Investigation of the Effects of Fosfomycin in Kidney Damage Caused by CLP-Induced Sepsis
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
Experimental Protocol
2.2. Cecal Ligation and Puncture-Induced Sepsis Model
2.3. Biochemical Procedure
Homogenization of Kidney Tissue
2.4. Malondialdehyde (MDA) Analyses
2.5. Glutathione (GSH) Analyses
2.6. IL-1β ve IL6 Analyses
2.7. Statistical Analysis
2.8. Histopathological Analyses
2.9. Immunohistochemical (IHC) Analysis
2.10. Semi-Quantative Analyses
3. Biochemical Analysis Results
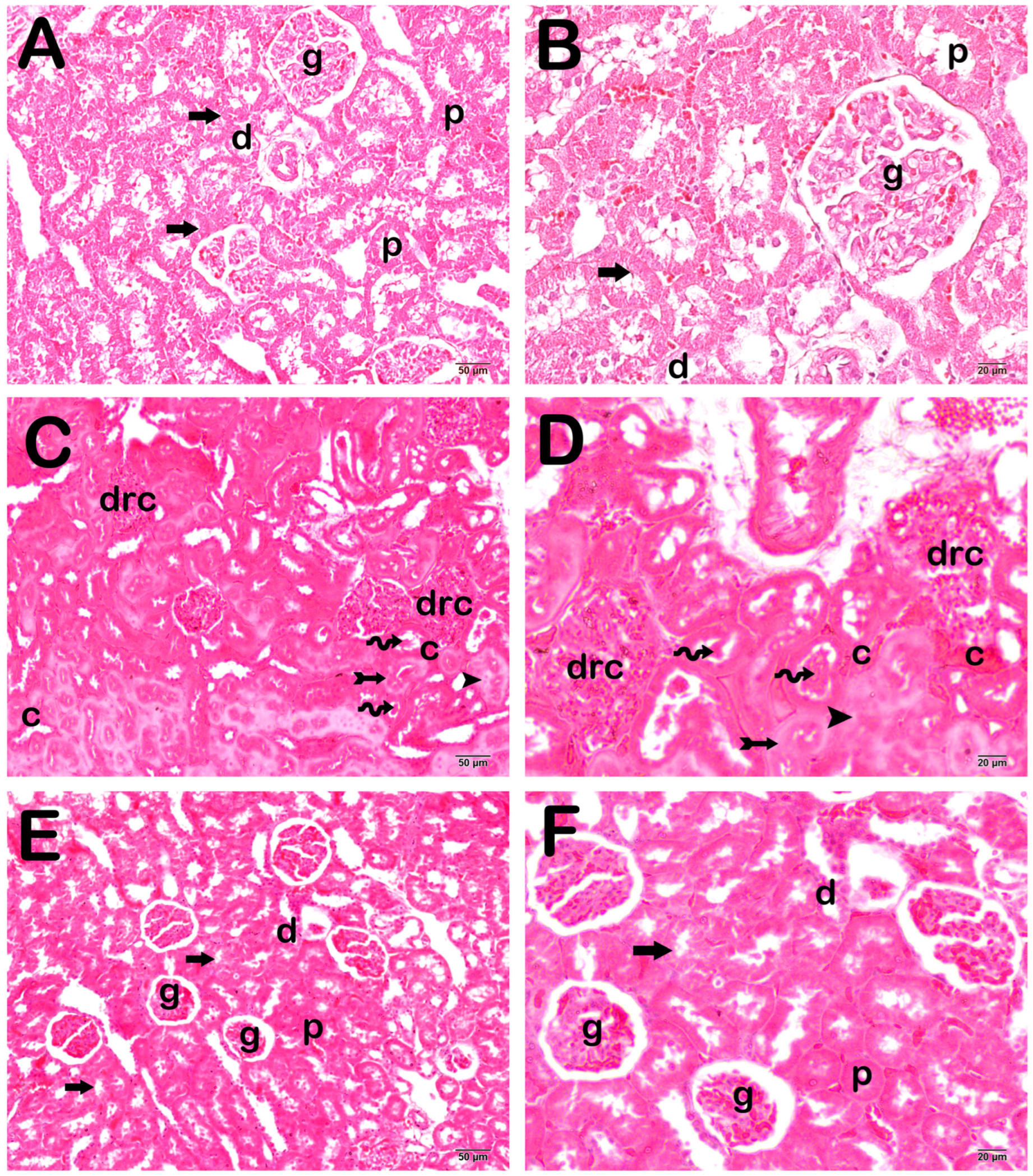
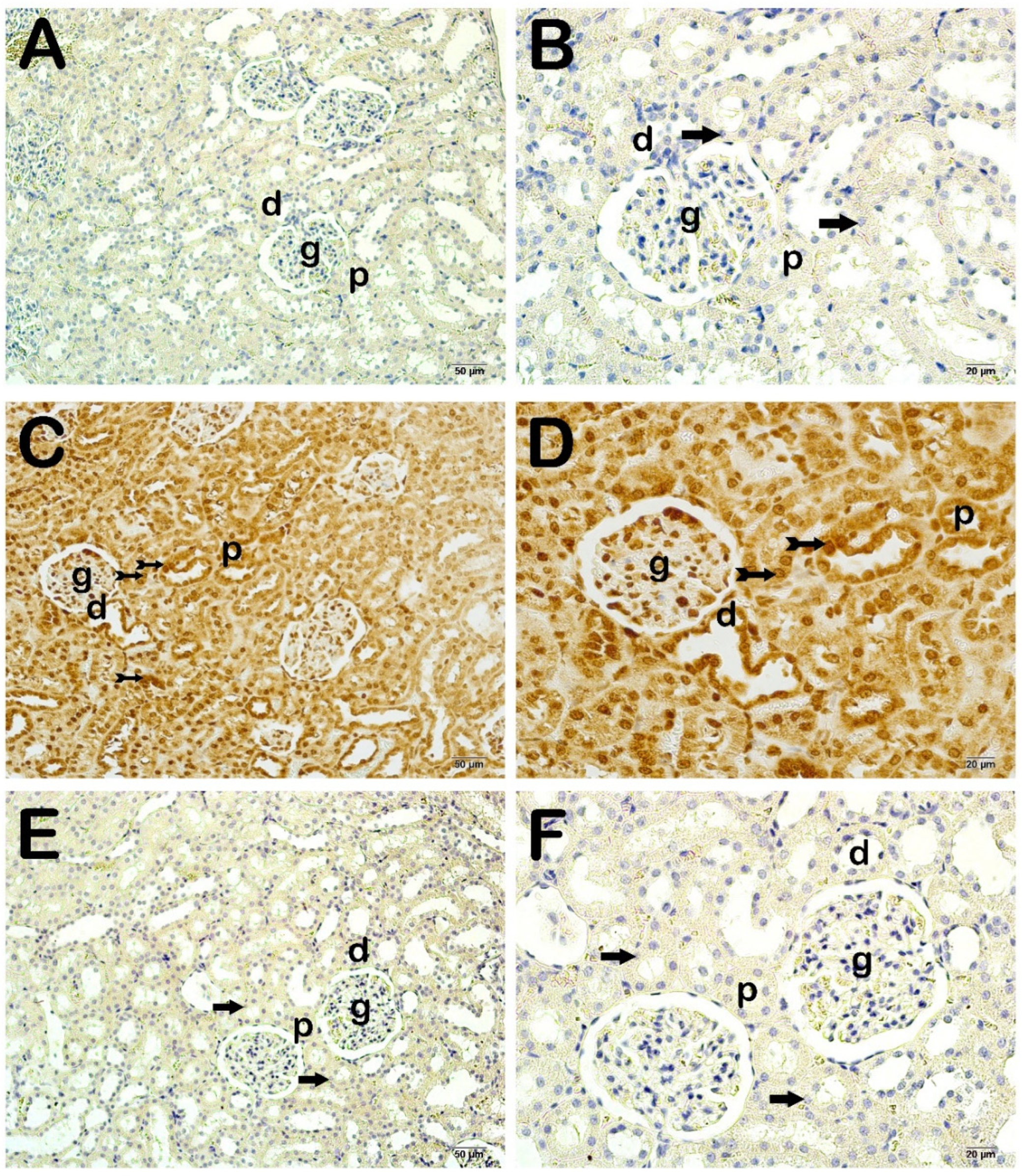
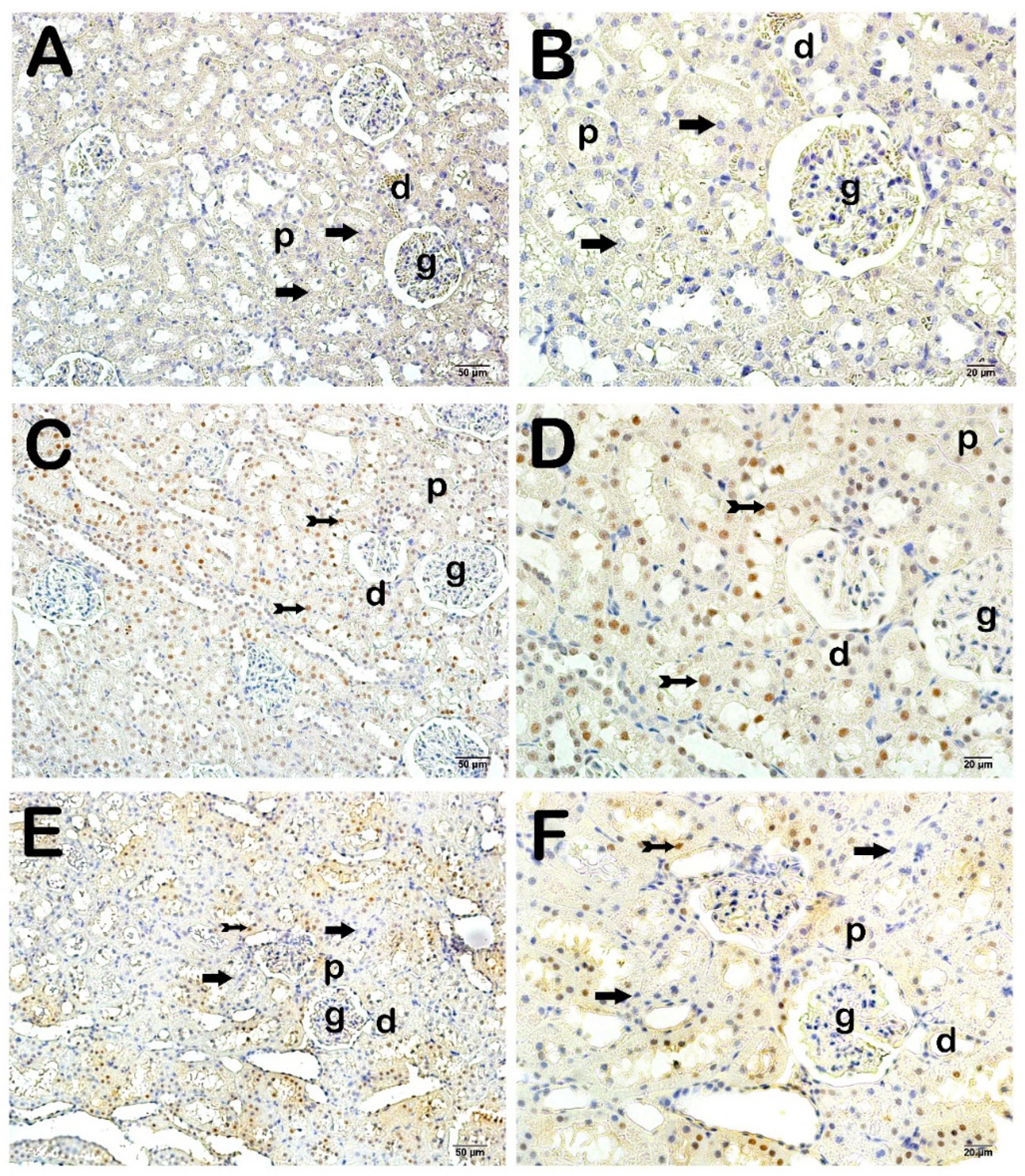
4. Histopathological Analysis Results
Semi-Quantitative Analysis
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. Available online: https://pubmed.ncbi.nlm.nih.gov/31954465/ (accessed on 28 December 2023). [CrossRef] [PubMed]
- Hoste, E.A.; Bagshaw, S.M.; Bellomo, R.; Cely, C.M.; Colman, R.; Cruz, D.N.; Edipidis, K.; Forni, L.G.; Gomersall, C.D.; Govil, D.; et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med. 2015, 41, 1411–1423. Available online: https://pubmed.ncbi.nlm.nih.gov/26162677/ (accessed on 28 December 2023). [CrossRef] [PubMed]
- Peerapornratana, S.; Manrique-Caballero, C.L.; Gómez, H.; Kellum, J.A. Acute kidney injury from sepsis: Current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019, 96, 1083–1099. Available online: https://pubmed.ncbi.nlm.nih.gov/31443997/ (accessed on 28 December 2023). [CrossRef] [PubMed]
- Caraballo, C.; Jaimes, F. Focus: Death: Organ Dysfunction in Sepsis: An Ominous Trajectory From Infection to Death. Yale J. Biol. Med. 2019, 92, 629. Available online: https://pubmed.ncbi.nlm.nih.gov/31866778/ (accessed on 28 December 2023).
- Yildiz, I.E.; Topcu, A.; Bahceci, I.; Arpa, M.; Tumkaya, L.; Mercantepe, T.; Batcik, S.; Yildiz, Y. The protective role of fosfomycin in lung injury due to oxidative stress and inflammation caused by sepsis. Life Sci. 2021, 279, 119662. Available online: https://pubmed.ncbi.nlm.nih.gov/34081989/ (accessed on 28 December 2023). [CrossRef]
- Topcu, A.; Saral, S.; Mercantepe, T.; Akyildiz, K.; Tumkaya, L.; Yilmaz, A. The effects of apelin-13 against cisplatin-induced nephrotoxicity in rats. Drug Chem. Toxicol. 2023, 46, 77–87. Available online: https://pubmed.ncbi.nlm.nih.gov/34894944/ (accessed on 28 December 2023). [CrossRef]
- Topcu, A.; Ozturk, A.; Deniz, E.; Duman Ozturk, S.; Arpa, M.; Atak, M. The effects of amiodarone in ovarian injury due to oxidative stress and inflammation caused by ischemia-reperfusion. Immunopharmacol. Immunotoxicol. 2022, 44, 1022–1031. Available online: https://pubmed.ncbi.nlm.nih.gov/35838634/ (accessed on 28 December 2023). [CrossRef]
- Li, Q.; Wang, B.; Lin, K.W.; Deng, T.; Huang, Q.F.; Xu, S.Q.; Wang, H.F.; Wu, X.X.; Li, N.; Yi, Y.; et al. Anthrahydroquinone-2,6-disulfonate alleviates paraquat-induced kidney injury via the apelin-APJ pathway in rats. Asian Pac. J. Trop. Biomed. 2022, 12, 333–342. [Google Scholar] [CrossRef]
- Dil, E.; Topcu, A.; Mercantepe, T.; Tumkaya, L.; Akyildiz, K.; Saral, S.; Yilmaz, A. Agomelatine on cisplatin-induced nephrotoxicity via oxidative stress and apoptosis. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 396, 2753–2764. Available online: https://pubmed.ncbi.nlm.nih.gov/37480488/ (accessed on 28 December 2023). [CrossRef]
- An, Y.; Wang, Y.; Zhan, J.; Tang, X.; Shen, K.; Shen, F.; Wang, C.; Luan, W.; Wang, X.; Liu, M.; et al. Fosfomycin Protects Mice From Staphylococcus aureus Pneumonia Caused by α-Hemolysin in Extracellular Vesicles by Inhibiting MAPK-Regulated NLRP3 Inflammasomes. Front. Cell Infect. Microbiol. 2019, 9, 253. Available online: https://pubmed.ncbi.nlm.nih.gov/31380296/ (accessed on 28 December 2023). [CrossRef]
- Kitada, M.; Ogura, Y.; Monno, I.; Xu, J.; Koya, D. Effect of Methionine Restriction on Aging: Its Relationship to Oxidative Stress. Biomedicines 2021, 9, 130. Available online: https://pubmed.ncbi.nlm.nih.gov/33572965/ (accessed on 28 December 2023). [CrossRef] [PubMed]
- Allameh, H.; Fatemi, I.; Malayeri, A.R.; Nesari, A.; Mehrzadi, S.; Goudarzi, M. Pretreatment with berberine protects against cisplatin-induced renal injury in male Wistar rats. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 1825–1833. Available online: https://pubmed.ncbi.nlm.nih.gov/32410067/ (accessed on 28 December 2023). [CrossRef] [PubMed]
- Yanagida, C.; Ito, K.; Komiya, I.; Horie, T. Protective effect of fosfomycin on gentamicin-induced lipid peroxidation of rat renal tissue. Chem. Biol. Interact. 2004, 148, 139–147. Available online: https://pubmed.ncbi.nlm.nih.gov/15276870/ (accessed on 28 December 2023). [CrossRef] [PubMed]
- Altıntaş, N.D. Sepsis ve Böbrekler. Türkiye Klin. Yoğun Bakım—Özel Konular 2021, 7, 56–61. Available online: https://www.turkiyeklinikleri.com/article/tr-sepsis-ve-bobrekler-93293.html (accessed on 28 December 2023).
- Rittirsch, D.; Huber-Lang, M.S.; Flierl, M.A.; Ward, P.A. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat. Protoc. 2009, 4, 31–36. Available online: https://pubmed.ncbi.nlm.nih.gov/19131954/ (accessed on 28 December 2023). [CrossRef] [PubMed]
- Cinar, I.; Sirin, B.; Aydin, P.; Toktay, E.; Cadirci, E.; Halici, I.; Halici, Z. Ameliorative effect of gossypin against acute lung injury in experimental sepsis model of rats. Life Sci. 2019, 221, 327–334. Available online: https://pubmed.ncbi.nlm.nih.gov/30797018/ (accessed on 28 December 2023). [CrossRef] [PubMed]
- Rojas, D.B.; Gemelli, T.; De Andrade, R.B.; Campos, A.G.; Dutra-Filho, C.S.; Wannmacher, C.M.D. Administration of histidine to female rats induces changes in oxidative status in cortex and hippocampus of the offspring. Neurochem. Res. 2012, 37, 1031–1036. Available online: https://pubmed.ncbi.nlm.nih.gov/22237970/ (accessed on 28 December 2023). [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Atici, A.E.; Arabacı Tamer, S.; Levent, H.N.; Peker Eyüboğlu, İ.; Ercan, F.; Akkiprik, M.; Yeğen, B.Ç. Neuropeptide W Attenuates Oxidative Multi-Organ Injury in Rats Induced with Intra-Abdominal Sepsis. Inflammation 2022, 45, 279–296. Available online: https://pubmed.ncbi.nlm.nih.gov/34564825/ (accessed on 28 December 2023). [CrossRef] [PubMed]
- Cai, D.; Duan, H.; Fu, Y.; Cheng, Z. Renal Tissue Damage Induced by Acute Kidney Injury in Sepsis Rat Model Is Inhibited by Cynaropicrin via IL-1β and TNF-α Down-Regulation. Dokl. Biochem. Biophys. 2021, 497, 151–157. Available online: https://pubmed.ncbi.nlm.nih.gov/33895932/ (accessed on 28 December 2023). [CrossRef] [PubMed]
- Juan, C.X.; Mao, Y.; Cao, Q.; Chen, Y.; Zhou, L.B.; Li, S.; Chen, H.; Chen, J.H.; Zhou, G.P.; Jin, R. Exosome-mediated pyroptosis of miR-93-TXNIP-NLRP3 leads to functional difference between M1 and M2 macrophages in sepsis-induced acute kidney injury. J. Cell Mol. Med. 2021, 25, 4786–4799. Available online: https://pubmed.ncbi.nlm.nih.gov/33745232/ (accessed on 28 December 2023). [CrossRef]
- Deng, Z.; Sun, M.; Wu, J.; Fang, H.; Cai, S.; An, S.; Huang, Q.; Chen, Z.; Wu, C.; Zhou, Z.; et al. SIRT1 attenuates sepsis-induced acute kidney injury via Beclin1 deacetylation-mediated autophagy activation. Cell Death Dis. 2021, 12, 217. Available online: https://pubmed.ncbi.nlm.nih.gov/33637691/ (accessed on 28 December 2023). [CrossRef]
- Mercantepe, F.; Mercantepe, T.; Topcu, A.; Yılmaz, A.; Tumkaya, L. Protective effects of amifostine, curcumin, and melatonin against cisplatin-induced acute kidney injury. Naunyn. Schmiedebergs Arch. Pharmacol. 2018, 391, 915–931. Available online: https://pubmed.ncbi.nlm.nih.gov/29860655/ (accessed on 28 December 2023). [CrossRef] [PubMed]
- Clementi, A.; Virzì, G.M.; Milan Manani, S.; Battaglia, G.G.; Ronco, C.; Zanella, M. Eryptosis in Patients with Chronic Kidney Disease: A Possible Relationship with Oxidative Stress and Inflammatory Markers. J. Clin. Med. 2022, 11, 7167. Available online: https://pubmed.ncbi.nlm.nih.gov/36498741/ (accessed on 28 December 2023). [CrossRef] [PubMed]
- Mercantepe, F.; Tumkaya, L.; Mercantepe, T.; Rakici, S. Histopathological evaluation of the effects of dexmedetomidine against pituitary damage ınduced by X-ray irradiation. Biomarkers 2023, 28, 168–176. Available online: https://pubmed.ncbi.nlm.nih.gov/36453587/ (accessed on 28 December 2023). [CrossRef] [PubMed]
- Bellomo, R.; Kellum, J.A.; Ronco, C.; Wald, R.; Martensson, J.; Maiden, M.; Bagshaw, S.M.; Glassford, N.J.; Lankadeva, Y.; Vaara, S.T.; et al. Acute kidney injury in sepsis. Intensive Care Med. 2017, 43, 816–828. Available online: https://pubmed.ncbi.nlm.nih.gov/28364303/ (accessed on 28 December 2023). [CrossRef]
- Wu, L.L.; Chiou, C.C.; Chang, P.Y.; Wu, J.T. Urinary 8-OHdG: A marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clinica Chimica Acta 2004, 339, 1–9. Available online: https://pubmed.ncbi.nlm.nih.gov/14687888/ (accessed on 28 December 2023). [CrossRef] [PubMed]
- Morris, S.; Cerceo, E. Trends, Epidemiology, and Management of Multi-Drug Resistant Gram-Negative Bacterial Infections in the Hospitalized Setting. Antibiotics 2020, 9, 196. Available online: https://pubmed.ncbi.nlm.nih.gov/32326058/ (accessed on 28 December 2023). [CrossRef]
- Lacquaniti, A.; Ceresa, F.; Campo, S.; Barbera, G.; Caruso, D.; Palazzo, E.; Patanè, F.; Monardo, P. Acute Kidney Injury and Sepsis after Cardiac Surgery: The Roles of Tissue Inhibitor Metalloproteinase-2, Insulin-like Growth Factor Binding Protein-7, and Mid-Regional Pro-Adrenomedullin. J. Clin. Med. 2023, 12, 5193. Available online: https://pubmed.ncbi.nlm.nih.gov/37629236/ (accessed on 28 December 2023). [CrossRef]
- Grabein, B.; Graninger, W.; Rodríguez Baño, J.; Dinh, A.; Liesenfeld, D.B. Intravenous fosfomycin-back to the future. Systematic review and meta-analysis of the clinical literature. Clin. Microbiol. Infect. 2017, 23, 363–372. Available online: https://pubmed.ncbi.nlm.nih.gov/27956267/ (accessed on 28 December 2023). [CrossRef]
- Iarikov, D.; Wassel, R.; Farley, J.; Nambiar, S. Adverse Events Associated with Fosfomycin Use: Review of the Literature and Analyses of the FDA Adverse Event Reporting System Database. Infect. Dis. Ther. 2015, 4, 433–458. Available online: https://pubmed.ncbi.nlm.nih.gov/26437630/ (accessed on 28 December 2023). [CrossRef] [PubMed]
- Candel, F.J.; Matesanz David, M.; Barberán, J. New perspectives for reassessing fosfomycin: Applicability in current clinical practice. Rev. Española De. Quimioter. 2019, 32 (Suppl. S1), p. 1. Available online: https://pubmed.ncbi.nlm.nih.gov/31131586/ (accessed on 28 December 2023).
- Michalopoulos, A.S.; Livaditis, I.G.; Gougoutas, V. The revival of fosfomycin. Int. J. Infect. Dis. 2011, 15, e732–e739. Available online: https://pubmed.ncbi.nlm.nih.gov/21945848/ (accessed on 28 December 2023). [CrossRef] [PubMed]
- Sirijatuphat, R.; Thamlikitkul, V. Preliminary study of colistin versus colistin plus fosfomycin for treatment of carbapenem-resistant Acinetobacter baumannii infections. Antimicrob. Agents Chemother. 2014, 58, 5598–5601. Available online: https://pubmed.ncbi.nlm.nih.gov/24982065/ (accessed on 28 December 2023). [CrossRef] [PubMed]
- Putensen, C.; Ellger, B.; Sakka, S.G.; Weyland, A.; Schmidt, K.; Zoller, M.; Weiler, N.; Kindgen-Milles, D.; Jaschinski, U.; Weile, J.; et al. Current clinical use of intravenous fosfomycin in ICU patients in two European countries. Infection 2019, 47, 827–836. Available online: https://pubmed.ncbi.nlm.nih.gov/31190298/ (accessed on 28 December 2023). [CrossRef]
- Sastry, S.; Doi, Y. Fosfomycin: Resurgence of an old companion. J. Infect. Chemother. 2016, 22, 273–280. Available online: https://pubmed.ncbi.nlm.nih.gov/26923259/ (accessed on 28 December 2023). [CrossRef] [PubMed]
- López-Montesinos, I.; Horcajada, J.P. Oral and intravenous fosfomycin in complicated urinary tract infections. Rev. Española De Quimioter. 2019, 32 (Suppl. S1), 37. Available online: https://pubmed.ncbi.nlm.nih.gov/31131591/ (accessed on 28 December 2023).
- Russo, A.; Bassetti, M.; Bellelli, V.; Bianchi, L.; Marincola Cattaneo, F.; Mazzocchetti, S.; Paciacconi, E.; Cottini, F.; Schiattarella, A.; Tufaro, G.; et al. Efficacy of a Fosfomycin-Containing Regimen for Treatment of Severe Pneumonia Caused by Multidrug-Resistant Acinetobacter baumannii: A Prospective, Observational Study. Infect. Dis. Ther. 2021, 10, 187–200. Available online: https://pubmed.ncbi.nlm.nih.gov/33068255/ (accessed on 28 December 2023). [CrossRef]
- Tan, J.; Yu, W.; Wu, G.; Shen, J.; Fang, Y.; Zhu, H.; Xiao, Q.; Peng, W.; Lan, Y.; Gong, Y. A Real-World Study Comparing Various Antimicrobial Regimens for Bloodstream Infections Caused by Carbapenem-Resistant Gram-Negative Bacilli in a Tertiary Hospital, Shanghai, China, from 2010 to 2017. Infect. Drug Resist. 2020, 13, 2453–2463. Available online: https://pubmed.ncbi.nlm.nih.gov/32765019/ (accessed on 28 December 2023). [CrossRef] [PubMed]
- Lima, E.M.; Cid, P.A.; Beck, D.S.; Pinheiro, L.H.Z.; Tonhá, J.P.S.; Alves, M.Z.O.; Lourenço, N.D.; Santos, R.Q.; Asensi, M.D.; Marques, J.A.; et al. Predictive factors for sepsis by carbapenem resistant Gram-negative bacilli in adult critical patients in Rio de Janeiro: A case-case-control design in a prospective cohort study. Antimicrob. Resist. Infect. Control 2020, 9, 132. Available online: https://pubmed.ncbi.nlm.nih.gov/32795380/ (accessed on 28 December 2023). [CrossRef]
| Score | Findings |
|---|---|
| Renal Corpuscle Degeneration | |
| 0 | ≤5% |
| 1 | Between 6–25% |
| 2 | Between 26–50% |
| 3 | ≥50% |
| Tubular Necrosis | |
| 0 | ≤5% |
| 1 | Between 6–25% |
| 2 | Between 26–50% |
| 3 | ≥50% |
| Excessive Protein Exudation | |
| 0 | ≤5% |
| 1 | Between 6–25% |
| 2 | Between 26–50% |
| 3 | ≥50% |
| Intraluminar Necrotic Cellular Debris | |
| 0 | ≤5% |
| 1 | Between 6–25% |
| 2 | Between 26–50% |
| 3 | ≥50% |
| Score | |
|---|---|
| 0 | None (less than 5%) |
| 1 | Mild (between 6% and 25%) |
| 2 | Moderate (between 26% and 50%) |
| 3 | Severe (more than 51%) |
| Groups | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | GP1: CLP-Induced Septic | GP2: CLP + FOS | p | |||||||
| Median | Minimum | Maximum | Median | Minimum | Maximum | Median | Minimum | Maximum | ||
| IL6 (pg/gr tissue) | 69.1 | 53.8 | 92.3 | 79.0 | 55.1 | 103.4 | 63.6 | 60.6 | 99.6 | 0.707 |
| IL1β (pg/gr tissue) | 575.3 | 525.0 | 602.6 | 551.5 | 500.3 | 622.6 | 536.5 | 471.5 | 595.1 | 0.463 |
| GSH (mmol/gr tissue) | 6041 | 5452 | 6829 | 5953 | 4184 | 7178 | 7287 * | 6281 | 7617 | 0.049 |
| MDA (nmol gr tissue) | 83.3 | 76.6 | 91.6 | 91.6 | 88.6 | 97.1 | 86.4 | 67.2 | 91.9 | 0.176 |
| Group | Renal Corpuscle Degeneration | Tubular Necrosis | Excessive Protein Exudation | Intraluminar Necrotic Cellular Debris | KHDS |
|---|---|---|---|---|---|
| Control | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| CLP | 2 (2–2) a | 2 (2–3) a | 2 (1–2) a | 1 (1–2) a | 9 (9–10) a |
| CLP + FOS | 0 (0–1) b,c | 1 (1–2) a,c | 1 (1–1) a,c | 1 (1–1) a,d | 4.5 (4–6) a,c |
| Group | Cleaved Caspase-3 Positivity | 8-OHdG Posivitiy |
|---|---|---|
| Control | 0 (0–0) | 0 (0–0) |
| CLP | 2 (2–3) a | 3 (2–3) a |
| CLP + FOS | 0 (0–1) b,c | 0.5 (0–1) b,d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yildiz, I.E.; Mercantepe, T.; Bahceci, I.; Arpa, M.; Batcik, S.; Yildiz, Y.; Tumkaya, L. Investigation of the Effects of Fosfomycin in Kidney Damage Caused by CLP-Induced Sepsis. Life 2025, 15, 2. https://doi.org/10.3390/life15010002
Yildiz IE, Mercantepe T, Bahceci I, Arpa M, Batcik S, Yildiz Y, Tumkaya L. Investigation of the Effects of Fosfomycin in Kidney Damage Caused by CLP-Induced Sepsis. Life. 2025; 15(1):2. https://doi.org/10.3390/life15010002
Chicago/Turabian StyleYildiz, Ilknur Esen, Tolga Mercantepe, Ilkay Bahceci, Medeni Arpa, Sule Batcik, Yasin Yildiz, and Levent Tumkaya. 2025. "Investigation of the Effects of Fosfomycin in Kidney Damage Caused by CLP-Induced Sepsis" Life 15, no. 1: 2. https://doi.org/10.3390/life15010002
APA StyleYildiz, I. E., Mercantepe, T., Bahceci, I., Arpa, M., Batcik, S., Yildiz, Y., & Tumkaya, L. (2025). Investigation of the Effects of Fosfomycin in Kidney Damage Caused by CLP-Induced Sepsis. Life, 15(1), 2. https://doi.org/10.3390/life15010002







