Increased Susceptibility to Salmonella Infection in Systemic Lupus Erythematosus Compared with Other Systemic Autoimmune Diseases: Insights from a Retrospective Cohort Study from the Largest Health Care System in Taiwan
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Ethical Statement
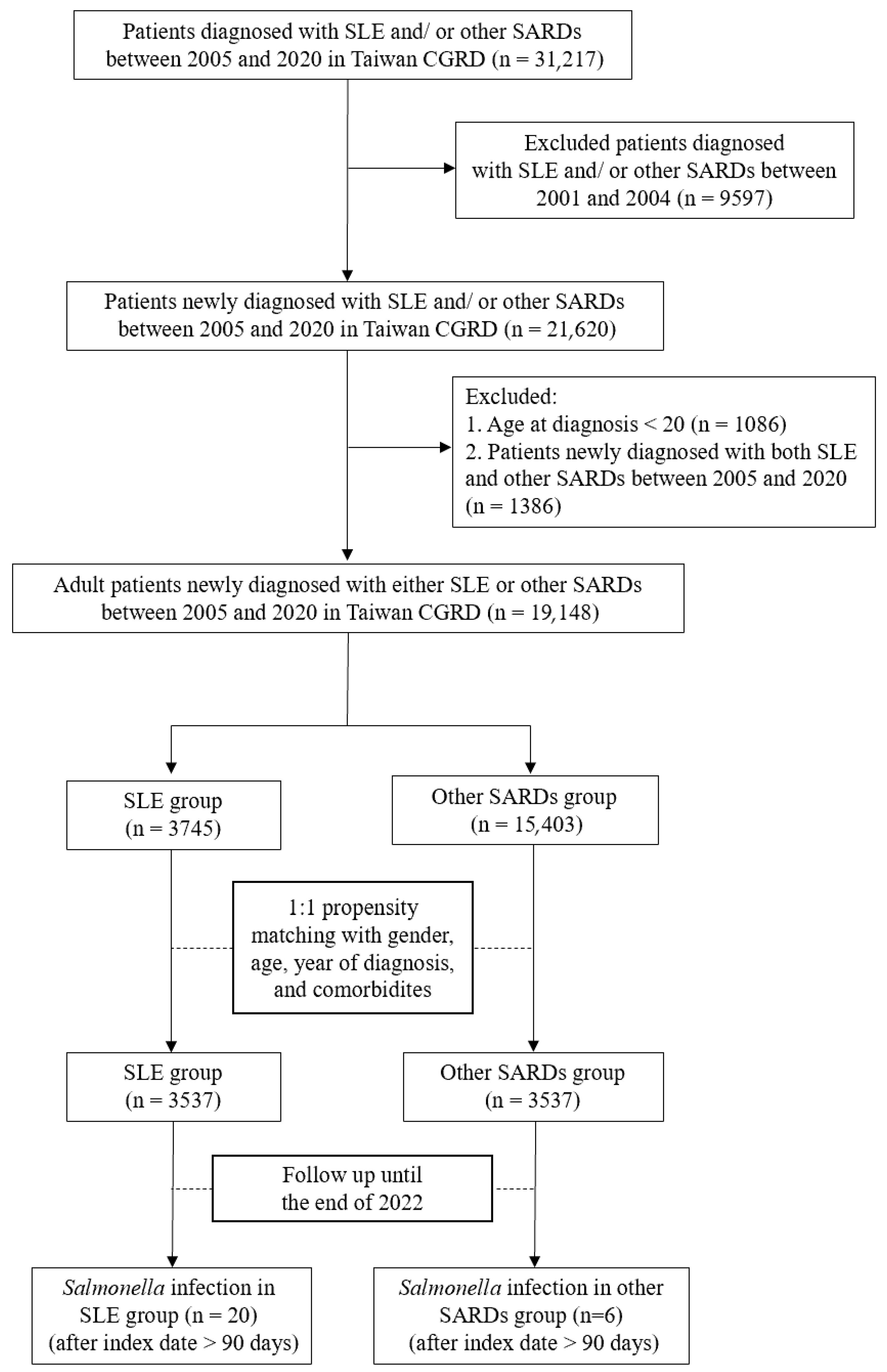
2.3. Study Design
2.4. Study Variables
2.5. Statistical Analysis
3. Results
3.1. Baseline Demographic Characteristics
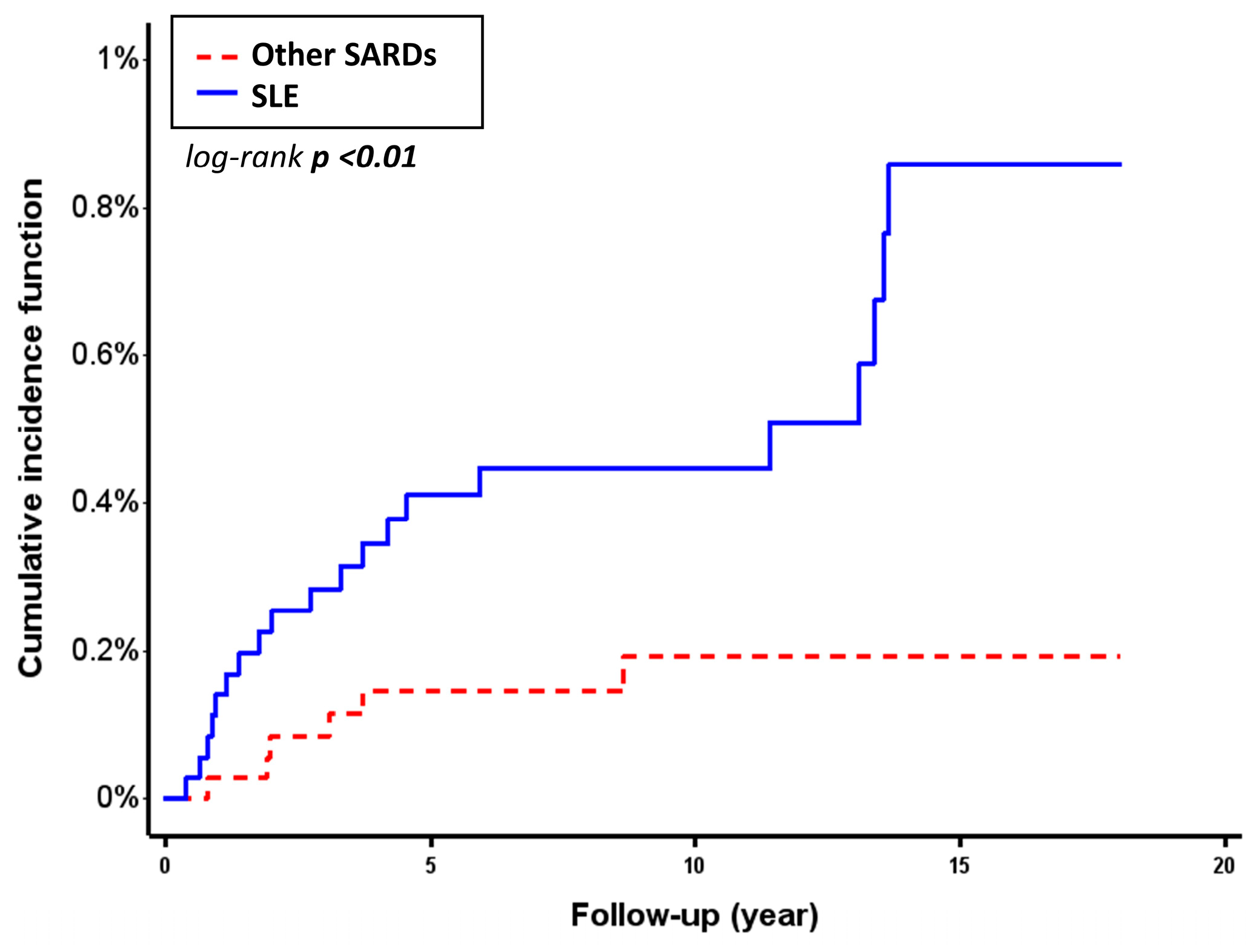
3.2. Incidence and Risk Comparison of Salmonella Infection in SLE and Other SARDs
3.3. Risk Factors for Salmonella Infection in the Patients with SLE
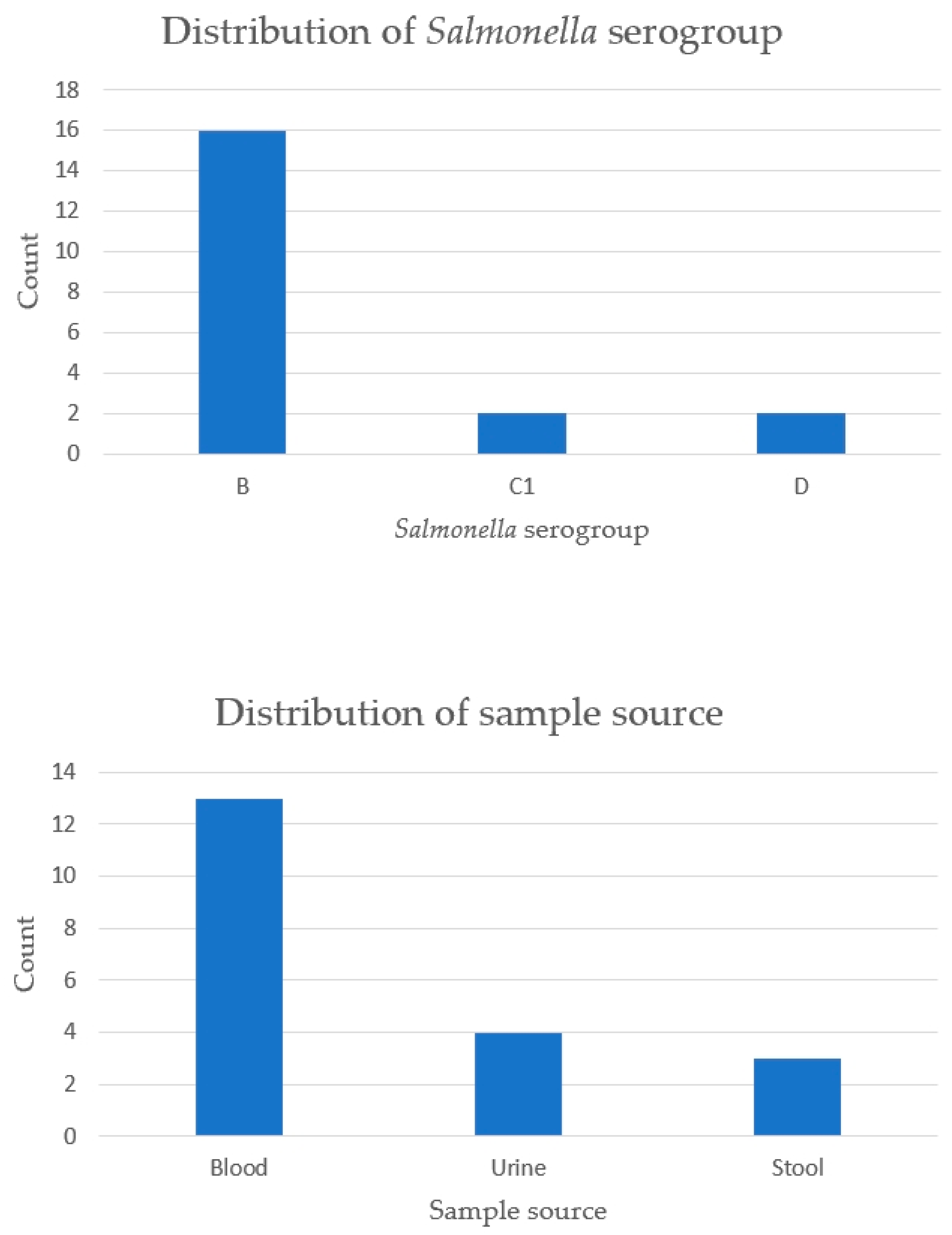
3.4. Serogroup and Sample Source of Salmonella in SLE
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Anti-dsDNA | Antibody to double-stranded deoxyribonucleic acid |
| C3 | Complement 3 |
| C4 | Complement 4 |
| CI | Confidence interval |
| CGRD | Chang Gung Research Database |
| DMARDs | Disease-modifying antirheumatic drugs |
| ESR | Erythrocyte sedimentation rate |
| HR | Hazard ratio |
| ICD | International Classification of Diseases |
| IgM | Immunoglobulin M |
| SARDs | Systemic autoimmune rheumatic diseases |
| SLE | Systemic lupus erythematosus |
References
- Burmester, G.R.; Bijlsma, J.W.J.; Cutolo, M.; McInnes, I.B. Managing rheumatic and musculoskeletal diseases—Past, present and future. Nat. Rev. Rheumatol. 2017, 13, 443–448. [Google Scholar] [CrossRef]
- Fanouriakis, A.; Tziolos, N.; Bertsias, G.; Boumpas, D.T. Update οn the diagnosis and management of systemic lupus erythematosus. Ann. Rheum. Dis. 2021, 80, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Tektonidou, M.G.; Wang, Z.; Dasgupta, A.; Ward, M.M. Burden of Serious Infections in Adults With Systemic Lupus Erythematosus: A National Population-Based Study, 1996–2011. Arthritis Care Res. 2015, 67, 1078–1085. [Google Scholar] [CrossRef]
- Consani Fernández, S.A.; Díaz Cuña, C.L.; Fernández Rey, L.; Rostán Sellanes, S.; Maciel Oleggini, G.; Facal Castro, J.A. Infections in systemic autoimmune diseases. Reumatol. Clínica (Engl. Ed.) 2021, 17, 582–587. [Google Scholar] [CrossRef]
- Crum, N.F.; Lederman, E.R.; Wallace, M.R. Infections associated with tumor necrosis factor-alpha antagonists. Medicine 2005, 84, 291–302. [Google Scholar] [CrossRef]
- Gordon, M.A. Salmonella infections in immunocompromised adults. J. Infect. 2008, 56, 413–422. [Google Scholar] [CrossRef]
- Tu, T.Y.; Yeh, C.Y.; Hung, Y.M.; Chang, R.; Chen, H.H.; Wei, J.C. Association Between a History of Nontyphoidal Salmonella and the Risk of Systemic Lupus Erythematosus: A Population-Based, Case-Control Study. Front. Immunol. 2021, 12, 725996. [Google Scholar] [CrossRef]
- Chen, J.Y.; Luo, S.F.; Wu, Y.J.; Wang, C.M.; Ho, H.H. Salmonella septic arthritis in systemic lupus erythematosus and other systemic diseases. Clin. Rheumatol. 1998, 17, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Pablos, J.L.; Aragon, A.; Gomez-Reino, J.J. Salmonellosis and systemic lupus erythematosus. Report of ten cases. Br. J. Rheumatol. 1994, 33, 129–132. [Google Scholar] [CrossRef]
- Shahram, F.; Akbarian, M.; Davatchi, F. Salmonella infection in systemic lupus erythematosus. Lupus 1993, 2, 55–59. [Google Scholar] [CrossRef]
- Abramson, S.; Kramer, S.B.; Radin, A.; Holzman, R. Salmonella bacteremia in systemic lupus erythematosus. Eight-year experience at a municipal hospital. Arthritis Rheum. 1985, 28, 75–79. [Google Scholar] [CrossRef]
- Li, M.; Wu, C.; Yin, P.; Qian, J.; Zhao, J.; Wang, Q.; Xu, D.; Su, J.; Leng, X.; Zheng, W.; et al. Mortality-related health metrics in systemic autoimmune diseases: An epidemiological analysis of a nationwide register-based cohort. Sci. Bull. 2025, 70, 492–495. [Google Scholar] [CrossRef] [PubMed]
- Yeh, K.W.; Yu, C.H.; Chan, P.C.; Horng, J.T.; Huang, J.L. Burden of systemic lupus erythematosus in Taiwan: A population-based survey. Rheumatol. Int. 2013, 33, 1805–1811. [Google Scholar] [CrossRef] [PubMed]
- Elsisi, G.H.; Hsieh, S.-C.; Chen, D.-Y. The economic burden of systemic lupus erythematosus in Taiwan. J. Med. Econ. 2024, 27, 56–66. [Google Scholar] [CrossRef]
- Cummings, P. Arguments for and against standardized mean differences (effect sizes). Arch. Pediatr. Adolesc. Med. 2011, 165, 592–596. [Google Scholar] [CrossRef]
- Wu, M.Y.; Huang, M.C.; Liao, H.H.; Chiang, J.H.; Lee, Y.C.; Hsu, C.Y.; Sun, M.F.; Yen, H.R. Acupuncture decreased the risk of coronary heart disease in patients with rheumatoid arthritis in Taiwan: A Nationwide propensity score-matched study. BMC Complement. Altern. Med. 2018, 18, 341. [Google Scholar] [CrossRef] [PubMed]
- Zandman-Goddard, G.; Shoenfeld, Y. Infections and SLE. Autoimmunity 2005, 38, 473–485. [Google Scholar] [CrossRef]
- Alarcón, G.S. Infections in systemic connective tissue diseases: Systemic lupus erythematosus, scleroderma, and polymyositis/dermatomyositis. Infect. Dis. Clin. North. Am. 2006, 20, 849–875. [Google Scholar] [CrossRef]
- Gallo, P.M.; Rapsinski, G.J.; Wilson, R.P.; Oppong, G.O.; Sriram, U.; Goulian, M.; Buttaro, B.; Caricchio, R.; Gallucci, S.; Tükel, Ç. Amyloid-DNA Composites of Bacterial Biofilms Stimulate Autoimmunity. Immunity 2015, 42, 1171–1184. [Google Scholar] [CrossRef]
- Qiu, C.C.; Caricchio, R.; Gallucci, S. Triggers of Autoimmunity: The Role of Bacterial Infections in the Extracellular Exposure of Lupus Nuclear Autoantigens. Front. Immunol. 2019, 10, 2608. [Google Scholar] [CrossRef]
- Ktsoyan, Z.; Budaghyan, L.; Agababova, M.; Mnatsakanyan, A.; Arakelova, K.; Gevorgyan, Z.; Sedrakyan, A.; Hovhannisyan, A.; Mkrtchyan, M.; Zakharyan, M.; et al. Potential Involvement of Salmonella Infection in Autoimmunity. Pathogens 2019, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.; Koh, W.H.; Loh, S.F.; Lam, M.S.; Howe, H.S. Non-thyphoidal salmonellosis in patients with systemic lupus erythematosus. A study of fifty patients and a review of the literature. Lupus 2001, 10, 87–92. [Google Scholar] [CrossRef]
- Albrecht, K.; Troll, W.; Callhoff, J.; Strangfeld, A.; Ohrndorf, S.; Mucke, J. Sex- and gender-related differences in systemic lupus erythematosus: A scoping review. Rheumatol. Int. 2025, 45, 160. [Google Scholar] [CrossRef]
- Gui, Y.; Bai, W.; Xu, J.; Duan, X.; Zhan, F.; Zhao, C.; Jiang, Z.; Li, Z.; Wu, L.; Liu, S.; et al. Sex differences in systemic lupus erythematosus (SLE): An inception cohort of the Chinese SLE Treatment and Research Group (CSTAR) registry XVII. Chin. Med. J. 2022, 135, 2191–2199. [Google Scholar] [CrossRef]
- Jung, J.Y.; Yoon, D.; Choi, Y.; Kim, H.A.; Suh, C.H. Associated clinical factors for serious infections in patients with systemic lupus erythematosus. Sci. Rep. 2019, 9, 9704. [Google Scholar] [CrossRef] [PubMed]
- Pimentel-Quiroz, V.R.; Ugarte-Gil, M.F.; Harvey, G.B.; Wojdyla, D.; Pons-Estel, G.J.; Quintana, R.; Esposto, A.; García, M.A.; Catoggio, L.J.; Cardiel, M.H.; et al. Factors predictive of serious infections over time in systemic lupus erythematosus patients: Data from a multi-ethnic, multi-national, Latin American lupus cohort. Lupus 2019, 28, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.H.; Chen, C.Y.; Ou, L.S.; Huang, J.L. Risk factors of mortality for Salmonella infection in systemic lupus erythematosus. J. Rheumatol. 2002, 29, 1214–1218. [Google Scholar]
- Merayo-Chalico, J.; Gómez-Martín, D.; Piñeirúa-Menéndez, A.; Santana-De Anda, K.; Alcocer-Varela, J. Lymphopenia as risk factor for development of severe infections in patients with systemic lupus erythematosus: A case-control study. Qjm 2013, 106, 451–457. [Google Scholar] [CrossRef]
- Martin, M.; Guffroy, A.; Argemi, X.; Martin, T. Systemic lupus erythematosus and lymphopenia: Clinical and pathophysiological features. Rev. Med. Interne 2017, 38, 603–613. [Google Scholar] [CrossRef]
- Sobhy, N.; Niazy, M.H.; Kamal, A. Lymphopenia in systemic lupus erythematosus patients: Is it more than a laboratory finding? Egypt. Rheumatol. 2020, 42, 23–26. [Google Scholar] [CrossRef]
- Yu, S.-F.; Cheng, T.-T.; Chen, Y.-C.; Wu, C.-H.; Chiu, C.-K.; Lai, H.-M.; Lee, C.-H. Salmonella infection in systemic lupus erythematosus: Seventeen-year experience at a medical center in southern Taiwan. Formos. J. Rheumatol. 2004, 18, 35–46. (In Chinese) [Google Scholar] [CrossRef]
| SLE (n = 3537) | Other SARDs (n = 3537) | p-Value a | Standardized Difference b | |
|---|---|---|---|---|
| Age | 42.93 ± 15.02 | 44.00 ± 14.31 | <0.01 | 0.07 |
| Male | 445 (12.58) | 445 (12.58) | 1 | 0 |
| Comorbidity | ||||
| Diabetes mellitus | 166 (4.69) | 216 (6.11) | 0.01 | 0.06 |
| Chronic kidney disease | 522 (14.76) | 444 (12.55) | 0.01 | 0.06 |
| Cirrhosis | 64 (1.81) | 63 (1.78) | 0.93 | <0.01 |
| Lymphoproliferative disorder/cancer | 187 (5.29) | 219 (6.19) | 0.10 | 0.04 |
| Drug | ||||
| Steroids | 1263 (35.71) | 1047 (29.60) | <0.001 | 0.13 |
| DMARDS | ||||
| Hydroxychloroquine | 1040 (29.40) | 1041 (29.43) | 0.98 | <0.001 |
| Azathioprine | 238 (6.73) | 78 (2.21) | <0.001 | 0.22 |
| Methotrexate | 17 (0.48) | 567 (16.03) | <0.001 | 0.59 |
| Leflunomide | 2 (0.06) | 61 (1.72) | <0.001 | 0.18 |
| Mycophenolic acid | 78 (2.21) | 12 (0.34) | <0.001 | 0.17 |
| Cyclosporin | 27 (0.76) | 49 (1.39) | 0.11 | 0.09 |
| Cyclophosphamide | 17 (0.48) | 9 (0.25) | 0.12 | 0.03 |
| Sulfasalazine | 14 (0.40) | 395 (11.17) | <0.001 | 0.50 |
| Tofacitinib | 0 (0.00) | 0 (0.00) | - | - |
| Baricitinib | 0 (0.00) | 0 (0.00) | - | - |
| Upadacitinib | 0 (0.00) | 0 (0.00) | - | - |
| Rituximab | 7 (0.20) | 8 (0.23) | 0.80 | 0.01 |
| Etanercept | 1 (0.03) | 13 (0.37) | <0.01 | 0.08 |
| Adalimumab | 0 (0.00) | 7 (0.20) | 0.02 | 0.06 |
| Golimumab | 0 (0.00) | 2 (0.06) | 0.50 | 0.03 |
| Certolizumab | 0 (0.00) | 7 (0.20) | 0.02 | 0.06 |
| Infliximab | 0 (0.00) | 0 (0.00) | - | - |
| Tocilizumab | 0 (0.00) | 14 (0.40) | <0.001 | 0.09 |
| Abatacept | 0 (0.00) | 3 (0.08) | 0.25 | 0.04 |
| Belimumab | 0 (0.00) | 0 (0.00) | - | - |
| Number | Follow-Up (Years) | Person-Years of Follow-Up | Incidence Rate, Per 1000 Person-Years | Crude HR (95% CI) | Adjusted HR a (95% CI) | Competing Risk Regression Model b Adjusted HR a (95% CI) | |
|---|---|---|---|---|---|---|---|
| SLE | 20/3537 | 10.38 ± 4.75 | 36,703.71 | 0.54 (0.31–0.78) | 3.28 (1.32–8.18) | 2.47 (0.95–6.38) | 2.58 (1.06–6.29) |
| Other SARDs | 6/3537 | 10.19 ± 4.57 | 36,059.53 | 0.17 (0.03–0.30) | - | - | - |
| p-value | <0.01 | - | - | - | 0.01 | 0.06 | 0.04 |
| Variable | Univariate Analysis | Multivariate Analysis | ||
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age ≥ 50 | 0.86 (0.36–2.05) | 0.74 | - | |
| Male | 2.55 (1.07–6.07) | 0.03 | - | |
| Comorbidity | ||||
| Diabetes mellitus | 2.38 (0.72–7.94) | 0.16 | - | |
| Chronic kidney disease | 1.08 (0.37–3.13) | 0.89 | - | |
| Cirrhosis | 2.33 (0.32–17.16) | 0.41 | - | |
| Lymphoproliferative disorder/cancer | 3.08 (1.06–8.94) | 0.04 | - | |
| DMARDS | ||||
| Hydroxychloroquine | 1.30 (0.58–2.92) | 0.53 | - | |
| Azathioprine | 3.03 (0.91–10.12) | 0.07 | - | |
| Mycophenolic acid | 3.91 (0.53–28.90) | 0.18 | - | |
| Cyclosporin | 4.22 (0.57–31.15) | 0.16 | - | |
| Laboratory data | ||||
| Leukopenia | 2.67 (1.19–6.02) | 0.02 | - | |
| Lymphopenia | 3.98 (1.83–8.68) | <0.001 | 3.98 (1.83–8.68) | <0.001 |
| Decreased IgM | 5.63 (0.76–41.56) | 0.09 | - | |
| Decreased C3 | 2.48 (1.13–5.47) | 0.02 | - | |
| Decreased C4 | 2.41 (1.02–5.74) | 0.05 | - | |
| Positivity of anti-dsDNA | 1.02 (0.45–2.28) | 0.97 | - | |
| Increased ferritin | 2.66 (0.63–11.28) | 0.18 | - | |
| Proteinuria | 4.06 (1.39–11.92) | 0.01 | - | |
| Hepatitis | 3.17 (1.20–8.41) | 0.02 | - | |
| Renal impairment | 1.87 (0.71–4.96) | 0.21 | - | |
| Hypoalbuminemia | 1.68 (0.68–4.19) | 0.26 | - | |
| Elevated ESR | 0.37 (0.13–1.06) | 0.06 | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, C.-Y.; Yu, H.-H.; Cheng, P.-Y.; Chen, Y.-F. Increased Susceptibility to Salmonella Infection in Systemic Lupus Erythematosus Compared with Other Systemic Autoimmune Diseases: Insights from a Retrospective Cohort Study from the Largest Health Care System in Taiwan. Life 2025, 15, 1522. https://doi.org/10.3390/life15101522
Wei C-Y, Yu H-H, Cheng P-Y, Chen Y-F. Increased Susceptibility to Salmonella Infection in Systemic Lupus Erythematosus Compared with Other Systemic Autoimmune Diseases: Insights from a Retrospective Cohort Study from the Largest Health Care System in Taiwan. Life. 2025; 15(10):1522. https://doi.org/10.3390/life15101522
Chicago/Turabian StyleWei, Chen-Ying, Han-Hua Yu, Pei-Yi Cheng, and Yen-Fu Chen. 2025. "Increased Susceptibility to Salmonella Infection in Systemic Lupus Erythematosus Compared with Other Systemic Autoimmune Diseases: Insights from a Retrospective Cohort Study from the Largest Health Care System in Taiwan" Life 15, no. 10: 1522. https://doi.org/10.3390/life15101522
APA StyleWei, C.-Y., Yu, H.-H., Cheng, P.-Y., & Chen, Y.-F. (2025). Increased Susceptibility to Salmonella Infection in Systemic Lupus Erythematosus Compared with Other Systemic Autoimmune Diseases: Insights from a Retrospective Cohort Study from the Largest Health Care System in Taiwan. Life, 15(10), 1522. https://doi.org/10.3390/life15101522






