N6-Methyladenosine Modification-Related Genes Express Differentially in Sterile Male Cattle-Yaks
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Animals
2.3. Testes Measurement
2.4. Hematoxylin and Eosin (H&E) Staining Assays
2.5. Reverse-Transcription Quantitative Polymerase Chain Reaction (RT-qPCR) Assays
2.6. Immunohistochemistry (IHC) Assays
2.7. Western Blotting Assays
2.8. Immunofluorescence Assays
2.9. Statistical Analyses
3. Results
3.1. Morphological and Histological Differences in Testes
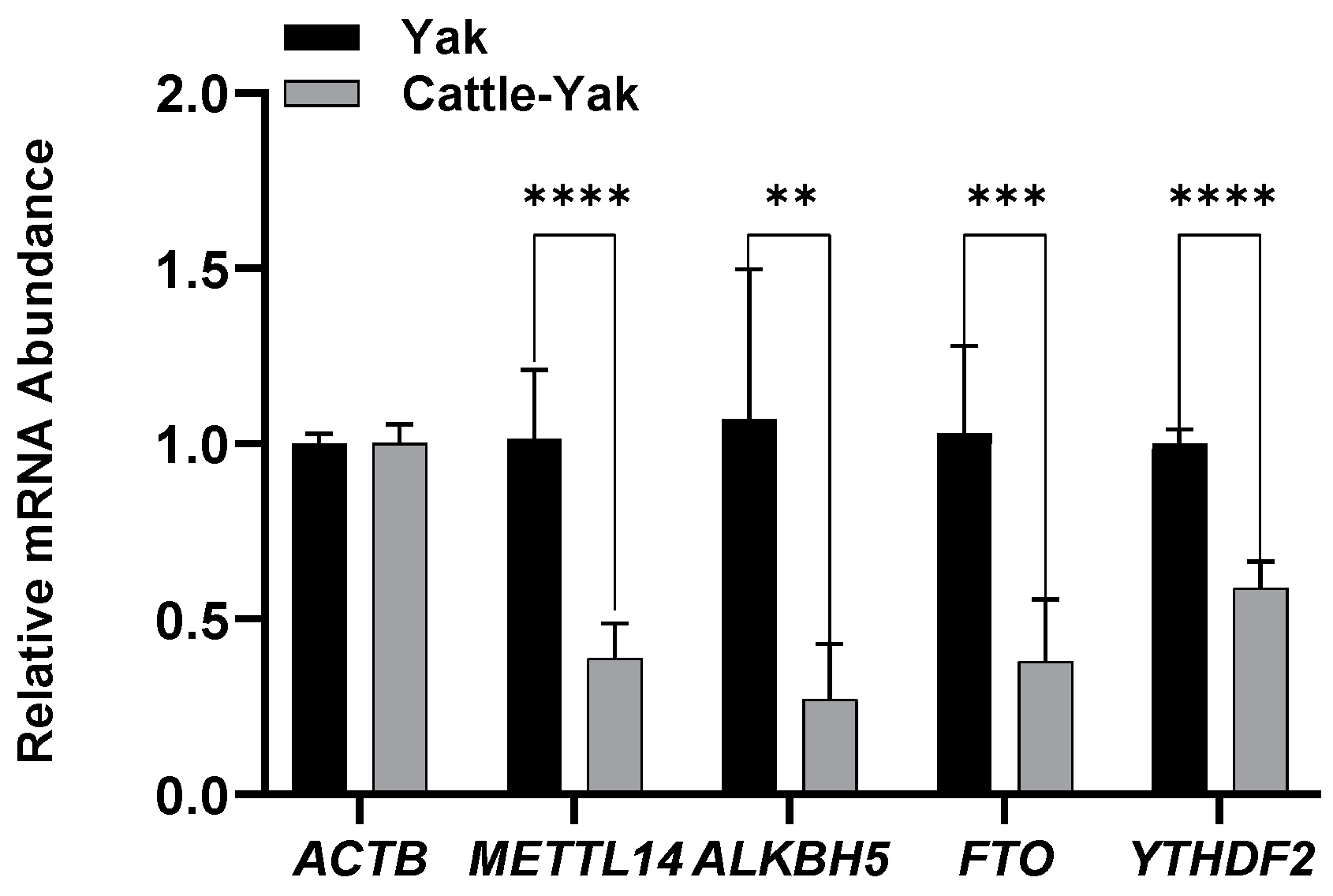
3.2. Expression of m6A-Associated Genes in the Testicular Tissue
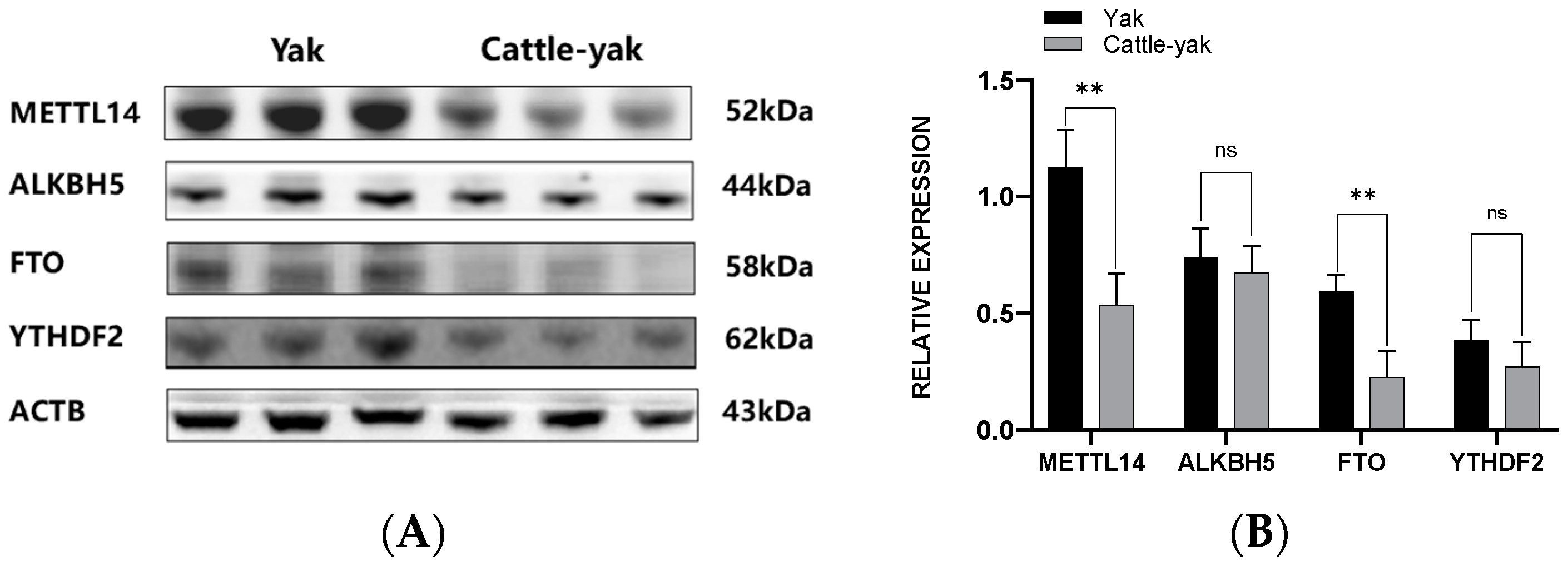
3.3. Protein Expression of m6A-Associated Genes
3.3.1. Western Blotting Assay
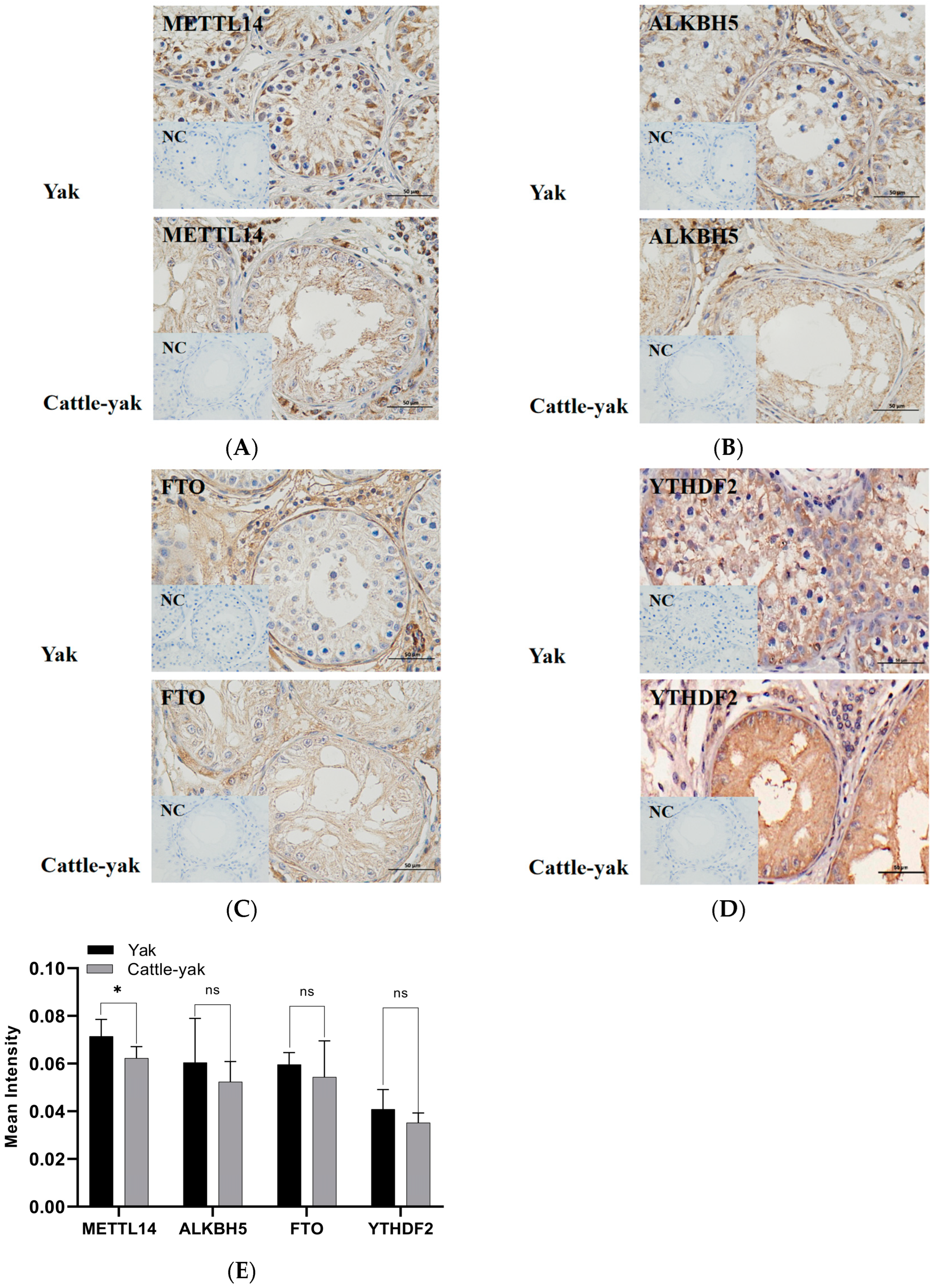
3.3.2. Immunohistochemistry (IHC) Assay
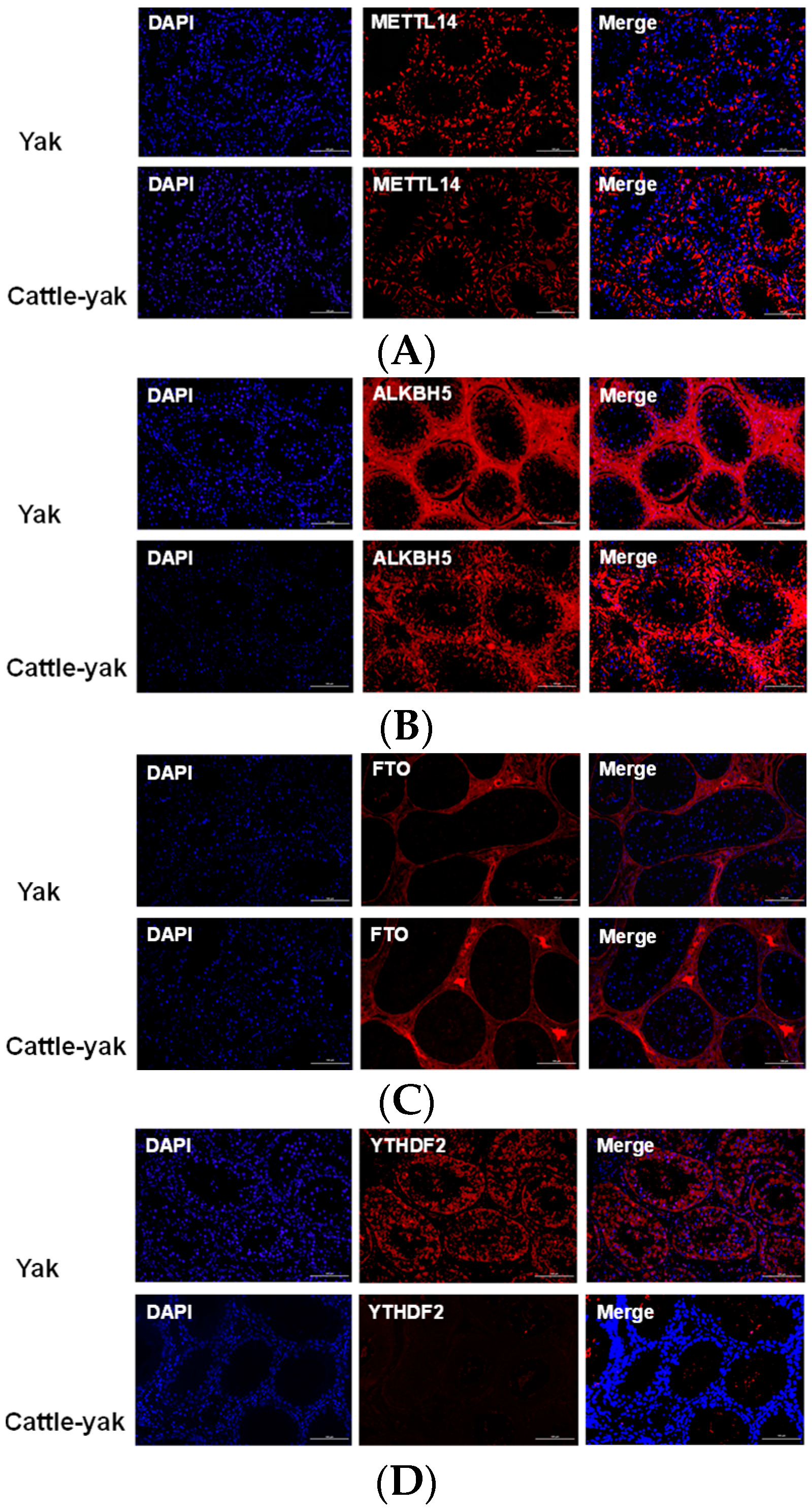
3.3.3. Immunofluorescence Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Min, X.Y.; Xiong, X.R.; Xiong, Y.; Zhang, H.; Hao, Z.Y.; Li, J. Research progress on sterility mechanisms of male cattle-yak. Chin. J. Anim. Sci. 2021, 57, 46–52. [Google Scholar]
- Jin, Y.C.; Yan, Z.X.; Li, S.S.; Liu, L.J. The research progress on male sterility of cattle-yak. Chin. Qinghai J. Anim. Vet. Sci. 2017, 47, 41–44. [Google Scholar]
- Qiu, Q.; Zhang, G.; Ma, T.; Qian, W.; Wang, J.; Ye, Z.; Cao, C.; Hu, Q.; Kim, J.; Larkin, M.D.; et al. The yak genome and adaptation to life at high altitude. Nat. Genet. 2012, 44, 946–949. [Google Scholar] [CrossRef] [PubMed]
- Mallet, J. Hybrid speciation. Nature 2007, 446, 279–283. [Google Scholar] [CrossRef]
- Gray, A.P. Mammalians Hybrids Commonwealth; Cambridge University Press: Cambridge, UK, 1954. [Google Scholar]
- Wodsedalek, J.E. Cause of the sterility in the mule. Biol. Bull. 1916, 30, 56. [Google Scholar] [CrossRef]
- Sato, Y.; Kuriwaki, R.; Hagino, S.; Shimazaki, M.; Sambuu, R.; Hirata, M.; Tanihara, F.; Takagi, M.; Taniguchi, M.; Otoi, T. Abnormal functions of leydig cells in crossbred cattle-yak showing infertility. Reprod. Domest. Anim. 2020, 55, 209–216. [Google Scholar] [CrossRef]
- Liu, H.; Wang, L.; Zheng, Y. Quantitative histological study of the distal cells of yak, yellow cattle and yak pituitary. Chin. Yak 1994, 1, 34–36. [Google Scholar]
- Luo, X.; Wu, K.; Yang, R. The physiological mechanism of normal sexual behaviour of male yak from the diurnal changes of LH, T, P4, 17B-E2 in blood. Herbivore 1996, 2, 41–43, 48. [Google Scholar]
- Niayale, R.; Cui, Y.; Adzitey, F. Male hybrid sterility in the cattle-yak and other bovines: A review. Biol. Reprod. 2021, 104, 495–507. [Google Scholar] [CrossRef]
- Gupta, N.; Yadav, B.R.; Gupta, S.C.; Kumar, P.; Sahai, R. Chromosomes of domestic yak (Bos grunniens). Indian J. Anim. Sci. 1996, 66, 742–745. [Google Scholar]
- Zhou, J.P.; Zhang, Z.P.; Hu, Y.; Qiu, G.F. Relationship between meiosis and sterility of yak outbreeding progeny. China Cattle Sci. 1999, 25, 12–15. [Google Scholar]
- Hu, O.M.; Zhong, J.C.; Zh, C.; Cai, L. A study on relationship between synaptonemal complex and male sterility of cattleyak. J. Southwest Natl. Coll. 2000, 26, 61–66. [Google Scholar]
- Zhang, Q.; Li, J.; Li, Q.; Li, X.; Liu, Z.; Song, D.; Xie, Z. Cloning and characterization of the gene encoding the bovine BOULE protein. Mol. Genet. Genom. 2009, 281, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Hsu, P.J.; Xing, X.; Fang, J.; Lu, Z.; Zou, Q.; Zhang, K.J.; Zhang, X.; Zhou, Y.; Zhang, T.; et al. Mettl3-/Mettl14-mediated mRNA N6-methyladenosine modulates murine spermatogenesis. Cell Res. 2017, 27, 1216–1230. [Google Scholar] [CrossRef]
- Zheng, G.; Dahl, J.A.; Niu, Y.; Fedorcsak, P.; Huang, C.M.; Li, C.J.; Vågbø, C.B.; Shi, Y.; Wang, W.L.; Song, S.H.; et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell 2013, 49, 18–29. [Google Scholar] [CrossRef]
- Zhao, X.; Lin, Z.; Fan, Y.; Li, W.; Zhang, Y.; Li, F.; Hong, T.; Feng, H.; Tong, M.; Wang, N.; et al. YTHDF2 is essential for spermatogenesis and fertility by mediating a wave of transcriptional transition in spermatogenic cells. Acta Biochim. Biophys. Sin. 2021, 53, 1702–1712. [Google Scholar] [CrossRef]
- Hsu, P.J.; Zhu, Y.; Ma, H.; Guo, Y.; Shi, X.; Liu, Y.; Qi, M.; Lu, Z.; Shi, H.; Wang, J.; et al. Ythdc2 is an N6-methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Res. 2017, 27, 1115–1127. [Google Scholar] [CrossRef]
- Zhang, J.M. The Relationship between the m6A Demethylase ALKBH5 and Yattle Male Sterility and the Targeted Regulation Analysis of miRNA-200a. Master Thesis, Southwest University, Chongqing, China, 2021. [Google Scholar]
- Chen, H.; Zhang, J.; Yan, Y.; Zhu, C.; Wang, L.; Fu, S.; Zuo, F.; Zhang, G.W. N6-methyladenosine RNA demethylase ALKBH5 is testis-specifically downregulated in hybrid male sterile dzo and is a target gene of bta-miR-200a. Theriogenology 2022, 187, 51–57. [Google Scholar] [CrossRef]
- Shen, M.; Guo, M.; Li, Y.; Wang, Y.; Qiu, Y.; Shao, J.; Zhang, F.; Xu, X.; Yin, G.; Wang, S.; et al. m6A methylation is required for dihydroartemisinin to alleviate liver fibrosis by inducing ferroptosis in hepatic stellate cells. Free Radic. Biol. Med. 2022, 182, 246–259. [Google Scholar] [CrossRef]
- Wang, P.; Doxtader, K.A.; Nam, Y. Structural Basis for Cooperative Function of Mettl3 and Mettl14 Methyltransferases. Mol. Cell 2016, 63, 306–317. [Google Scholar] [CrossRef]
- Ping, X.L.; Sun, B.F.; Wang, L.; Xiao, W.; Yang, X.; Wang, W.J.; Adhikari, S.; Shi, Y.; Lv, Y.; Chen, Y.S.; et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014, 24, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Fu, Y.; Zhao, X.; Dai, Q.; Zheng, G.; Yang, Y.; Yi, C.; Lindahl, T.; Pan, T.; Yang, Y.G.; et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 2011, 7, 885–887. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Zhao, Y.; He, J.; Zhang, Y.; Xi, H.; Liu, M.; Ma, J.; Wu, L. YTHDF2 destabilizes m(6)A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat. Commun. 2016, 7, 12626. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Kong, S.; Tao, M.; Ju, S. The potential role of RNA N6-methyladenosine in cancer progression. Mol. Cancer 2020, 19, 88. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, Y.; Yang, Q.; Xu, S.; Ma, S.; Yan, R.; Zhang, R.; Jia, G.; Ai, D.; Yang, Q. Gene expression dynamics during the gonocyte to spermatogonia transition and spermatogenesis in the domestic yak. J. Anim. Sci. Biotechnol. 2019, 10, 64. [Google Scholar] [CrossRef]
- Cai, X.; Yu, S.; Mipam, T.; Yang, F.; Zhao, W.; Liu, W.; Cao, S.; Shen, L.; Zhao, F.; Sun, L.; et al. Comparative analysis of testis transcriptomes associated with male infertility in cattleyak. Theriogenology 2017, 88, 28–42. [Google Scholar] [CrossRef]
- Wu, S.; Mipam, T.; Xu, C.; Zhao, W.; Shah, M.A.; Yi, C.; Luo, H.; Cai, X.; Zhong, J. Testis transcriptome profiling identified genes involved in spermatogenic arrest of cattleyak. PLoS ONE 2020, 15, e0229503. [Google Scholar] [CrossRef]
- Civetta, A. Misregulation of Gene Expression and Sterility in Interspecies Hybrids: Causal Links and Alternative Hypotheses. J. Mol. Evol. 2016, 82, 176–182. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Wu, S.X.; Wang, G.W.; Wan, R.D.; Yang, Q.E. Single-cell analysis identifies critical regulators of spermatogonial development and differentiation in cattle-yak bulls. J. Dairy. Sci. 2024, 107, 7317–7336. [Google Scholar] [CrossRef]
- Fu, R.; Wei, Y.P.; Meng, R.; Wu, K.X.; Chen, S.M.; Zhang, L.C.; Wang, X.Z. Establishment of a real-time PCR assay for detection of Dazl in cattle, yak and cattle-yak testis. Acta Agric. Boreali-Occident. Sin. 2013, 22, 12–18. [Google Scholar]
- Ruggiu, M.; Speed, R.; Taggart, M.; McKay, S.J.; Kilanowski, F.; Saunders, P.; Dorin, J.; Cooke, H.J. The mouse Dazla gene encodes a cytoplasmic protein essential for gametogenesis. Nature 1997, 389, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wei, Y.P.; Chen, S.M. Expression level of Boule gene mRNA in testes of yellow cattle, yak and Dzo. Chin. J. Anim. Sci. 2013, 49, 10–13. [Google Scholar]
- Luo, H.; Jia, C.; Qu, X.G.; Zhao, X.B.; Zhong, J.C.; Xie, Z.; Li, Q.F. Cloning of b-Sycp2 gene and expression level of mRNA in testis of yellow cattle, yak and Dzo. Sci. Agric. Sin. 2013, 46, 367–375. [Google Scholar]
- Wang, S.; Pan, Z.; Zhang, Q.; Xie, Z.; Liu, H.; Li, Q. Differential mRNA expression and promoter methylation status of SYCP3 gene in testes of yaks and cattle-yaks. Reprod. Domest. Anim. 2012, 47, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Li, B.J.; Luo, H.; Qu, X.G.; Pan, Z.X.; Xie, Z.; Liu, H.L.; Li, Q.F. Analysis of expression characteristics of synaptonemal complex protein b-FKBP6 gene in yak and Dzo. J. Nanjing Agric. Univ. 2013, 36, 129–132. [Google Scholar]
- Zhou, Y.; Luo, H.; Li, B.J.; Jia, C.; Xie, Z.; Zhao, X.B.; Zhong, J.C.; Li, Q.F. mRNA expression level and promoter methylation of DDX4 gene in testes of yak and cattle-yak. Sci. Agric. Sin. 2013, 46, 630–638. [Google Scholar]
- Dai, L.S. Regulation of Testis-Specific miRNA-469 by GRTH/DDX25 Controls Male Germ Cells Development. PhD Thesis, Jilin University, Jilin, China, 2011. [Google Scholar]
- Zhao, S.S.; Wu, S.X.; Jia, G.X.; Abulizi, W.; Yang, Q.E. Localization and expression of SLX4 in the testis of sterile male cattle-yak. Reprod. Domest. Anim. 2023, 58, 679–687. [Google Scholar] [CrossRef]
- Yang, Q.; Xie, Y.; Pan, B.; Cheng, Y.; Zhu, Y.; Fei, X.; Li, X.; Yu, J.; Chen, Z.; Li, J.; et al. The Expression and Epigenetic Characteristics of the HSF2 Gene in Cattle-Yak and the Correlation with Its Male Sterility. Animals 2024, 14, 1410. [Google Scholar] [CrossRef]
- Wu, S.-X.; Wang, G.W.; Fang, Y.-G.; Chen, Y.-W.; Jin, Y.-Y.; Liu, X.-T.; Jia, G.-X.; Yang, Q.-E. Transcriptome analysis reveals dysregulated gene expression networks in Sertoli cells of cattle-yak hybrids. Theriogenology 2023, 203, 33–42. [Google Scholar] [CrossRef]
- Mipam, T.; Chen, X.; Zhao, W.; Zhang, P.; Chai, Z.; Yue, B.; Luo, H.; Wang, J.; Wang, H.; Wu, Z.; et al. Single-cell transcriptome analysis and in vitro differentiation of testicular cells reveal novel insights into male sterility of the interspecific hybrid cattle-yak. BMC Genom. 2023, 24, 149. [Google Scholar] [CrossRef]
- Wang, X.; Pei, J.; Xiong, L.; Kang, Y.; Guo, S.; Cao, M.; Ding, Z.; Bao, P.; Chu, M.; Liang, C.; et al. Single-cell RNA sequencing and UPHLC-MS/MS targeted metabolomics offer new insights into the etiological basis for male cattle-yak sterility. Int. J. Biol. Macromol. 2023, 253 Pt 3, 126831. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Klukovich, R.; Peng, H.; Wang, Z.; Yu, T.; Zhang, Y.; Zheng, H.; Klungland, A.; Yan, W. ALKBH5-dependent m6A demethylation controls splicing and stability of long 3′-UTR mRNAs in male germ cells. Proc. Natl. Acad. Sci. USA 2018, 115, E325–E333. [Google Scholar] [CrossRef] [PubMed]
- Soh, Y.Q.S.; Mikedis, M.M.; Kojima, M.; Godfrey, A.K.; de Rooij, D.G.; Page, D.C. Meioc maintains an extended meiotic prophase I in mice. PLoS Genet. 2017, 13, e1006704. [Google Scholar] [CrossRef] [PubMed]
- Landfors, M.; Nakken, S.; Fusser, M.; Dahl, J.A.; Klungland, A.; Fedorcsak, P. Sequencing of FTO and ALKBH5 in men undergoing infertility work-up identifies an infertility-associated variant and two missense mutations. Fertil. Steril. 2016, 105, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Zhong, C.; Wu, X.; Chen, J.; Tao, B.; Xia, X.; Shi, M.; Zhu, Z.; Trudeau, V.L.; Hu, W. Mettl3 Mutation Disrupts Gamete Maturation and Reduces Fertility in Zebrafish. Genetics 2018, 208, 729–743. [Google Scholar] [CrossRef]
- Wang, X.; Pei, J.; Guo, S.; Cao, M.; Kang, Y.; Xiong, L.; La, Y.; Bao, P.; Liang, C.; Yan, P.; et al. Characterization of N6-methyladenosine in cattle-yak testis tissue. Front. Vet. Sci. 2022, 9, 971515. [Google Scholar] [CrossRef]

| Testicular Length (cm) | Yak | Cattle-Yak |
|---|---|---|
| 1 | 7.3 | 6.4 |
| 2 | 7.1 | 6 |
| 3 | 6.6 | 5.5 |
| 4 | 6.8 | 5.2 |
| 5 | 7.5 | 6.4 |
| 6 | 7.6 | 6.1 |
| mean ± SD | 7.150 ± 0.3937 | 5.933 ± 0.4885 *** |
| Primer Name | Primer Sequences (5′–3′) |
|---|---|
| METTL14-F | GCAGAAGTTACGTCGACAGC |
| METTL14-R | AGGTATCATAGGAAGCCCTGC |
| ALKBH5-F | CGTGACTGTGCTCAGTGGATA |
| ALKBH5-R | CGGGGTGCATCTAATCTCGT |
| FTO-F | GAGCGCGAAGCTAAGAAAAGA |
| FTO-R | TCTGTGCATCAAGGATGGCT |
| YTHDF2-F | GGCAGTGGGTTCGGTCATAA |
| YTHDF2-R | ACCGAAGCTTCTCCAAGACG |
| ACTB-F | CACAGGCCTCTCGCCTTC |
| ACTB-R | ATCATCCATGGCGAACTGGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Chen, L.; Jiang, H.; Wang, H.; Zhang, Y.; Yuan, Z.; Ma, Y. N6-Methyladenosine Modification-Related Genes Express Differentially in Sterile Male Cattle-Yaks. Life 2024, 14, 1155. https://doi.org/10.3390/life14091155
Liu Y, Chen L, Jiang H, Wang H, Zhang Y, Yuan Z, Ma Y. N6-Methyladenosine Modification-Related Genes Express Differentially in Sterile Male Cattle-Yaks. Life. 2024; 14(9):1155. https://doi.org/10.3390/life14091155
Chicago/Turabian StyleLiu, Yuxin, Lili Chen, Hui Jiang, Hongzhuang Wang, Yujiao Zhang, Zhengrong Yuan, and Yi Ma. 2024. "N6-Methyladenosine Modification-Related Genes Express Differentially in Sterile Male Cattle-Yaks" Life 14, no. 9: 1155. https://doi.org/10.3390/life14091155
APA StyleLiu, Y., Chen, L., Jiang, H., Wang, H., Zhang, Y., Yuan, Z., & Ma, Y. (2024). N6-Methyladenosine Modification-Related Genes Express Differentially in Sterile Male Cattle-Yaks. Life, 14(9), 1155. https://doi.org/10.3390/life14091155







