In Vitro Effects of PRP, Ozonized PRP, Hyaluronic Acid, Paracetamol, and Polyacrylamide on Equine Synovial Fluid-Derived Mesenchymal Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Examination and Selection of Horses
2.1.1. Experimental Animals
2.1.2. Clinical and Orthopedic Examination
2.2. Collection and Analysis of Synovial Fluid
2.2.1. Synovial Fluid Collection and Processing
2.2.2. Synovial Fluid Analysis
2.3. Mesenchymal Stem Cell Isolation and Morphological Evaluation
Flow Cytometry Immunophenotyping
2.4. Preparation of Platelet-Enriched Plasma and Ozonized PRP
2.5. Determination of the Effects of Regenerative Preparations on Stem Cell Cultures
2.5.1. Supplementation of Stem Cells with Regenerative Preparations
2.5.2. Determination of Stem Cells Viability
2.6. Statistical Assessment
3. Results
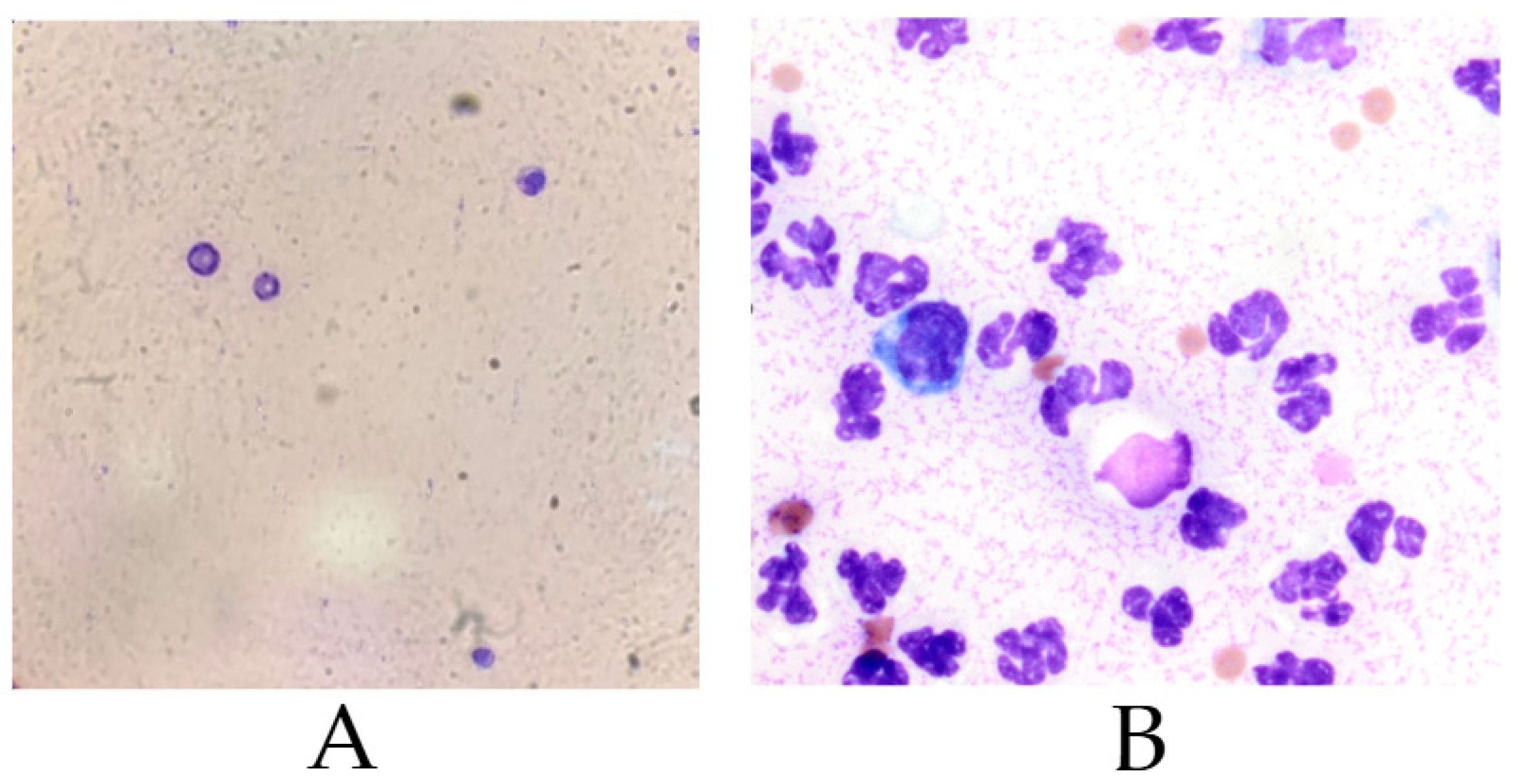
3.1. Cytological Analysis of Synovial Fluid
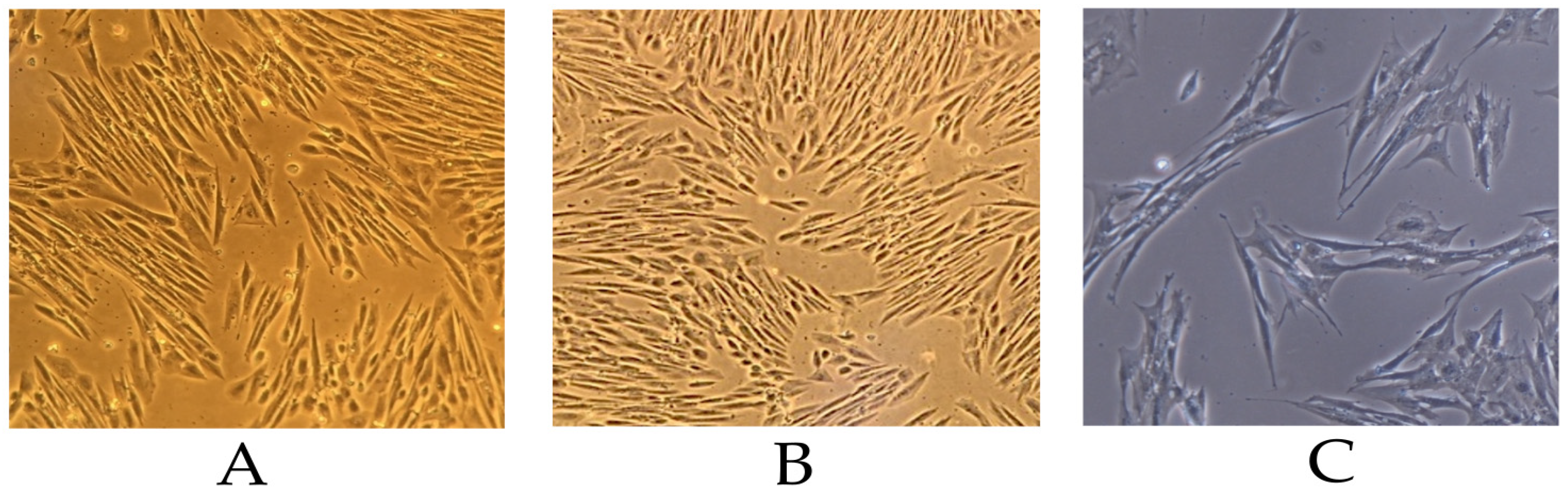
3.2. Morphology of Isolated Mesenchymal Stem Cells
Immunophenotypic Characterization
3.3. Effects of Regenerative Preparations on Stem Cell Cultures
3.3.1. Effect of PRP
3.3.2. Effect of Sodium Hyaluronate
3.3.3. Effect of Paracetamol
3.3.4. Effect of Polyacrylamide Gel
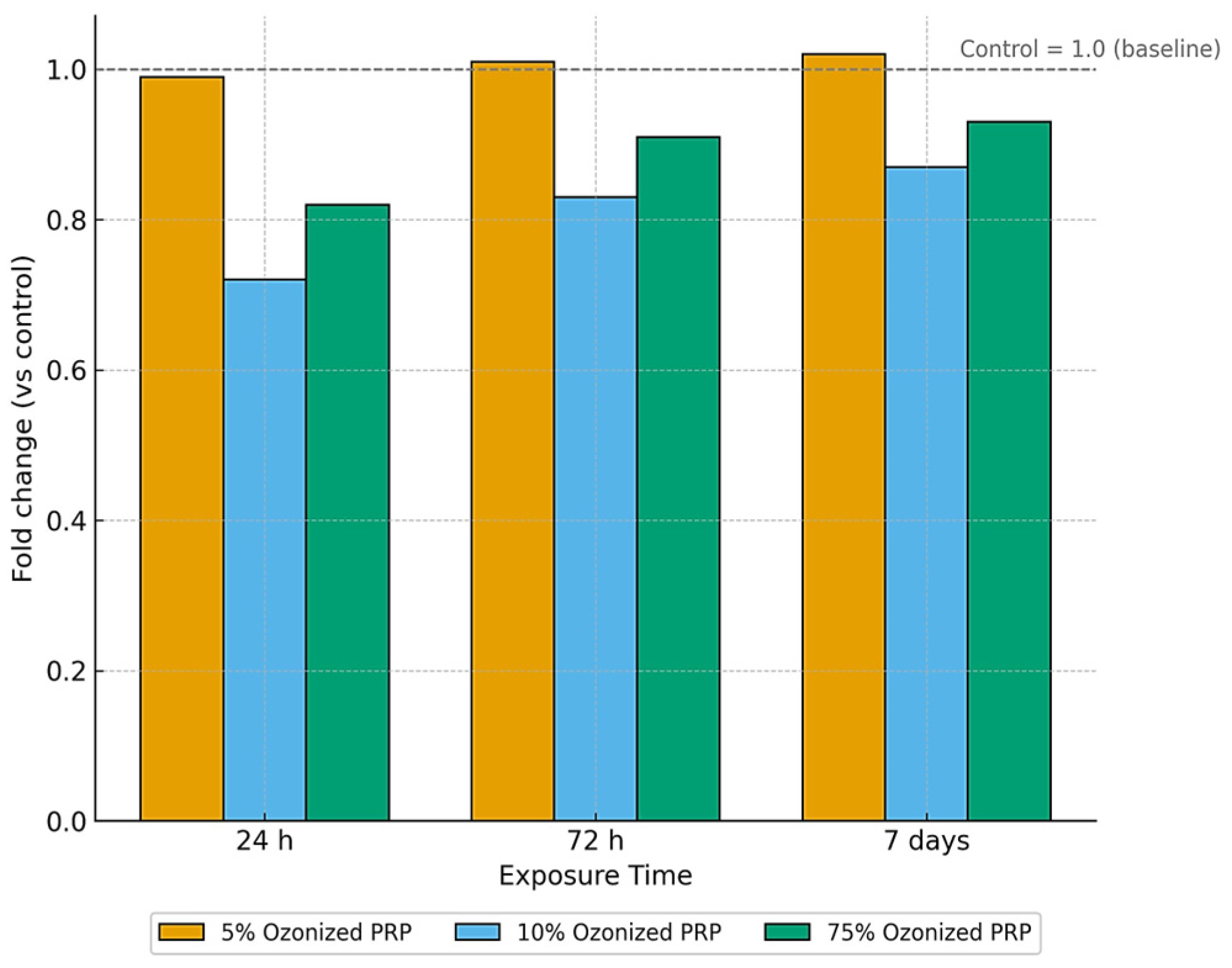
3.3.5. Effect of Ozonized PRP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MSC | Mesenchymal Stem Cells |
| PRP | Platelet-Rich Plasma |
| HA | Hyaluronic Acid |
| SVF | Stromal Vascular Fraction |
| ACS | Autologous Conditioned Serum |
| CPC | Chondroprogenitor Cells |
| ROS | Reactive Oxygen Species |
| RSD% | Relative Standard Deviation Percentage |
| ANOVA | Analysis of Variance |
References
- Ribitsch, I.; Oreff, G.L.; Jenner, F. Regenerative Medicine for Equine Musculoskeletal Diseases. Animals 2021, 11, 234. [Google Scholar] [CrossRef]
- Murray, R.C.; Walters, J.M.; Snart, H.; Dyson, S.J.; Parkin, T.D.H. Identification of Risk Factors for Lameness in Dressage Horses. Vet. J. 2010, 184, 27–36. [Google Scholar] [CrossRef]
- Guidoni, K.; Chiaradia, E.; Pepe, M.; Di Meo, A.; Tognoloni, A.; Seccaroni, M.; Beccati, F. The Combined Use of Triamcinolone and Platelet-Rich Plasma in Equine Metacarpophalangeal Joint Osteoarthritis Treatments: An In Vivo and In Vitro Study. Animals 2024, 14, 3645. [Google Scholar] [CrossRef]
- Garbin, L.C.; Olver, C.S. Platelet-Rich Products and Their Application to Osteoarthritis. J. Equine Vet. Sci. 2020, 86, 102820. [Google Scholar] [CrossRef] [PubMed]
- Sakata, R.; Reddi, A.H. Platelet-Rich Plasma Modulates Actions on Articular Cartilage Lubrication and Regeneration. Tissue Eng. Part B Rev. 2016, 22, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yuan, M.; Meng, H.Y.; Wang, A.Y.; Guo, Q.Y.; Wang, Y.; Peng, J. Basic Science and Clinical Application of Platelet-Rich Plasma for Cartilage Defects and Osteoarthritis: A Review. Osteoarthr. Cartil. 2013, 21, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Textor, J.A.; Willits, N.H.; Tablin, F. Synovial Fluid Growth Factor and Cytokine Concentrations after Intra-Articular Injection of a Platelet-Rich Product in Horses. Vet. J. 2013, 198, 217–223. [Google Scholar] [CrossRef]
- Camargo Garbin, L.; Lopez, C.; Carmona, J.U. A Critical Overview of the Use of Platelet-Rich Plasma in Equine Medicine Over the Last Decade. Front. Vet. Sci. 2021, 8, 641818. [Google Scholar] [CrossRef]
- Peng, C.; Yang, L.; Labens, R.; Gao, Y.; Zhu, Y.; Li, J. A Systematic Review and Meta-analysis of the Efficacy of Platelet-rich Plasma Products for Treatment of Equine Joint Disease. Equine Vet. J. 2024, 56, 858–869. [Google Scholar] [CrossRef]
- Mehrabani, D.; Seghatchian, J.; Acker, J.P. Platelet Rich Plasma in Treatment of Musculoskeletal Pathologies. Transfus. Apher. Sci. 2019, 58, 102675. [Google Scholar] [CrossRef]
- Fotouhi, A.; Maleki, A.; Dolati, S.; Aghebati-Maleki, A.; Aghebati-Maleki, L. Platelet Rich Plasma, Stromal Vascular Fraction and Autologous Conditioned Serum in Treatment of Knee Osteoarthritis. Biomed. Pharmacother. 2018, 104, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Muiños-López, E.; Delgado, D.; Sánchez, P.; Paiva, B.; Anitua, E.; Fiz, N.; Aizpurua, B.; Guadilla, J.; Padilla, S.; Granero-Moltó, F.; et al. Modulation of Synovial Fluid-Derived Mesenchymal Stem Cells by Intra-Articular and Intraosseous Platelet Rich Plasma Administration. Stem Cells Int. 2016, 2016, 1247950. [Google Scholar] [CrossRef]
- Colbath, A.C.; Frisbie, D.D.; Dow, S.W.; Kisiday, J.D.; McIlwraith, C.W.; Goodrich, L.R. Equine Models for the Investigation of Mesenchymal Stem Cell Therapies in Orthopaedic Disease. Oper. Tech. Sports Med. 2017, 25, 41–49. [Google Scholar] [CrossRef]
- Gugjoo, M.B.; Amarpal; Makhdoomi, D.M.; Sharma, G.T. Equine Mesenchymal Stem Cells: Properties, Sources, Characterization, and Potential Therapeutic Applications. J. Equine Vet. Sci. 2019, 72, 16–27. [Google Scholar] [CrossRef]
- Tang, H.-C.; Chen, W.-C.; Chiang, C.-W.; Chen, L.-Y.; Chang, Y.-C.; Chen, C.-H. Differentiation Effects of Platelet-Rich Plasma Concentrations on Synovial Fluid Mesenchymal Stem Cells from Pigs Cultivated in Alginate Complex Hydrogel. Int. J. Mol. Sci. 2015, 16, 18507–18521. [Google Scholar] [CrossRef]
- Tjandra, K.C.; Novriansyah, R.; Sudiasa, I.N.S.; Ar, A.; Rahmawati, N.A.D.; Dilogo, I.H. Modified Mesenchymal Stem Cell, Platelet-Rich Plasma, and Hyaluronic Acid Intervention in Early Stage Osteoarthritis: A Systematic Review, Meta-Analysis, and Meta-Regression of Arthroscopic-Guided Intra-Articular Approaches. PLoS ONE 2024, 19, e0295876. [Google Scholar] [CrossRef]
- Zayed, M.; Adair, S.; Ursini, T.; Schumacher, J.; Misk, N.; Dhar, M. Concepts and Challenges in the Use of Mesenchymal Stem Cells as a Treatment for Cartilage Damage in the Horse. Res. Vet. Sci. 2018, 118, 317–323. [Google Scholar] [CrossRef]
- Dernek, B.; Kesiktas, F.N. Efficacy of Combined Ozone and Platelet-Rich-Plasma Treatment versus Platelet-Rich-Plasma Treatment Alone in Early Stage Knee Osteoarthritis. J. Back Musculoskelet. Rehabil. 2019, 32, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Vendruscolo, C.D.P.; Moreira, J.J.; Seidel, S.R.T.;; Fülber, J.; Neuenschwander, H.M.; Bonagura, G.; Agreste, F.R.; Baccarin, R.Y.A. Effects of Medical Ozone upon Healthy Equine Joints: Clinical and Laboratorial Aspects. PLoS ONE 2018, 13, e0197736. [Google Scholar] [CrossRef]
- Sciorsci, R.L.; Lillo, E.; Occhiogrosso, L.; Rizzo, A. Ozone Therapy in Veterinary Medicine: A Review. Res. Vet. Sci. 2020, 130, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Carrade, D.D.; Owens, S.D.; Galuppo, L.D.; Vidal, M.A.; Ferraro, G.L.; Librach, F.; Buerchler, S.; Friedman, M.S.; Walker, N.J.; Borjesson, D.L. Clinicopathologic Findings Following Intra-Articular Injection of Autologous and Allogeneic Placentally Derived Equine Mesenchymal Stem Cells in Horses. Cytotherapy 2011, 13, 419–430. [Google Scholar] [CrossRef]
- Barrachina, L.; Romero, A.; Zaragoza, P.; Rodellar, C.; Vázquez, F.J. Practical Considerations for Clinical Use of Mesenchymal Stem Cells: From the Laboratory to the Horse. Vet. J. 2018, 238, 49–57. [Google Scholar] [CrossRef]
- Vinod, E.; Padmaja, K.; Ramasamy, B.; Sathishkumar, S. Systematic Review of Articular Cartilage Derived Chondroprogenitors for Cartilage Repair in Animal Models. J. Orthop. 2023, 35, 43–53. [Google Scholar] [CrossRef]
- Cassano, J.M.; Marycz, K.; Horna, M.; Nogues, M.P.; Morgan, J.M.; Herrmann, D.B.; Galuppo, L.D.; Vapniarsky, N. Evaluating the Safety of Intra-Articular Mitotherapy in the Equine Model: A Potential Novel Treatment for Osteoarthritis. J. Equine Vet. Sci. 2023, 120, 104164. [Google Scholar] [CrossRef]
- Pereira, M.F.; Ribeiro, G.; Gonzales, A.; Arantes, J.A.; Dória, R.G.S. Effects of Intra-Articular Administration of Hyaluronic Acid or Platelet-Rich Plasma as a Complementary Treatment to Arthroscopy in Horses with Osteochondritis Dissecans. Vet. Anim. Sci. 2024, 23, 100330. [Google Scholar] [CrossRef] [PubMed]
- Smit, Y.; Marais, H.J.; Thompson, P.N.; Mahne, A.T.; Goddard, A. Clinical Findings, Synovial Fluid Cytology and Growth Factor Concentrations after Intra-Articular Use of a Platelet-Rich Product in Horses with Osteoarthritis. J. S. Afr. Vet. Assoc. 2019, 90, e1–e9. [Google Scholar] [CrossRef]
- Conrado, F.O.; Beatty, S.S.K. Fluid Analysis in the Equine Patient. Vet. Clin. North Am. Equine Pract. 2021, 36, e1–e28. [Google Scholar] [CrossRef] [PubMed]
- Steel, C.M. Equine Synovial Fluid Analysis. Vet. Clin. North Am. Equine Pract. 2008, 24, 437–454. [Google Scholar] [CrossRef] [PubMed]
- Murata, D.; Miyakoshi, D.; Hatazoe, T.; Miura, N.; Tokunaga, S.; Fujiki, M.; Nakayama, K.; Misumi, K. Multipotency of Equine Mesenchymal Stem Cells Derived from Synovial Fluid. Vet. J. 2014, 202, 53–61. [Google Scholar] [CrossRef]
- Crecan, C.; Oprean, L.S.; Tripon, M.A.; Bodnariuc, A.; Luciana, R.; Pall, E.; Oros, D.C.; Morar, I. Equestrian Synovial Fluid Mesenchymal Stem Cells, a Potential Experimental Model for Osteoarticular Therapies. Farmacia 2019, 67, 458–466. [Google Scholar] [CrossRef]
- Segabinazzi, L.G.T.M.; Podico, G.; Rosser, M.F.; Nanjappa, S.G.; Alvarenga, M.A.; Canisso, I.F. Three Manual Noncommercial Methods to Prepare Equine Platelet-Rich Plasma. Animals 2021, 11, 1478. [Google Scholar] [CrossRef]
- Radtke, A.V.; Goodale, M.B.; Fortier, L.A. Platelet and Leukocyte Concentration in Equine Autologous Conditioned Plasma Are Inversely Distributed by Layer and Are Not Affected by Centrifugation Rate. Front. Vet. Sci. 2020, 7, 00173. [Google Scholar] [CrossRef] [PubMed]
- Ionita, J.-C.; Kissich, C.; Gottschalk, J.; Einspanier, A.; Köller, G.; Winter, K.; Brehm, W. Comparison of Cellular and Growth Factor Concentrations in Equine Autologous Conditioned Plasma® (ACP) and Manually Prepared Platelet Rich Plasma (MPRP). Pferdeheilkd. Equine Med. 2014, 30, 195–201. [Google Scholar] [CrossRef]
- Hauschild, G.; Geburek, F.; Gosheger, G.; Eveslage, M.; Serrano, D.; Streitbürger, A.; Johannlükens, S.; Menzel, D.; Mischke, R. Short Term Storage Stability at Room Temperature of Two Different Platelet-Rich Plasma Preparations from Equine Donors and Potential Impact on Growth Factor Concentrations. BMC Vet. Res. 2016, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhong, Y.; Wang, D.; Lu, Z. A Simple Colorimetric Method for Viable Bacteria Detection Based on Cell Counting Kit-8. Anal. Methods 2021, 13, 5211–5215. [Google Scholar] [CrossRef]
- Chanda, M.; Klinphayom, C.; Sungsuwan, T.; Senarat, W.; Thongkham, E.; Kamlangdee, A.; Senarat, N. Diagnostic Imaging Features, Cytological Examination, and Treatment of Lymphocytic Tenosynovitis of the Common Digital Extensor Tendon Sheath in an Eventing Horse. Vet. Anim. Sci. 2021, 14, 100209. [Google Scholar] [CrossRef]
- Lapjit, C.; Charoenchanikran, P.; Petchkaew, P.; Sukpipattanamongkol, S.; Yodsheewan, R.; Theerapan, W.; Chanda, M. Diagnostic Imaging and Cytological Analysis Aid the Clinical Investigation of Long Digital Extensor Tendon Subtendinous Bursitis in a Horse. J. Equine Vet. Sci. 2021, 101, 103449. [Google Scholar] [CrossRef]
- Sanchez-Teran, A.F.; Bracamonte, J.L.; Hendrick, S.; Riddell, L.; Musil, K.; Hoff, B.; Rubio-Martínez, L.M. Effect of Repeated Through-and-through Joint Lavage on Serum Amyloid A in Synovial Fluid from Healthy Horses. Vet. J. 2016, 210, 30–33. [Google Scholar] [CrossRef]
- Greve, L.; Pfau, T.; Dyson, S. Thoracolumbar Movement in Sound Horses Trotting in Straight Lines in Hand and on the Lunge and the Relationship with Hind Limb Symmetry or Asymmetry. Vet. J. 2017, 220, 95–104. [Google Scholar] [CrossRef]
- Beck, A.A.; Paz, L.B.; Frank, M.I.; Engelmann, A.M.; Krause, A.; Côrte, F.D.D.L. Safety and Synovial Inflammatory Response After Intra-Articular Injection of Botulinum Toxin Type A in Healthy Horses. J. Equine Vet. Sci. 2022, 110, 103865. [Google Scholar] [CrossRef]
- Rinnovati, R.; Bonelli, F.; Tognetti, R.; Gallo, C.; Bassini, R.F.; Marchetti, V.; Sgorbini, M. Effect of Repeated Arthrocentesis on Cytology of Synovial Fluid. J. Equine Vet. Sci. 2017, 57, 112–115. [Google Scholar] [CrossRef]
- Jiang, D.; Muschhammer, J.; Qi, Y.; Kügler, A.; de Vries, J.C.; Saffarzadeh, M.; Sindrilaru, A.; Vander Beken, S.; Wlaschek, M.; Kluth, M.A.; et al. Suppression of Neutrophil-Mediated Tissue Damage—A Novel Skill of Mesenchymal Stem Cells. Stem Cells 2016, 34, 2393–2406. [Google Scholar] [CrossRef]
- Fiala-Rechsteiner, S.M.; Amaral, M.G.; Cruz, L.A.; Rodrigues, R.F.; Pimentel, A.M.; Mattos, R.C. Inflammatory Lesions in the Oviducts and Its Relationship With Endometrial Inflammation and Ovarian Activity in Criollo Mares. J. Equine Vet. Sci. 2015, 35, 731–734. [Google Scholar] [CrossRef]
- Frisbie, D.D.; Al-Sobayil, F.; Billinghurst, R.C.; Kawcak, C.E.; McIlwraith, C.W. Changes in Synovial Fluid and Serum Biomarkers with Exercise and Early Osteoarthritis in Horses. Osteoarthr. Cartil. 2008, 16, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Walters, M.; Skovgaard, K.; Andersen, P.H.; Heegaard, P.M.H.; Jacobsen, S. Dynamics of Local Gene Regulations in Synovial Fluid Leukocytes from Horses with Lipopolysaccharide-Induced Arthritis. Vet. Immunol. Immunopathol. 2021, 241, 110325. [Google Scholar] [CrossRef]
- Dumoulin, M.; Martens, A.; Van den Abeele, A.-M.; Boyen, F.; Oosterlinck, M.; Wilderjans, H.; Gasthuys, F.; Haesebrouck, F.; Pille, F. Evaluation of Direct Etest for Antimicrobial Susceptibility Testing of Bacteria Isolated from Synovial Fluid of Horses Using Enrichment Bottles. Vet. J. 2017, 220, 55–62. [Google Scholar] [CrossRef]
- Soares, C.S.; Babo, P.S.; Reis, R.L.; Carvalho, P.P.; Gomes, M.E. Platelet-Derived Products in Veterinary Medicine: A New Trend or an Effective Therapy? Trends Biotechnol. 2021, 39, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, Y.; Fu, S.C.; Shen, J.; Zhang, H.; Jiang, C.; Shu-Hang Yung, P. Platelet-Rich Plasma Pretreatment Protects Anterior Cruciate Ligament Fibroblasts Correlated with PI3K-Akt-MTOR Pathway under Hypoxia Condition. J. Orthop. Transl. 2022, 34, 102–112. [Google Scholar] [CrossRef]
- Naor, D. Editorial: Interaction Between Hyaluronic Acid and Its Receptors (CD44, RHAMM) Regulates the Activity of Inflammation and Cancer. Front. Immunol. 2016, 7, 00039. [Google Scholar] [CrossRef] [PubMed]
- Mercer, M.A.; McKenzie, H.C.; Byron, C.R.; Pleasant, R.S.; Bogers, S.H.; Council-Troche, R.M.; Werre, S.R.; Burns, T.; Davis, J.L. Pharmacokinetics and Clinical Efficacy of Acetaminophen (Paracetamol) in Adult Horses with Mechanically Induced Lameness. Equine Vet. J. 2023, 55, 524–533. [Google Scholar] [CrossRef]
- Almaawi, A.; Wang, H.T.; Ciobanu, O.; Rowas, S.A.L.; Rampersad, S.; Antoniou, J.; Mwale, F. Effect of Acetaminophen and Nonsteroidal Anti-Inflammatory Drugs on Gene Expression of Mesenchymal Stem Cells. Tissue Eng. Part A 2013, 19, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, Q.; Liu, A.; Anadón, A.; Rodríguez, J.-L.; Martínez-Larrañaga, M.-R.; Yuan, Z.; Martínez, M.-A. Paracetamol: Overdose-Induced Oxidative Stress Toxicity, Metabolism, and Protective Effects of Various Compounds in Vivo and in Vitro. Drug Metab. Rev. 2017, 49, 395–437. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Cui, Y.; Wang, J.; Liu, D.; Tian, Y.; Liu, K.; Wang, X.; Liu, L.; He, Y.; Pei, Y.; et al. Mesenchymal Stem Cells Protect against Acetaminophen Hepatotoxicity by Secreting Regenerative Cytokine Hepatocyte Growth Factor. Stem Cell Res. Ther. 2022, 13, 94. [Google Scholar] [CrossRef]
- Yiang, G.; Yu, Y.; Lin, K.; Chen, J.; Chang, W.; Wei, C. Acetaminophen Induces JNK/P38 Signaling and Activates the Caspase-9-3-Dependent Cell Death Pathway in Human Mesenchymal Stem Cells. Int. J. Mol. Med. 2015, 36, 485–492. [Google Scholar] [CrossRef] [PubMed]
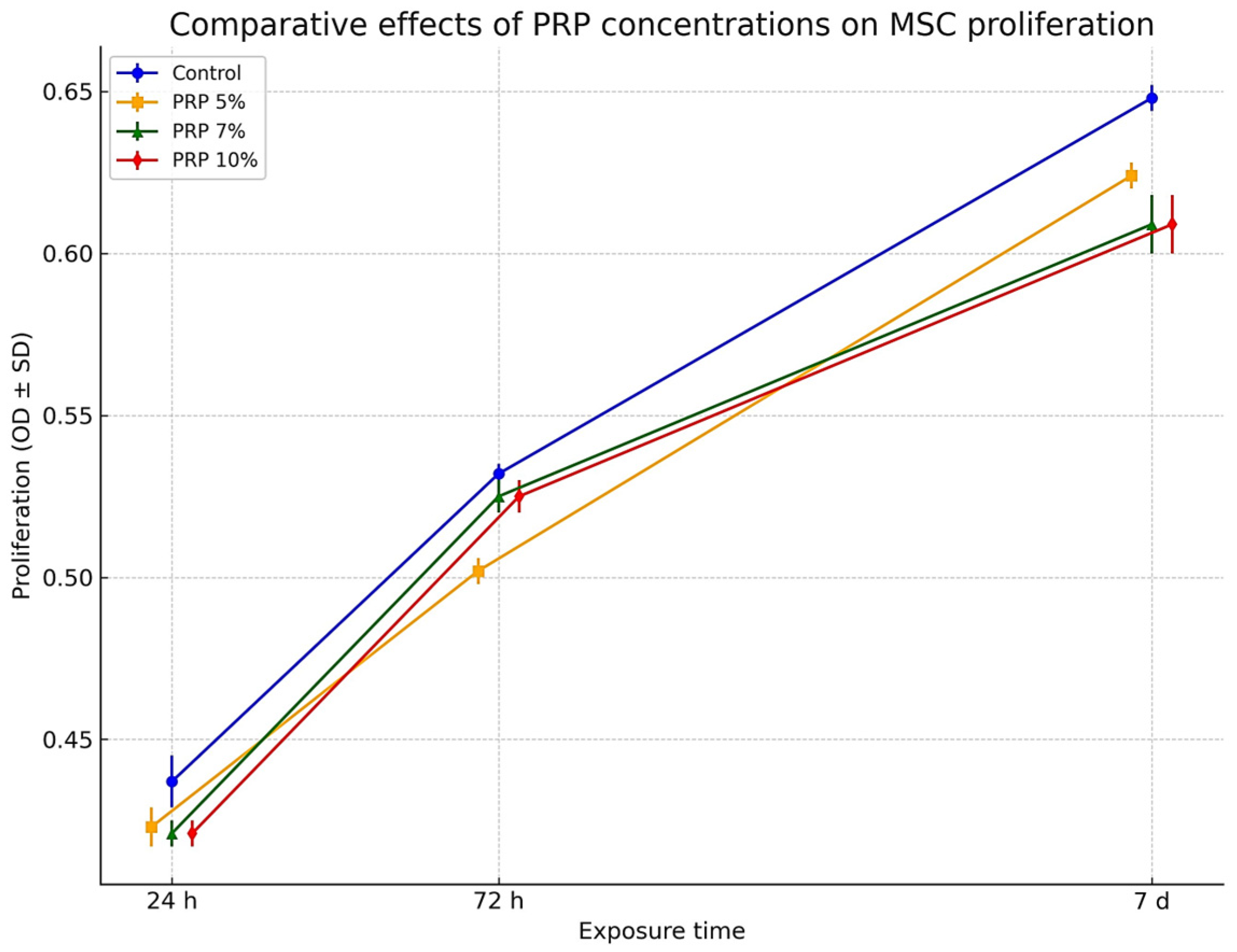
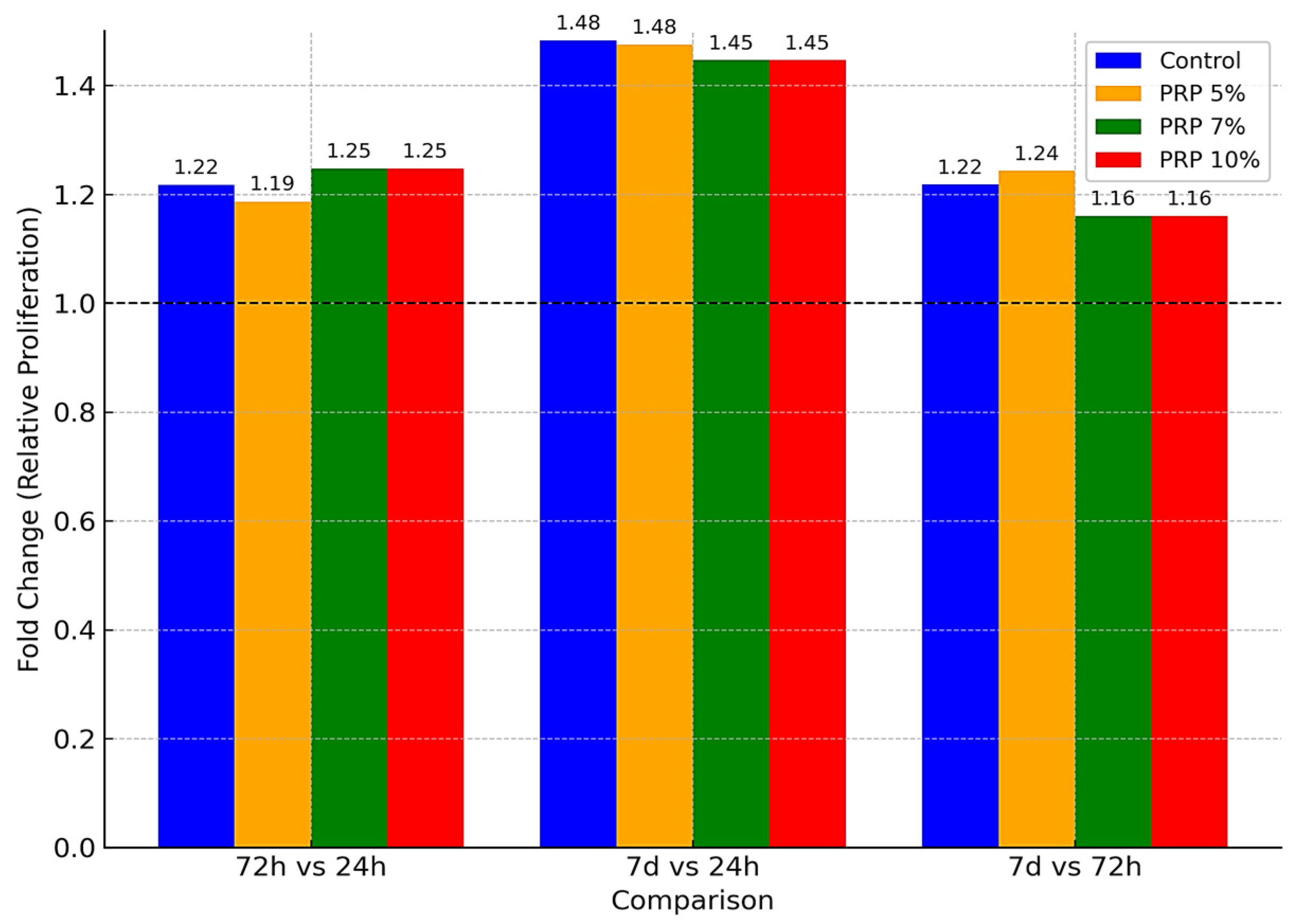


| Concentration | Exposure Time | Mean ± SD (Replicate 1) | RSD% | Mean ± SD (Replicate 2) | RSD% | Mean ± SD (Replicate 3) | RSD% | F-Value | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Control | 24 h | 0.430 ± 0.008 i | 1.873 | 0.435 ± 0.008 i | 1.873 | 0.446 ± 0.008 i | 1.873 | 5.54 | 0.043 |
| Control | 72 h | 0.530 ± 0.003 e | 0.497 | 0.531 ± 0.003 e | 0.497 | 0.535 ± 0.003 e | 0.497 | 0.43 | 0.668 |
| Control | 7 d | 0.645 ± 0.004 a | 0.556 | 0.647 ± 0.004 a | 0.556 | 0.652 ± 0.004 a | 0.556 | 3.67 | 0.091 |
| PRP 5% | 24 h | 0.418 ± 0.006 j | 1.316 | 0.422 ± 0.006 j | 1.316 | 0.429 ± 0.006 j | 1.316 | 5.05 | 0.052 |
| PRP 5% | 72 h | 0.499 ± 0.004 h | 0.718 | 0.501 ± 0.004 h | 0.718 | 0.506 ± 0.004 h | 0.718 | 2.21 | 0.191 |
| PRP 5% | 7 d | 0.620 ± 0.004 b | 0.578 | 0.625 ± 0.004 b | 0.578 | 0.627 ± 0.004 b | 0.578 | 6.13 | 0.036 |
| PRP 7% | 24 h | 0.418 ± 0.004 k | 0.856 | 0.420 ± 0.004 k | 0.856 | 0.425 ± 0.004 k | 0.856 | 11.42 | 0.009 |
| PRP 7% | 72 h | 0.520 ± 0.005 f | 0.959 | 0.530 ± 0.005 f | 0.959 | 0.524 ± 0.005 f | 0.959 | 2.96 | 0.127 |
| PRP 7% | 7 d | 0.600 ± 0.009 c | 1.403 | 0.610 ± 0.009 c | 1.403 | 0.617 ± 0.009 c | 1.403 | 7.84 | 0.021 |
| PRP 10% | 24 h | 0.418 ± 0.004 l | 0.856 | 0.420 ± 0.004 l | 0.856 | 0.425 ± 0.004 l | 0.856 | 3.39 | 0.103 |
| PRP 10% | 72 h | 0.520 ± 0.005 g | 0.959 | 0.530 ± 0.005 g | 0.959 | 0.524 ± 0.005 g | 0.959 | 4.84 | 0.056 |
| PRP 10% | 7 d | 0.600 ± 0.009 d | 1.403 | 0.610 ± 0.009 d | 1.403 | 0.617 ± 0.009 d | 1.403 | 9.76 | 0.013 |
| Concentration (mg) | Exposure Time | Mean ± SD (Replicate 1) | RSD% | Mean ± SD (Replicate 2) | RSD% | Mean ± SD (Replicate 3) | RSD% | F-Value | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Control | 24 h | 0.412 ± 0.006 a | 1.3 | 0.412 ± 0.006 a | 1.3 | 0.412 ± 0.006 a | 1.3 | F = 9.58 | p = 0.002 |
| 72 h | 0.624 ± 0.004 b | 0.6 | 0.624 ± 0.004 b | 0.6 | 0.624 ± 0.004 b | 0.6 | F = 11.50 | p = 0.001 | |
| 7 days | 0.750 ± 0.007 c | 0.9 | 0.750 ± 0.007 c | 0.9 | 0.750 ± 0.007 c | 0.9 | F = 23.43 | p = 0.001 | |
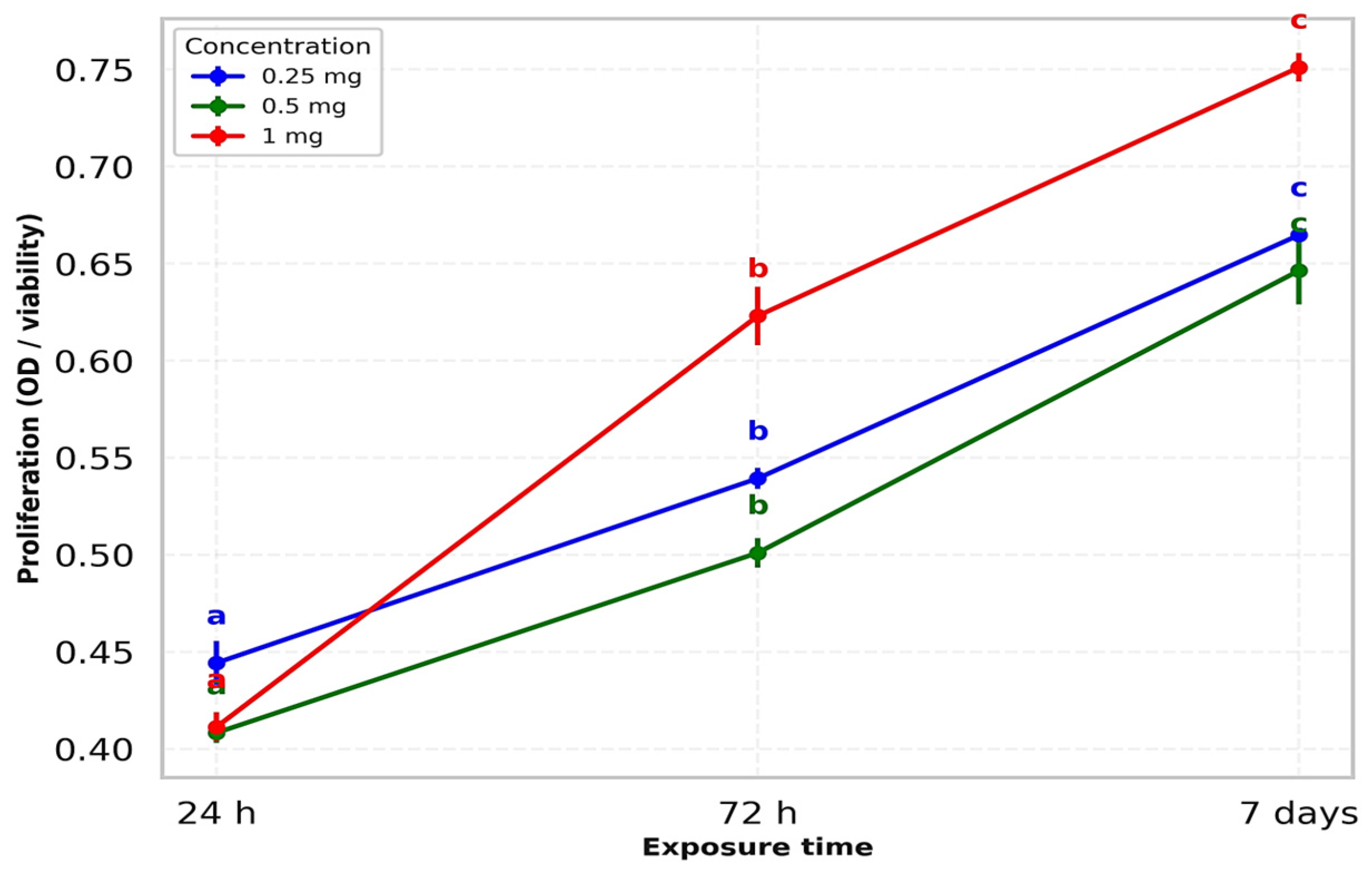
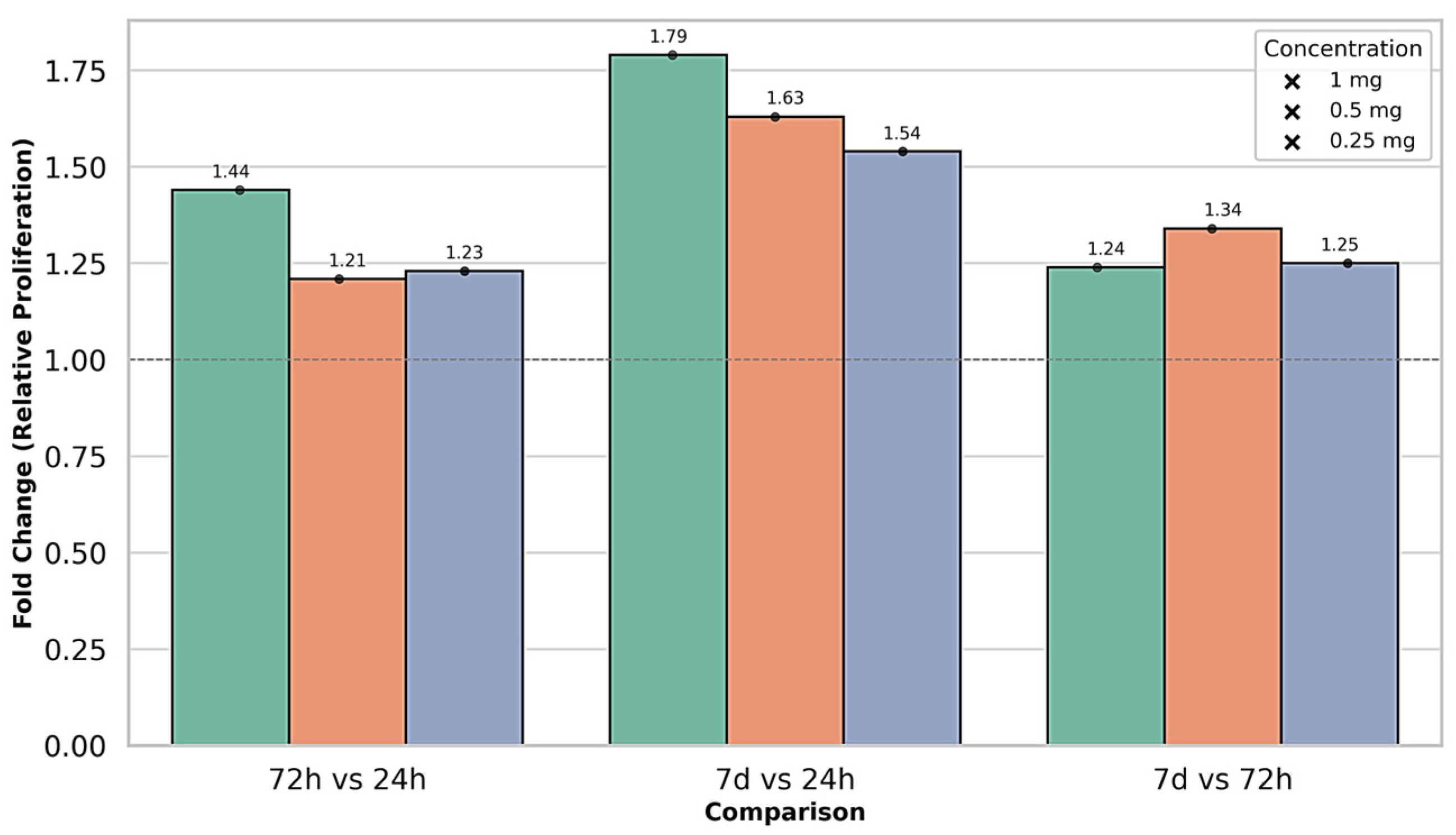
| 1 mg | 24 h | 0.421 ± 0.003 a | 0.713 | 0.407 ± 0.002 a | 0.491 | 0.406 ± 0.003 a | 0.739 | F = 18.95 | p = 0.002 |
| 72 h | 0.607 ± 0.002 b | 0.329 | 0.621 ± 0.003 b | 0.483 | 0.641 ± 0.003 b | 0.468 | F = 22.54 | p = 0.001 | |
| 7 days | 0.754 ± 0.004 c | 0.478 | 0.743 ± 0.004 c | 0.485 | 0.756 ± 0.007 c | 0.867 | F = 19.73 | p = 0.002 | |
| 0.5 mg | 24 h | 0.411 ± 0.003 a | 0.730 | 0.407 ± 0.007 a | 1.611 | 0.407 ± 0.007 a | 1.611 | F = 7.21 | p = 0.025 |
| 72 h | 0.497 ± 0.002 b | 0.402 | 0.497 ± 0.002 b | 0.402 | 0.509 ± 0.009 b | 1.679 | F = 6.58 | p = 0.030 | |
| 7 days | 0.668 ± 0.007 c | 1.080 | 0.637 ± 0.008 c | 1.285 | 0.634 ± 0.005 c | 0.723 | F = 12.97 | p = 0.007 | |
| 0.25 mg | 24 h | 0.432 ± 0.003 a | 0.694 | 0.445 ± 0.006 a | 1.251 | 0.456 ± 0.007 a | 1.438 | F = 18.36 | p = 0.002 |
| 72 h | 0.533 ± 0.004 b | 0.676 | 0.542 ± 0.003 b | 0.488 | 0.543 ± 0.003 b | 0.552 | F = 21.65 | p = 0.001 | |
| 7 days | 0.664 ± 0.002 c | 0.301 | 0.665 ± 0.005 c | 0.752 | 0.665 ± 0.005 c | 0.752 | F = 16.74 | p = 0.003 |
| Concentration (μg/mL) | Exposure Time | Mean ± SD (Replicate 1) | RSD% | Mean ± SD (Replicate 2) | RSD% | Mean ± SD (Replicate 3) | RSD% | F-Value | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Control | 24 h | 0.398 ± 0.004 a | 1.0 | 0.401 ± 0.005 a | 1.2 | 0.405 ± 0.003 a | 0.8 | F = 10.12 | p = 0.012 |
| 72 h | 0.512 ± 0.006 b | 1.1 | 0.516 ± 0.004 b | 0.9 | 0.518 ± 0.005 b | 1.0 | F = 12.45 | p = 0.009 | |
| 7 days | 0.603 ± 0.004 c | 0.7 | 0.609 ± 0.006 c | 1.0 | 0.611 ± 0.005 c | 0.8 | F = 15.78 | p = 0.006 | |
| 50 | 24 h | 0.403 ± 0.002 a | 0.517 | 0.402 ± 0.003 a | 0.658 | 0.407 ± 0.002 a | 0.491 | F = 9.84 | p = 0.013 |
| 50 | 72 h | 0.517 ± 0.006 b | 1.177 | 0.516 ± 0.002 b | 0.388 | 0.514 ± 0.004 b | 0.701 | F = 8.92 | p = 0.017 |
| 50 | 7 days | 0.612 ± 0.003 c | 0.432 | 0.609 ± 0.005 c | 0.752 | 0.601 ± 0.007 c | 1.091 | F = 11.26 | p = 0.010 |
| 100 | 24 h | 0.463 ± 0.002 a | 0.432 | 0.453 ± 0.002 a | 0.442 | 0.445 ± 0.005 a | 1.124 | F = 15.47 | p = 0.004 |
| 100 | 72 h | 0.541 ± 0.007 b | 1.212 | 0.554 ± 0.004 b | 0.651 | 0.521 ± 0.003 b | 0.576 | F = 18.63 | p = 0.002 |
| 100 | 7 days | 0.651 ± 0.001 c | 0.154 | 0.654 ± 0.004 c | 0.551 | 0.657 ± 0.002 c | 0.304 | F = 20.15 | p = 0.001 |
| Concentration | Exposure Time | Mean ± SD (Replicate 1) | RSD% | Mean ± SD (Replicate 2) | RSD% | Mean ± SD (Replicate 3) | RSD% | F-Value | p-Value |
|---|---|---|---|---|---|---|---|---|---|
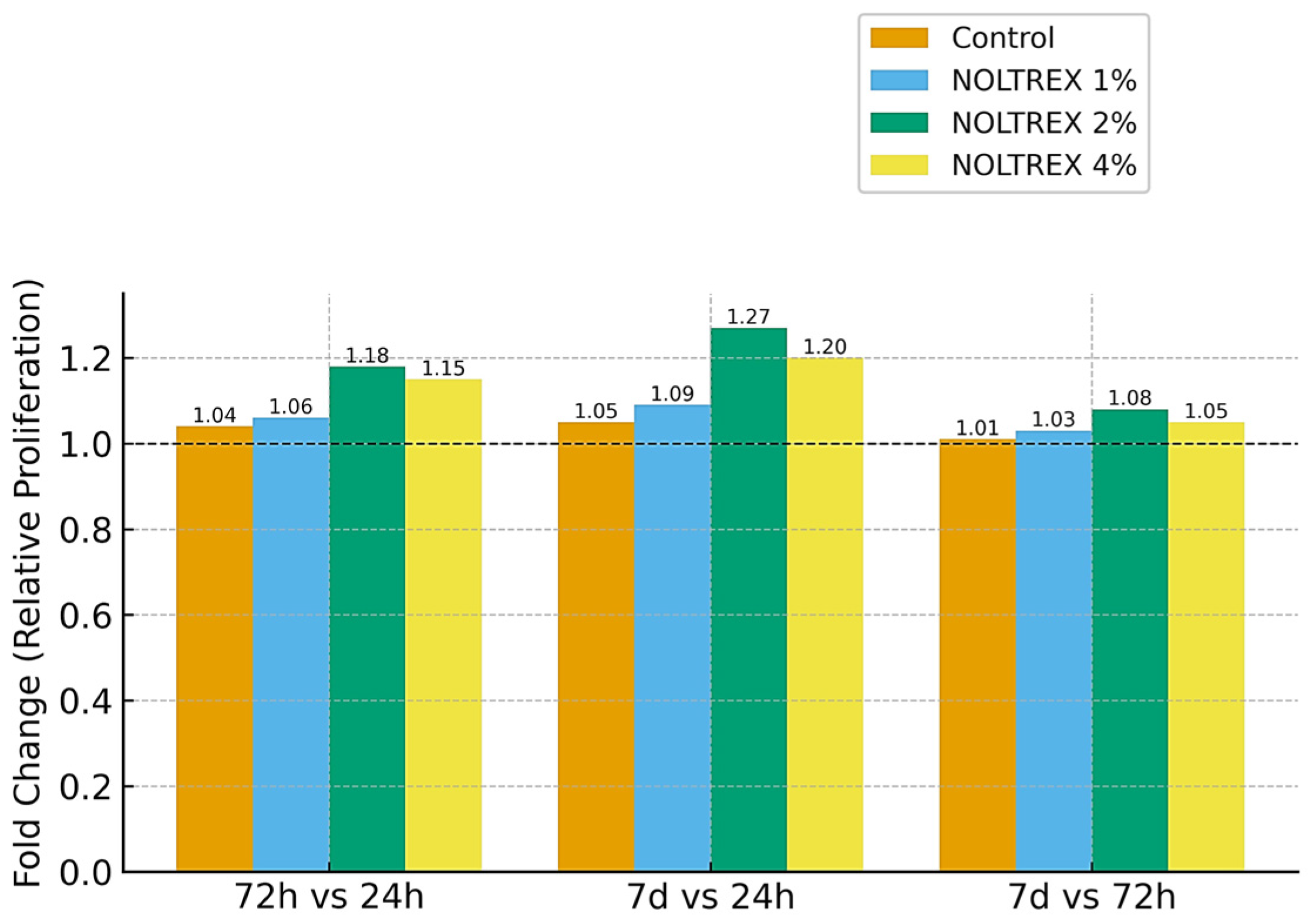
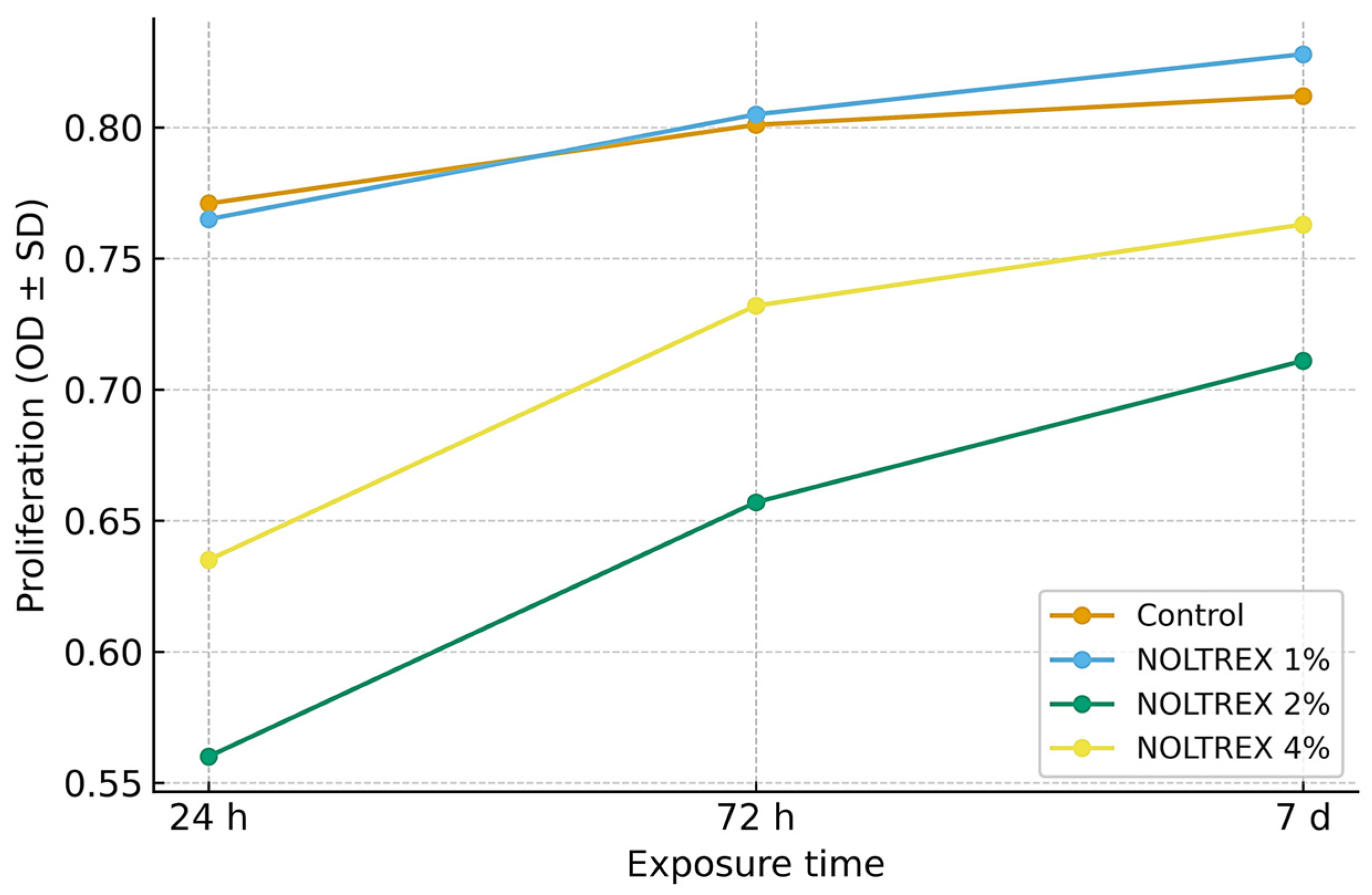
| Control | 24 h | 0.770 ± 0.005 g | 0.649 | 0.772 ± 0.003 e | 0.389 | 0.769 ± 0.003 f | 0.390 | 8.94 | p = 0.017 |
| Control | 72 h | 0.801 ± 0.003 c | 0.375 | 0.797 ± 0.003 d | 0.376 | 0.795 ± 0.003 d | 0.377 | 9.76 | p = 0.014 |
| Control | 7 d | 0.811 ± 0.003 b | 0.370 | 0.821 ± 0.003 b | 0.365 | 0.816 ± 0.003 b | 0.368 | 10.53 | p = 0.011 |
| 1% | 24 h | 0.791 ± 0.003 e | 0.379 | 0.771 ± 0.003 f | 0.389 | 0.719 ± 0.004 h | 0.556 | 11.87 | p = 0.008 |
| 1% | 72 h | 0.800 ± 0.005 d | 0.625 | 0.811 ± 0.003 c | 0.370 | 0.799 ± 0.003 c | 0.375 | 12.59 | p = 0.007 |
| 1% | 7 d | 0.821 ± 0.003 a | 0.365 | 0.841 ± 0.003 a | 0.357 | 0.821 ± 0.003 a | 0.365 | 13.45 | p = 0.006 |
| 2% | 24 h | 0.595 ± 0.003 l | 0.504 | 0.525 ± 0.005 l | 0.952 | 0.554 ± 0.003 l | 0.542 | 16.74 | p = 0.003 |
| 2% | 72 h | 0.661 ± 0.003 j | 0.454 | 0.643 ± 0.003 j | 0.467 | 0.671 ± 0.003 j | 0.447 | 17.94 | p = 0.002 |
| 2% | 7 d | 0.708 ± 0.003 i | 0.424 | 0.709 ± 0.003 i | 0.423 | 0.713 ± 0.003 i | 0.421 | 19.74 | p = 0.001 |
| 4% | 24 h | 0.607 ± 0.003 k | 0.494 | 0.632 ± 0.003 k | 0.475 | 0.662 ± 0.003 k | 0.453 | 20.79 | p = 0.001 |
| 4% | 72 h | 0.712 ± 0.002 h | 0.281 | 0.721 ± 0.003 h | 0.416 | 0.751 ± 0.003 g | 0.399 | 22.15 | p = 0.001 |
| 4% | 7 d | 0.776 ± 0.003 f | 0.387 | 0.729 ± 0.003 g | 0.412 | 0.782 ± 0.003 e | 0.384 | 23.54 | p = 0.001 |
| Concentration | Exposure Time | Mean ± SD (Replicate 1) | RSD% | Mean ± SD (Replicate 2) | RSD% | Mean ± SD (Replicate 3) | RSD% | F-Value | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Control | 24 h | 0.770 ± 0.005 c | 0.20 | 0.772 ± 0.003 a | 0.389 | 0.769 ± 0.003 a | 0.390 | F = 8.94 | p = 0.017 |
| Control | 72 h | 0.798 ± 0.003 b | 0.38 | 0.797 ± 0.003 b | 0.376 | 0.795 ± 0.003 b | 0.377 | F = 9.76 | p = 0.014 |
| Control | 7 days | 0.816 ± 0.005 a | 0.61 | 0.821 ± 0.003 c | 0.365 | 0.816 ± 0.003 c | 0.368 | F = 10.53 | p = 0.011 |
| 5% | 24 h | 0.760 ± 0.037 c | 4.89 | 0.771 ± 0.003 a | 0.389 | 0.719 ± 0.004 a | 0.556 | F = 11.87 | p = 0.008 |
| 5% | 72 h | 0.803 ± 0.007 b | 0.83 | 0.811 ± 0.003 b | 0.370 | 0.799 ± 0.003 b | 0.375 | F = 12.59 | p = 0.007 |
| 5% | 7 days | 0.828 ± 0.012 a | 1.40 | 0.841 ± 0.003 c | 0.357 | 0.821 ± 0.003 c | 0.365 | F = 13.43 | p = 0.006 |
| 10% | 24 h | 0.558 ± 0.035 c | 6.30 | 0.525 ± 0.005 a | 0.952 | 0.554 ± 0.003 a | 0.542 | F = 16.70 | p = 0.003 |
| 10% | 72 h | 0.658 ± 0.014 b | 2.16 | 0.643 ± 0.003 b | 0.467 | 0.671 ± 0.003 b | 0.447 | F = 17.96 | p = 0.002 |
| 10% | 7 days | 0.710 ± 0.003 a | 0.37 | 0.709 ± 0.003 c | 0.423 | 0.713 ± 0.003 c | 0.421 | F = 19.21 | p = 0.001 |
| 75% | 24 h | 0.634 ± 0.028 c | 4.35 | 0.632 ± 0.003 a | 0.475 | 0.662 ± 0.003 a | 0.453 | F = 20.73 | p = 0.001 |
| 75% | 72 h | 0.728 ± 0.020 b | 2.81 | 0.721 ± 0.003 b | 0.416 | 0.751 ± 0.003 b | 0.399 | F = 22.18 | p = 0.001 |
| 75% | 7 days | 0.762 ± 0.029 a | 3.81 | 0.729 ± 0.003 c | 0.412 | 0.782 ± 0.003 c | 0.384 | F = 23.56 | p = 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bungărdean, D.; Pall, E.; Daradics, Z.; Popescu, M.; Tripon, M.A.; Lupșan, A.F.; Crecan, C.M.; Morar, I.A.; Nicolescu, A.; Bora, F.D.; et al. In Vitro Effects of PRP, Ozonized PRP, Hyaluronic Acid, Paracetamol, and Polyacrylamide on Equine Synovial Fluid-Derived Mesenchymal Stem Cells. Life 2025, 15, 1558. https://doi.org/10.3390/life15101558
Bungărdean D, Pall E, Daradics Z, Popescu M, Tripon MA, Lupșan AF, Crecan CM, Morar IA, Nicolescu A, Bora FD, et al. In Vitro Effects of PRP, Ozonized PRP, Hyaluronic Acid, Paracetamol, and Polyacrylamide on Equine Synovial Fluid-Derived Mesenchymal Stem Cells. Life. 2025; 15(10):1558. https://doi.org/10.3390/life15101558
Chicago/Turabian StyleBungărdean, Denisa, Emoke Pall, Zsofia Daradics, Maria Popescu, Mirela Alexandra Tripon, Alexandru Florin Lupșan, Cristian Mihăiță Crecan, Ianu Adrian Morar, Alexandru Nicolescu, Florin Dumitru Bora, and et al. 2025. "In Vitro Effects of PRP, Ozonized PRP, Hyaluronic Acid, Paracetamol, and Polyacrylamide on Equine Synovial Fluid-Derived Mesenchymal Stem Cells" Life 15, no. 10: 1558. https://doi.org/10.3390/life15101558
APA StyleBungărdean, D., Pall, E., Daradics, Z., Popescu, M., Tripon, M. A., Lupșan, A. F., Crecan, C. M., Morar, I. A., Nicolescu, A., Bora, F. D., & Marcus, I. (2025). In Vitro Effects of PRP, Ozonized PRP, Hyaluronic Acid, Paracetamol, and Polyacrylamide on Equine Synovial Fluid-Derived Mesenchymal Stem Cells. Life, 15(10), 1558. https://doi.org/10.3390/life15101558







