Metabolic Shift and Hyperosmolarity Underlie Age-Related Macular Degeneration
Abstract
1. Introduction
2. Inflammation Is the Driving Force in AMD
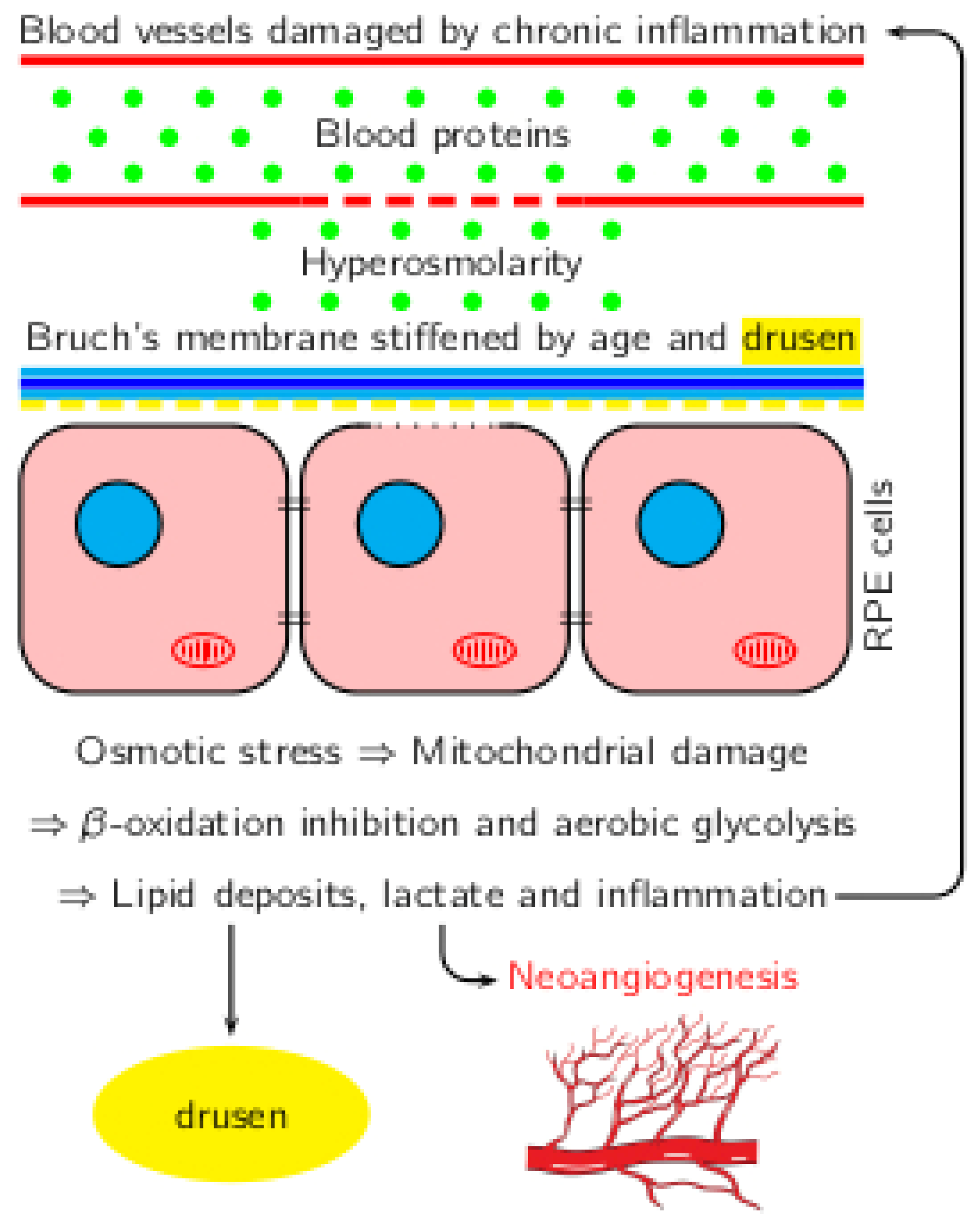
3. Increased Osmolality as the Driving Force for AMD
4. Osmosis in the Posterior Segment
5. Aging of the Posterior Segment: Vascular Changes
6. Increased Osmolarity and AMD
7. Metabolic Shift Induced by Increased Pressure
8. Novel Therapeutic Approaches to AMD
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fleckenstein, M.; Schmitz-Valckenberg, S.; Chakravarthy, U. Age-Related Macular Degeneration: A Review. JAMA 2024, 331, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Colijn, J.M.; Buitendijk, G.H.; Prokofyeva, E.; Alves, D.; Cachulo, M.L.; Khawaja, A.P.; Cougnard-Gregoire, A.; Merle, B.M.; Korb, C.; Erke, M.G. Prevalence of Age-Related Macular Degeneration in Europe: The Past and the Future. Ophthalmology 2017, 124, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Al-Zamil, W.M.; Yassin, S.A. Recent Developments in Age-Related Macular Degeneration: A Review. Clin. Interv. Aging 2017, 12, 1313–1330. [Google Scholar] [CrossRef] [PubMed]
- Buitendijk, G.H.; Rochtchina, E.; Myers, C.; van Duijn, C.M.; Lee, K.E.; Klein, B.E.; Meuer, S.M.; de Jong, P.T.; Holliday, E.G.; Tan, A.G. Prediction of Age-Related Macular Degeneration in the General Population: The Three Continent AMD Consortium. Ophthalmology 2013, 120, 2644–2655. [Google Scholar] [CrossRef]
- Hrishikesh, V.; Pranaykumar, S. Age-Related Macular Degeneration: Epidemiology, Pathophysiology, Diagnosis, and Treatment. Cureus 2022, 14, e29583. [Google Scholar]
- Madonna, R.; Giovannelli, G.; Confalone, P.; Renna, F.V.; Geng, Y.-J.; De Caterina, R. High Glucose-Induced Hyperosmolarity Contributes to COX-2 Expression and Angiogenesis: Implications for Diabetic Retinopathy. Cardiovasc. Diabetol. 2016, 15, 18. [Google Scholar] [CrossRef]
- Veltmann, M.; Hollborn, M.; Reichenbach, A.; Wiedemann, P.; Kohen, L.; Bringmann, A. Osmotic Induction of Angiogenic Growth Factor Expression in Human Retinal Pigment Epithelial Cells. PLoS ONE 2016, 11, e0147312. [Google Scholar] [CrossRef][Green Version]
- Arsenijevic, T.; Vujovic, A.; Libert, F.; Op de Beeck, A.; Hebrant, A.; Janssens, S.; Grégoire, F.; Lefort, A.; Bolaky, N.; Perret, J. Hyperosmotic Stress Induces Cell Cycle Arrest in Retinal Pigmented Epithelial Cells. Cell Death Dis. 2013, 4, e662. [Google Scholar] [CrossRef]
- Haddad, S.; Chen, C.A.; Santangelo, S.L.; Seddon, J.M. The Genetics of Age-Related Macular Degeneration: A Review of Progress to Date. Surv. Ophthalmol. 2006, 51, 316–363. [Google Scholar] [CrossRef]
- Klein, R.; Peto, T.; Bird, A.; Vannewkirk, M.R. The Epidemiology of Age-Related Macular Degeneration. Am. J. Ophthalmol. 2004, 137, 486–495. [Google Scholar] [CrossRef]
- Pawelec, G.; Goldeck, D.; Derhovanessian, E. Inflammation, Ageing and Chronic Disease. Curr. Opin. Immunol. 2014, 29, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Ginaldi, L.; Mengoli, L.P.; De Martinis, M. Osteoporosis, Inflammation and Ageing. Handb. Immunosenescence Basic Underst. Clin. Appl. 2009, 1329–1352. [Google Scholar]
- Sparrow, J.R. Bisretinoids of RPE Lipofuscin: Trigger for Complement Activation in Age-Related Macular Degeneration. Inflamm. Retin. Dis. Complement Biol. Pathol. 2010, 63–74. [Google Scholar]
- Telander, D.G. Inflammation and Age-Related Macular Degeneration (AMD); Taylor & Francis: Abingdon, UK, 2011; Volume 26, pp. 192–197. [Google Scholar]
- Dentchev, T.; Milam, A.H.; Lee, V.M.-Y.; Trojanowski, J.Q.; Dunaief, J.L. Amyloid-β Is Found in Drusen from Some Age-Related Macular Degeneration Retinas, but Not in Drusen from Normal Retinas. Am. J. Ophthalmol. 2003, 136, 787. [Google Scholar] [CrossRef]
- Malsy, J.; Alvarado, A.C.; Lamontagne, J.O.; Strittmatter, K.; Marneros, A.G. Distinct Effects of Complement and of NLRP3-and Non-NLRP3 Inflammasomes for Choroidal Neovascularization. eLife 2020, 9, e60194. [Google Scholar] [CrossRef]
- Braig, D.; Nero, T.L.; Koch, H.-G.; Kaiser, B.; Wang, X.; Thiele, J.R.; Morton, C.J.; Zeller, J.; Kiefer, J.; Potempa, L.A. Transitional Changes in the CRP Structure Lead to the Exposure of Proinflammatory Binding Sites. Nat. Commun. 2017, 8, 14188. [Google Scholar] [CrossRef]
- Chu, L.; Bi, C.; Wang, C.; Zhou, H. The Relationship between Complements and Age-Related Macular Degeneration and Its Pathogenesis. J. Ophthalmol. 2024, 2024, 6416773. [Google Scholar] [CrossRef]
- Roubeix, C.; Nous, C.; Augustin, S.; Ronning, K.E.; Mathis, T.; Blond, F.; Lagouge-Roussey, P.; Crespo-Garcia, S.; Sullivan, P.M.; Gautier, E.L. Splenic Monocytes Drive Pathogenic Subretinal Inflammation in Age-Related Macular Degeneration. J. Neuroinflammation 2024, 21, 22. [Google Scholar] [CrossRef]
- Schnabolk, G.; Obert, E.; Singh, S.; Guzman, W.; Husain, S. Effect of HDAC Inhibition on a Model of Dry AMD in the Presence of Systemic Inflammation. Investig. Ophthalmol. Vis. Sci. 2023, 64, 3909. [Google Scholar]
- Cotran, R.S.; Majno, G. The Delayed and Prolonged Vascular Leakage in Inflammation: I. Topography of the Leaking Vessels after Thermal Injury. Am. J. Pathol. 1964, 45, 261. [Google Scholar]
- Roviezzo, F.; Tsigkos, S.; Kotanidou, A.; Bucci, M.; Brancaleone, V.; Cirino, G.; Papapetropoulos, A. Angiopoietin-2 Causes Inflammation in Vivo by Promoting Vascular Leakage. J. Pharmacol. Exp. Ther. 2005, 314, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Abolhassani, M.; Wertz, X.; Pooya, M.; Chaumet-Riffaud, P.; Guais, A.; Schwartz, L. Hyperosmolarity Causes Inflammation through the Methylation of Protein Phosphatase 2A. Inflamm. Res. 2008, 57, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.; Guais, A.; Pooya, M.; Abolhassani, M. Is Inflammation a Consequence of Extracellular Hyperosmolarity? J. Inflamm. 2009, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Darwish, A.; Lui, F. Physiology, Colloid Osmotic Pressure; StatPearls: Tampa, FL, USA, 2019. [Google Scholar]
- Lüke, C.; Widder, R.A.; Walter, P.; Brunner, R.; Kirchhof, B.; Borberg, H. The Effect of Membrane Differential Filtration on the Colloid Osmotic Pressure in Patients with Age-related Macular Degeneration: Significance to Visual Function? Ther. Apher. Dial. 2003, 7, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Willermain, F.; Libert, S.; Motulsky, E.; Salik, D.; Caspers, L.; Perret, J.; Delporte, C. Origins and Consequences of Hyperosmolar Stress in Retinal Pigmented Epithelial Cells. Front. Physiol. 2014, 5, 199. [Google Scholar] [CrossRef] [PubMed]
- Henry, D.N.; Frank, R.N.; Hootman, S.R.; Rood, S.E.; Heilig, C.W.; Busik, J.V. Glucose-Specific Regulation of Aldose Reductase in Human Retinal Pigment Epithelial Cells in Vitro. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1554–1560. [Google Scholar]
- Omori, K.; Fujiseki, Y.; Omori, K.; Suzukawa, J.; Inagaki, C. Regulation of the Expression of Lysyl Oxidase mRNA in Cultured Rabbit Retinal Pigment Epithelium Cells. Matrix Biol. 2002, 21, 337–348. [Google Scholar] [CrossRef]
- Civan, M.; Marano, C.; Matschinsky, F.; Peterson-Yantorno, K. Prolonged Incubation with Elevated Glucose Inhibits the Regulatory Response to Shrinkage of Cultured Human Retinal Pigment Epithelial Cells. J. Membr. Biol. 1994, 139, 1–13. [Google Scholar] [CrossRef]
- Orgül, S.; Reuter, U.; Kain, H. Osmotic Stress in an in Vitro Model of the Outer Blood-Retinal Barrier. Ger. J. Ophthalmol. 1993, 2, 436–443. [Google Scholar]
- Carpene, G.; Onorato, D.; Nocini, R.; Fortunato, G.; Rizk, J.G.; Henry, B.M.; Lippi, G. Blood Lactate Concentration in COVID-19: A Systematic Literature Review. Clin. Chem. Lab. Med. 2022, 60, 332–337. [Google Scholar] [CrossRef]
- Adorante, J.S.; Miller, S.S. Potassium-Dependent Volume Regulation in Retinal Pigment Epithelium Is Mediated by Na, K, Cl Cotransport. J. Gen. Physiol. 1990, 96, 1153–1176. [Google Scholar] [CrossRef] [PubMed]
- Obika, L.O.; Amabebe, E.; Ozoene, J.; Inneh, C. Thirst Perception, Plasma Osmolality and Estimated Plasma Arginine Vasopressin Concentration in Dehydrated and Oral Saline Loaded Subjects. Niger. J. Physiol. Sci. 2013, 28, 83–89. [Google Scholar] [PubMed]
- He, F.J.; Markandu, N.D.; Sagnella, G.A.; de Wardener, H.E.; MacGregor, G.A. Plasma Sodium: Ignored and Underestimated. Hypertension 2005, 45, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Hollborn, M.; Vogler, S.; Reichenbach, A.; Wiedemann, P.; Bringmann, A.; Kohen, L. Regulation of the Hyperosmotic Induction of Aquaporin 5 and VEGF in Retinal Pigment Epithelial Cells: Involvement of NFAT5. Mol. Vis. 2015, 21, 360. [Google Scholar] [PubMed]
- Lemp, M.A.; Bron, A.J.; Baudouin, C.; Del Castillo, J.M.B.; Geffen, D.; Tauber, J.; Foulks, G.N.; Pepose, J.S.; Sullivan, B.D. Tear Osmolarity in the Diagnosis and Management of Dry Eye Disease. Am. J. Ophthalmol. 2011, 151, 792–798. [Google Scholar] [CrossRef]
- Gilbard, J.P.; Farris, R.L. Ocular Surface Drying and Tear Film Osmolarity in Thyroid Eye Disease. Acta Ophthalmol. 1983, 61, 108–116. [Google Scholar] [CrossRef]
- Anwar, Z.; Wellik, S.R.; Galor, A. Glaucoma Therapy and Ocular Surface Disease: Current Literature and Recommendations. Curr. Opin. Ophthalmol. 2013, 24, 136–143. [Google Scholar] [CrossRef]
- Jacob, T.; Duncan, G. Osmotic Influences on Lens Membrane Characteristics. Exp. Eye Res. 1980, 31, 505–512. [Google Scholar] [CrossRef]
- Asiedu, K.; Abu, S.L. The Impact of Topical Intraocular Pressure Lowering Medications on the Ocular Surface of Glaucoma Patients: A Review. J. Curr. Ophthalmol. 2019, 31, 8–15. [Google Scholar] [CrossRef]
- Joyal, J.-S.; Gantner, M.L.; Smith, L.E. Retinal Energy Demands Control Vascular Supply of the Retina in Development and Disease: The Role of Neuronal Lipid and Glucose Metabolism. Prog. Retin. Eye Res. 2018, 64, 131–156. [Google Scholar] [CrossRef]
- Alm, A.; Bill, A. Ocular and Optic Nerve Blood Flow at Normal and Increased Intraocular Pressures in Monkeys (Macaca Irus): A Study with Radioactively Labelled Microspheres Including Flow Determinations in Brain and Some Other Tissues. Exp. Eye Res. 1973, 15, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.J.; Clover, G.M. The Effect of Age on the Macromolecular Permeability of Human Bruch’s Membrane. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2970–2975. [Google Scholar]
- Pournaras, C.J.; Rungger-Brändle, E.; Riva, C.E.; Hardarson, S.H.; Stefansson, E. Regulation of Retinal Blood Flow in Health and Disease. Prog. Retin. Eye Res. 2008, 27, 284–330. [Google Scholar] [CrossRef] [PubMed]
- Hoseini-Yazdi, H.; Vincent, S.J.; Collins, M.J.; Read, S.A.; Alonso-Caneiro, D. Wide-Field Choroidal Thickness in Myopes and Emmetropes. Sci. Rep. 2019, 9, 3474. [Google Scholar] [CrossRef]
- Spaide, R.F.; Koizumi, H.; Pozonni, M.C. Enhanced Depth Imaging Spectral-Domain Optical Coherence Tomography. Am. J. Ophthalmol. 2008, 146, 496–500. [Google Scholar] [CrossRef]
- Margolis, R.; Spaide, R.F. A Pilot Study of Enhanced Depth Imaging Optical Coherence Tomography of the Choroid in Normal Eyes. Am. J. Ophthalmol. 2009, 147, 811–815. [Google Scholar] [CrossRef]
- Tokuda, S.; Yu, A.S. Regulation of Epithelial Cell Functions by the Osmolality and Hydrostatic Pressure Gradients: A Possible Role of the Tight Junction as a Sensor. Int. J. Mol. Sci. 2019, 20, 3513. [Google Scholar] [CrossRef]
- Shirao, Y.; Steinberg, R.H. Mechanisms of Effects of Small Hyperosmotic Gradients on the Chick RPE. Investig. Ophthalmol. Vis. Sci. 1987, 28, 2015–2025. [Google Scholar]
- Kvanta, A.; Algvere, P.; Berglin, L.; Seregard, S. Subfoveal Fibrovascular Membranes in Age-Related Macular Degeneration Express Vascular Endothelial Growth Factor. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1929–1934. [Google Scholar] [CrossRef]
- Marmor, M.F. Retinal Detachment from Hyperosmotic Intravitreal Injection. Investig. Ophthalmol. Vis. Sci. 1979, 18, 1237–1244. [Google Scholar]
- Turner, J.L.; Bierman, E.L. Effects of Glucose and Sorbitol on Proliferation of Cultured Human Skin Fibroblasts and Arterial Smooth-Muscle Cells. Diabetes 1978, 27, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Pennesi, M.E.; Neuringer, M.; Courtney, R.J. Animal Models of Age Related Macular Degeneration. Mol. Asp. Med. 2012, 33, 487–509. [Google Scholar] [CrossRef] [PubMed]
- Lyzogubov, V.V.; Tytarenko, R.G.; Liu, J.; Bora, N.S.; Bora, P.S. Polyethylene Glycol (PEG)-Induced Mouse Model of Choroidal Neovascularization. J. Biol. Chem. 2011, 286, 16229–16237. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.; Logan, C.; Lyzogubov, V.V.; Bora, N.S.; Bora, P.S. Wet and Dry Age-Related Macular Degeneration Induced by Polyethylene Glycol. Preprints 2023, 2023071318, V1. [Google Scholar]
- Christen, R.; Schackmann, R.; Shapiro, B. Metabolism of Sea Urchin Sperm. Interrelationships between Intracellular pH, ATPase Activity, and Mitochondrial Respiration. J. Biol. Chem. 1983, 258, 5392–5399. [Google Scholar] [CrossRef]
- Sinning, A.; Hübner, C.A. Minireview: pH and Synaptic Transmission. FEBS Lett. 2013, 587, 1923–1928. [Google Scholar] [CrossRef]
- Schwartz, L.; Peres, S.; Jolicoeur, M.; da Veiga Moreira, J. Cancer and Alzheimer’s Disease: Intracellular pH Scales the Metabolic Disorders. Biogerontology 2020, 21, 683–694. [Google Scholar] [CrossRef]
- Zebhauser, P.T.; Berthele, A.; Goldhardt, O.; Diehl-Schmid, J.; Priller, J.; Ortner, M.; Grimmer, T. Cerebrospinal Fluid Lactate Levels along the Alzheimer’s Disease Continuum and Associations with Blood-Brain Barrier Integrity, Age, Cognition, and Biomarkers. Alzheimer Res. Ther. 2022, 14, 1–8. [Google Scholar]
- Demetrius, L.A.; Simon, D.K. An Inverse-Warburg Effect and the Origin of Alzheimer’s Disease. Biogerontology 2012, 13, 583–594. [Google Scholar] [CrossRef]
- Poitry-Yamate, C.L.; Poitry, S.; Tsacopoulos, M. Lactate Released by Muller Glial Cells Is Metabolized by Photoreceptors from Mammalian Retina. J. Neurosci. 1995, 15, 5179–5191. [Google Scholar] [CrossRef]
- Ng, S.K.; Wood, J.P.; Chidlow, G.; Han, G.; Kittipassorn, T.; Peet, D.J.; Casson, R.J. Cancer-like Metabolism of the Mammalian Retina. Clin. Exp. Ophthalmol. 2015, 43, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, A.; Reichenbach, A. Role of Muller Cells in Retinal Degenerations. Front. Biosci. 2001, 6, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Yokosako, K.; Mimura, T.; Funatsu, H.; Noma, H.; Goto, M.; Kamei, Y.; Kondo, A.; Matsubara, M. Glycolysis in Patients with Age-Related Macular Degeneration. Open Ophthalmol. J. 2014, 8, 39. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dhup, S.; Kumar Dadhich, R.; Ettore Porporato, P.; Sonveaux, P. Multiple Biological Activities of Lactic Acid in Cancer: Influences on Tumor Growth, Angiogenesis and Metastasis. Curr. Pharm. Des. 2012, 18, 1319–1330. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, J.; Sun, X. Resistance to Anti-VEGF Therapy in Neovascular Age-Related Macular Degeneration: A Comprehensive Review. Drug Des. Dev. Ther. 2016, 10, 1857–1867. [Google Scholar]
- Reber, F.; Kasper, M.; Siegner, A.; Kniep, E.; Seigel, G.; Funk, R.H. Alteration of the Intracellular pH and Apoptosis Induction in a Retinal Cell Line by the AGE-Inducing Agent Glyoxal. Graefe’s Arch. Clin. Exp. Ophthalmol. 2002, 240, 1022–1032. [Google Scholar] [CrossRef]
- Kumar, A.; Haery, C.; Paladugu, B.; Kumar, A.; Symeoneides, S.; Taiberg, L.; Osman, J.; Trenholme, G.; Opal, S.M.; Goldfarb, R. The Duration of Hypotension before the Initiation of Antibiotic Treatment Is a Critical Determinant of Survival in a Murine Model of Escherichia Coli Septic Shock: Association with Serum Lactate and Inflammatory Cytokine Levels. J. Infect. Dis. 2006, 193, 251–258. [Google Scholar] [CrossRef]
- Marcoux, J.; McArthur, D.A.; Miller, C.; Glenn, T.C.; Villablanca, P.; Martin, N.A.; Hovda, D.A.; Alger, J.R.; Vespa, P.M. Persistent Metabolic Crisis as Measured by Elevated Cerebral Microdialysis Lactate-Pyruvate Ratio Predicts Chronic Frontal Lobe Brain Atrophy after Traumatic Brain Injury. Crit. Care Med. 2008, 36, 2871–2877. [Google Scholar] [CrossRef]
- Ikizawa, T.; Ikeda, K.; Arita, M.; Kitajima, S.; Soga, T.; Ichijo, H.; Naguro, I. Mitochondria Directly Sense Osmotic Stress to Trigger Rapid Metabolic Remodeling via Regulation of Pyruvate Dehydrogenase Phosphorylation. J. Biol. Chem. 2023, 299, 102837. [Google Scholar] [CrossRef]
- Samra, Y.A.; Zaidi, Y.; Rajpurohit, P.; Raghavan, R.; Cai, L.; Kaddour-Djebbar, I.; Tawfik, A. Warburg Effect as a Novel Mechanism for Homocysteine-Induced Features of Age-Related Macular Degeneration. Int. J. Mol. Sci. 2023, 24, 1071. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Chien, Y.; Yarmishyn, A.A.; Lim, L.-Y.; Tsai, H.-Y.; Kuo, W.-C.; Tsai, P.-H.; Yang, S.-H.; Hong, S.-I.; Chen, S.-J. Inhibition of Oxidative Stress-Induced Epithelial-Mesenchymal Transition in Retinal Pigment Epithelial Cells of Age-Related Macular Degeneration Model by Suppressing ERK Activation. J. Adv. Res. 2024, 60, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Macular Photocoagulation Study Group. Laser Photocoagulation of Subfoveal Neovascular Lesions in Age-Related Macular Degeneration: Results of a Randomized Clinical Trial. Arch. Ophthalmol. 1991, 109, 1220–1231. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.; Korobelnik, J.-F.; Lanzetta, P.; Holz, F.G.; Pruente, C.; Schmidt-Erfurth, U.; Tano, Y.; Wolf, S. Ranibizumab (Lucentis) in Neovascular Age-Related Macular Degeneration: Evidence from Clinical Trials. Br. J. Ophthalmol. 2010, 94, 2–13. [Google Scholar] [CrossRef] [PubMed]
- de Guimaraes, T.A.C.; Varela, M.D.; Georgiou, M.; Michaelides, M. Treatments for Dry Age-Related Macular Degeneration: Therapeutic Avenues, Clinical Trials and Future Directions. Br. J. Ophthalmol. 2022, 106, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Evangeliou, A.; Vlassopoulos, D. Carnitine Metabolism and Deficit-When Supplementation Is Necessary? Curr. Pharm. Biotechnol. 2003, 4, 211–219. [Google Scholar] [CrossRef]
- Napoli, E.; Dueñas, N.; Giulivi, C. Potential Therapeutic Use of the Ketogenic Diet in Autism Spectrum Disorders. Front. Pediatr. 2014, 2, 69. [Google Scholar] [CrossRef]
- Gough, S.; Casella, A.; Ortega, K.; Hackam, A. Neuroprotection by the Ketogenic Diet: Evidence and Controversies. Front. Nutr. 2021, 8, 782657. [Google Scholar] [CrossRef]
- Gough, S.M. Beneficial Effects of the Ketogenic Diet and Ketone Body Metabolism in Different Models of Neurodegeneration. Ph.D. Thesis, University of Miami, Miami, FL, USA, 2021. [Google Scholar]
- Ryals, R.C.; Huang, S.J.; Wafai, D.; Bernert, C.; Steele, W.; Six, M.; Bonthala, S.; Titus, H.; Yang, P.; Gillingham, M. A Ketogenic & Low-Protein Diet Slows Retinal Degeneration in Rd10 Mice. Transl. Vis. Sci. Technol. 2020, 9, 18. [Google Scholar]
- Tao, Y.; Jiang, P.; Wei, Y.; Wang, P.; Sun, X.; Wang, H. α-Lipoic Acid Treatment Improves Vision-Related Quality of Life in Patients with Dry Age-Related Macular Degeneration. Tohoku J. Exp. Med. 2016, 240, 209–214. [Google Scholar] [CrossRef]
- Yang, S.-H.; Li, W.; Sumien, N.; Forster, M.; Simpkins, J.W.; Liu, R. Alternative Mitochondrial Electron Transfer for the Treatment of Neurodegenerative Diseases and Cancers: Methylene Blue Connects the Dots. Prog. Neurobiol. 2017, 157, 273–291. [Google Scholar] [CrossRef]
- Bruchey, A.K.; Gonzalez-Lima, F. Behavioral, Physiological and Biochemical Hormetic Responses to the Autoxidizable Dye Methylene Blue. Am. J. Pharmacol. Toxicol. 2008, 3, 72. [Google Scholar] [CrossRef] [PubMed]
- Montegut, L.; Martinez-Basilio, P.C.; da Veiga Moreira, J.; Schwartz, L.; Jolicoeur, M. Combining Lipoic Acid to Methylene Blue Reduces the Warburg Effect in CHO Cells: From TCA Cycle Activation to Enhancing Monoclonal Antibody Production. PLoS ONE 2020, 15, e0231770. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Rojas, J.C.; Gonzalez-Lima, F. Methylene Blue Prevents Neurodegeneration Caused by Rotenone in the Retina. Neurotox. Res. 2006, 9, 47–57. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Macular Degeneration |
|---|---|
| Extracellular osmolarity | Increases |
| Oxidative phosphorylation | Decreases in photoreceptors |
| Extracellular lactate concentration | Increases |
| Intracellular pH | Decreases in photoreceptors |
| Cell ecosystem | Photoreceptors + Müller cells |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwartz, L.; Schwartz, J.; Henry, M.; Bakkar, A. Metabolic Shift and Hyperosmolarity Underlie Age-Related Macular Degeneration. Life 2024, 14, 1189. https://doi.org/10.3390/life14091189
Schwartz L, Schwartz J, Henry M, Bakkar A. Metabolic Shift and Hyperosmolarity Underlie Age-Related Macular Degeneration. Life. 2024; 14(9):1189. https://doi.org/10.3390/life14091189
Chicago/Turabian StyleSchwartz, Laurent, Jules Schwartz, Marc Henry, and Ashraf Bakkar. 2024. "Metabolic Shift and Hyperosmolarity Underlie Age-Related Macular Degeneration" Life 14, no. 9: 1189. https://doi.org/10.3390/life14091189
APA StyleSchwartz, L., Schwartz, J., Henry, M., & Bakkar, A. (2024). Metabolic Shift and Hyperosmolarity Underlie Age-Related Macular Degeneration. Life, 14(9), 1189. https://doi.org/10.3390/life14091189







