Exploring the Potential of Micro-Immunotherapy in the Treatment of Periodontitis
Abstract
1. Introduction
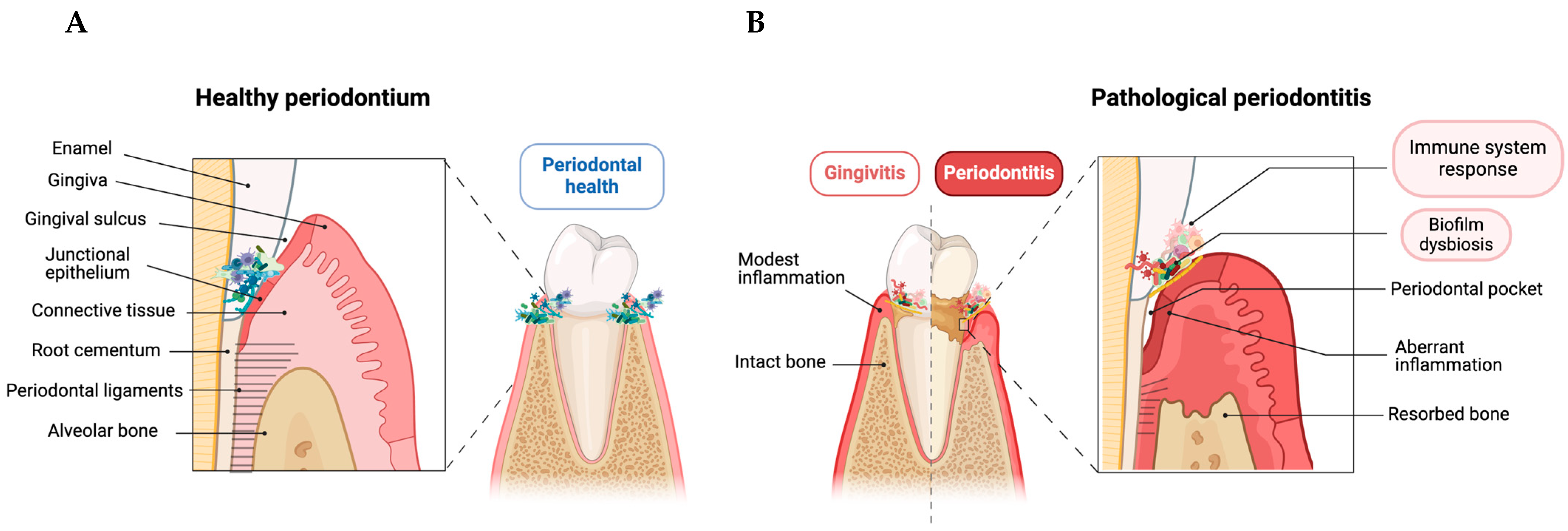
2. Periodontitis Immunopathology
2.1. Innate Response
2.2. Adaptative Response
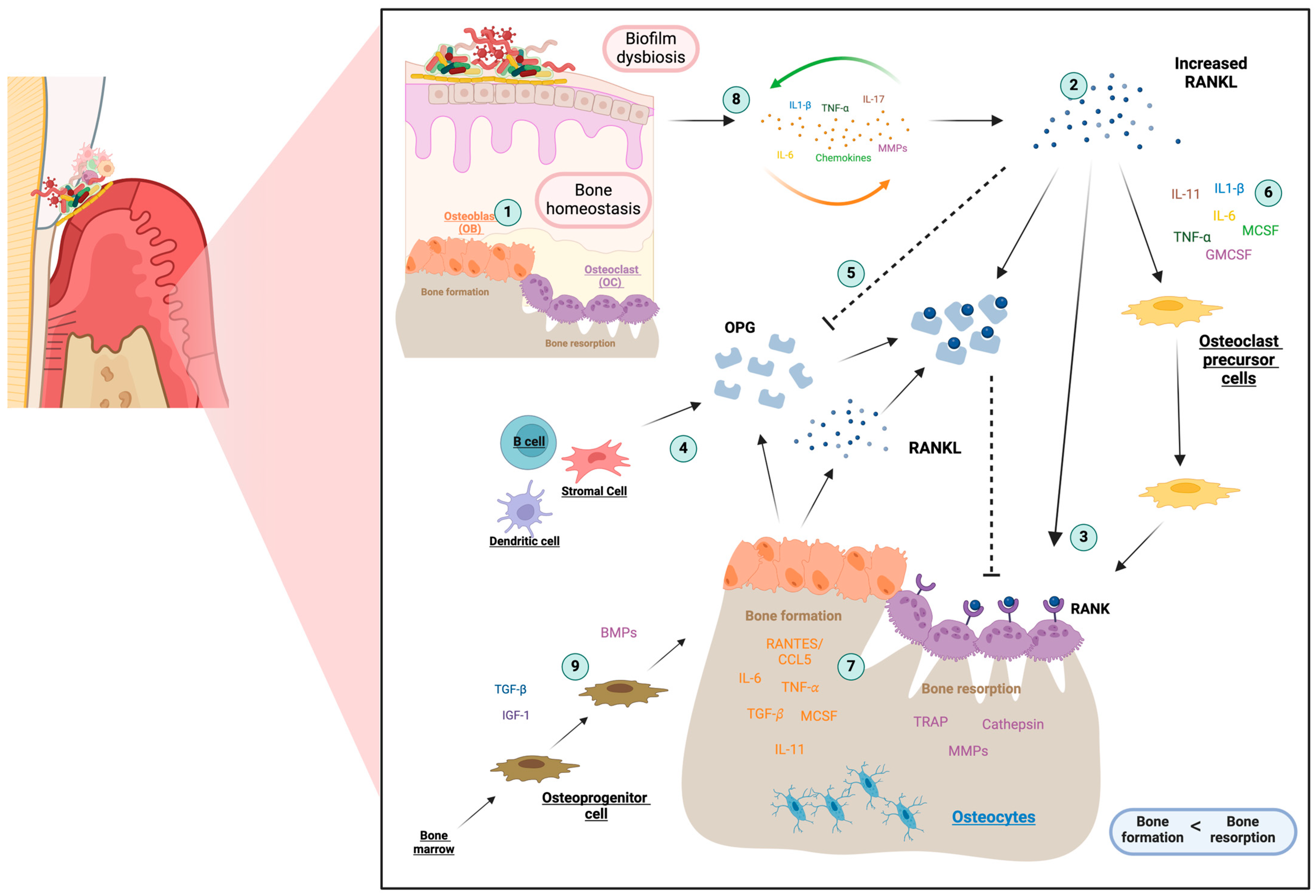
2.3. Osteoimmunology
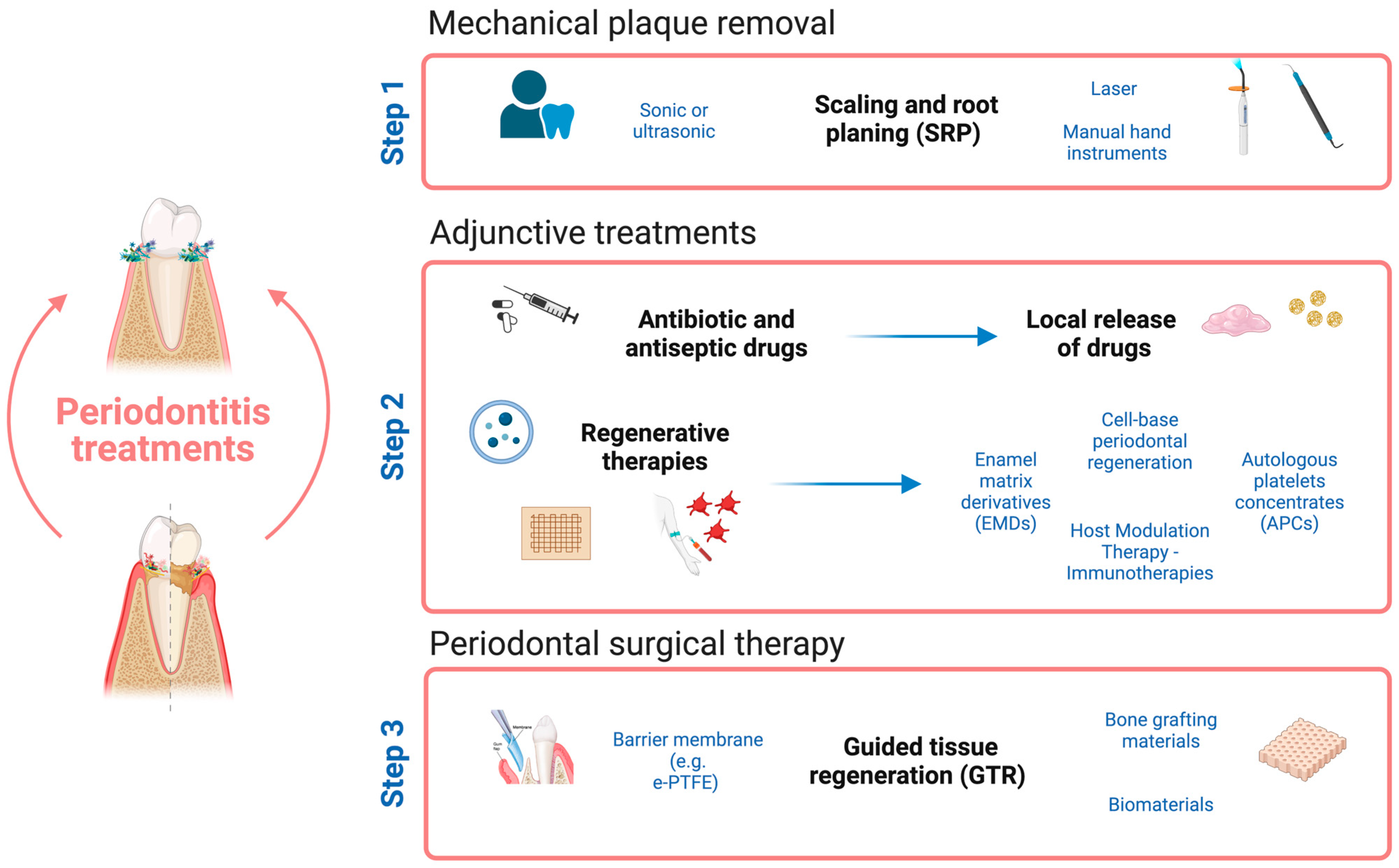
3. Approaches in Periodontal Therapy
3.1. Strategies in Periodontal Treatment
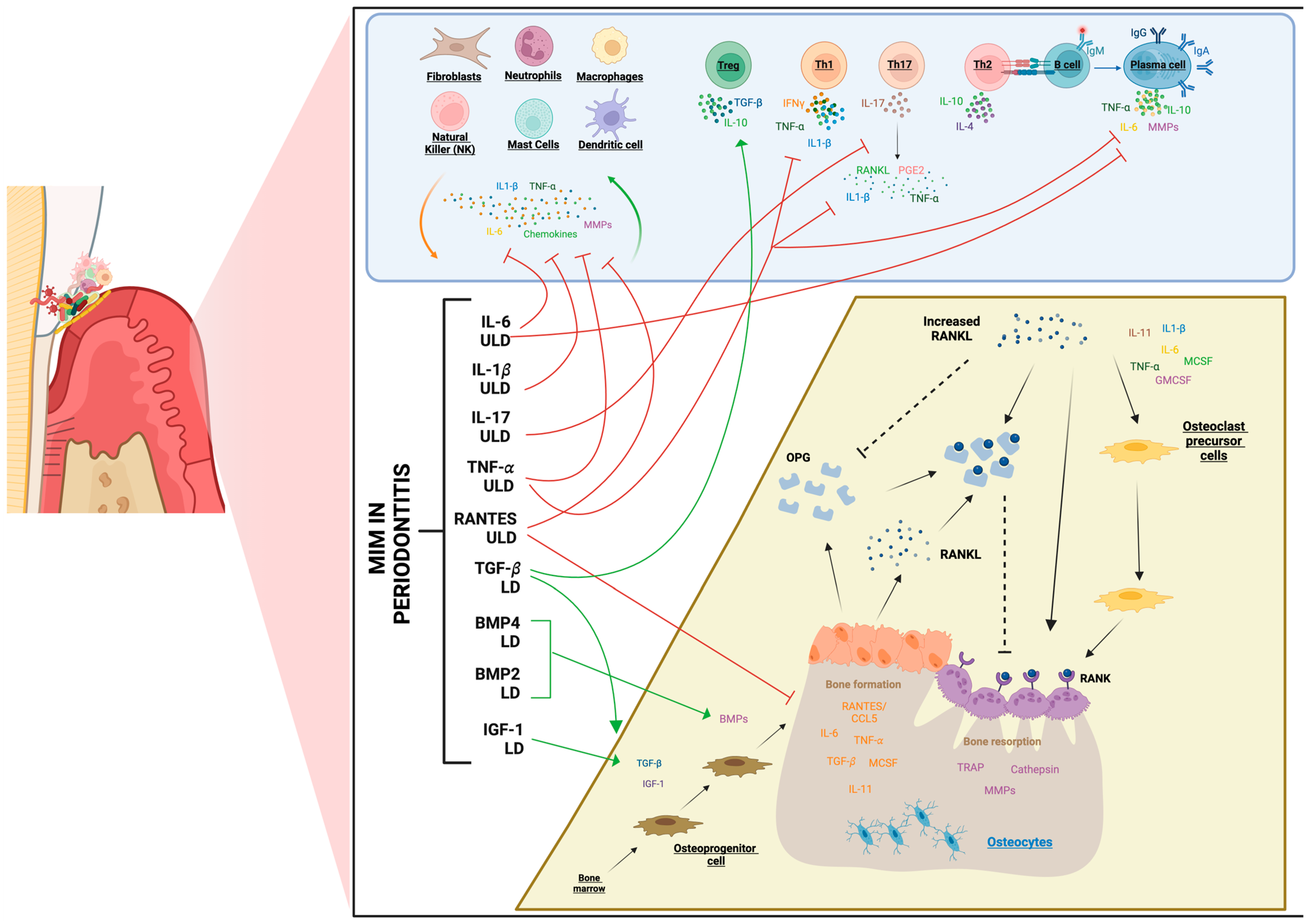
3.2. Micro-Immunotherapy Therapeutic Landscape and Its Potential in Periodontitis
4. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sedghi, L.M.; Bacino, M.; Kapila, Y.L. Periodontal Disease: The Good, The Bad, and The Unknown. Front. Cell Infect. Microbiol. 2021, 11, 766944. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Ruiz, J.S.; Guerrero-Velázquez, C.; Martínez-Esquivias, F.; Martínez-Pérez, L.A.; Guzmán-Flores, J.M. Innate and Adaptive Immunity of Periodontal Disease. From Etiology to Alveolar Bone Loss. Oral Dis. 2022, 28, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal Diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.A. Prevalence of Periodontal Disease, Its Association with Systemic Diseases and Prevention. Int. J. Health Sci. 2017, 11, 72–80. [Google Scholar]
- Shazam, H.; Shaikh, F.; Hussain, Z. Bone Turnover Markers in Chronic Periodontitis: A Literature Review. Cureus 2020, 12, e6699. [Google Scholar] [CrossRef]
- Nazir, M.; Al-Ansari, A.; Al-Khalifa, K.; Alhareky, M.; Gaffar, B.; Almas, K. Global Prevalence of Periodontal Disease and Lack of Its Surveillance. Sci. World J. 2020, 2020, 2146160. [Google Scholar] [CrossRef] [PubMed]
- Botelho, J.; Machado, V.; Leira, Y.; Proença, L.; Chambrone, L.; Mendes, J.J. Economic burden of periodontitis in the United States and Europe: An updated estimation. J. Periodontol. 2022, 93, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Velsko, I.M.; Fellows Yates, J.A.; Aron, F.; Hagan, R.W.; Frantz, L.A.F.; Loe, L.; Martinez, J.B.R.; Chaves, E.; Gosden, C.; Larson, G.; et al. Microbial Differences between Dental Plaque and Historic Dental Calculus Are Related to Oral Biofilm Maturation Stage. Microbiome 2019, 7, 102. [Google Scholar] [CrossRef]
- Abusleme, L.; Dupuy, A.K.; Dutzan, N.; Silva, N.; Burleson, J.A.; Strausbaugh, L.D.; Gamonal, J.; Diaz, P.I. The Subgingival Microbiome in Health and Periodontitis and Its Relationship with Community Biomass and Inflammation. ISME J. 2013, 7, 1016–1025. [Google Scholar] [CrossRef]
- Linhartova, P.B.; Danek, Z.; Deissova, T.; Hromcik, F.; Lipovy, B.; Szaraz, D.; Janos, J.; Fassmann, A.; Bartova, J.; Drizhal, I.; et al. Interleukin Gene Variability and Periodontal Bacteria in Patients with Generalized Aggressive Form of Periodontitis. Int. J. Mol. Sci. 2020, 21, 4728. [Google Scholar] [CrossRef]
- Ferreira, M.C.; Dias-Pereira, A.C.; Branco-de-Almeida, L.S.; Martins, C.C.; Paiva, S.M. Impact of Periodontal Disease on Quality of Life: A Systematic Review. J. Periodontal. Res. 2017, 52, 651–665. [Google Scholar] [CrossRef] [PubMed]
- Könönen, E.; Gursoy, M.; Gursoy, U. Periodontitis: A Multifaceted Disease of Tooth-Supporting Tissues. J. Clin. Med. 2019, 8, 1135. [Google Scholar] [CrossRef] [PubMed]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S.; et al. A New Classification Scheme for Periodontal and Peri-Implant Diseases and Conditions-Introduction and Key Changes from the 1999 Classification. J. Periodontol. J. Clin. Periodontol. 2018, 89, S1–S8. [Google Scholar] [CrossRef] [PubMed]
- Kurgan, S.; Kantarci, A. Molecular Basis for Immunohistochemical and Inflammatory Changes during Progression of Gingivitis to Periodontitis. Periodontology 2000 2018, 76, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Zhang, D.; Wang, C. Identifying Periodontitis Risk Factors through a Retrospective Analysis of 80 Cases. Pak. J. Med. Sci. 2021, 38, 293. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Chavakis, T. Local and Systemic Mechanisms Linking Periodontal Disease and Inflammatory Comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440. [Google Scholar] [CrossRef]
- Hajishengallis, G. Interconnection of Periodontal Disease and Comorbidities: Evidence, Mechanisms, and Implications. Periodontology 2000 2022, 89, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Periodontitis: From Microbial Immune Subversion to Systemic Inflammation. Nat. Rev. Immunol. 2015, 15, 30. [Google Scholar] [CrossRef]
- Di Stefano, M.; Polizzi, A.; Santonocito, S.; Romano, A.; Lombardi, T.; Isola, G. Impact of Oral Microbiome in Periodontal Health and Periodontitis: A Critical Review on Prevention and Treatment. Int. J. Mol. Sci. 2022, 23, 5142. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Q.; Yan, X.; Shan, Y.; Xing, L.; Li, M.; Long, H.; Lai, W. Immune Landscape of Periodontitis Unveils Alterations of Infiltrating Immunocytes and Molecular Networks-Aggregating into an Interactive Web-Tool for Periodontitis Related Immune Analysis and Visualization. J. Transl. Med. 2020, 18, 438. [Google Scholar] [CrossRef]
- Jacques, C.; Marchand, F.; Chatelais, M.; Brulefert, A.; Floris, I. Understanding the Mode of Action of a Micro-Immunotherapy Formulation: Pre-Clinical Evidence from the Study of 2LEBV® Active Ingredients. Life 2024, 14, 102. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dan, R.; Zhou, X.; Xiang, J.; Wang, J.; Liu, J. Immune Senescence and Periodontitis: From Mechanism to Therapy. J. Leukoc. Biol. 2022, 112, 1025–1040. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Immunomicrobial Pathogenesis of Periodontitis: Keystones, Pathobionts, and Host Response. Trends Immunol. 2014, 35, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.-Y.; Lee, D.-S.; Cho, Y.K.; Lee, J.-Y.; Park, J.-H.; Lee, S.H. Daphne Jejudoensis Attenuates LPS-Induced Inflammation by Inhibiting TNF-α, IL-1β, IL-6, INOS, and COX-2 Expression in Periodontal Ligament Cells. Pharmaceuticals 2022, 15, 387. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Wang, Q.; Chen, Q. The Cytokine Network Involved in the Host Immune Response to Periodontitis. Int. J. Oral Sci. 2019, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Sirisereephap, K.; Maekawa, T.; Tamura, H.; Hiyoshi, T.; Domon, H.; Isono, T.; Terao, Y.; Maeda, T.; Tabeta, K. Osteoimmunology in Periodontitis: Local Proteins and Compounds to Alleviate Periodontitis. Int. J. Mol. Sci. 2022, 23, 5540. [Google Scholar] [CrossRef] [PubMed]
- Damgaard, C.; Holmstrup, P.; Van Dyke, T.E.; Nielsen, C.H. The Complement System and Its Role in the Pathogenesis of Periodontitis: Current Concepts. J. Periodontal. Res. 2015, 50, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Bosshardt, D.D. The Periodontal Pocket: Pathogenesis, Histopathology and Consequences. Periodontology 2000 2018, 76, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Sun, X.; Zhao, J.; Xia, L.; Li, J.; Xu, M.; Wang, B.; Guo, H.; Yu, C.; Gao, Y.; et al. CCL5 Deficiency Promotes Liver Repair by Improving Inflammation Resolution and Liver Regeneration through M2 Macrophage Polarization. Cell. Mol. Immunol. 2020, 17, 753–764. [Google Scholar] [CrossRef]
- Sahingur, S.E.; Yeudall, W.A. Chemokine Function in Periodontal Disease and Oral Cavity Cancer. Front. Immunol. 2015, 6, 140295. [Google Scholar] [CrossRef]
- Xu, X.W.; Liu, X.; Shi, C.; Sun, H.C. Roles of Immune Cells and Mechanisms of Immune Responses in Periodontitis. Chin. J. Dent. Res. 2021, 24, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, G.; Shaik-Dasthagirisaheb, Y.B.; Huang, N.; Viglianti, G.A.; Henderson, A.J.; Kantarci, A.; Gibson, F.C. Immunologic Environment Influences Macrophage Response to Porphyromonas Gingivalis. Mol. Oral Microbiol. 2017, 32, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Tripathi, A.; Singh, S.; Sharan Sinha, N. Mast Cell: A Periodontal Inflammatory Marker. Int. J. Appl. Dent. Sci. 2019, 5, 225–228. [Google Scholar]
- Lagdive, S.; Lagdive, S.; Mani, A.; Anarthe, R.; Pendyala, G.; Pawar, B.; Marawar, P. Correlation of Mast Cells in Periodontal Diseases. J. Indian Soc. Periodontol. 2013, 17, 63. [Google Scholar] [CrossRef] [PubMed]
- Vahabi, S.; Rezazadeh, F.; Movaghar, S.E.; Nazemisalman, B. Relationship between Mast Cell Counts and Different Types of Periodontitis. J. Periodontol. Implant. Dent. 2011, 2, 56–60. [Google Scholar] [CrossRef]
- Luchian, I.; Goriuc, A.; Sandu, D.; Covasa, M. The Role of Matrix Metalloproteinases (MMP-8, MMP-9, MMP-13) in Periodontal and Peri-Implant Pathological Processes. Int. J. Mol. Sci. 2022, 23, 1806. [Google Scholar] [CrossRef] [PubMed]
- Beklen, A.; Ainola, M.; Hukkanen, M.; Gürgan, C.; Sorsa, T.; Konttinen, Y.T. MMPs, IL-1, and TNF Are Regulated by IL-17 in Periodontitis. J. Dent. Res. 2007, 86, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Yucel-Lindberg, T.; Båge, T. Inflammatory Mediators in the Pathogenesis of Periodontitis. Expert Rev. Mol. Med. 2013, 15, e7. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.A.; Passarelli, P.C.; Godino, E.; Lombardo, N.; Altamura, F.R.; Speranza, A.; Lopez, A.; Papi, P.; Pompa, G.; D’addona, A. Antibiotics The Treatment of Peri-Implant Diseases: A New Approach Using HYBENX® as a Decontaminant for Implant Surface and Oral Tissues. Antibiotics 2021, 10, 512. [Google Scholar] [CrossRef]
- Graves, D. Cytokines That Promote Periodontal Tissue Destruction. J. Periodontol. 2008, 79, 1585–1591. [Google Scholar] [CrossRef]
- Ozer Yucel, O. Inflammatory Cytokines and the Pathogenesis of Periodontal Disease. Immunome Res. 2015, 11, 11–13. [Google Scholar] [CrossRef]
- Takayanagi, H. Osteoimmunology: Shared Mechanisms and Crosstalk between the Immune and Bone Systems. Nat. Rev. Immunol. 2007, 7, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.; Horowitz, M.; Choi, Y. Osteoimmunology: Interactions of the Bone and Immune System. Endocr. Rev. 2008, 29, 403–440. [Google Scholar] [CrossRef]
- Graves, D.T.; Kayal, R.A.; Oates, T.; Garlet, G.P. Osteoimmunology in the Oral Cavity (Periodontal Disease, Lesions of Endodontic Origin and Orthodontic Tooth Movement), 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2011; ISBN 9780128005712. [Google Scholar]
- Usui, M.; Onizuka, S.; Sato, T.; Kokabu, S.; Ariyoshi, W.; Nakashima, K. Mechanism of Alveolar Bone Destruction in Periodontitis—Periodontal Bacteria and Inflammation. Jpn. Dent. Sci. Rev. 2021, 57, 201–208. [Google Scholar] [CrossRef]
- Lechner, J.; Rudi, T.; von Baehr, V. Osteoimmunology of Tumor Necrosis Factor-Alpha, IL-6, and RANTES/CCL5: A Review of Known and Poorly Understood Inflammatory Patterns in Osteonecrosis. Clin. Cosmet. Investig. Dent. 2018, 10, 251–262. [Google Scholar] [CrossRef]
- Yu, X.; Huang, Y.; Collin-Osdoby, P.; Osdoby, P. CCR1 Chemokines Promote the Chemotactic Recruitment, RANKL Development, and Motility of Osteoclasts and Are Induced by Inflammatory Cytokines in Osteoblasts. J. Bone Miner. Res. 2004, 19, 2065–2077. [Google Scholar] [CrossRef]
- Yano, S.; Mentaverri, R.; Kanuparthi, D.; Bandyopadhyay, S.; Rivera, A.; Brown, E.M.; Chattopadhyay, N. Functional Expression of β-Chemokine Receptors in Osteoblasts: Role of Regulated upon Activation, Normal T Cell Expressed and Secreted (RANTES) in Osteoblasts and Regulation of Its Secretion by Osteoblasts and Osteoclasts. Endocrinology 2005, 146, 2324–2335. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, X.; Wang, D.; Zheng, J.; Chen, L.; Xie, Q.; Liu, X.; Niu, S.; Qu, G.; Lan, J.; et al. Periodontal Inflammation-Triggered by Periodontal Ligament Stem Cell Pyroptosis Exacerbates Periodontitis. Front. Cell Dev. Biol. 2021, 9, 663037. [Google Scholar] [CrossRef]
- Bordukalo-Nikšić, T.; Kufner, V.; Vukičević, S. The Role Of BMPs in the Regulation of Osteoclasts Resorption and Bone Remodeling: From Experimental Models to Clinical Applications. Front. Immunol. 2022, 13, 1. [Google Scholar] [CrossRef]
- Dhawan, U.; Jaffery, H.; Salmeron-Sanchez, M.; Dalby, M.J. An Ossifying Landscape: Materials and Growth Factor Strategies for Osteogenic Signalling and Bone Regeneration. Curr. Opin. Biotechnol. 2022, 73, 355–363. [Google Scholar] [CrossRef]
- Luu, H.H.; Song, W.X.; Luo, X.; Manning, D.; Luo, J.; Deng, Z.L.; Sharff, K.A.; Montag, A.G.; Haydon, R.C.; He, T.C. Distinct Roles of Bone Morphogenetic Proteins in Osteogenic Differentiation of Mesenchymal Stem Cells. J. Orthop. Res. 2007, 25, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Sconocchia, T.; Sconocchia, G. Regulation of the Immune System in Health and Disease by Members of the Bone Morphogenetic Protein Family. Front. Immunol. 2021, 12, 802346. [Google Scholar] [CrossRef] [PubMed]
- Lindhe, J. Clinical Periodontology and Implant Dentistry, 4th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2003; Volume 82, ISBN 1405102365. [Google Scholar]
- Kwon, T.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2020, 71, 462–476. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.A.; Lang, N.P. Surgical and Nonsurgical Periodontal Therapy. Learned and Unlearned Concepts. Periodontology 2000 2013, 62, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Tariq, M.; Iqbal, Z.; Ali, J.; Baboota, S.; Talegaonkar, S.; Ahmad, Z.; Sahni, J.K. Treatment Modalities and Evaluation Models for Periodontitis. Int. J. Pharm. Investig. 2012, 2, 106. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Berglundh, T.; Sculean, A.; Tonetti, M.S.; Merete Aass, A.; Aimetti, M.; et al. Treatment of Stage I–III Periodontitis—The EFP S3 Level Clinical Practice Guideline. J. Clin. Periodontol. 2020, 47, 4–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Greenwell, H. Surgical Periodontal Therapy. Periodontology 2000 2001, 25, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Golub, L.M.; Lee, H. Periodontal Therapeutics: Current Host-modulation Agents and Future Directions. Periodontology 2000 2020, 82, 186–204. [Google Scholar] [CrossRef] [PubMed]
- Preshaw, P.M. Host Modulation Therapy with Anti-Inflammatory Agents. Periodontology 2000 2018, 76, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Zoltowska, A.; Machut, K.; Pawlowska, E.; Derwich, M. Plasma Rich in Growth Factors in the Treatment of Endodontic Periapical Lesions in Adult Patients: A Narrative Review. Pharmaceuticals 2021, 14, 1041. [Google Scholar] [CrossRef]
- Miron, R.J.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Hernandez, M.; Choukroun, J. Platelet-Rich Fibrin and Soft Tissue Wound Healing: A Systematic Review. Tissue Eng. Part B Rev. 2017, 23, 83–99. [Google Scholar] [CrossRef]
- Mohan, S.; Jaishangar, N.; Devy, S.; Narayanan, A.; Cherian, D.; Madhavan, S. Platelet-Rich Plasma and Platelet-Rich Fibrin in Periodontal Regeneration: A Review. J. Pharm. Bioallied Sci. 2019, 11, 126. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, F.; Dong, F.; Zhang, Z.; Song, P.; Chen, H.; Wang, J. CGF Membrane Promotes Periodontal Tissue Regeneration Mediated by HUCMSCs through Upregulating TAZ and Osteogenic Differentiation Genes. Stem Cells Int. 2021, 2021, 6644366. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Duan, J.; Zhang, Y.; Chu, Y.; Sun, C. The Effect of Concentrated Growth Factors in the Treatment of Periodontal Intrabony Defects. Future Sci. OA 2016, 2, FS136. [Google Scholar] [CrossRef] [PubMed]
- Venezia, E.; Goldstein, M.; Boyan, B.D.; Schwartz, Z. The Use of Enamel Matrix Derivative in the Treatment of Periodontal Defects: A Literature Review and Meta-Analysis. Crit. Rev. Oral Biol. Med. 2004, 15, 382–402. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.R.; Acharya, L.D.; Udupa, N. Evaluation of Antiplaque Activity of Azadirachta Indica Leaf Extract Gel—A 6-Week Clinical Study. J. Ethnopharmacol. 2004, 90, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Preshaw, P.M.; Hefti, A.F.; Jepsen, S.; Etienne, D.; Walker, C.; Bradshaw, M.H. Subantimicrobial Dose Doxycycline as Adjunctive Treatment for Periodontitis. A Review. J. Clin. Periodontol. 2004, 31, 697–707. [Google Scholar] [CrossRef]
- Warner, A.J.; Hathaway-Schrader, J.D.; Lubker, R.; Davies, C.; Novince, C.M. Tetracyclines and Bone: Unclear Actions with Potentially Lasting Effects. Bone 2022, 159, 116377. [Google Scholar] [CrossRef]
- Hughes, F.J.; Bartold, P.M. Periodontal Complications of Prescription and Recreational Drugs. Periodontology 2000 2018, 78, 47–58. [Google Scholar] [CrossRef]
- Ader, R.; Cohen, N.; Felten, D. Psychoneuroimmunology: Interactions between the Nervous System and the Immune System. Lancet 1995, 345, 99–103. [Google Scholar] [CrossRef]
- Ader, R.; Cohen, N. Psychoneuroimmunology: Conditioning and Stress. Annu. Rev. Psychol. 1993, 44, 53–85. [Google Scholar] [CrossRef] [PubMed]
- Manchanda, R.K.; Gupta, M.; Gupta, A.; van Haselen, R. The Clinical and Biological Effects of Homeopathically Prepared Signaling Molecules: A Scoping Review. Homeopathy 2022, 111, 010–021. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Kroemer, G. Hallmarks of Health. Cell 2021, 184, 33–63. [Google Scholar] [CrossRef] [PubMed]
- Jacques, C.; Marchand, F.; Chatelais, M.; Floris, I. Actives from the Micro-Immunotherapy Medicine 2LMIREG® Reduce the Expression of Cytokines and Immune-Related Markers Including Interleukin-2 and HLA-II While Modulating Oxidative Stress and Mitochondrial Function. J. Inflamm. Res. 2024, 17, 1161–1181. [Google Scholar] [CrossRef] [PubMed]
- Jacques, C.; Floris, I.; Lejeune, B. Ultra-Low Dose Cytokines in Rheumatoid Arthritis, Three Birds with One Stone as the Rationale of the 2LARTH® Micro-Immunotherapy Treatment. Int. J. Mol. Sci. 2021, 22, 6717. [Google Scholar] [CrossRef]
- Jacques, C.; Floris, I. Special Focus on the Cellular Anti-Inflammatory Effects of Several Micro-Immunotherapy Formulations: Considerations Regarding Intestinal-, Immune-Axis-Related- and Neuronal-Inflammation Contexts. J. Inflamm. Res. 2022, 15, 6695–6717. [Google Scholar] [CrossRef] [PubMed]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Klatzmann, D.; Abbas, A.K. The Promise of Low-Dose Interleukin-2 Therapy for Autoimmune and Inflammatory Diseases. Nat. Rev. Immunol. 2015, 15, 283–294. [Google Scholar] [CrossRef]
- Bernasconi, S. Low Dose Medicine: Theoretical Background and Scientific Evidence. Ital. J. Pediatr. 2018, 44, 23. [Google Scholar] [CrossRef]
- Floris, I.; Appel, K.; Rose, T.; Lejeune, B. 2LARTH®, a Micro-Immunotherapy Medicine, Exerts Anti-Inflammatory Effects in Vitro and Reduces TNF-α and IL-1β Secretion. J. Inflamm. Res. 2018, 11, 397–405. [Google Scholar] [CrossRef]
- Jacques, C.; Marchesi, I.; Fiorentino, F.P.; Chatelais, M.; Lilli, N.L.; Appel, K.; Lejeune, B.; Floris, I. A Micro-Immunotherapy Sequential Medicine MIM-Seq Displays Immunomodulatory Effects on Human Macrophages and Anti-Tumor Properties towards In Vitro 2D and 3D Models of Colon Carcinoma and in an In Vivo Subcutaneous Xenograft Colon Carcinoma Model. Int. J. Mol. Sci. 2022, 23, 6059. [Google Scholar] [CrossRef]
- Ferrà-Cañellas, M.D.M.; Munar-Bestard, M.; Floris, I.; Ramis, J.M.; Monjo, M.; Garcia-Sureda, L. A Sequential Micro-Immunotherapy Medicine Increases Collagen Deposition in Human Gingival Fibroblasts and in an Engineered 3D Gingival Model under Inflammatory Conditions. Int. J. Mol. Sci. 2023, 24, 10484. [Google Scholar] [CrossRef] [PubMed]
- Jacques, C.; Floris, I. How an Immune-Factor-Based Formulation of Micro-Immunotherapy Could Interfere with the Physiological Processes Involved in the Atopic March. Int. J. Mol. Sci. 2023, 24, 1483. [Google Scholar] [CrossRef]
- Biancotto, A.; Wank, A.; Perl, S.; Cook, W.; Olnes, M.J.; Dagur, P.K.; Fuchs, J.C.; Langweiler, M.; Wang, E.; McCoy, J.P. Baseline Levels and Temporal Stability of 27 Multiplexed Serum Cytokine Concentrations in Healthy Subjects. PLoS ONE 2013, 8, e76091. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Giordano, J. Ultra Low Doses and Biological Amplification: Approaching Avogadro’s Number. Pharmacol. Res. 2021, 170, 105738. [Google Scholar] [CrossRef] [PubMed]
- Floris, I.; Rose, T.; Rojas, J.A.C.; Appel, K.; Roesch, C.; Lejeune, B. Pro-Inflammatory Cytokines at Ultra-Low Dose Exert Anti-Inflammatory Effect In Vitro: A Possible Mode of Action Involving Sub-Micron Particles? Dose-Response 2020, 18, 155932582096172. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Rai, U. Dual Mode of Catecholamine Action on Splenic Macrophage Phagocytosis in Wall Lizard, Hemidactylus Flaviviridis. Gen. Comp. Endocrinol. 2004, 136, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Csaba, G.; Kovács, P.; Tóthfalusi, L.; Pállinger, É. Effects of Extremely Low Concentrations of Hormones on the Insulin Binding of Tetrahymena. Cell Biol. Int. 2006, 30, 957–962. [Google Scholar] [CrossRef]
- Ershova, E.S.; Sergeeva, V.A.; Tabakov, V.J.; Kameneva, L.A.; Porokhovnik, L.N.; Voronov, I.I.; Khakina, E.A.; Troshin, P.A.; Kutsev, S.I.; Veiko, N.N.; et al. Functionalized Fullerene Increases NF-ΚB Activity and Blocks Genotoxic Effect of Oxidative Stress in Serum-Starving Human Embryo Lung Diploid Fibroblasts. Oxidative Med. Cell. Longev. 2016, 2016, 9895245. [Google Scholar] [CrossRef]
- Civciristov, S.; Ellisdon, A.M.; Suderman, R.; Pon, C.K.; Evans, B.A.; Kleifeld, O.; Charlton, S.J.; Hlavacek, W.S.; Canals, M.; Halls, M.L. Preassembled GPCR Signaling Complexes Mediate Distinct Cellular Responses to Ultralow Ligand Concentrations. Sci. Signal. 2018, 11, eaan1188. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Agathokleous, E. Hormesis: Transforming Disciplines That Rely on the Dose Response. IUBMB Life 2022, 74, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J. Hormesis: Path and Progression to Significance. Int. J. Mol. Sci. 2018, 19, 2871. [Google Scholar] [CrossRef]
- Agathokleous, E.; Calabrese, E.J. Hormesis: A General Biological Principle. Chem. Res. Toxicol. 2022, 35, 547–549. [Google Scholar] [CrossRef] [PubMed]
- Kamrin, M.A. The “Low Dose” Hypothesis: Validity and Implications for Human Risk. Int. J. Toxicol. 2007, 26, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. Hormesis and Disease Resistance: Activation of Cellular Stress Response Pathways. Hum. Exp. Toxicol. 2008, 27, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Forcina, L.; Franceschi, C.; Musarò, A. The Hormetic and Hermetic Role of IL-6. Ageing Res. Rev. 2022, 80, 101697. [Google Scholar] [CrossRef] [PubMed]
- Cardani, D.; Dusio, G.F.; Luchini, P.; Sciarabba, M.; Solimene, U.; Rumio, C. Cardani Oral Administration of Interleukin-10 and Anti-IL-1 Antibody Ameliorates Experimental Intestinal Inflammation. Gastroenterol. Res. 2013, 6, 124. [Google Scholar] [CrossRef] [PubMed]
- Iuliano, M.; Santilli, V.; Mineo, A.; Paoloni, M.; Rosa, P.; Mangino, G.; Romeo, G. Inflammatory Response Modulation by Low-Dose Anti-Inflammatory Drugs Treatment in an In Vitro Osteoarthritis Cellular Model. Curr. Med. Chem. 2024, 31, 1740–1753. [Google Scholar] [CrossRef] [PubMed]
- Martin-Martin, L.S.; Giovannangeli, F.; Bizzi, E.; Massafra, U.; Ballanti, E.; Cassol, M.; Migliore, A. An Open Randomized Active-Controlled Clinical Trial with Low-Dose SKA Cytokines versus DMARDs Evaluating Low Disease Activity Maintenance in Patients with Rheumatoid Arthritis. Drug Des. Devel. Ther. 2017, 11, 985–994. [Google Scholar] [CrossRef]
- Mancini, F.; Milardi, D.; Carfagna, P.; Grande, G.; Miranda, V.; De Cicco Nardone, A.; Ricciardi, D.; Pontecorvi, A.; Marana, R.; De Cicco Nardone, F. Low-Dose SKA Progesterone and Interleukin-10 Modulate the Inflammatory Pathway in Endometriotic Cell Lines. Int. Immunopharmacol. 2018, 55, 223–230. [Google Scholar] [CrossRef]
- Lilli, N.L.; Révy, D.; Robelet, S.; Lejeune, B. Effect of the Micro-Immunotherapy Medicine 2LPARK® on Rat Primary Dopaminergic Neurons after 6-OHDA Injury: Oxidative Stress and Survival Evaluation in an in Vitro Model of Parkinson’s Disease. Degener. Neurol. Neuromuscul. Dis. 2019, 9, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Jacques, C.; Chatelais, M.; Fekir, K.; Fauconnier, L.; Mellier, M.; Togbe, D.; Floris, I. The Micro-Immunotherapy Medicine 2LEID Exhibits an Immunostimulant Effect by Boosting Both Innate and Adaptive Immune Responses. Int. J. Mol. Sci. 2021, 23, 110. [Google Scholar] [CrossRef] [PubMed]
- Floris, I.; García-González, V.; Palomares, B.; Appel, K.; Lejeune, B. The Micro-Immunotherapy Medicine 2LARTH® Reduces Inflammation and Symptoms of Rheumatoid Arthritis In Vivo. Int. J. Rheumatol. 2020, 2020, 1594573. [Google Scholar] [CrossRef] [PubMed]
- Floris, I.; Chenuet, P.; Togbe, D.; Volteau, C.; Lejeune, B. Potential Role of the Micro-Immunotherapy Medicine 2LALERG in the Treatment of Pollen-Induced Allergic Inflammation. Dose-Response 2020, 18, 155932582091409. [Google Scholar] [CrossRef] [PubMed]
- Jacques, C.; Chatelais, M.; Fekir, K.; Brulefert, A.; Floris, I. The Unitary Micro-Immunotherapy Medicine Interferon-γ (4 CH) Displays Similar Immunostimulatory and Immunomodulatory Effects than Those of Biologically Active Human Interferon-γ on Various Cell Types. Int. J. Mol. Sci. 2022, 23, 2314. [Google Scholar] [CrossRef] [PubMed]
- Ferrà-Cañellas, M.D.M.; Munar-Bestard, M.; Garcia-Sureda, L.; Lejeune, B.; Ramis, J.M.; Monjo, M. BMP4 Micro-immunotherapy Increases Collagen Deposition and Reduces PGE2 Release in Human Gingival Fibroblasts and Increases Tissue Viability of Engineered 3D Gingiva under Inflammatory Conditions. J. Periodontol. 2021, 92, 1448–1459. [Google Scholar] [CrossRef] [PubMed]
- Floris, I.; Lechner, J.; Lejeune, B. Follow-up of Patients with Systemic Immunological Diseases Undergoing Fatty-Degenerative Osteolysis of the Jawbone Surgery and Treated with RANTES 27CH. J. Biol. Regul. Homeost. Agents 2018, 32, 37–45. [Google Scholar] [PubMed]
- Grayson, M.H.; Holtzman, M.J. Chemokine Complexity: The Case for CCL5. Am. J. Respir. Cell Mol. Biol. 2006, 35, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Lan, T.; Wei, Y.; Wei, X. CCL5/CCR5 Axis in Human Diseases and Related Treatments. Genes Dis. 2021, 9, 12–27. [Google Scholar] [CrossRef]
- Lechner, J.; Schulz, T.; Lejeune, B.; von Baehr, V. Jawbone Cavitation Expressed RANTES/CCL5: Case Studies Linking Silent Inflammation in the Jawbone with Epistemology of Breast Cancer. Breast Cancer Targets Ther. 2021, 13, 225–240. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrà-Cañellas, M.d.M.; Garcia-Sureda, L. Exploring the Potential of Micro-Immunotherapy in the Treatment of Periodontitis. Life 2024, 14, 552. https://doi.org/10.3390/life14050552
Ferrà-Cañellas MdM, Garcia-Sureda L. Exploring the Potential of Micro-Immunotherapy in the Treatment of Periodontitis. Life. 2024; 14(5):552. https://doi.org/10.3390/life14050552
Chicago/Turabian StyleFerrà-Cañellas, Maria del Mar, and Laura Garcia-Sureda. 2024. "Exploring the Potential of Micro-Immunotherapy in the Treatment of Periodontitis" Life 14, no. 5: 552. https://doi.org/10.3390/life14050552
APA StyleFerrà-Cañellas, M. d. M., & Garcia-Sureda, L. (2024). Exploring the Potential of Micro-Immunotherapy in the Treatment of Periodontitis. Life, 14(5), 552. https://doi.org/10.3390/life14050552





