.; Serezani, C.H.; Garg, S.K.; Jatzek, A.; Swanson, M.D.; Gonzalez-Hernandez, M.J.; Teitz-Tennenbaum, S.; Punturieri, A.; et al. Murine colitis is mediated by vimentin. Sci. Rep. 2013, 3, 1045. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, G.; Rogel, M.R.; Baker, M.A.; Troken, J.R.; Urich, D.; Morales-Nebreda, L.; Sennello, J.A.; Kutuzov, M.A.; Sitikov, A.; Davis, J.M.; et al. Vimentin regulates activation of the NLRP3 inflammasome. Nat. Commun. 2015, 6, 6574. [Google Scholar] [CrossRef] [PubMed]
- McDonald-Hyman, C.; Muller, J.T.; Loschi, M.; Thangavelu, G.; Saha, A.; Kumari, S.; Reichenbach, D.K.; Smith, M.J.; Zhang, G.; Koehn, B.H.; et al. The vimentin intermediate filament network restrains regulatory T cell suppression of graft-versus-host disease. J. Clin. Investig. 2018, 128, 4604–4621. [Google Scholar] [CrossRef]
- van Beijnum, J.R.; Huijbers, E.J.M.; van Loon, K.; Blanas, A.; Akbari, P.; Roos, A.; Wong, T.J.; Denisov, S.S.; Hackeng, T.M.; Jimenez, C.R.; et al. Extracellular vimentin mimics VEGF and is a target for anti-angiogenic immunotherapy. Nat. Commun. 2022, 13, 2842. [Google Scholar] [CrossRef]
- Da, Q.; Behymer, M.; Correa, J.I.; Vijayan, K.V.; Cruz, M.A. Platelet adhesion involves a novel interaction between vimentin and von Willebrand factor under high shear stress. Blood 2014, 123, 2715–2721. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Vargas, M.; Cebula, A.; Brubaker, L.S.; Seshadri, N.; Lam, F.W.; Loor, M.; Rosengart, T.K.; Yee, A.; Rumbaut, R.E.; Cruz, M.A. A novel interaction between extracellular vimentin and fibrinogen in fibrin formation. Thromb. Res. 2023, 221, 97–104. [Google Scholar] [CrossRef]
- Mohanasundaram, P.; Coelho-Rato, L.S.; Modi, M.K.; Urbanska, M.; Lautenschläger, F.; Cheng, F.; Eriksson, J.E. Cytoskeletal vimentin regulates cell size and autophagy through mTORC1 signaling. PLoS Biol. 2022, 20, e3001737. [Google Scholar] [CrossRef]
- Su, L.; Pan, P.; Yan, P.; Long, Y.; Zhou, X.; Wang, X.; Zhou, R.; Wen, B.; Xie, L.; Liu, D. Role of vimentin in modulating immune cell apoptosis and inflammatory responses in sepsis. Sci. Rep. 2019, 9, 5747. [Google Scholar] [CrossRef]
- Zhang, L.; Qu, Z.; Wu, J.; Yao, S.; Zhang, Q.; Zhang, T.; Mo, L.; Yao, Q.; Xu, Y.; Chen, R. SARs of a novel series of s-triazine compounds targeting vimentin to induce methuotic phenotype. Eur. J. Med. Chem. 2021, 214, 113188. [Google Scholar] [CrossRef]
- Kim, H.R.; Warrington, S.J.; López-Guajardo, A.; Al Hennawi, K.; Cook, S.L.; Griffith, Z.D.J.; Symmes, D.; Zhang, T.; Qu, Z.; Xu, Y.; et al. ALD-R491 regulates vimentin filament stability and solubility, cell contractile force, cell migration speed and directionality. Front. Cell Dev. Biol. 2022, 10, 926283. [Google Scholar] [CrossRef]
- Li, Z.; Wu, J.; Zhou, J.; Yuan, B.; Chen, J.; Wu, W.; Mo, L.; Qu, Z.; Zhou, F.; Dong, Y.; et al. A Vimentin-Targeting Oral Compound with Host-Directed Antiviral and Anti-Inflammatory Actions Addresses Multiple Features of COVID-19 and Related Diseases. mBio 2021, 12, e0254221. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Shen, Y.; Mohanasundaram, P.; Lindström, M.; Ivaska, J.; Ny, T.; Eriksson, J.E. Vimentin coordinates fibroblast proliferation and keratinocyte differentiation in wound healing via TGF-β-Slug signaling. Proc. Natl. Acad. Sci. USA 2016, 113, E4320–E4327. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Wong, I.Y.; Moore, A.S.; Medalia, O.; Lippincott-Schwartz, J.; Weitz, D.A.; Goldman, R.D. Vimentin intermediate filaments as structural and mechanical coordinators of mesenchymal cells. Nat. Cell Biol. 2025, 27, 1210–1218. [Google Scholar] [CrossRef]
- Rosier, M.; Krstulović, A.; Kim, H.R.; Kaur, N.; Enakireru, E.M.; Symmes, D.; Dobra, K.; Chen, R.; Evans, C.A.; Gad, A.K.B. The Vimentin-Targeting Drug ALD-R491 Partially Reverts the Epithelial-to-Mesenchymal Transition and Vimentin Interactome of Lung Cancer Cells. Cancers 2024, 17, 81. [Google Scholar] [CrossRef]
- Wu, J.; Xie, Q.; Liu, Y.; Gao, Y.; Qu, Z.; Mo, L.; Xu, Y.; Chen, R.; Shi, L. A Small Vimentin-Binding Molecule Blocks Cancer Exosome Release and Reduces Cancer Cell Mobility. Front. Pharmacol. 2021, 12, 627394. [Google Scholar] [CrossRef]
- Wu, J.; Wu, X.; Cheng, C.; Liu, L.; Xu, L.; Xu, Z.; Wang, S.; Symmes, D.; Mo, L.; Chen, R.; et al. Therapeutic targeting of vimentin by ALD-R491 impacts multiple pathogenic processes to attenuate acute and chronic colitis in mice. Biomed. Pharmacother. 2023, 168, 115648. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Wen, H. Sepsis Induced by Cecal Ligation and Puncture. Methods Mol. Biol. 2019, 1960, 249–255. [Google Scholar] [CrossRef]
- Jensen, I.J.; McGonagill, P.W.; Berton, R.R.; Wagner, B.A.; Silva, E.E.; Buettner, G.R.; Griffith, T.S.; Badovinac, V.P. Prolonged Reactive Oxygen Species Production following Septic Insult. Immunohorizons 2021, 5, 477–488. [Google Scholar] [CrossRef]
- Rittirsch, D.; Huber-Lang, M.S.; Flierl, M.A.; Ward, P.A. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat. Protoc. 2009, 4, 31–36. [Google Scholar] [CrossRef]
- Casey, L.C.; Balk, R.A.; Bone, R.C. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann. Intern. Med. 1993, 119, 771–778. [Google Scholar] [CrossRef]
- Michie, H.R.; Manogue, K.R.; Spriggs, D.R.; Revhaug, A.; O’Dwyer, S.; Dinarello, C.A.; Cerami, A.; Wolff, S.M.; Wilmore, D.W. Detection of circulating tumor necrosis factor after endotoxin administration. N. Engl. J. Med. 1988, 318, 1481–1486. [Google Scholar] [CrossRef]
- van der Poll, T.; van Deventer, S.J.H. Cytokines and Anticytokines in the Pathogenesis of Sepsis. Infect. Dis. Clin. N. Am. 1999, 13, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.J., Jr.; Agosti, J.M.; Opal, S.M.; Lowry, S.F.; Balk, R.A.; Sadoff, J.C.; Abraham, E.; Schein, R.M.; Benjamin, E.; The Soluble TNF Receptor Sepsis Study Group. Treatment of septic shock with the tumor necrosis factor receptor:Fc fusion protein. N. Engl. J. Med. 1996, 334, 1697–1702. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Yin, X.; Tan, B.; Wang, J.; Chen, J.; Zhao, B.; Yang, Q.; Li, Z. Drug-induced liver injury following the use of tocilizumab or sarilumab in patients with coronavirus disease 2019. BMC Infect. Dis. 2022, 22, 929. [Google Scholar] [CrossRef]
- Opal, S.M.; Fisher, C.J., Jr.; Dhainaut, J.F.; Vincent, J.L.; Brase, R.; Lowry, S.F.; Sadoff, J.C.; Slotman, G.J.; Levy, H.; Balk, R.A.; et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: A phase III, randomized, double-blind, placebo-controlled, multicenter trial. Crit. Care Med. 1997, 25, 1115–1124. [Google Scholar] [CrossRef]
- Lelubre, C.; Vincent, J.L. Mechanisms and treatment of organ failure in sepsis. Nat. Rev. Nephrol. 2018, 14, 417–427. [Google Scholar] [CrossRef]
- Sakr, Y.; Jaschinski, U.; Wittebole, X.; Szakmany, T.; Lipman, J.; Ñamendys-Silva, S.A.; Martin-Loeches, I.; Leone, M.; Lupu, M.N.; Vincent, J.L.; et al. Sepsis in Intensive Care Unit Patients: Worldwide Data from the Intensive Care over Nations Audit. Open Forum Infect. Dis. 2018, 5, ofy313. [Google Scholar] [CrossRef]
- Disselkamp, M.; Coz Yataco, A.O.; Simpson, S.Q. POINT: Should Broad-Spectrum Antibiotics Be Routinely Administered to All Patients with Sepsis as Soon as Possible? Yes. Chest 2019, 156, 645–647. [Google Scholar] [CrossRef]
- Patel, J.J.; Bergl, P.A. Rebuttal from Drs Patel and Bergl. Chest 2019, 156, 651–652. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Gao, Y.; Shou, S.; Chai, Y. The roles of macrophage polarization in the host immune response to sepsis. Int. Immunopharmacol. 2021, 96, 107791. [Google Scholar] [CrossRef] [PubMed]
- Soto-Heredero, G.; Desdín-Micó, G.; Mittelbrunn, M. Mitochondrial dysfunction defines T cell exhaustion. Cell Metab. 2021, 33, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Luperto, M.; Zafrani, L. T cell dysregulation in inflammatory diseases in ICU. Intensive Care Med. Exp. 2022, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Leng, F.Y.; Liu, J.L.; Liu, Z.J.; Yin, J.Y.; Qu, H.P. Increased proportion of CD4+CD25+Foxp3+ regulatory T cells during early-stage sepsis in ICU patients. J. Microbiol. Immunol. Infect. 2013, 46, 338–344. [Google Scholar] [CrossRef]
- Venet, F.; Chung, C.S.; Kherouf, H.; Geeraert, A.; Malcus, C.; Poitevin, F.; Bohé, J.; Lepape, A.; Ayala, A.; Monneret, G. Increased circulating regulatory T cells (CD4+CD25+CD127−) contribute to lymphocyte anergy in septic shock patients. Intensive Care Med. 2009, 35, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Xu, J.Y.; Shi, X.Y.; Huang, W.; Ruan, T.Y.; Xie, P.; Ding, J.L. M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab. Investig. 2013, 93, 844–854. [Google Scholar] [CrossRef]
- Moisan, E.; Girard, D. Cell surface expression of intermediate filament proteins vimentin and lamin B1 in human neutrophil spontaneous apoptosis. J. Leukoc. Biol. 2006, 79, 489–498. [Google Scholar] [CrossRef]
- Lamontagne, F.; Rochwerg, B.; Lytvyn, L.; Guyatt, G.H.; Møller, M.H.; Annane, D.; Kho, M.E.; Adhikari, N.K.J.; Machado, F.; Vandvik, P.O.; et al. Corticosteroid therapy for sepsis: A clinical practice guideline. BMJ 2018, 362, k3284. [Google Scholar] [CrossRef]
- Kim, J.; Yang, C.; Kim, E.J.; Jang, J.; Kim, S.J.; Kang, S.M.; Kim, M.G.; Jung, H.; Park, D.; Kim, C. Vimentin filaments regulate integrin-ligand interactions by binding to the cytoplasmic tail of integrin β3. J. Cell Sci. 2016, 129, 2030–2042. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Wang, S.; Yu, K.; Xu, Z.; Wu, X.; Symmes, D.; Mo, L.; Cheng, C.; Chen, R.; Zhang, J. Oral Treatment with the Vimentin-Targeting Compound ALD-R491 Mitigates Hyperinflammation, Multi-Organ Injury, and Mortality in CLP-Induced Septic Mice. Life 2025, 15, 1563. https://doi.org/10.3390/life15101563
Wu J, Wang S, Yu K, Xu Z, Wu X, Symmes D, Mo L, Cheng C, Chen R, Zhang J. Oral Treatment with the Vimentin-Targeting Compound ALD-R491 Mitigates Hyperinflammation, Multi-Organ Injury, and Mortality in CLP-Induced Septic Mice. Life. 2025; 15(10):1563. https://doi.org/10.3390/life15101563
Chicago/Turabian StyleWu, Jianping, Shuaishuai Wang, Kuai Yu, Zijing Xu, Xueting Wu, Deebie Symmes, Lian Mo, Chun Cheng, Ruihuan Chen, and Junfeng Zhang. 2025. "Oral Treatment with the Vimentin-Targeting Compound ALD-R491 Mitigates Hyperinflammation, Multi-Organ Injury, and Mortality in CLP-Induced Septic Mice" Life 15, no. 10: 1563. https://doi.org/10.3390/life15101563
APA StyleWu, J., Wang, S., Yu, K., Xu, Z., Wu, X., Symmes, D., Mo, L., Cheng, C., Chen, R., & Zhang, J. (2025). Oral Treatment with the Vimentin-Targeting Compound ALD-R491 Mitigates Hyperinflammation, Multi-Organ Injury, and Mortality in CLP-Induced Septic Mice. Life, 15(10), 1563. https://doi.org/10.3390/life15101563








