A Sole Case of the FGF23 Gene Mutation c.202A>G (p.Thr68Ala) Associated with Multiple Severe Vascular Aneurysms and a Hyperphosphatemic Variant of Tumoral Calcinosis—A Case Report
Abstract
1. Introduction
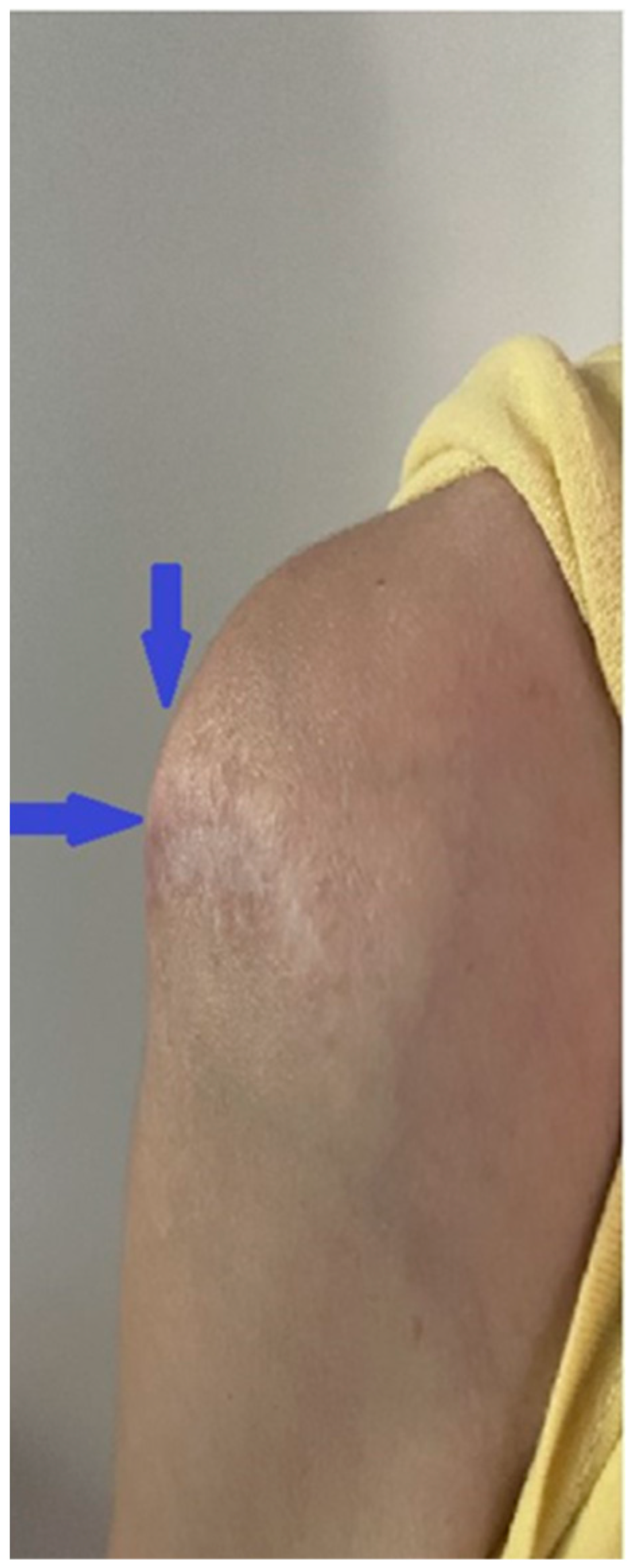
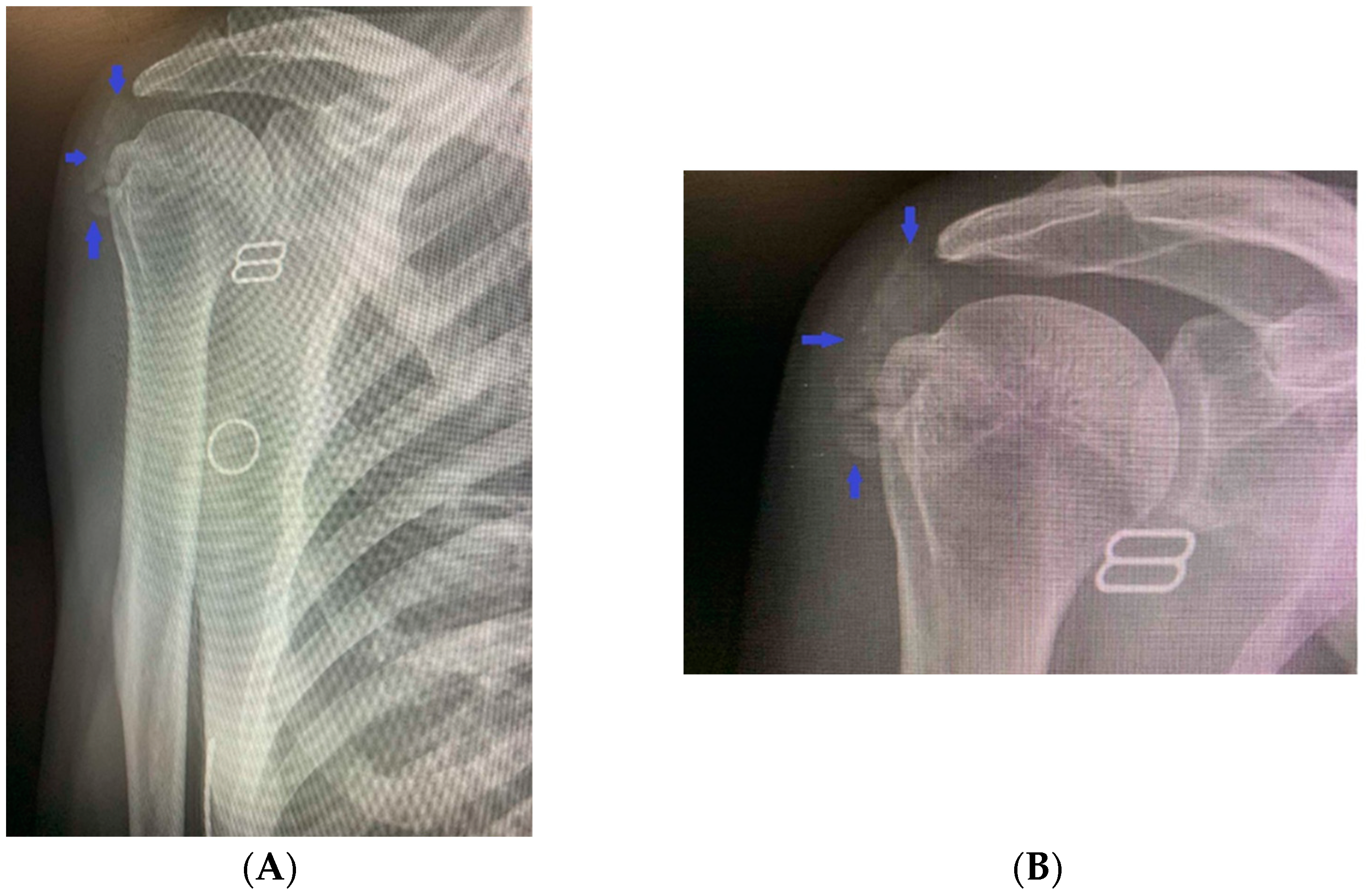
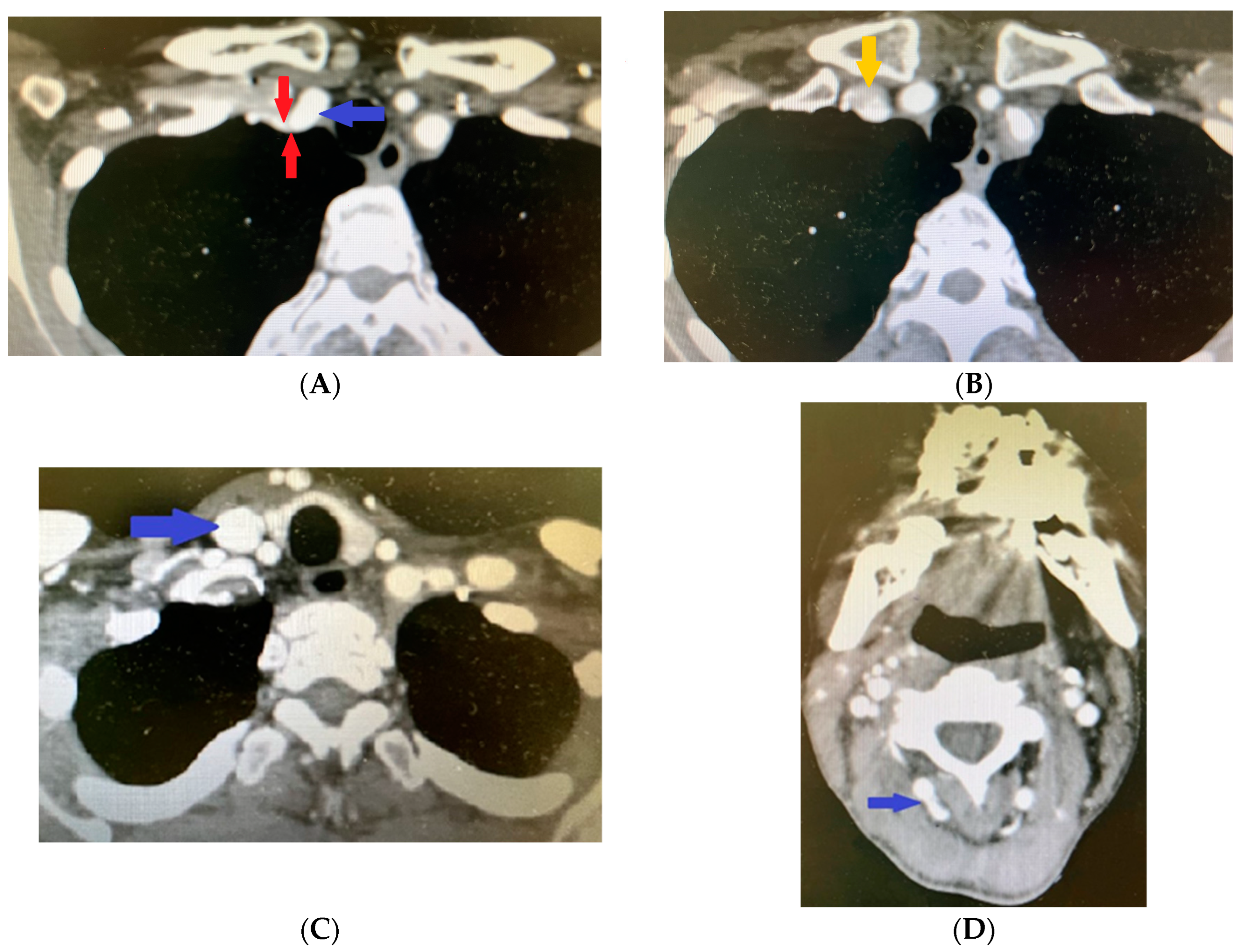
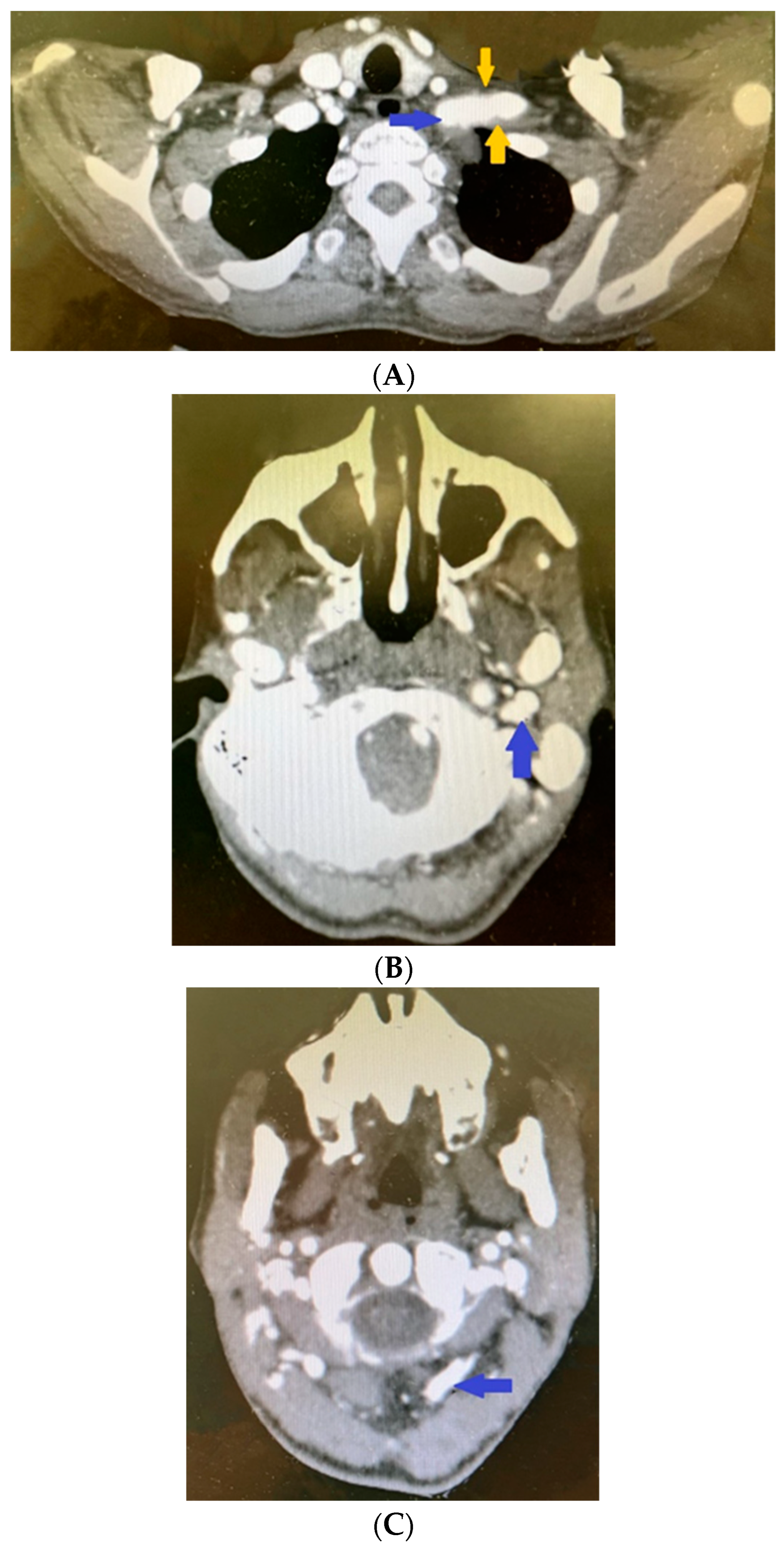
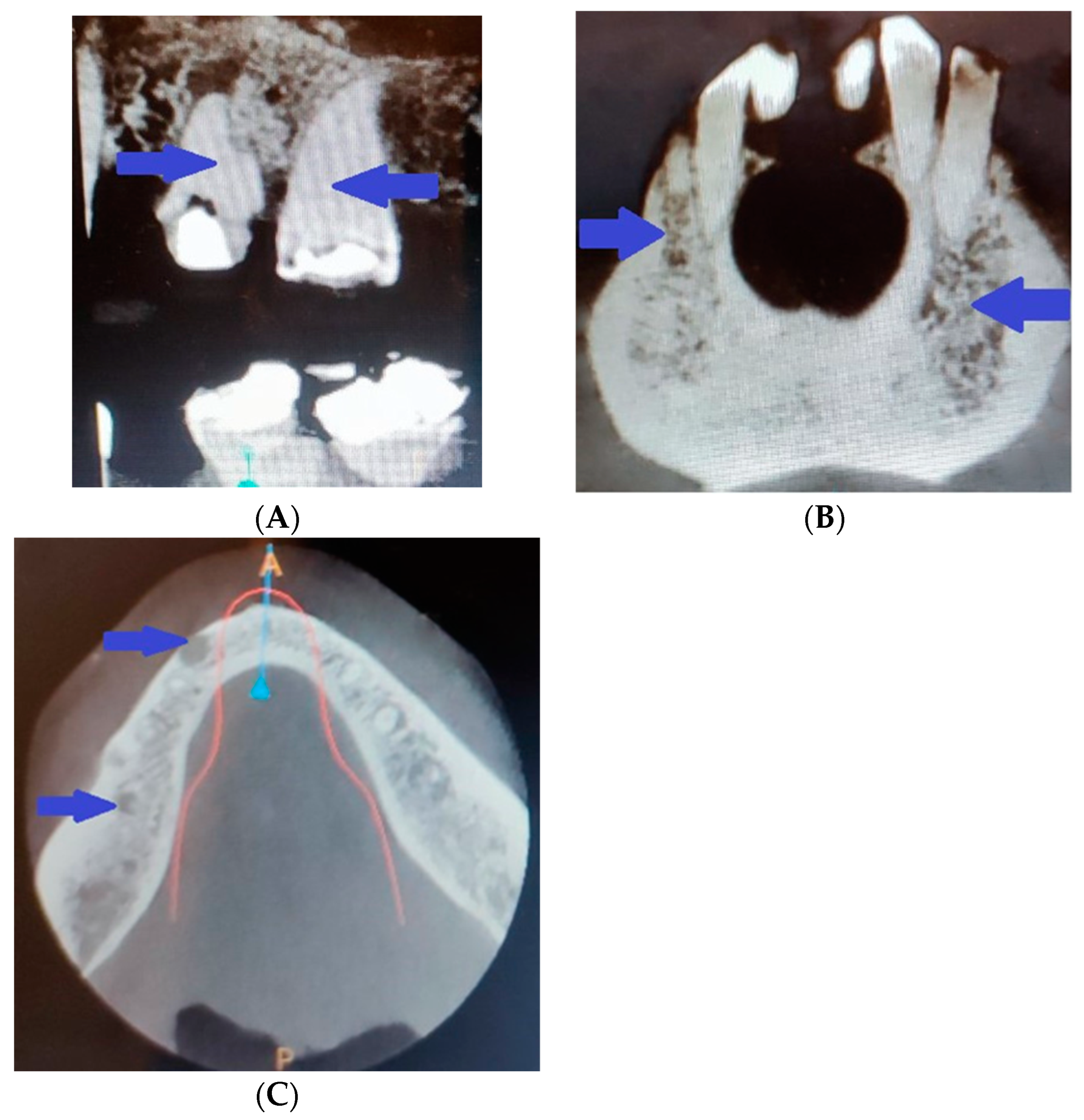
2. Clinical Case Presentation
3. Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Inclan, A.; Leon, P.P.; Camejo, M. Tumoral calcinosis. J. Am. Med. Ass. 1943, 121, 490–495. [Google Scholar] [CrossRef]
- Fathi, I.; Sakr, M. Review of tumoral calcinosis: A rare clinico-pathological entity. World J. Clin. Cases 2014, 2, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.J.; Schaefer, P.C.; Goldsmith, R.S.; Chausmer, A.B. Hyperphosphatemic tumoral calcinosis: Association with elevation of serum 1,25-dihydroxycholecalciferol concentrations. Ann. Intern. Med. 1982, 96, 586–591. [Google Scholar] [CrossRef]
- Sprecher, E. Familial tumoral calcinosis: From characterization of a rare phenotype to the pathogenesis of ectopic calcification. J. Investig. Dermatol. 2010, 130, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Specktor, P.; Cooper, J.G.; Indelman, M.; Sprecher, E. Hyperphosphatemic familial tumoral calcinosis caused by a mutation in GALNT3 in a European kindred. J. Hum. Genet. 2006, 51, 487–490. [Google Scholar] [CrossRef]
- Topaz, O.; Shurman, D.L.; Bergman, R.; Indelman, M.; Ratajczak, P.; Mizrachi, M.; Khamaysi, Z.; Behar, D.; Petronius, D.; Friedman, V.; et al. Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat. Genet. 2004, 36, 579–581. [Google Scholar] [CrossRef]
- Benet-Pagès, A.; Orlik, P.; Strom, T.M.; Lorenz-Depiereux, B. An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum. Mol. Genet. 2005, 14, 385–390. [Google Scholar] [CrossRef]
- Boyce, A.M.; Lee, A.E.; Roszko, K.L.; Gafni, R.I. Hyperphosphatemic Tumoral Calcinosis: Pathogenesis, Clinical Presentation, and Challenges in Management. Front. Endocrinol. 2020, 11, 293. [Google Scholar] [CrossRef]
- McClatchie, S.; Bremner, A.D. Tumoral calcinosis--An unrecognized disease. BMJ 1969, 1, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Lafferty, F.; Reynolds, E.; Pearson, O. Tumoral calcinosis: A metabolic disease of obscure etiology. Am. J. Med. 1965, 38, 105–118. [Google Scholar] [CrossRef]
- Olsen, K.M.; Chew, F.S. Tumoral calcinosis: Pearls, polemics, and alternative possibilities. RadioGraphics 2006, 26, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, C.C.; Fishman, E.K.; Scott, W.W. Tumoral calcinosis causing bone erosion in a renal dialysis patient. Clin. Imaging 1992, 16, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.-I.; Ito, M.; Tatsumi, S.; Kuwahata, M.; Segawa, H. New aspect of renal phosphate reabsorption: The type IIc sodium-dependent phosphate transporter. Am. J. Nephrol. 2007, 27, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Memon, F.; El-Abbadi, M.; Nakatani, T.; Taguchi, T.; Lanske, B.; Razzaque, M.S. Does Fgf23–klotho activity influence vascular and soft tissue calcification through regulating mineral ion metabolism? Kidney Int. 2008, 74, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Ärnlöv, J.; Carlsson, A.C.; Sundström, J.; Ingelsson, E.; Larsson, A.; Lind, L.; Larsson, T.E. Serum FGF23 and risk of cardiovascular events in relation to mineral metabolism and cardiovascular pathology. Clin. J. Am. Soc. Nephrol. 2013, 8, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Adams, W.M.; Laitt, R.D.; Davies, M.; O’Donovan, D.G. Familial tumoral calcinosis: Association with cerebral and peripheral aneurysm formation. Neuroradiology 1999, 41, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Maiorano, E.; Favia, G.; Lacaita, M.G.; Limongelli, L.; Tempesta, A.; Laforgia, N.; Cazzolla, A.P. Hyperphosphatemic familial tumoral calcinosis: Odontostomatologic management and pathological features. Am. J. Case Rep. 2014, 15, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Burkes, E.J., Jr.; Lyles, K.W.; Dolan, E.A.; Giammara, B.; Hanker, J. Dental lesions in tumoral calcinosis. J. Oral. Pathol. Med. 1991, 20, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, S.; Baujat, G.; Seyahi, A.; Garoufali, A.G.; Imel, E.A.; Padgett, L.R.; Austin, A.M.; Sorenson, A.H.; Pejin, Z.; Topouchian, V.; et al. Clinical variability of familial tumoral calcinosis caused by novel GALNT3 mutations. Am. J. Med. Genet. Part. A 2010, 152A, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Miller, C.J.; Nast, C.C.; Adams, M.D.; Truitt, B.; Tayek, J.A.; Tong, L.; Mehtani, P.; Monteon, F.; Sedor, J.R.; et al. Severe vascular calcification and tumoral calcinosis in a family with hyperphosphatemia: A fibroblast growth factor 23 mutation identified by exome sequencing. Nephrol. Dial. Transplant. 2014, 29, 2235–2243. [Google Scholar] [CrossRef] [PubMed]
- Tagliabracci, V.S.; Engel, J.L.; Wiley, S.E.; Xiao, J.; Gonzalez, D.J.; Appaiah, H.N.; Koller, A.; Nizet, V.; White, K.E.; Dixon, J.E. Dynamic regulation of FGF23 by Fam20C phosphorylation, GalNAc-T3 glycosylation, and furin proteolysis. Proc. Natl. Acad. Sci. USA 2014, 111, 5520–5525. [Google Scholar] [CrossRef] [PubMed]
- Raman, J.; Guan, Y.; Perrine, C.L.; Gerken, T.A.; A. Tabak, L. UDP-N-acetyl-α-d-galactosamine:polypeptide N-acetylgalactosaminyltransferases: Completion of the family tree. Glycobiology 2015, 22, 768–777, Erratum in Glycobiology 2015, 25, 465. [Google Scholar] [CrossRef] [PubMed]
- Melhem, R.E.; Najjar, S.S.; Khachadurian, A.K. Cortical hyperostosis with hyperphosphatemia: A new syndrome? J. Pediatr. 1970, 77, 986–990. [Google Scholar] [CrossRef] [PubMed]
- Sprecher, E. Tumoral calcinosis: New insights for the rheumatologist into a familial crystal deposition disease. Curr. Rheumatol. Rep. 2007, 9, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, S.; Imel, E.A.; Kreiter, M.L.; Yu, X.; Mackenzie, D.S.; Sorenson, A.H.; Goetz, R.; Mohammadi, M.; White, K.E.; Econs, M.J. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J. Clin. Investig. 2007, 117, 2684–2691. [Google Scholar] [CrossRef]
- Riminucci, M.; Collins, M.T.; Fedarko, N.S.; Cherman, N.; Corsi, A.; White, K.E.; Waguespack, S.; Gupta, A.; Hannon, T.; Econs, M.J.; et al. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J. Clin. Investig. 2003, 112, 683–692. [Google Scholar] [CrossRef]
- Yamamoto, H.; Ramos-Molina, B.; Lick, A.N.; Prideaux, M.; Albornoz, V.; Bonewald, L.; Lindberg, I. Posttranslational processing of FGF23 in osteocytes during the osteoblast to osteocyte transition. Bone 2016, 84, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Toro, L.; Barrientos, V.; León, P.; Rojas, M.; Gonzalez, M.; González-Ibáñez, A.; Illanes, S.; Sugikawa, K.; Abarzúa, N.; Bascuñán, C.; et al. Erythropoietin induces bone marrow and plasma fibroblast growth factor 23 during acute kidney injury. Kidney Int. 2018, 93, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, J.; Bardet, C.; Prié, D. Physiology of FGF23 and overview of genetic diseases associated with renal phosphate wasting. Metabolism 2019, 103, 153865. [Google Scholar] [CrossRef] [PubMed]
- Ben-Dov, I.Z.; Galitzer, H.; Lavi-Moshayoff, V.; Goetz, R.; Kuro-O, M.; Mohammadi, M.; Sirkis, R.; Naveh-Many, T.; Silver, J. The parathyroid is a target organ for FGF23 in rats. J. Clin. Investig. 2007, 117, 4003–4008. [Google Scholar] [CrossRef]
- Ramnitz, M.S.; Gafni, R.I.; Collins, M.T. Hyperphosphatemic Familial Tumoral Calcinosis. 2018 Feb 1. In GeneReviews® [Internet]; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993–2024. [Google Scholar] [PubMed]
- Razzaque, M.S. The FGF23–Klotho axis: Endocrine regulation of phosphate homeostasis. Nat. Rev. Endocrinol. 2009, 5, 611–619. [Google Scholar] [CrossRef]
- Smack, D.P.; Norton, S.A.; Fitzpatrick, J.E. Proposal for a pathogenesis-based classification of tumoral calcinosis. Int. J. Dermatol. 1996, 35, 265–271. [Google Scholar] [CrossRef]
- Thomson, J.G. Calcifying collagenolysis (tumoural calcinosis). Br. J. Radiol. 1966, 39, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Pakasa, N.; Kalengayi, R. Tumoral calcinosis: A clinicopathological study of 111 cases with emphasis on the earliest changes. Histopathology 1997, 31, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Slavin, R.E.; Wen, J.; Kumar, D.; Evans, E.B. Familial tumoral calcinosis. A clinical, histopathologic, and ultrastructural study with an analysis of its calcifying process and pathogenesis. Am. J. Surg. Pathol. 1993, 17, 788–802. [Google Scholar] [CrossRef]
- Boskey, A.L.; Vigorita, V.J.; Sencer, O.; Stuchin, S.A.; Lane, J.M. Chemical, microscopic, and ultrastructural characterization of the mineral deposits in tumoral calcinosis. Clin. Orthop. Relat. Res. 1983, 178, 258–269. [Google Scholar] [CrossRef]
- Mallick, S.; Ahmad, Z.; Gupta, A.K.; Mathur, S.R. Hyperphosphatemic tumoral calcinosis. BMJ Case Rep. 2013, 2013, bcr2013008728. [Google Scholar] [CrossRef]
- Laasri, K.; El Houss, S.; Halfi, I.M.; Nassar, I.; Billah, N.M. A rare case of idiopathic tumoral calcinosis: Case report. Radiol. Case Rep. 2022, 17, 4350–4353. [Google Scholar] [CrossRef] [PubMed]
- Hug, I.; Gunçaga, J. Tumoral calcinosis with sedimentation sign. Br. J. Radiol. 1974, 47, 734–736. [Google Scholar] [CrossRef]
- Naikmasur, V.; Guttal, K.; Burde, K.; Sattur, A.; Nandimath, K. Tumoral calcinosis with dental manifestations—A case report. Dent. Updat. 2008, 35, 134–138. [Google Scholar] [CrossRef]
- Ramnitz, M.S.; Gourh, P.; Goldbach-Mansky, R.; Wodajo, F.; Ichikawa, S.; Econs, M.J.; White, K.E.; Molinolo, A.; Chen, M.Y.; Heller, T.; et al. Phenotypic and genotypic characterization and treatment of a cohort with familial tumoral calcinosis/hyperostosis-hyperphosphatemia syndrome. J. Bone Miner. Res. 2016, 31, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, S.; Imel, E.A.; Sorenson, A.H.; Severe, R.; Knudson, P.; Harris, G.J.; Shaker, J.L.; Econs, M.J. Tumoral calcinosis presenting with eyelid calcifications due to novel missense mutations in the glycosyl transferase domain of Thegalnt3gene. J. Clin. Endocrinol. Metab. 2006, 91, 4472–4475. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bruns, D.E.; Lieb, W.; Conway, B.P.; Savory, J.; Wills, M.R.; Boskey, A.L. Band keratopathy and calcific lid lesions in tumoral calcinosis. Arch. Ophthalmol. 1988, 106, 725–726. [Google Scholar] [CrossRef]
- McPhaul, J.J., Jr.; Engel, F.L. Heterotopic calcification, hyperphosphatemia and angioid streaks of the retina. Am. J. Med. 1961, 31, 488–492. [Google Scholar] [CrossRef]
- Foley, R.N.; Collins, A.J.; Herzog, C.A.; Ishani, A.; Kalra, P.A. Serum phosphorus levels associate with coronary atherosclerosis in young adults. J. Am. Soc. Nephrol. 2009, 20, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Adeney, K.L.; Siscovick, D.S.; Ix, J.H.; Seliger, S.L.; Shlipak, M.G.; Jenny, N.S.; Kestenbaum, B.R. Association of serum phosphate with vascular and valvular calcification in moderate CKD. J. Am. Soc. Nephrol. 2009, 20, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Tamei, N.; Ogawa, T.; Ishida, H.; Ando, Y.; Nitta, K. Serum fibroblast growth factor-23 levels and progression of aortic arch calcification in non-diabetic patients on chronic hemodialysis. J. Atheroscler. Thromb. 2011, 18, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Román-García, P.; Carrillo-López, N.; Fernández-Martín, J.L.; Naves-Díaz, M.; Ruiz-Torres, M.P.; Cannata-Andía, J.B. High phosphorus diet induces vascular calcification, a related decrease in bone mass and changes in the aortic gene expression. Bone 2010, 46, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Demer, L.L.; Tintut, Y. Vascular Calcification: Pathobiology of a multifaceted disease. Circulation 2008, 117, 2938–2948. [Google Scholar] [CrossRef] [PubMed]
- Leoncini, G.; Ratto, E.; Viazzi, F.; Vaccaro, V.; Parodi, A.; Falqui, V.; Conti, N.; Tomolillo, C.; Deferrari, G.; Pontremoli, R.; et al. Increased ambulatory arterial stiffness index is associated with target organ damage in primary hypertension. Hypertension 2006, 48, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Wanga, S.; Hibender, S.; Ridwan, Y.; van Roomen, C.; Vos, M.; van der Made, I.; van Vliet, N.; Franken, R.; van Riel, L.A.; Groenink, M.; et al. Aortic microcalcification is associated with elastin fragmentation in Marfan syndrome. J. Pathol. 2017, 243, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Shoab, E.; Sho, M.; Singh, T.M.; Nanjob, H.; Komatsub, M.; Xua, C.; Masudab, H.; Zarins, C.K. Arterial enlargement in response to high flow requires early expression of matrix metalloproteinases to degrade extracellular matrix. Exp. Mol. Pathol. 2002, 73, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Tromp, G.; Gatalica, Z.; Skunca, M.; Berguer, R.; Siegel, T.; Kline, R.A.; Kuivaniemi, H. Elevated Expression of Matrix Metalloproteinase-13 in Abdominal Aortic Aneurysms. Ann. Vasc. Surg. 2004, 18, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Taneda, M.; Asai, T.; Kinoshita, A.; Ito, M.; Kuroda, R.; Redekop, G.J.; Björkman, J.; Frösen, J.; Tähtinen, O.; et al. Structural Fragility and Inflammatory Response of Ruptured Cerebral Aneurysms. A comparative study between ruptured and unruptured cerebral aneurysms. Stroke 1999, 30, 1396–1401. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Hashimoto, N.; Naritomi, H.; Nagata, I.; Nozaki, K.; Kondo, S.; Kurino, M.; Kikuchi, H. Prevention of rat cerebral aneurysm formation by inhibition of nitric oxide synthase. Circulation 2000, 101, 2532–2538. [Google Scholar] [CrossRef] [PubMed]
- Speer, M.Y.; Chien, Y.-C.; Quan, M.; Yang, H.-Y.; Vali, H.; McKee, M.D.; Giachelli, C.M. Smooth muscle cells deficient in osteopontin have enhanced susceptibility to calcification in vitro. Cardiovasc. Res. 2005, 66, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Mackenzie, N.C.W.; Millán, J.L.; Farquharson, C.; MacRae, V.E. The appearance and modulation of osteocyte marker expression during calcification of vascular smooth muscle cells. PLoS ONE 2011, 6, e19595. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Mackenzie, N.C.; Millan, J.L.; Farquharson, C.; MacRae, V.E. A protective role for FGF-23 in local defence against disrupted arterial wall integrity? Mol. Cell. Endocrinol. 2013, 372, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Speer, M.Y.; Yang, H.-Y.; Brabb, T.; Leaf, E.; Look, A.; Lin, W.-L.; Frutkin, A.; Dichek, D.; Giachelli, C.M. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ. Res. 2009, 104, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Claramunt-Taberner, D.; Bertholet-Thomas, A.; Carlier, M.-C.; Dijoud, F.; Chotel, F.; Silve, C.; Bacchetta, J. Hyperphosphatemic tumoral calcinosis caused by FGF23 compound heterozygous mutations: What are the therapeutic options for a better control of phosphatemia? Pediatr. Nephrol. 2018, 33, 1263–1267. [Google Scholar] [CrossRef] [PubMed]
- Farrow, E.G.; Imel, E.A.; White, K.E. Miscellaneous non-inflammatory musculoskeletal conditions. Hyperphosphatemic familial tumoral calcinosis (FGF23, GALNT3 and αKlotho). Best. Pract. Res. Clin. Rheumatol. 2011, 25, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Gregosiewicz, A.; Warda, E. Tumoral calcinosis: Successful medical treatment. A case report. J. Bone Jt. Surg. Am. 1989, 71, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, G.; Lafferty, F.W.; Pearson, O.H. Treatment of tumoral calcinosis with phosphorus deprivation. Ann. Intern. Med. 1972, 77, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Kirk, T.S.; A Simon, M. Tumoral calcinosis. Report. of a case with successful medical management. J. Bone Jt. Surg. 1981, 63, 1167–1169. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.; Clements, M.R.; Mawer, E.B.; Freemont, A.J. Tumoral calcinosis: Clinical and metabolic response to phosphorus deprivation. Q J. Med. 1987, 63, 493–503. [Google Scholar] [PubMed]
- Yamaguchi, T.; Sugimoto, T.; Imai, Y.; Fukase, M.; Fujita, T.; Chihara, K. Successful treatment of hyperphosphatemic tumoral calcinosis with long-term acetazolamide. Bone 1995, 16 (Suppl. S4), 247S–250S. [Google Scholar] [CrossRef] [PubMed]
- Jost, J.; Bahans, C.; Courbebaisse, M.; Tran, T.-A.; Linglart, A.; Benistan, K.; Lienhardt, A.; Mutar, H.; Pfender, E.; Ratsimbazafy, V.; et al. Topical Sodium Thiosulfate: A Treatment for Calcifications in Hyperphosphatemic Familial Tumoral Calcinosis? J. Clin. Endocrinol. Metab. 2016, 101, 2810–2815. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.; Boutignon, H.; Mallet, E.; Linglart, A.; Guillozo, H.; Jehan, F.; Garabedian, M. Infantile hypercalcemia and hypercalciuria: New insights into a vitamin d-dependent mechanism and response to ketoconazole treatment. J. Pediatr. 2010, 157, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Tezelman, S.; Siperstein, A.E.; Duh, Q.-Y.; Clark, O.H. Tumoral Calcinosis. Controversies in the etiology and alternatives in the treatment. Arch. Surg. 1993, 128, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Noyez, J.F.; Murphree, S.M.; Chen, K. Tumoral calcinosis, a clinical report of eleven cases. Acta Orthop. Belg. 1993, 59, 249–254. [Google Scholar] [PubMed]
- Kisembo, H.; Kiguli-Malwadde, E.; Kawooya, M.G. Tumoral calcinosis: Report of nine cases. East Afr. Med. J. 2000, 77, 574–575. [Google Scholar] [PubMed]
- Neeman, Z.; Wood, B.J. Angiographic findings in tumoral calcinosis. Clin. Imaging 2003, 27, 184–186. [Google Scholar] [CrossRef] [PubMed]
- Calloway, D.M.; Saldaña, M.J. Combined modality treatment for tumoral calcinosis. Orthop Rev. 1993, 22, 365–369. [Google Scholar] [PubMed]
- Genome Aggregation Database. Available online: https://gnomad.broadinstitute.org/about (accessed on 1 April 2024).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, N.G. A Sole Case of the FGF23 Gene Mutation c.202A>G (p.Thr68Ala) Associated with Multiple Severe Vascular Aneurysms and a Hyperphosphatemic Variant of Tumoral Calcinosis—A Case Report. Life 2024, 14, 613. https://doi.org/10.3390/life14050613
Ivanova NG. A Sole Case of the FGF23 Gene Mutation c.202A>G (p.Thr68Ala) Associated with Multiple Severe Vascular Aneurysms and a Hyperphosphatemic Variant of Tumoral Calcinosis—A Case Report. Life. 2024; 14(5):613. https://doi.org/10.3390/life14050613
Chicago/Turabian StyleIvanova, Nevena Georgieva. 2024. "A Sole Case of the FGF23 Gene Mutation c.202A>G (p.Thr68Ala) Associated with Multiple Severe Vascular Aneurysms and a Hyperphosphatemic Variant of Tumoral Calcinosis—A Case Report" Life 14, no. 5: 613. https://doi.org/10.3390/life14050613
APA StyleIvanova, N. G. (2024). A Sole Case of the FGF23 Gene Mutation c.202A>G (p.Thr68Ala) Associated with Multiple Severe Vascular Aneurysms and a Hyperphosphatemic Variant of Tumoral Calcinosis—A Case Report. Life, 14(5), 613. https://doi.org/10.3390/life14050613





