Chlamydia Pneumoniae Pericarditis Complicated with Guillain–Barré Syndrome
Abstract
1. Introduction
2. Case Report
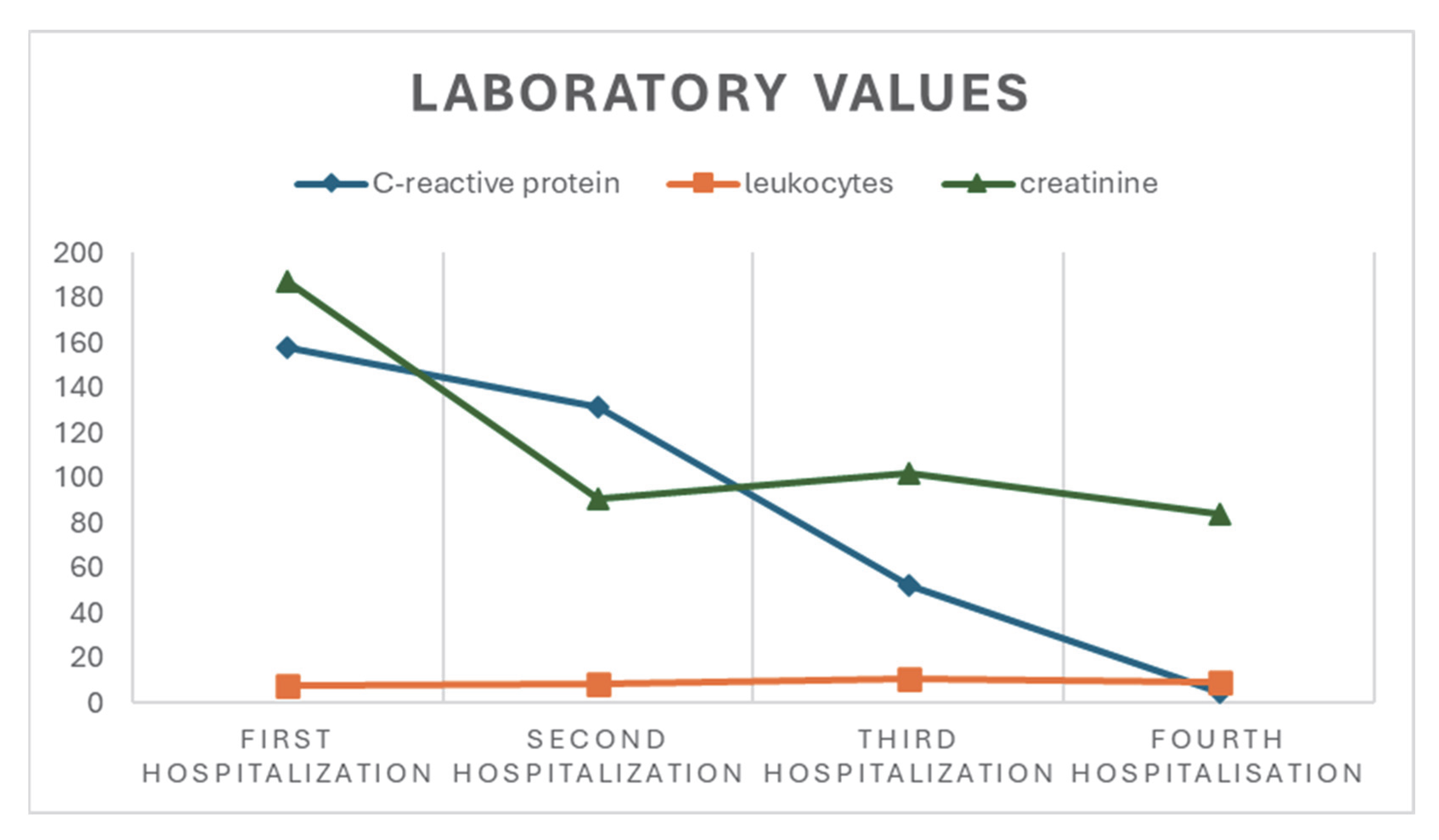
2.1. First Hospitalization
2.2. Second Hospitalization
2.3. Third Hospitalization
2.4. Fourth Hospitalization, Neurology Department
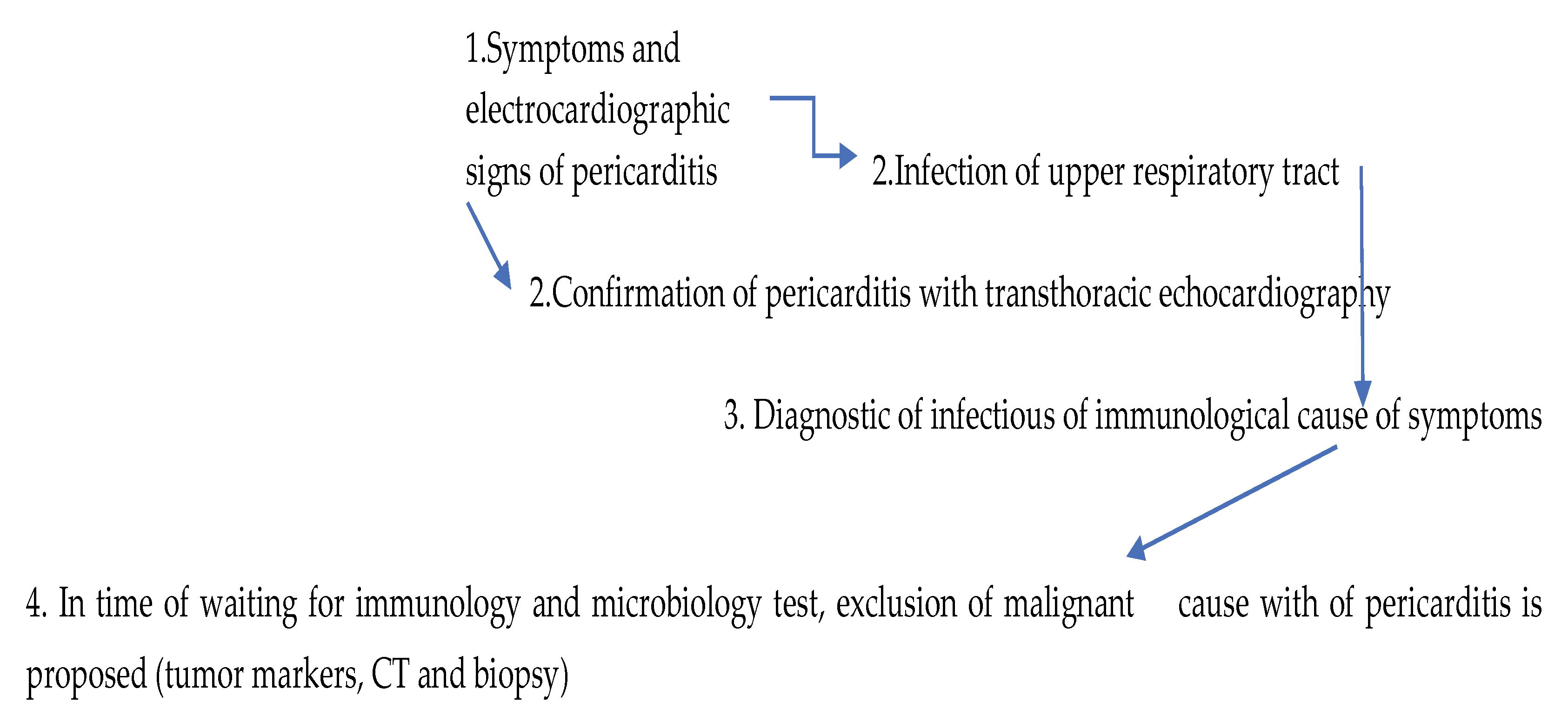
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CT | Computed tomography |
| CSF | Cerebrospinal fluid |
| DOAJ | Directory of open-access journals |
| ENMG | Electroneuromyography |
| GBS | Guillain–Barré syndrome |
| IVIg | Intravenous immunoglobulin |
| MRC | Medical Research Council |
| MRI | Magnetic resonance imaging |
| NSAID | Non-steroidal anti-inflammatory drugs |
| PET-CT | Positron emission tomography |
| PTSD | Post-traumatic stress disorder |
| PCR | Polymerase chain reaction |
| TLA | Three-letter acronym |
References
- Adler, Y.; Charron, P.; Imazio, M.; Badano, L.; Barón-Esquivias, G.; Bogaert, J.; Brucato, A.; Gueret, P.; Klingel, K.; Lionis, C.; et al. 2015 ESC Guidelines for the Diagnosis and Management of Pericardial Diseases. Eur. Heart J. 2015, 36, 2921–2964. [Google Scholar] [CrossRef]
- Chen, W.-H.; Guo, Y.-S.; Zhang, D.-H.; Zhang, H.-J. Long-Term Prognosis of Suspected Myocarditis and Cardiomyopathy Associated with Viral Infection of the Myocardial Tissue: A Meta-Analysis of Cohort Studies. Cardiovasc. Ther. 2019, 2019, 9342792. [Google Scholar] [CrossRef]
- Imazio, M.; Gaita, F.; LeWinter, M. Evaluation and Treatment of Pericarditis: A Systematic Review. JAMA J. Am. Med. Assoc. 2015, 314, 1498–1506. [Google Scholar] [CrossRef]
- Burillo, A.; Bouza, E. Chlamydophila Pneumoniae. Infect. Dis. Clin. N. Am. 2010, 24, 61–71. [Google Scholar] [CrossRef]
- Grayston, J.T. Background and Current Knowledge of Chlamydia Pneumoniae and Atherosclerosis. J. Infect. Dis. 2000, 3, 402–410. [Google Scholar] [CrossRef]
- Assar, O.; Nejatizadeh, A.; Dehghan, F.; Kargar, M.; Zolghadri, N. Association of Chlamydia Pneumoniae Infection with Atherosclerotic Plaque Formation. Glob. J. Health Sci. 2015, 8, 260–267. [Google Scholar] [CrossRef]
- Player, M.S.; Mainous, A.G.; Everett, C.J.; Diaz, V.A.; Knoll, M.E.; Wright, R.U. Chlamydia Pneumoniae and Progression of Subclinical Atherosclerosis. Eur. J. Prev. Cardiol. 2014, 21, 559–565. [Google Scholar] [CrossRef]
- Saikku, P. Chlamydia Pneumoniae and Cardiovascular Diseases. Clin. Microbiol. Infect. 1996, 1 (Suppl. 1), S19–S22. [Google Scholar] [CrossRef] [PubMed]
- Oztek Celebi, F.Z.; Fettah, A.; Yesil, S.; Yoldas, T.; Tanir, G.; Akcaboy, M.; Senel, S. Acute Haemorrhagic Pericarditis: An Unusual Presentation of Chlamydophila Pneumoniae Pneumonia Infection. Paediatr. Int. Child. Health 2020, 40, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Biberić, M.; Zrna, S.; Juranić, J.; Tokmadžić, V.S.; Kurtović, B.; Župan, Ž. Acute Fulminant Chlamydia Pneumoniae Myocarditis Treated with Mechanical Circulatory Support in a Female Adult: A Case Report. Anesth. Pain. Res. 2018, 2, 1–5. [Google Scholar] [CrossRef]
- De Meyst, A.; Alexiou, Z.; Lernout, T.; Morré, S.A.; Vanrompay, D. Challenges in Chlamydial Serology: Insights from a Belgian and a Dutch Population Cohort. Microorganisms 2024, 12, 658. [Google Scholar] [CrossRef]
- Gnarpe, H.G.; Gnarpe, J. Chlamydia Pneumoniae and Myocarditis. In Infectious Agents and Pathogenesis; Springer: Berlin/Heidelberg, Germany, 2005; pp. 187–197. [Google Scholar]
- Ruts, L.; Drenthen, J.; Jacobs, B.C.; Van Doorn, P.A. Distinguishing Acute-Onset CIDP from Fluctuating Guillain-Barré Syndrome. Neurology 2010, 74, 1680–1686. [Google Scholar] [CrossRef]
- Ropper, A.H. Further Regional Variants of Acute Immune Polyneuropathy. Arch. Neurol. 1994, 51, 671–675. [Google Scholar] [CrossRef]
- Ropper, A.H. The Guillain-Barré Syndrome. N. Engl. J. Med. 1992, 326, 1130–1136. [Google Scholar]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the Management of Infective Endocarditis. Eur Heart J 2015, 36, 3075–3123. [Google Scholar] [CrossRef]
- Cunha, B.A. The Atypical Pneumonias: Clinical Diagnosis and Importance. Clin. Microbiol. Infect. 2006, 12, 12–24. [Google Scholar] [CrossRef]
- Haider, M.; Rizvi, M.; Malik, A.; Azam, M.; Rabbani, M.U. Acute and Chronic Chlamydia Pneumoniae Infection and Inflammatory Markers in Coronary Artery Disease Patients. J. Infect. Dev. Ctries. 2011, 5, 580–586. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hoefer, D.; Poelzl, G.; Kilo, J.; Hoermann, C.; Mueller, L.; Laufer, G.; Antretter, H. Early Detection and Successful Therapy of Fulminant Chlamydia Pneumoniae Myocarditis. ASAIO J. 2005, 51, 480–481. [Google Scholar] [CrossRef] [PubMed]
- Bachmaier, K.; Neu, N.; de la Maza, L.M.; Pal, S.; Hessel, A.; Penninger, J.M. Chlamydia Infections and Heart Disease Linked through Antigenic Mimicry. Science 1999, 283, 1335–1339. [Google Scholar] [CrossRef]
- Freund, O.; Eviatar, T.; Bornstein, G. Concurrent Myopathy and Inflammatory Cardiac Disease in COVID-19 Patients: A Case Series and Literature Review. Rheumatol. Int. 2022, 42, 905–912. [Google Scholar] [CrossRef] [PubMed]
- St Louis, E.K.; Jacobson, M.D. Unilateral Third Nerve Palsy Caused by Guillain-Barré Syndrome. Neurocrit Care 2004, 1, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.B.; Chaudhry, R.; Tabassum, I.; Hussain Ahmed, N.; Kumar Sahu, J.; Dhawan, B.; Kalra, V. The Presence of Mycoplasma Pneumoniae Infection and GM1 Ganglioside Antibodies in Guillain-Barré Syndrome. J. Infect. Dev. Ctries. 2011, 5, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Rac, H.; Av, S.; Doorn, V. Intravenous Immunoglobulin for Guillain-Barré SyndromE. Cochrane Libr. 2014, 2014, CD002063. [Google Scholar]
| Laboratory, Microbiological, and Serological Tests | Results |
|---|---|
| Sputum for Mycobacterium tuberc. and QuantiFeron test | negative |
| Urine and blood cultures | sterile |
| Nasal swab for adenovirus antigen | negative |
| Throat swab (microbiology) | normal mucosal flora |
| Hepatitis B serology | anti-HBs positive; anti-HBc, HBaAg neg. |
| Hepatitis C serology | anti-HCV neg. |
| HIV serology | negative |
| Borrelia burgdorferi serology | IgG pos.; IgM negative |
| Mycoplasma pneumoniae serology | IgG pos.; IgM negative |
| Parvovirus B19 serology | IgG pos.; IgM negative |
| Cytomegalovirus serology | IgG pos.; IgM negative |
| Epstein–Barr virus serology | negative |
| Chlamydia pneumoniae serology | IgA (128) and IgG positive (>512) |
| ANA, anti-dsDNA, ENA screening, anti-SS-A-/Ro, Anti-SS-B_7La, Anti-SM, Anti-SM/RNP, Anti-JO, Anti-Scl-70, Anti ribosomal antibodies, Anticentromere antibodies, Anti-U1-RNP, Anti-PM-Scl, ANTI-PCNA, Anti-histone, Anti-MPO (Panca), Anti-PR-3 (Canca), Anti-CCP antibodies | negative |
| Type of Laboratory Test | Measured Value and Meaning |
|---|---|
| Total CSF protein | 1.57 g/L (above reference values) |
| CSF albumin | 883 mg/L (above reference values) |
| Serum albumin | 34.3 g/L (within reference values) |
| Q (CSF/serum) | 25.8 (above reference values) |
| CSF IgG | 302 mg/L (above reference values) |
| HSV serology, serum | IgM negative, IgG positive |
| HSV serology, CSF | IgG negative |
| Borrelia burgdorferi serology, CSF | IgM and IgG neg. |
| Anti-myelin-associated glycoprotein (anti-MAG) | negative |
| Anti-ganglioside antibodies (GM1, GT1a, GD1a, GD1b, GQ1b) | negative |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ćosić, V.; Pojatić, Đ.; Bakalar, I.; Moser, N.; Miškić, K.; Miškić, B. Chlamydia Pneumoniae Pericarditis Complicated with Guillain–Barré Syndrome. Life 2025, 15, 1532. https://doi.org/10.3390/life15101532
Ćosić V, Pojatić Đ, Bakalar I, Moser N, Miškić K, Miškić B. Chlamydia Pneumoniae Pericarditis Complicated with Guillain–Barré Syndrome. Life. 2025; 15(10):1532. https://doi.org/10.3390/life15101532
Chicago/Turabian StyleĆosić, Vesna, Đorđe Pojatić, Iva Bakalar, Nataša Moser, Karla Miškić, and Blaženka Miškić. 2025. "Chlamydia Pneumoniae Pericarditis Complicated with Guillain–Barré Syndrome" Life 15, no. 10: 1532. https://doi.org/10.3390/life15101532
APA StyleĆosić, V., Pojatić, Đ., Bakalar, I., Moser, N., Miškić, K., & Miškić, B. (2025). Chlamydia Pneumoniae Pericarditis Complicated with Guillain–Barré Syndrome. Life, 15(10), 1532. https://doi.org/10.3390/life15101532






