Preliminary Evaluation of the Diagnostic Performance of OvMANE1 and OvMCBL02 Multiepitope Antigens for the Non-Invasive Diagnosis of Onchocerciasis Exposure
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Study Site, Population, and Sample Collection
2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. Data Analysis
3. Results
Antibody Capture ELISA to Determine Exposure of O. volvulus Infection
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Onchocerciasis Key Facts. Geneva. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/onchocerciasis#:~:text=Key%20facts,the%20parasitic%20worm%20Onchocerca%20volvulus.&text=Symptoms%20include%20severe%20itching%2C%20disfiguring,live%20in%2031%20African%20countries (accessed on 25 April 2025).
- Shintouo, C.M.; Shey, R.A.; Mets, T.; Vanhamme, L.; Souopgui, J.; Ghogomu, S.M.; Njemini, R. Onchocerciasis Fingerprints in the Geriatric Population: Does Host Immunity Play a Role? Trop. Med. Infect. Dis. 2021, 6, 153. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Casulli, A. New global targets for NTDs in the WHO roadmap 2021–2030. PLoS Negl. Trop. Dis. 2021, 15, e0009373. [Google Scholar] [CrossRef] [PubMed]
- Rosa, B.A.; Curtis, K.; Gilmore, P.E.; Martin, J.; Zhang, Q.; Sprung, R.; Weil, G.J.; Townsend, R.R.; Fischer, P.U.; Mitreva, M. Direct Proteomic Detection and Prioritization of 19 Onchocerciasis Biomarker Candidates in Humans. Mol. Cell. Proteom. 2022, 22, 100454. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peeling, R.W.; Mabey, D. Diagnostics for the control and elimination of neglected tropical diseases. Parasitology 2014, 141, 1789–1794. [Google Scholar] [CrossRef] [PubMed]
- Albiez, E.J. Studies on nodules and adult Onchocerca volvulus during a nodulectomy trial in hyperendemic villages in Liberia and Upper Volta. I. Palpable and impalpable onchocercomata. Tropenmed. Parasitol. 1983, 34, 54–60. [Google Scholar] [PubMed]
- Hotterbeekx, A.; Perneel, J.; Mandro, M.; Abhafule, G.; Siewe Fodjo, J.N.; Dusabimana, A.; Abrams, S.; Kumar-Singh, S.; Colebunders, R. Comparison of Diagnostic Tests for Onchocerca volvulus in the Democratic Republic of Congo. Pathogens 2020, 9, 435. [Google Scholar] [CrossRef] [PubMed]
- Shintouo, C.M.; Ghogomu, S.M.; Shey, R.A.; Hotterbeekx, A.; Yagmur, E.; Mets, T.; Vanhamme, L.; Colebunders, R.; Souopgui, J.; Njemini, R. Tandem Use of OvMANE1 and Ov-16 ELISA Tests Increases the Sensitivity for the Diagnosis of Human Onchocerciasis. Life 2021, 11, 1284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dieye, Y.; Storey, H.L.; Barrett, K.L.; Gerth-Guyette, E.; Di Giorgio, L.; Golden, A.; Faulx, D.; Kalnoky, M.; Ndiaye, M.K.N.; Sy, N.; et al. Feasibility of utilizing the SD BIOLINE Onchocerciasis IgG4 rapid test in onchocerciasis surveillance in Senegal. PLoS Negl. Trop. Dis. 2017, 11, e0005884. [Google Scholar] [CrossRef]
- Taylor, H.R.; Munoz, B.; Keyvan-Larijani, E.; Greene, B.M. Reliability of detection of microfilariae in skin snips in the diagnosis of onchocerciasis. Am. J. Trop. Med. Hyg. 1989, 41, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Vlaminck, J.; Fischer, P.U.; Weil, G.J. Diagnostic Tools for Onchocerciasis Elimination Programs. Trends Parasitol. 2015, 31, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, G.E.; Awadzi, K.; Opoku, N.; Narayanan, R.B.; Akuffo, H.O. Comparison between the skin snip test and simple dot blot assay as potential rapid assessment tools for Onchocerciasis in the postcontrol era in Ghana. Clin. Diagn. Lab. Immunol. 2002, 9, 1014–1020. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Itoh, M.; Weerasooriya, M.V.; Yahathugoda, T.C.; Takagi, H.; Samarawickrema, W.A.; Nagaoka, F.; Kimura, E. Effects of 5 rounds of mass drug administration with diethylcarbamazine and albendazole on filaria-specific IgG4 titers in urine: 6-year follow-up study in Sri Lanka. Parasitol. Int. 2011, 60, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Ejazi, S.A.; Bhattacharya, P.; Bakhteyar, M.A.; Mumtaz, A.A.; Pandey, K.; Das, V.N.; Das, P.; Rahaman, M.; Goswami, R.P.; Ali, N. Noninvasive Diagnosis of Visceral Leishmaniasis: Development and Evaluation of Two Urine-Based Immunoassays for Detection of Leishmania donovani Infection in India. PLoS Negl. Trop. Dis. 2016, 10, e0005035. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Corstjens, P.L.A.M.; Nyakundi, R.K.; de Dood, C.J.; Kariuki, T.M.; Ochola, E.A.; Karanja, D.M.S.; Mwinzi, P.N.M.; van Dam, G.J. Improved sensitivity of the urine CAA lateral-flow assay for diagnosing active Schistosoma infections by using larger sample volumes. Parasites Vectors 2015, 8, 241. [Google Scholar] [CrossRef]
- Adriko, M.; Standley, C.J.; Tinkitina, B.; Tukahebwa, E.M.; Fenwick, A.; Fleming, F.M.; Sousa-Figueiredo, J.C.; Stothard, J.R.; Kabatereine, N.B. Evaluation of circulating cathodic antigen (CCA) urine-cassette assay as a survey tool for Schistosoma mansoni in different transmission settings within Bugiri District, Uganda. Acta Trop. 2014, 136, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.Z.; Itoh, M.; Ul Islam, M.A.; Saifuddin Ekram, A.R.; Rahman, M.A.; Takagi, H.; Takesue, A.; Hashiguchi, Y.; Kimura, E. ELISA with recombinant rKRP42 antigen using urine samples: A tool for predicting clinical visceral leishmaniasis cases and its outbreak. Am. J. Trop. Med. Hyg. 2012, 87, 658–662. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, H.; Qiu, S.; Hong, W.-X.; Song, K.-Y.; Wang, J.; Yang, H.-Q.; Deng, Y.-Q.; Zhu, S.-Y.; Zhang, F.-C.; Qin, C.-F. Dengue Specific Immunoglobulin A Antibody is Present in Urine and Associated with Disease Severity. Sci. Rep. 2016, 6, 27298. [Google Scholar] [CrossRef] [PubMed]
- Ambe, L.A.; Limunga, E.; Mbah, C.E.; Adela, N.; Eric, N.; Ngoe, M.; Sone, B.; Lochnit, G.; Tachu, J.B.; Wanji, S.; et al. Identification and Characterization of Onchocerca volvulus Heat Shock Protein 70 (OvHSP70) as Novel Diagnostic Marker of Onchocerciasis in Human Urine. Pathogens 2024, 13, 293. [Google Scholar] [CrossRef]
- Shintouo, C.M.; Shey, R.A.; Nebangwa, D.N.; Esoh, K.K.; Nongley, N.F.; Nguve, J.E.; Giron, P.; Mutesa, L.; Vanhamme, L.; Souopgui, J.; et al. In Silico Design and Validation of OvMANE1, a Chimeric Antigen for Human Onchocerciasis Diagnosis. Pathogens 2020, 9, 495. [Google Scholar] [CrossRef] [PubMed]
- Yengo, B.N.; Shintouo, C.M.; Hotterbeekx, A.; Yaah, N.E.; Shey, R.A.; Quanico, J.; Baggerman, G.; Ayong, L.; Vanhamme, L.; Njemini, R.; et al. Immunoinformatics Design and Assessment of a Multiepitope Antigen (OvMCBL02) for Onchocerciasis Diagnosis and Monitoring. Diagnostics 2022, 12, 1440. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Bhaskar, K.R.; Hossain, F.; Khan, M.A.; Vallur, A.C.; Duthie, M.S.; Hamano, S.; Salam, M.A.; Huda, M.M.; Khan, M.G.; et al. Evaluation of diagnostic performance of rK28 ELISA using urine for diagnosis of visceral leishmaniasis. Parasites Vectors 2016, 9, 383. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takagi, A. Detection of H. pylori by serum and urine-based ELISA. Nihon Rinsho 2003, 61, 88–91. [Google Scholar] [PubMed]
- Aydin, S.; Emre, E.; Ugur, K.; Aydin, M.A.; Sahin, İ.; Cinar, V.; Akbulut, T. An overview of ELISA: A review and update on best laboratory practices for quantifying peptides and proteins in biological fluids. J. Int. Med. Res. 2025, 53, 3000605251315913. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Itoh, M.; Sako, Y.; Itoh, S.; Ishikawa, Y.; Akabane, H.; Nakaya, K.; Nagaoka, F.; Ito, A. Immunodiagnosis of alveolar echinococcosis using urine samples. Parasitol. Int. 2013, 62, 514–516. [Google Scholar] [CrossRef] [PubMed]
- Noma, M.; Zouré, H.G.M.; Tekle, A.H.; Enyong, P.A.I.; Nwoke, B.E.B.; Remme, J.H.F. The geographic distribution of onchocerciasis in the 20 participating countries of the African Programme for Onchocerciasis Control: (1) priority areas for ivermectin treatment. Parasites Vectors 2014, 7, 325. [Google Scholar] [CrossRef] [PubMed]
- Shintouo, C.M.; Nguve, J.E.; Asa, F.B.; Shey, R.A.; Kamga, J.; Souopgui, J.; Ghogomu, S.M.; Njemini, R. Entomological Assessment of Onchocerca Species Transmission by Black Flies in Selected Communities in the West Region of Cameroon. Pathogens 2020, 9, 722. [Google Scholar] [CrossRef] [PubMed]
- Aza’ah, R.A.; Sumo, L.; Ntonifor, N.H.; Bopda, J.; Bamou, R.H.; Nana-Djeunga, H.C. Point prevalence mapping reveals hotspot for onchocerciasis transmission in the Ndikinimeki Health District, Centre Region, Cameroon. Parasites Vectors 2020, 13, 519. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Biamonte, M.A.; Cantey, P.T.; Coulibaly, Y.I.; Gass, K.M.; Hamill, L.C.; Hanna, C.; Lammie, P.J.; Kamgno, J.; Nutman, T.B.; Oguttu, D.W.; et al. Onchocerciasis: Target product profiles of in vitro diagnostics to support onchocerciasis elimination mapping and mass drug administration stopping decisions. PLoS Negl. Trop. Dis. 2022, 16, e0010682. [Google Scholar] [CrossRef]
- Njume, F.N.; Ghogomu, S.M.; Shey, R.A.; Gainkam, L.O.T.; Poelvoorde, P.; Humblet, P.; Kamgno, J.; Robert, A.; Mutesa, L.; Lelubre, C.; et al. Identification and characterization of the Onchocerca volvulus Excretory Secretory Product Ov28CRP, a putative GM2 activator protein. PLoS Negl. Trop. Dis. 2019, 13, e0007591. [Google Scholar] [CrossRef] [PubMed]
- Lagatie, O.; Granjon, E.; Odiere, M.R.; Zrein, M.; Stuyver, L.J. Assessment of multiplex Onchocerca volvulus peptide ELISA in non-endemic tropical regions. Parasites Vectors 2019, 12, 570. [Google Scholar] [CrossRef]
- Lustigman, S.; Brotman, B.; Johnson, E.H.; Smith, A.B.; Huima, T.; Prince, A.M. Identification and characterization of an Onchocerca volvulus cDNA clone encoding a microfilarial surface-associated antigen. Mol. Biochem. Parasitol. 1992, 50, 79–93. [Google Scholar] [CrossRef] [PubMed]

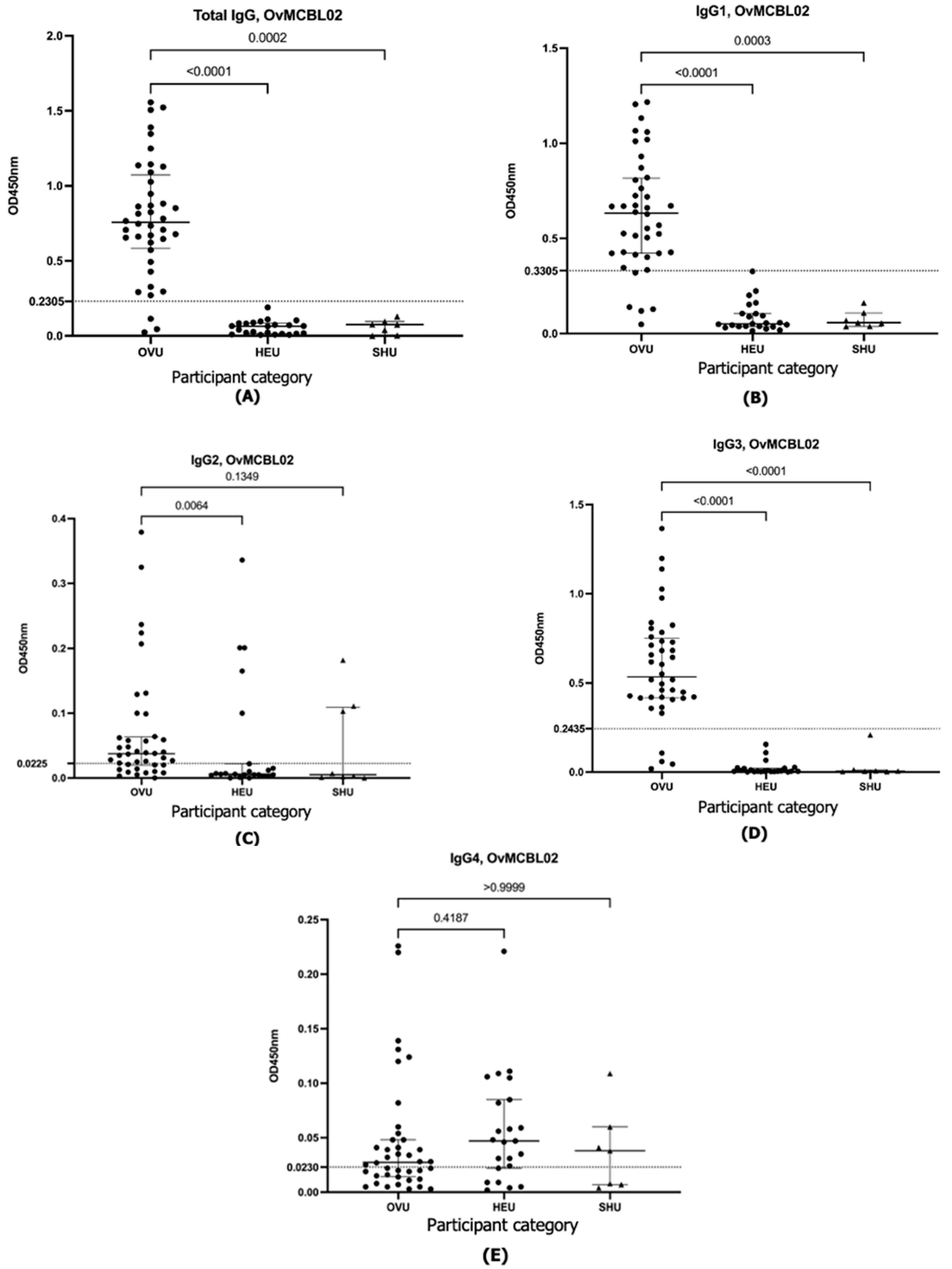
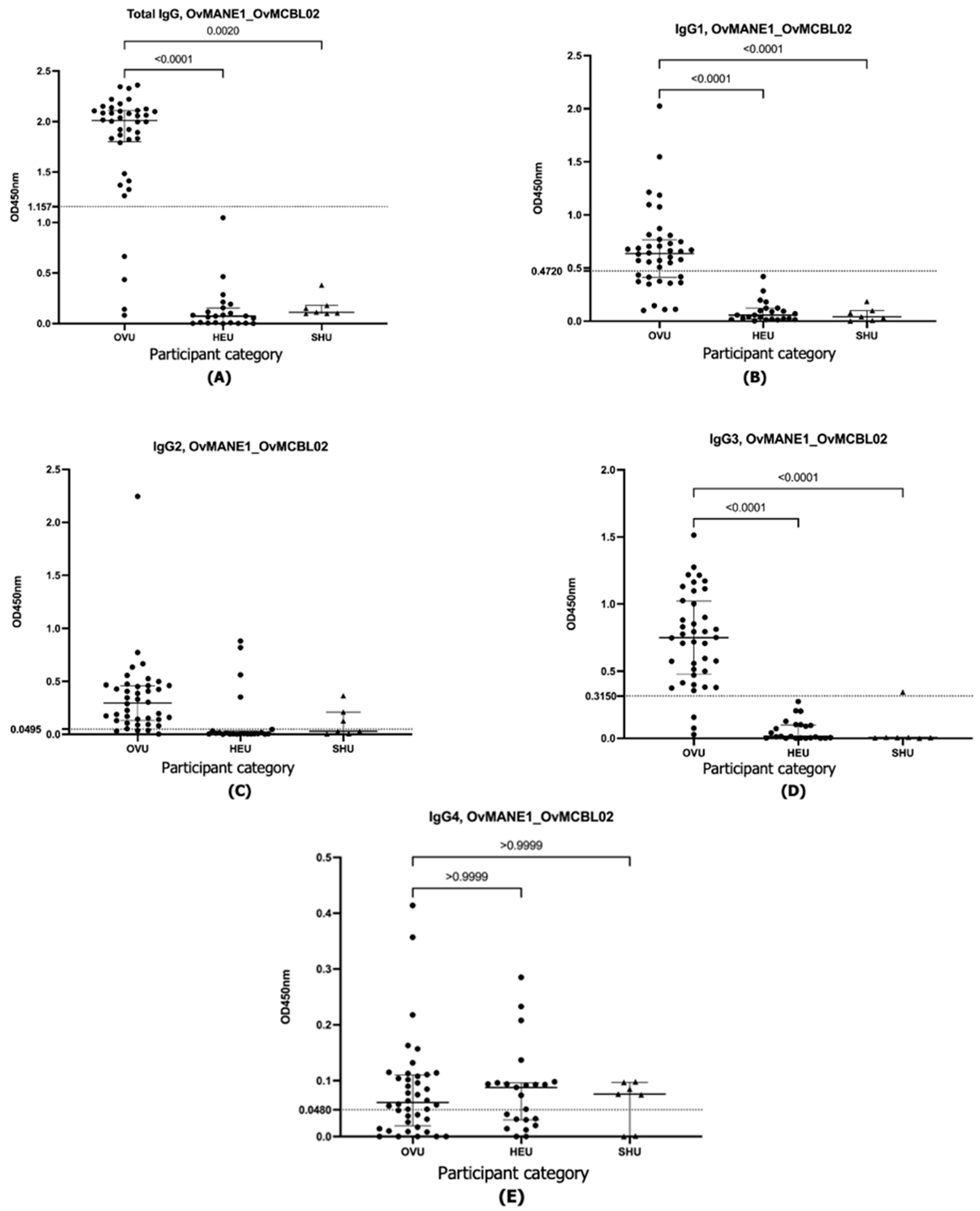
| OvMANE1 | Total IgG | IgG1 | IgG2 | IgG3 | IgG4 | |
|---|---|---|---|---|---|---|
| ROC analysis | ROC area (AUC) | 0.9880 | 0.8864 | 0.8000 | 0.9853 | 0.6321 |
| 95% CI of AUC | 0.9687 to 1.000 | 0.8063 to 0.9665 | 0.6706 to 0.9294 | 0.9645 to 1.000 | 0.4853 to 0.7788 | |
| p-value (against AUC = 0.5) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0828 | |
| Diagnostic accuracy parameter | Cutoff value | 1.209 | 0.3140 | 0.5965 | 0.1525 | 0.0945 |
| Sensitivity (%) (95% CI) | 85.0 (70.93% to 92.94%) | 77.5 (62.50% to 87.68%) | 32.5 (20.08% to 47.98%) | 87.5 (73.89% to 94.54%) | 47.5 (32.94% to 62.50%) | |
| Specificity (%) (95% CI) | 100.0 (85.69% to 100.0%) | 91.3 (73.20% to 98.45%) | 91.30 (73.20% to 98.45%) | 100.0 (85.69% to 100.0%) | 73.9 (53.53% to 87.45%) | |
| False negative | 15.0% | 22.5% | 67.5% | 12.5% | 52.5% | |
| False positive | 0.0% | 8.7% | 8.7% | 0.0% | 26.1% | |
| OvMCBL02 | ||||||
| ROC analysis | ROC area (AUC) | 0.9707 | 0.9685 | 0.7391 | 0.9837 | 0.5973 |
| 95% CI of AUC | 0.9304 to 1.000 | 0.9315 to 1.000 | 0.5902 to 0.8881 | 0.9612 to 1.000 | 0.4465 to 0.7481 | |
| p-value (against AUC = 0.5) | <0.0001 | <0.0001 | 0.0017 | <0.0001 | 0.2014 | |
| Diagnostic accuracy parameter | Cutoff value | 0.2305 | 0.3305 | 0.0225 | 0.2435 | 0.0230 |
| Sensitivity (%) (95% CI) | 92.5 (80.14% to 97.42%) | 87.5 (73.89% to 94.54%) | 70.0% (54.57% to 81.93%) | 90.0 (76.95% to 96.04%) | 45.0 (30.71% to 60.17%) | |
| Specificity (%) (95% CI) | 100.0 (85.69% to 100.0%) | 100.0 (85.69% to 100.0%) | 78.3 (58.10% to 90.34%) | 100.0 (85.69% to 100.0%) | 73.9 (53.53% to 87.45%) | |
| False negative | 7.5% | 12.5% | 30.0% | 10.0% | 55.0% | |
| False positive | 0.0% | 0.0% | 21.7% | 0.0% | 26.1% | |
| OvMANE1_OvMCBL02 | ||||||
| ROC analysis | ROC area (AUC) | 0.9793 | 0.9685 | 0.8304 | 0.9772 | 0.5158 |
| 95% CI of AUC | 0.9513 to 1.000 | 0.9328 to 1.000 | 0.6932 to 0.9676 | 0.9467 to 1.000 | 0.3677 to 0.6638 | |
| p-value (against AUC = 0.5) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.8360 | |
| Diagnostic accuracy parameter | Cutoff value | 1.157 | 0.4275 | 0.0495 | 0.3150 | 0.0480 |
| Sensitivity (%) (95% CI) | 90.0 (76.95% to 96.04%) | 72.5 (57.17% to 83.89%) | 90.0 (76.95% to 96.04%) | 92.5 (80.14% to 97.42%) | 37.5 (24.22% to 52.97%) | |
| Specificity (%) (95% CI) | 100.0 (85.69% to 100.0%) | 100.0 (85.69% to 100.0%) | 82.6 (62.86% to 93.02%) | 100.0 (85.69% to 100.0%) | 60.8 (40.79% to 77.84%) | |
| False negative | 10.0% | 27.5% | 10.0% | 7.5% | 62.5% | |
| False positive | 0.0% | 0.0% | 17.4% | 0.0% | 39.2% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yengo, B.N.; Shintouo, C.M.; Shey, R.A.; Yaah, N.E.; Vanhamme, L.; Njemini, R.; Souopgui, J.; Ghogomu, S.M. Preliminary Evaluation of the Diagnostic Performance of OvMANE1 and OvMCBL02 Multiepitope Antigens for the Non-Invasive Diagnosis of Onchocerciasis Exposure. Life 2025, 15, 1515. https://doi.org/10.3390/life15101515
Yengo BN, Shintouo CM, Shey RA, Yaah NE, Vanhamme L, Njemini R, Souopgui J, Ghogomu SM. Preliminary Evaluation of the Diagnostic Performance of OvMANE1 and OvMCBL02 Multiepitope Antigens for the Non-Invasive Diagnosis of Onchocerciasis Exposure. Life. 2025; 15(10):1515. https://doi.org/10.3390/life15101515
Chicago/Turabian StyleYengo, Bernis Neneyoh, Cabirou Mounchili Shintouo, Robert Adamu Shey, Ntang Emmaculate Yaah, Luc Vanhamme, Rose Njemini, Jacob Souopgui, and Stephen Mbigha Ghogomu. 2025. "Preliminary Evaluation of the Diagnostic Performance of OvMANE1 and OvMCBL02 Multiepitope Antigens for the Non-Invasive Diagnosis of Onchocerciasis Exposure" Life 15, no. 10: 1515. https://doi.org/10.3390/life15101515
APA StyleYengo, B. N., Shintouo, C. M., Shey, R. A., Yaah, N. E., Vanhamme, L., Njemini, R., Souopgui, J., & Ghogomu, S. M. (2025). Preliminary Evaluation of the Diagnostic Performance of OvMANE1 and OvMCBL02 Multiepitope Antigens for the Non-Invasive Diagnosis of Onchocerciasis Exposure. Life, 15(10), 1515. https://doi.org/10.3390/life15101515










