Management of Refractory Chronic Obstructive Pulmonary Disease: A Review
Abstract
1. Introduction
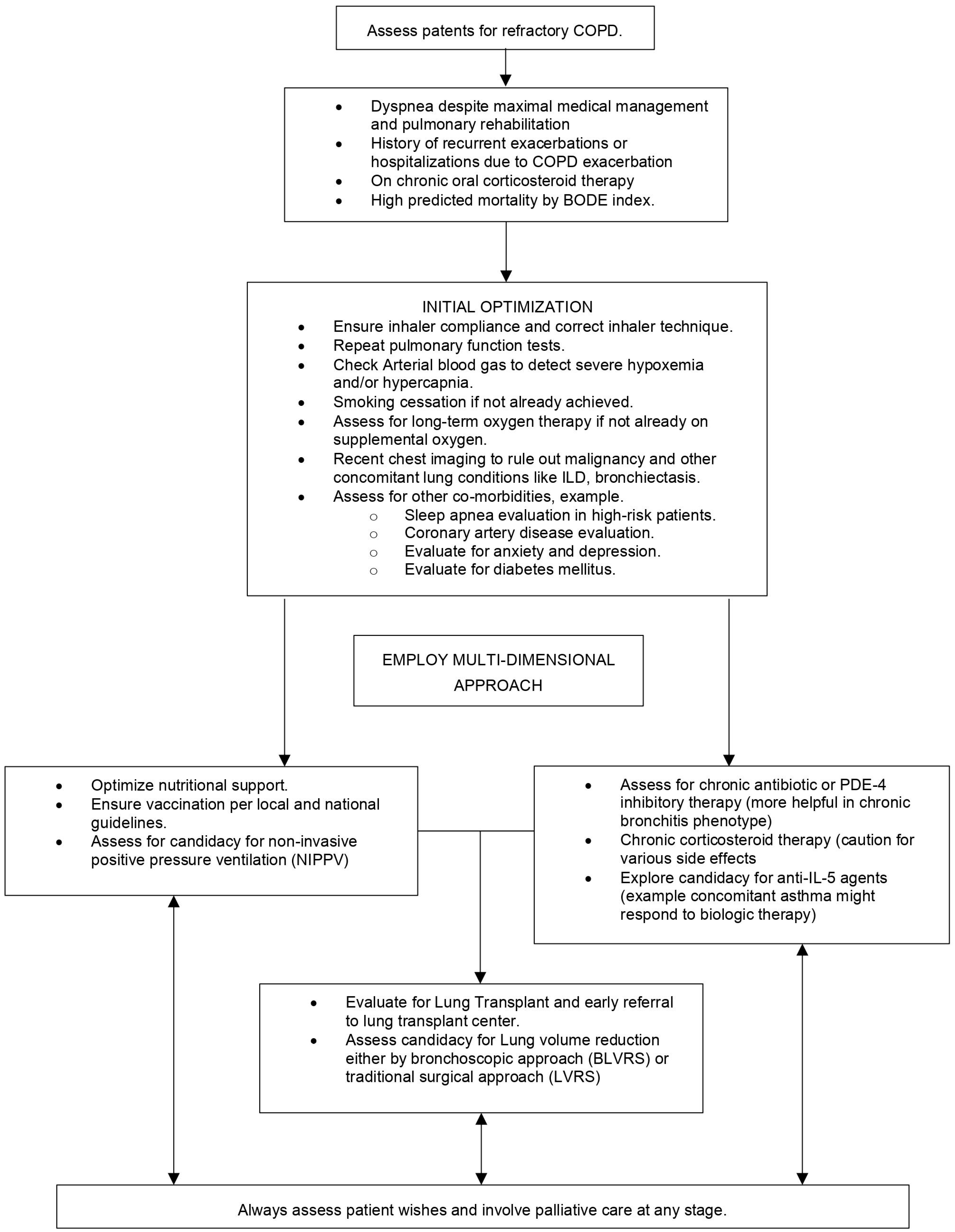
2. Initial Assessment of Patients with Refractory COPD
2.1. Evaluation of Dyspnea, Exacerbation History, and Smoking History
2.2. Optimizing Inhaler Technique
2.3. Pulmonary Function Testing, Arterial Blood Gas Analysis, and Cardiothoracic Imaging
2.4. Evaluating Co-Morbidities
3. Non-Pharmacological Approaches for Refractory COPD Management
3.1. Nutrition
3.2. Pulmonary Rehabilitation
3.3. Non-Invasive Positive Pressure Ventilation
3.4. Managing Co-Morbidities
4. Pharmacological Approaches for Refractory COPD Management
4.1. Long-Term Oxygen Therapy (LTOT)
4.2. Chronic Suppressive Antibiotic Therapy
4.3. Phosphodiesterase Inhibitors
4.4. Chronic Glucocorticoids
4.5. Role of Biologics in COPD
4.6. Anti-Interleukin-5/Interleukin-5 Receptor Monoclonal Antibody
4.7. Anti-Interleukin-4 Monoclonal Antibody
5. Surgical Approaches for Refractory COPD Management
5.1. Lung Volume Reduction Surgery
5.2. Bronchoscopic Lung Volume Reduction Surgery (BLVRS)
6. Lung Transplantation
7. Palliative Care
8. Future Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Celli, B.; Fabbri, L.; Criner, G.; Martinez, F.J.; Mannino, D.; Vogelmeier, C.; Montes de Oca, M.; Papi, A.; Sin, D.D.; Han, M.K.; et al. Definition and Nomenclature of Chronic Obstructive Pulmonary Disease: Time for Its Revision. Am. J. Respir. Crit. Care Med. 2022, 206, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Yang, I.A.; Jenkins, C.R.; Salvi, S.S. Chronic obstructive pulmonary disease in never-smokers: Risk factors, pathogenesis, and implications for prevention and treatment. Lancet Respir. Med. 2022, 10, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Agustí, A.; Melén, E.; DeMeo, D.L.; Breyer-Kohansal, R.; Faner, R. Pathogenesis of chronic obstructive pulmonary disease: Understanding the contributions of gene-environment interactions across the lifespan. Lancet Respir. Med. 2022, 10, 512–524. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.H.; Hobbs, B.D.; Silverman, E.K. Genetics of chronic obstructive pulmonary disease: Understanding the pathobiology and heterogeneity of a complex disorder. Lancet Respir. Med. 2022, 10, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef] [PubMed]
- Adeloye, D.; Song, P.; Zhu, Y.; Campbell, H.; Sheikh, A.; Rudan, I. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: A systematic review and modelling analysis. Lancet Respir. Med. 2022, 10, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Adeloye, D.; Chua, S.; Lee, C.; Basquill, C.; Papana, A.; Theodoratou, E.; Nair, H.; Gasevic, D.; Sridhar, D.; Campbell, H.; et al. Global and regional estimates of COPD prevalence: Systematic review and meta-analysis. J. Glob. Health 2015, 5, 020415. [Google Scholar] [CrossRef] [PubMed]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef] [PubMed]
- Tashkin, D.P.; Altose, M.D.; Bleecker, E.R.; Connett, J.E.; Kanner, R.E.; Lee, W.W.; Wise, R. The lung health study: Airway responsiveness to inhaled methacholine in smokers with mild to moderate airflow limitation. The Lung Health Study Research Group. Am. Rev. Respir. Dis. 1992, 145, 301–310. [Google Scholar] [CrossRef]
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar] [CrossRef]
- Syamlal, G.; Kurth, L.M.; Dodd, K.E.; Blackley, D.J.; Hall, N.B.; Mazurek, J.M. Chronic Obstructive Pulmonary Disease Mortality by Industry and Occupation—United States, 2020. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1550–1554. [Google Scholar] [CrossRef] [PubMed]
- Wedzicha, J.A.; Seemungal, T.A. COPD exacerbations: Defining their cause and prevention. Lancet 2007, 370, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Safka, K.A.; McIvor, R.A. Non-pharmacological management of chronic obstructive pulmonary disease. Ulster Med. J. 2015, 84, 13–21. [Google Scholar] [PubMed]
- Khan, K.S.; Jawaid, S.; Memon, U.A.; Perera, T.; Khan, U.; Farwa, U.E.; Jindal, U.; Afzal, M.S.; Razzaq, W.; Abdin, Z.U.; et al. Management of Chronic Obstructive Pulmonary Disease (COPD) Exacerbations in Hospitalized Patients from Admission to Discharge: A Comprehensive Review of Therapeutic Interventions. Cureus 2023, 15, e43694. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, K.; Murthi, S.; Elango, K.; Rahi, M.S.; Thilagar, B.; Ramalingam, S.; Voruganti, D.; Paramasivam, V.K.; Kolandaivel, K.P.; Arora, A.; et al. The Impact of Diabetes Mellitus in Patients with Chronic Obstructive Pulmonary Disease (COPD) Hospitalization. J. Clin. Med. 2021, 10, 235. [Google Scholar] [CrossRef] [PubMed]
- Rahi, M.S.; Thilagar, B.; Balaji, S.; Prabhakaran, S.Y.; Mudgal, M.; Rajoo, S.; Yella, P.R.; Satija, P.; Zagorulko, A.; Gunasekaran, K. The Impact of Anxiety and Depression in Chronic Obstructive Pulmonary Disease. Adv. Respir. Med. 2023, 91, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. Available online: www.goldcopd.org (accessed on 28 January 2024).
- Gunasekaran, K.; Voruganti, D.C.; Singh Rahi, M.; Elango, K.; Ramalingam, S.; Geeti, A.; Kwon, J. Trends in Prevalence and Outcomes of Cannabis Use Among Chronic Obstructive Pulmonary Disease Hospitalizations: A Nationwide Population-Based Study 2005–2014. Cannabis Cannabinoid Res. 2021, 6, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, K.; Singh Rahi, M.; Rajasurya, V.; Wolff, A. Trends in E-Cigarette Use Among Various Subgroups. Am. J. Med. 2020, 133, e607. [Google Scholar] [CrossRef] [PubMed]
- Rigotti, N.A.; Kruse, G.R.; Livingstone-Banks, J.; Hartmann-Boyce, J. Treatment of Tobacco Smoking: A Review. JAMA 2022, 327, 566–577. [Google Scholar] [CrossRef]
- Barjaktarevic, I.Z.; Milstone, A.P. Nebulized Therapies in COPD: Past, Present, and the Future. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 1665–1677. [Google Scholar] [CrossRef]
- Jardim, J.R.; Nascimento, O.A. The Importance of Inhaler Adherence to Prevent COPD Exacerbations. Med. Sci. 2019, 7, 54. [Google Scholar] [CrossRef]
- Sanchis, J.; Gich, I.; Pedersen, S. Systematic Review of Errors in Inhaler Use: Has Patient Technique Improved Over Time? Chest 2016, 150, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Turégano-Yedro, M.; Trillo-Calvo, E.; Navarro, I.R.F.; Maya-Viejo, J.D.; González Villaescusa, C.; Echave Sustaeta, J.M.; Doña, E.; Alcázar Navarrete, B. Inhaler Adherence in COPD: A Crucial Step Towards the Correct Treatment. Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 2887–2893. [Google Scholar] [CrossRef] [PubMed]
- Simoni-Wastila, L.; Wei, Y.J.; Qian, J.; Zuckerman, I.H.; Stuart, B.; Shaffer, T.; Dalal, A.A.; Bryant-Comstock, L. Association of chronic obstructive pulmonary disease maintenance medication adherence with all-cause hospitalization and spending in a Medicare population. Am. J. Geriatr. Pharmacother. 2012, 10, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, J.S.; Park, Y.; Hur, P.; Huang, T.Y.; Harris, I.; Netzer, G.; Lehmann, S.W.; Langenberg, P.; Khokhar, B.; Wei, Y.J.; et al. Adherence to Maintenance Medications among Older Adults with Chronic Obstructive Pulmonary Disease. The Role of Depression. Ann. Am. Thorac. Soc. 2016, 13, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- Lareau, S.C.; Yawn, B.P. Improving adherence with inhaler therapy in COPD. Int. J. Chronic Obstr. Pulm. Dis. 2010, 5, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, M.K. Nutrition and lung health. Proc. Nutr. Soc. 1999, 58, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Clini, E.M.; Ambrosino, N. Nonpharmacological treatment and relief of symptoms in COPD. Eur. Respir. J. 2008, 32, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Long, R.; Stracy, C.; Oliver, M.C. Nutritional care in Chronic Obstructive Pulmonary Disease. Br. J. Community Nurs. 2018, 23, S18–S26. [Google Scholar] [CrossRef]
- Pyszora, A.; Lewko, A. Non-pharmacological Management in Palliative Care for Patients With Advanced COPD. Front. Cardiovasc. Med. 2022, 9, 907664. [Google Scholar] [CrossRef]
- Schols, A.M.; Ferreira, I.M.; Franssen, F.M.; Gosker, H.R.; Janssens, W.; Muscaritoli, M.; Pison, C.; Rutten-van Mölken, M.; Slinde, F.; Steiner, M.C.; et al. Nutritional assessment and therapy in COPD: A European Respiratory Society statement. Eur. Respir. J. 2014, 44, 1504–1520. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, A.N.; Tingö, L.; Brummer, R.J. The Potential Effects of Probiotics and ω-3 Fatty Acids on Chronic Low-Grade Inflammation. Nutrients 2020, 12, 2402. [Google Scholar] [CrossRef] [PubMed]
- Spruit, M.A.; Singh, S.J.; Garvey, C.; ZuWallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.; et al. An official American Thoracic Society/European Respiratory Society statement: Key concepts and advances in pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.E.; Cox, N.S.; Houchen-Wolloff, L.; Rochester, C.L.; Garvey, C.; ZuWallack, R.; Nici, L.; Limberg, T.; Lareau, S.C.; Yawn, B.P.; et al. Defining Modern Pulmonary Rehabilitation. An Official American Thoracic Society Workshop Report. Ann. Am. Thorac. Soc. 2021, 18, e12–e29. [Google Scholar] [CrossRef] [PubMed]
- Pitta, F.; Troosters, T.; Probst, V.S.; Langer, D.; Decramer, M.; Gosselink, R. Are patients with COPD more active after pulmonary rehabilitation? Chest 2008, 134, 273–280. [Google Scholar] [CrossRef]
- McCarthy, B.; Casey, D.; Devane, D.; Murphy, K.; Murphy, E.; Lacasse, Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2015, 2015, CD003793. [Google Scholar] [CrossRef]
- Puhan, M.A.; Gimeno-Santos, E.; Cates, C.J.; Troosters, T. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2016, 12, Cd005305. [Google Scholar] [CrossRef]
- Ringbaek, T.; Brøndum, E.; Martinez, G.; Lange, P. Rehabilitation in COPD: The long-term effect of a supervised 7-week program succeeded by a self-monitored walking program. Chronic Respir. Dis. 2008, 5, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Rochester, D.F.; Braun, N.M.; Arora, N.S. Respiratory muscle strength in chronic obstructive pulmonary disease. Am. Rev. Respir. Dis. 1979, 119, 151–154. [Google Scholar]
- Schönhofer, B.; Polkey, M.I.; Suchi, S.; Köhler, D. Effect of home mechanical ventilation on inspiratory muscle strength in COPD. Chest 2006, 130, 1834–1838. [Google Scholar] [CrossRef]
- Garay, S.M.; Turino, G.M.; Goldring, R.M. Sustained reversal of chronic hypercapnia in patients with alveolar hypoventilation syndromes. Long-term maintenance with noninvasive nocturnal mechanical ventilation. Am. J. Med. 1981, 70, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Budweiser, S.; Heinemann, F.; Fischer, W.; Dobroschke, J.; Pfeifer, M. Long-term reduction of hyperinflation in stable COPD by non-invasive nocturnal home ventilation. Respir. Med. 2005, 99, 976–984. [Google Scholar] [CrossRef]
- Macrea, M.; Oczkowski, S.; Rochwerg, B.; Branson, R.D.; Celli, B.; Coleman, J.M., 3rd; Hess, D.R.; Knight, S.L.; Ohar, J.A.; Orr, J.E.; et al. Long-Term Noninvasive Ventilation in Chronic Stable Hypercapnic Chronic Obstructive Pulmonary Disease. An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2020, 202, e74–e87. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.B.; Rehal, S.; Arbane, G.; Bourke, S.; Calverley, P.M.A.; Crook, A.M.; Dowson, L.; Duffy, N.; Gibson, G.J.; Hughes, P.D.; et al. Effect of Home Noninvasive Ventilation With Oxygen Therapy vs Oxygen Therapy Alone on Hospital Readmission or Death After an Acute COPD Exacerbation: A Randomized Clinical Trial. JAMA 2017, 317, 2177–2186. [Google Scholar] [CrossRef]
- Struik, F.M.; Lacasse, Y.; Goldstein, R.; Kerstjens, H.M.; Wijkstra, P.J. Nocturnal non-invasive positive pressure ventilation for stable chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2013, 2013, Cd002878. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Sellarés, J.; Valencia, M.; Carrillo, A.; Gonzalez, G.; Badia, J.R.; Nicolas, J.M.; Torres, A. Non-invasive ventilation after extubation in hypercapnic patients with chronic respiratory disorders: Randomised controlled trial. Lancet 2009, 374, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Gay, P.C.; Hubmayr, R.D.; Stroetz, R.W. Efficacy of nocturnal nasal ventilation in stable, severe chronic obstructive pulmonary disease during a 3-month controlled trial. Mayo Clin. Proc. 1996, 71, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Meecham Jones, D.J.; Paul, E.A.; Jones, P.W.; Wedzicha, J.A. Nasal pressure support ventilation plus oxygen compared with oxygen therapy alone in hypercapnic COPD. Am. J. Respir. Crit. Care Med. 1995, 152, 538–544. [Google Scholar] [CrossRef]
- Köhnlein, T.; Windisch, W.; Köhler, D.; Drabik, A.; Geiseler, J.; Hartl, S.; Karg, O.; Laier-Groeneveld, G.; Nava, S.; Schönhofer, B.; et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: A prospective, multicentre, randomised, controlled clinical trial. Lancet Respir. Med. 2014, 2, 698–705. [Google Scholar] [CrossRef]
- McEvoy, R.D.; Pierce, R.J.; Hillman, D.; Esterman, A.; Ellis, E.E.; Catcheside, P.G.; O’Donoghue, F.J.; Barnes, D.J.; Grunstein, R.R. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: A randomised controlled trial. Thorax 2009, 64, 561–566. [Google Scholar] [CrossRef]
- Clini, E.; Sturani, C.; Rossi, A.; Viaggi, S.; Corrado, A.; Donner, C.F.; Ambrosino, N. The Italian multicentre study on noninvasive ventilation in chronic obstructive pulmonary disease patients. Eur. Respir. J. 2002, 20, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Horie, T.; Chohnabayashi, N.; Jinta, T.; Tsugitomi, R.; Shiraki, A.; Tokioka, F.; Kadowaki, T.; Watanabe, A.; Fukui, M.; et al. Home High-Flow Nasal Cannula Oxygen Therapy for Stable Hypercapnic COPD: A Randomized Clinical Trial. Am. J. Respir. Crit. Care Med. 2022, 206, 1326–1335. [Google Scholar] [CrossRef] [PubMed]
- Maclagan, L.C.; Croxford, R.; Chu, A.; Sin, D.D.; Udell, J.A.; Lee, D.S.; Austin, P.C.; Gershon, A.S. Quantifying COPD as a risk factor for cardiac disease in a primary prevention cohort. Eur. Respir. J. 2023, 62, 2202364. [Google Scholar] [CrossRef] [PubMed]
- Matamis, D.; Tsagourias, M.; Papathanasiou, A.; Sineffaki, H.; Lepida, D.; Galiatsou, E.; Nakos, G. Targeting occult heart failure in intensive care unit patients with acute chronic obstructive pulmonary disease exacerbation: Effect on outcome and quality of life. J. Crit. Care 2014, 29, 315.e7–315.e14. [Google Scholar] [CrossRef] [PubMed]
- Krist, A.H.; Davidson, K.W.; Mangione, C.M.; Barry, M.J.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Kubik, M.; et al. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 325, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Smalley, M.; Anusim, N.; Jindal, V.; Singh Rahi, M.; Gupta, S.; Gupta, S.; Jaiyesimi, I. Paradigm shift in the management of metastatic nonsmall cell lung cancer. Int. J. Clin. Pract. 2021, 75, e14533. [Google Scholar] [CrossRef]
- Ni, Y.; Shi, G.; Yu, Y.; Hao, J.; Chen, T.; Song, H. Clinical characteristics of patients with chronic obstructive pulmonary disease with comorbid bronchiectasis: A systemic review and meta-analysis. Int. J. Chronic Obstr. Pulm. Dis. 2015, 10, 1465–1475. [Google Scholar] [CrossRef]
- Young, T.; Palta, M.; Dempsey, J.; Skatrud, J.; Weber, S.; Badr, S. The occurrence of sleep-disordered breathing among middle-aged adults. N. Engl. J. Med. 1993, 328, 1230–1235. [Google Scholar] [CrossRef]
- Shepard, J.W., Jr.; Garrison, M.W.; Grither, D.A.; Evans, R.; Schweitzer, P.K. Relationship of ventricular ectopy to nocturnal oxygen desaturation in patients with chronic obstructive pulmonary disease. Am. J. Med. 1985, 78, 28–34. [Google Scholar] [CrossRef]
- Sterling, K.L.; Pépin, J.L.; Linde-Zwirble, W.; Chen, J.; Benjafield, A.V.; Cistulli, P.A.; Cole, K.V.; Emami, H.; Woodford, C.; Armitstead, J.P.; et al. Impact of Positive Airway Pressure Therapy Adherence on Outcomes in Patients with Obstructive Sleep Apnea and Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2022, 206, 197–205. [Google Scholar] [CrossRef]
- Wedzicha, J.A.; Calverley, P.M.A.; Albert, R.K.; Anzueto, A.; Criner, G.J.; Hurst, J.R.; Miravitlles, M.; Papi, A.; Rabe, K.F.; Rigau, D.; et al. Prevention of COPD exacerbations: A European Respiratory Society/American Thoracic Society guideline. Eur. Respir. J. 2017, 50, 1602265. [Google Scholar] [CrossRef] [PubMed]
- Branson, R.D. Oxygen Therapy in COPD. Respir. Care 2018, 63, 734–748. [Google Scholar] [CrossRef] [PubMed]
- Lacasse, Y.; Sériès, F.; Corbeil, F.; Baltzan, M.; Paradis, B.; Simão, P.; Abad Fernández, A.; Esteban, C.; Guimarães, M.; Bourbeau, J.; et al. Randomized Trial of Nocturnal Oxygen in Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2020, 383, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.B.; Blackford, A.L.; Benditt, J.O.; Make, B.J.; Sciurba, F.C.; McCormack, M.C.; Martinez, F.J.; Fessler, H.E.; Fishman, A.P.; Wise, R.A.; et al. Continuous oxygen use in nonhypoxemic emphysema patients identifies a high-risk subset of patients: Retrospective analysis of the National Emphysema Treatment Trial. Chest 2008, 134, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Criner, G.J. Ambulatory home oxygen: What is the evidence for benefit, and who does it help? Respir. Care 2013, 58, 48–64. [Google Scholar] [CrossRef]
- Matzneller, P.; Krasniqi, S.; Kinzig, M.; Sorgel, F.; Huttner, S.; Lackner, E.; Muller, M.; Zeitlinger, M. Blood, tissue, and intracellular concentrations of azithromycin during and after end of therapy. Antimicrob. Agents Chemother. 2013, 57, 1736–1742. [Google Scholar] [CrossRef]
- Sethi, S.; Murphy, T.F. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N. Engl. J. Med. 2008, 359, 2355–2365. [Google Scholar] [CrossRef]
- Martinez, F.J.; Curtis, J.L.; Albert, R. Role of macrolide therapy in chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2008, 3, 331–350. [Google Scholar] [CrossRef] [PubMed]
- Desaki, M.; Takizawa, H.; Ohtoshi, T.; Kasama, T.; Kobayashi, K.; Sunazuka, T.; Omura, S.; Yamamoto, K.; Ito, K. Erythromycin suppresses nuclear factor-kappaB and activator protein-1 activation in human bronchial epithelial cells. Biochem. Biophys. Res. Commun. 2000, 267, 124–128. [Google Scholar] [CrossRef]
- Desaki, M.; Okazaki, H.; Sunazuka, T.; Omura, S.; Yamamoto, K.; Takizawa, H. Molecular mechanisms of anti-inflammatory action of erythromycin in human bronchial epithelial cells: Possible role in the signaling pathway that regulates nuclear factor-kappaB activation. Antimicrob. Agents Chemother. 2004, 48, 1581–1585. [Google Scholar] [CrossRef]
- Joelsson, J.P.; Myszor, I.T.; Sigurdsson, S.; Lehmann, F.; Page, C.P.; Gudmundsson, G.H.; Gudjonsson, T.; Karason, S. Azithromycin has lung barrier protective effects in a cell model mimicking ventilator-induced lung injury. Altex 2020, 37, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Mammen, M.J.; Sethi, S. Macrolide therapy for the prevention of acute exacerbations in chronic obstructive pulmonary disease. Pol. Arch. Med. Wewnętrznej 2012, 122, 54–59. [Google Scholar] [CrossRef]
- Francis, R.S.; May, J.R.; Spicer, C.C. Chemotherapy of bronchitis. Influence of penicillin and tetracycline administered daily, or intermittently for exacerbations. A report to the Research Committee of the British Tuberculosis Association by its Bronchitis Subcommittee. Br. Med. J. 1961, 2, 979–985. [Google Scholar] [CrossRef]
- Francis, R.S.; Spicer, C.C. Chemotherapy in chronic bronchitis. Influence of daily penicillin and tetracycline on exacerbations and their cost. Br. Med. J. 1960, 1, 297–303. [Google Scholar] [CrossRef]
- Herath, S.C.; Poole, P. Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst. Rev. 2013, 11, CD009764. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Shao, X.; Cai, X.; Wei, C.; Cui, J.; Wang, R.; Liu, Y. Prophylactic use of macrolide antibiotics for the prevention of chronic obstructive pulmonary disease exacerbation: A meta-analysis. PLoS ONE 2015, 10, e0121257. [Google Scholar] [CrossRef] [PubMed]
- Herath, S.C.; Normansell, R.; Maisey, S.; Poole, P. Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst. Rev. 2018, 10, CD009764. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Jones, P.W.; Theron, M.S.; Miravitlles, M.; Rubinstein, E.; Wedzicha, J.A.; Wilson, R.; PULSE Study Group. Pulsed moxifloxacin for the prevention of exacerbations of chronic obstructive pulmonary disease: A randomized controlled trial. Respir. Res. 2010, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Brill, S.E.; Law, M.; El-Emir, E.; Allinson, J.P.; James, P.; Maddox, V.; Donaldson, G.C.; McHugh, T.D.; Cookson, W.O.; Moffatt, M.F.; et al. Effects of different antibiotic classes on airway bacteria in stable COPD using culture and molecular techniques: A randomised controlled trial. Thorax 2015, 70, 930–938. [Google Scholar] [CrossRef]
- Albert, R.K.; Connett, J.; Bailey, W.C.; Casaburi, R.; Cooper, J.A., Jr.; Criner, G.J.; Curtis, J.L.; Dransfield, M.T.; Han, M.K.; Lazarus, S.C.; et al. Azithromycin for prevention of exacerbations of COPD. N. Engl. J. Med. 2011, 365, 689–698. [Google Scholar] [CrossRef]
- Uzun, S.; Djamin, R.S.; Kluytmans, J.A.; Mulder, P.G.; van’t Veer, N.E.; Ermens, A.A.; Pelle, A.J.; Hoogsteden, H.C.; Aerts, J.G.; van der Eerden, M.M. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): A randomised, double-blind, placebo-controlled trial. Lancet Respir. Med. 2014, 2, 361–368. [Google Scholar] [CrossRef]
- Boswell-Smith, V.; Spina, D.; Page, C.P. Phosphodiesterase inhibitors. Br. J. Pharmacol. 2006, 147 (Suppl. S1), S252–S257. [Google Scholar] [CrossRef]
- Reid, P. Roflumilast Altana Pharma. Curr. Opin. Investig. Drugs 2002, 3, 1165–1170. [Google Scholar] [PubMed]
- Gamble, E.; Grootendorst, D.C.; Brightling, C.E.; Troy, S.; Qiu, Y.; Zhu, J.; Parker, D.; Matin, D.; Majumdar, S.; Vignola, A.M.; et al. Antiinflammatory effects of the phosphodiesterase-4 inhibitor cilomilast (Ariflo) in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2003, 168, 976–982. [Google Scholar] [CrossRef]
- DALIRESP®. (Roflumilast) Tablets [Package Insert]; AstraZeneca Pharmaceuticals LP: Wilmington, DE, USA, 2017. [Google Scholar]
- Janjua, S.; Fortescue, R.; Poole, P. Phosphodiesterase-4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2020, 5, CD002309. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.J.; Calverley, P.M.; Goehring, U.M.; Brose, M.; Fabbri, L.M.; Rabe, K.F. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): A multicentre randomised controlled trial. Lancet 2015, 385, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.F.; Lv, X.D.; Chen, W.Y.; Yang, Q.; Fang, Z.X.; Lu, W.F. Effect of roflumilast on chronic obstructive pulmonary disease: A systematic review and meta-analysis. Ir. J. Med. Sci. 2018, 187, 731–738. [Google Scholar] [CrossRef]
- US Food and Drug Administration Prescribing Information for Daliresp. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/022522s009lbl.pdf (accessed on 4 February 2024).
- Ram, F.S.; Jones, P.W.; Castro, A.A.; De Brito, J.A.; Atallah, A.N.; Lacasse, Y.; Mazzini, R.; Goldstein, R.; Cendon, S. Oral theophylline for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2002, 2002, Cd003902. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Jeffers, H.; Langford-Wiley, B.; Davies, J.; Thulborn, S.J.; Mahdi, M.; A’Court, C.; Binnian, I.; Bright, S.; Cartwright, S.; et al. Blood eosinophil-guided oral prednisolone for COPD exacerbations in primary care in the UK (STARR2): A non-inferiority, multicentre, double-blind, placebo-controlled, randomised controlled trial. Lancet Respir. Med. 2024, 12, 67–77. [Google Scholar] [CrossRef]
- Bafadhel, M.; McKenna, S.; Terry, S.; Mistry, V.; Pancholi, M.; Venge, P.; Lomas, D.A.; Barer, M.R.; Johnston, S.L.; Pavord, I.D.; et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: A randomized placebo-controlled trial. Am. J. Respir. Crit. Care Med. 2012, 186, 48–55. [Google Scholar] [CrossRef]
- Sivapalan, P.; Lapperre, T.S.; Janner, J.; Laub, R.R.; Moberg, M.; Bech, C.S.; Eklof, J.; Holm, F.S.; Armbruster, K.; Sivapalan, P.; et al. Eosinophil-guided corticosteroid therapy in patients admitted to hospital with COPD exacerbation (CORTICO-COP): A multicentre, randomised, controlled, open-label, non-inferiority trial. Lancet Respir. Med. 2019, 7, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Walters, J.A.; Walters, E.H.; Wood-Baker, R. Oral corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2005, 3, CD005374. [Google Scholar] [CrossRef] [PubMed]
- Horita, N.; Miyazawa, N.; Morita, S.; Kojima, R.; Inoue, M.; Ishigatsubo, Y.; Kaneko, T. Evidence suggesting that oral corticosteroids increase mortality in stable chronic obstructive pulmonary disease. Respir. Res. 2014, 15, 37. [Google Scholar] [CrossRef] [PubMed]
- Rice, K.L.; Rubins, J.B.; Lebahn, F.; Parenti, C.M.; Duane, P.G.; Kuskowski, M.; Joseph, A.M.; Niewoehner, D.E. Withdrawal of chronic systemic corticosteroids in patients with COPD: A randomized trial. Am. J. Respir. Crit. Care Med. 2000, 162, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Decramer, M.; Lacquet, L.M.; Fagard, R.; Rogiers, P. Corticosteroids contribute to muscle weakness in chronic airflow obstruction. Am. J. Respir. Crit. Care Med. 1994, 150, 11–16. [Google Scholar] [CrossRef]
- Sivapalan, P.; Ingebrigtsen, T.S.; Rasmussen, D.B.; Sorensen, R.; Rasmussen, C.M.; Jensen, C.B.; Allin, K.H.; Eklof, J.; Seersholm, N.; Vestbo, J.; et al. COPD exacerbations: The impact of long versus short courses of oral corticosteroids on mortality and pneumonia: Nationwide data on 67 000 patients with COPD followed for 12 months. BMJ Open Respir. Res. 2019, 6, e000407. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A.; Neighbour, H.; Nair, P. Targeted therapy of bronchitis in obstructive airway diseases. Pharmacol. Ther. 2013, 140, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Kolsum, U.; Brightling, C.E.; Locantore, N.; Agusti, A.; Tal-Singer, R.; ECLIPSE investigators. Eosinophilic inflammation in COPD: Prevalence and clinical characteristics. Eur. Respir. J. 2014, 44, 1697–1700. [Google Scholar] [CrossRef]
- Varricchi, G.; Bagnasco, D.; Borriello, F.; Heffler, E.; Canonica, G.W. Interleukin-5 pathway inhibition in the treatment of eosinophilic respiratory disorders: Evidence and unmet needs. Curr. Opin. Allergy Clin. Immunol. 2016, 16, 186–200. [Google Scholar] [CrossRef] [PubMed]
- Donovan, T.; Milan, S.J.; Wang, R.; Banchoff, E.; Bradley, P.; Crossingham, I. Anti-IL-5 therapies for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2020, 12, CD013432. [Google Scholar] [CrossRef]
- Gandhi, N.A.; Pirozzi, G.; Graham, N.M.H. Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev. Clin. Immunol. 2017, 13, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Le Floc’h, A.; Allinne, J.; Nagashima, K.; Scott, G.; Birchard, D.; Asrat, S.; Bai, Y.; Lim, W.K.; Martin, J.; Huang, T.; et al. Dual blockade of IL-4 and IL-13 with dupilumab, an IL-4Ralpha antibody, is required to broadly inhibit type 2 inflammation. Allergy 2020, 75, 1188–1204. [Google Scholar] [CrossRef] [PubMed]
- Pivotal Study to Assess the Efficacy, Safety and Tolerability of Dupilumab in Patients with Moderate to Severe COPD with Type 2 Inflammation. Available online: https://clinicaltrials.gov/study/NCT04456673 (accessed on 1 March 2024).
- Bhatt, S.P.; Rabe, K.F.; Hanania, N.A.; Vogelmeier, C.F.; Cole, J.; Bafadhel, M.; Christenson, S.A.; Papi, A.; Singh, D.; Laws, E.; et al. Dupilumab for COPD with Type 2 Inflammation Indicated by Eosinophil Counts. N. Engl. J. Med. 2023, 389, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.J.; de Oca, M.M.; Whyte, R.I.; Stetz, J.; Gay, S.E.; Celli, B.R. Lung-volume reduction improves dyspnea, dynamic hyperinflation, and respiratory muscle function. Am. J. Respir. Crit. Care Med. 1997, 155, 1984–1990. [Google Scholar] [CrossRef] [PubMed]
- Fessler, H.E.; Scharf, S.M.; Ingenito, E.P.; McKenna, R.J., Jr.; Sharafkhaneh, A. Physiologic basis for improved pulmonary function after lung volume reduction. Proc. Am. Thorac. Soc. 2008, 5, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Fessler, H.E.; Permutt, S. Lung volume reduction surgery and airflow limitation. Am. J. Respir. Crit. Care Med. 1998, 157, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Mineo, D.; Ambrogi, V.; Cufari, M.E.; Gambardella, S.; Pignotti, L.; Pompeo, E.; Mineo, T.C. Variations of inflammatory mediators and alpha1-antitrypsin levels after lung volume reduction surgery for emphysema. Am. J. Respir. Crit. Care Med. 2010, 181, 806–814. [Google Scholar] [CrossRef]
- Clarenbach, C.F.; Sievi, N.A.; Brock, M.; Schneiter, D.; Weder, W.; Kohler, M. Lung Volume Reduction Surgery and Improvement of Endothelial Function and Blood Pressure in Patients with Chronic Obstructive Pulmonary Disease. A Randomized Controlled Trial. Am. J. Respir. Crit. Care Med. 2015, 192, 307–314. [Google Scholar] [CrossRef]
- van Agteren, J.E.; Carson, K.V.; Tiong, L.U.; Smith, B.J. Lung volume reduction surgery for diffuse emphysema. Cochrane Database Syst. Rev. 2016, 10, Cd001001. [Google Scholar] [CrossRef]
- Criner, G.J.; Sue, R.; Wright, S.; Dransfield, M.; Rivas-Perez, H.; Wiese, T.; Sciurba, F.C.; Shah, P.L.; Wahidi, M.M.; de Oliveira, H.G.; et al. A Multicenter Randomized Controlled Trial of Zephyr Endobronchial Valve Treatment in Heterogeneous Emphysema (LIBERATE). Am. J. Respir. Crit. Care Med. 2018, 198, 1151–1164. [Google Scholar] [CrossRef]
- Kemp, S.V.; Slebos, D.J.; Kirk, A.; Kornaszewska, M.; Carron, K.; Ek, L.; Broman, G.; Hillerdal, G.; Mal, H.; Pison, C.; et al. A Multicenter Randomized Controlled Trial of Zephyr Endobronchial Valve Treatment in Heterogeneous Emphysema (TRANSFORM). Am. J. Respir. Crit. Care Med. 2017, 196, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Majid, A.; Labarca, G.; Uribe, J.P.; Kheir, F.; Pacheco, C.; Folch, E.; Jantz, M.A.; Mehta, H.J.; Patel, N.M.; Herth, F.J.F.; et al. Efficacy of the Spiration Valve System in Patients with Severe Heterogeneous Emphysema: A Systematic Review and Meta-Analysis. Respiration 2020, 99, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Fishman, A.; Martinez, F.; Naunheim, K.; Piantadosi, S.; Wise, R.; Ries, A.; Weinmann, G.; Wood, D.E. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N. Engl. J. Med. 2003, 348, 2059–2073. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, E.R.; Make, B.J. Maximum exercise as an outcome in COPD: Minimal clinically important difference. J. Chronic Obstr. Pulm. Dis. 2005, 2, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.; Sousa, I.; Shah, P.L.; Diggle, P.; Goldstraw, P. Lung Volume Reduction Surgery: Reinterpreted with Longitudinal Data Analyses Methodology. Ann. Thorac. Surg. 2020, 109, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- Fishman, A.; Fessler, H.; Martinez, F.; McKenna, R.J., Jr.; Naunheim, K.; Piantadosi, S.; Weinmann, G.; Wise, R. Patients at high risk of death after lung-volume-reduction surgery. N. Engl. J. Med. 2001, 345, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, A.M.; Meyers, B.F.; Guthrie, T.J.; Davis, G.E.; Yusen, R.D.; Lefrak, S.S.; Patterson, G.A.; Cooper, J.D. Long-term outcome of bilateral lung volume reduction in 250 consecutive patients with emphysema. J. Thorac. Cardiovasc. Surg. 2003, 125, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Naunheim, K.S.; Wood, D.E.; Krasna, M.J.; DeCamp, M.M., Jr.; Ginsburg, M.E.; McKenna, R.J., Jr.; Criner, G.J.; Hoffman, E.A.; Sternberg, A.L.; Deschamps, C. Predictors of operative mortality and cardiopulmonary morbidity in the National Emphysema Treatment Trial. J. Thorac. Cardiovasc. Surg. 2006, 131, 43–53. [Google Scholar] [CrossRef]
- Criner, G.J.; Delage, A.; Voelker, K.; Hogarth, D.K.; Majid, A.; Zgoda, M.; Lazarus, D.R.; Casal, R.; Benzaquen, S.B.; Holladay, R.C.; et al. Improving Lung Function in Severe Heterogenous Emphysema with the Spiration Valve System (EMPROVE). A Multicenter, Open-Label Randomized Controlled Clinical Trial. Am. J. Respir. Crit. Care Med. 2019, 200, 1354–1362. [Google Scholar] [CrossRef]
- US Food and Drug Administration. FDA approves novel device for treating breathing difficulty from severe emphysema. In FDA News Release; US Food and Drug Administration: Silver Spring, MD, USA, 2018. [Google Scholar]
- Garner, J.; Kemp, S.V.; Toma, T.P.; Hansell, D.M.; Polkey, M.I.; Shah, P.L.; Hopkinson, N.S. Survival after Endobronchial Valve Placement for Emphysema: A 10-Year Follow-up Study. Am. J. Respir. Crit. Care Med. 2016, 194, 519–521. [Google Scholar] [CrossRef]
- Gompelmann, D.; Benjamin, N.; Bischoff, E.; Kontogianni, K.; Schuhmann, M.; Hoffmann, H.; Heussel, C.P.; Herth, F.J.F.; Eberhardt, R. Survival after Endoscopic Valve Therapy in Patients with Severe Emphysema. Respiration 2019, 97, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Hartman, J.E.; Welling, J.B.A.; Klooster, K.; Carpaij, O.A.; Augustijn, S.W.S.; Slebos, D.J. Survival in COPD patients treated with bronchoscopic lung volume reduction. Respir. Med. 2022, 196, 106825. [Google Scholar] [CrossRef] [PubMed]
- Koster, T.D.; Klooster, K.; Ten Hacken, N.H.T.; van Dijk, M.; Slebos, D.J. Endobronchial valve therapy for severe emphysema: An overview of valve-related complications and its management. Expert Rev Respir. Med. 2020, 14, 1235–1247. [Google Scholar] [CrossRef] [PubMed]
- Fiorelli, A.; D’Andrilli, A.; Bezzi, M.; Ibrahim, M.; Anile, M.; Diso, D.; Cusumano, G.; Terminella, A.; Luzzi, V.; Innocenti, M.; et al. Complications related to endoscopic lung volume reduction for emphysema with endobronchial valves: Results of a multicenter study. J. Thorac. Dis. 2018, 10, S3315–S3325. [Google Scholar] [CrossRef] [PubMed]
- Todd, J.L.; Palmer, S.M. Lung transplantation in advanced COPD: Is it worth it? Semin. Respir. Crit. Care Med. 2010, 31, 365–372. [Google Scholar] [CrossRef][Green Version]
- Geert, M.V.; Jens, G. Lung transplantation for COPD/pulmonary emphysema. Eur. Respir. Rev. 2023, 32, 220116. [Google Scholar] [CrossRef] [PubMed]
- Weill, D.; Benden, C.; Corris, P.A.; Dark, J.H.; Davis, R.D.; Keshavjee, S.; Lederer, D.J.; Mulligan, M.J.; Patterson, G.A.; Singer, L.G.; et al. A consensus document for the selection of lung transplant candidates: 2014—An update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2015, 34, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Eskander, A.; Waddell, T.K.; Faughnan, M.E.; Chowdhury, N.; Singer, L.G. BODE index and quality of life in advanced chronic obstructive pulmonary disease before and after lung transplantation. J. Heart Lung Transplant. 2011, 30, 1334–1341. [Google Scholar] [CrossRef] [PubMed]
- Pochettino, A.; Kotloff, R.M.; Rosengard, B.R.; Arcasoy, S.M.; Blumenthal, N.P.; Kaiser, L.R.; Bavaria, J.E. Bilateral versus single lung transplantation for chronic obstructive pulmonary disease: Intermediate-term results. Ann. Thorac. Surg. 2000, 70, 1813–1818, discussion 1818–1819. [Google Scholar] [CrossRef]
- Thabut, G.; Mal, H. Outcomes after lung transplantation. J. Thorac. Dis. 2017, 9, 2684–2691. [Google Scholar] [CrossRef]
- Inci, I.; Iskender, I.; Ehrsam, J.; Caviezel, C.; Hillinger, S.; Opitz, I.; Schneiter, D.; Weder, W. Previous lung volume reduction surgery does not negatively affect survival after lung transplantation. Eur. J. Cardiothorac. Surg. 2018, 53, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, S.J.; Wilkinson-Maitland, C.; Saad, N.; Li, P.Z.; Smith, B.M.; Bourbeau, J.; Jensen, D. Effect of morphine on breathlessness and exercise endurance in advanced COPD: A randomised crossover trial. Eur. Respir. J. 2017, 50, 1701235. [Google Scholar] [CrossRef]
- Abernethy, A.P.; Currow, D.C.; Frith, P.; Fazekas, B.S.; McHugh, A.; Bui, C. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ 2003, 327, 523–528. [Google Scholar] [CrossRef]
- Johnson, M.A.; Woodcock, A.A.; Geddes, D.M. Dihydrocodeine for breathlessness in “pink puffers”. Br. Med. J. (Clin. Res. Ed.) 1983, 286, 675–677. [Google Scholar] [CrossRef] [PubMed]
- Ekstrom, M.; Nilsson, F.; Abernethy, A.A.; Currow, D.C. Effects of opioids on breathlessness and exercise capacity in chronic obstructive pulmonary disease. A systematic review. Ann. Am. Thorac. Soc. 2015, 12, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Currow, D.; Louw, S.; McCloud, P.; Fazekas, B.; Plummer, J.; McDonald, C.F.; Agar, M.; Clark, K.; McCaffrey, N.; Ekstrom, M.P.; et al. Regular, sustained-release morphine for chronic breathlessness: A multicentre, double-blind, randomised, placebo-controlled trial. Thorax 2020, 75, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Verberkt, C.A.; van den Beuken-van Everdingen, M.H.J.; Schols, J.; Hameleers, N.; Wouters, E.F.M.; Janssen, D.J.A. Effect of Sustained-Release Morphine for Refractory Breathlessness in Chronic Obstructive Pulmonary Disease on Health Status: A Randomized Clinical Trial. JAMA Intern. Med. 2020, 180, 1306–1314. [Google Scholar] [CrossRef]
- Ferreira, D.H.; Louw, S.; McCloud, P.; Fazekas, B.; McDonald, C.F.; Agar, M.R.; Clark, K.; McCaffrey, N.; Ekstrom, M.; Currow, D.C.; et al. Controlled-Release Oxycodone vs. Placebo in the Treatment of Chronic Breathlessness-A Multisite Randomized Placebo Controlled Trial. J. Pain Symptom Manag. 2020, 59, 581–589. [Google Scholar] [CrossRef]
- Ekstrom, M.; Ferreira, D.; Chang, S.; Louw, S.; Johnson, M.J.; Eckert, D.J.; Fazekas, B.; Clark, K.J.; Agar, M.R.; Currow, D.C.; et al. Effect of Regular, Low-Dose, Extended-release Morphine on Chronic Breathlessness in Chronic Obstructive Pulmonary Disease: The BEAMS Randomized Clinical Trial. JAMA 2022, 328, 2022–2032. [Google Scholar] [CrossRef]
- Maddocks, M.; Lovell, N.; Booth, S.; Man, W.D.; Higginson, I.J. Palliative care and management of troublesome symptoms for people with chronic obstructive pulmonary disease. Lancet 2017, 390, 988–1002. [Google Scholar] [CrossRef]
- Molassiotis, A.; Bailey, C.; Caress, A.; Tan, J.Y. Interventions for cough in cancer. Cochrane Database Syst. Rev. 2015, 5, CD007881. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.; Wang, G.; McGarvey, L.; Vertigan, A.E.; Altman, K.W.; Birring, S.S.; Panel, C.E.C. Treatment of Unexplained Chronic Cough: CHEST Guideline and Expert Panel Report. Chest 2016, 149, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Horton, R.; Rocker, G. Contemporary issues in refractory dyspnoea in advanced chronic obstructive pulmonary disease. Curr. Opin. Support. Palliat Care 2010, 4, 56–62. [Google Scholar] [CrossRef]
- Jordan, N.; Lee, T.A.; Valenstein, M.; Pirraglia, P.A.; Weiss, K.B. Effect of depression care on outcomes in COPD patients with depression. Chest 2009, 135, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Kunik, M.E.; Veazey, C.; Cully, J.A.; Souchek, J.; Graham, D.P.; Hopko, D.; Carter, R.; Sharafkhaneh, A.; Goepfert, E.J.; Wray, N.; et al. COPD education and cognitive behavioral therapy group treatment for clinically significant symptoms of depression and anxiety in COPD patients: A randomized controlled trial. Psychol. Med. 2008, 38, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Maurer, J.; Rebbapragada, V.; Borson, S.; Goldstein, R.; Kunik, M.E.; Yohannes, A.M.; Hanania, N.A. Anxiety and depression in COPD: Current understanding, unanswered questions, and research needs. Chest 2008, 134, 43S–56S. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.C.; Earnest, A.; Lu, S.J. A multidimensional grading system (BODE index) as predictor of hospitalization for COPD. Chest 2005, 128, 3810–3816. [Google Scholar] [CrossRef]
- Mathioudakis, A.G.; Vanfleteren, L.; Lahousse, L.; Higham, A.; Allinson, J.P.; Gotera, C.; Visca, D.; Singh, D.; Spanevello, A. Current developments and future directions in COPD. Eur. Respir. Rev. 2020, 29, 200289. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.E.K.; Bafadhel, M. What will Happen in the World of COPD 2030? Turk. Thorac. J. 2019, 20, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Miao, K.; Zhou, L.; Xiong, W.N. Stem cell therapy for chronic obstructive pulmonary disease. Chin. Med. J. 2021, 134, 1535–1545. [Google Scholar] [CrossRef]
- Estépar, R.S.J. Artificial Intelligence in COPD: New Venues to Study a Complex Disease. Barc. Respir. Netw. Rev. 2020, 6, 144–160. [Google Scholar] [CrossRef] [PubMed]
- Riera-Martínez, L.; Cànaves-Gómez, L.; Iglesias, A.; Martin-Medina, A.; Cosío, B.G. The Role of IL-33/ST2 in COPD and Its Future as an Antibody Therapy. Int. J. Mol. Sci. 2023, 24, 8702. [Google Scholar] [CrossRef] [PubMed]
| Author (Year) | Type of Study | Patients | Group 1 | Group 2 | Outcomes |
|---|---|---|---|---|---|
| Murphy et al. (2017) [45] | Randomized controlled trial | 116 patients with persistent hypercapnia (PaCO2 > 53 mm Hg) 2–4 weeks post COPD exacerbation | NIPPV plus oxygen therapy | Oxygen therapy alone | Median time to readmission lower in NIPPV group (4.3 months vs. 1.4 months; HR 0.49; p = 0.002). 17% absolute risk reduction in 12-month mortality or readmission rate in NIPPV group |
| Kohnlein et al. (2014) [50] | Randomized controlled trial | 195 patients with advanced COPD with PaCO2 > 52 mm Hg and pH > 7.35. NIPPV was targeted to reduce baseline PaCO2 by at least 20%. | NIPPV plus standard medical therapy | Standard medical therapy alone | Lower 1-year mortality in the NIPPV group (12% vs. 33%; HR 0.24 (95% CI 0.11–0.49; p = 0.0004) |
| McEvoy et al. (2009) [51] | Randomized controlled trial | 144 patients with severe oxygen dependent COPD and PaCO2 > 46 mm Hg | NIPPV plus LTOT | LTOT alone | Lower mortality was observed in NIPPV group (HR 0.63, 95% CI 0.40 to 0.99, p = 0.045) but had worsening quality of life. |
| Clini et al. (2002) [52] | Randomized controlled trial | 90 patients with severe oxygen depended COPD were used to assess NIPPV impact on QOL and resource utilization. | NIPPV plus LTOT | LTOT alone | At 2 years, no difference in mortality and hospital readmission. NIPPV group although noted significant decrease in ICU admission and improved HRQOL scores. |
| Nagata et al. (2022) [53] | Randomized controlled trial | 104 patients with severe oxygen dependent COPD and daytime hypercapnia used to assess efficacy of HFNC in reducing exacerbations | HFNC | LTOT with low flow/regular oxygen | Significant reduction in episodes and prolonged duration without acute exacerbations in HFNC group. HFNC group also showed improved HRQoL scores, PFT parameters, and peripheral oxygen saturation. |
| Parameters | Lung Volume Reduction Surgery | Bronchoscopic Lung Volume Reduction Surgery |
|---|---|---|
| Clinical | Age < 75 years | No typical age cut-off |
| Quit smoking > 6 months. | Quit smoking > 4 months | |
| Clinical exam indicative of emphysema | Clinical exam indicative of emphysema | |
| Uncontrolled symptoms despite maximal medical management and pulmonary rehabilitation | Symptomatic despite maximal medical therapy (stable on <20 mg prednisone or equivalent/day) | |
| BMI < 40 kg/m2 | BMI < 35 kg/m2 | |
| Physiological | Post-bronchodilator FEV1 < 45% of predicted | FEV1 15–45% of predicted |
| TLC > 100% of predicted, RV > 150% of predicted indicating hyperinflation | TLC > 100% of predicted. RV > 175% of predicted | |
| Post-pulmonary rehabilitation 6MWD > 140 m | 6MWD 100–500 m | |
| Imaging | CT chest confirming severe emphysema, ideally upper lobe predominant | CT chest confirming emphysema (could be homogenous) |
| Little to no collateral ventilation of the targeted lobe |
| Type of Procedure | General Contraindications/Exclusion Criteria |
|---|---|
| Lung volume reduction surgery (LVRS) | Age > 75 years |
| Active smoker | |
| Previous thoracic surgeries/procedures, chest wall deformity | |
| Pulmonary hypertension | |
| Clinically significant bronchiectasis | |
| Significant cardiac co-morbidities like heart failure (LVEF < 45%), uncontrolled hypertension, myocardial infarction | |
| FEV1 < 20% of predicted with either DLCO < 20% of predicted or homogenous emphysema | |
| Severe hypercapnia PaCO2 > 60 mm Hg | |
| Severe hypoxemia PaO2 < 45 mm Hg | |
| Significant pleuro-parenchymal interstitial lung disease | |
| Bronchoscopic lung volume reduction surgery (BLVRS) | Active pulmonary infection/pneumonia |
| Large bullae involving > 30% of either lung | |
| Severe hypercapnia PaCO2 > 60 mm Hg | |
| Severe hypoxemia PaO2 < 45 mm Hg | |
| Prior lung transplant, LVRS, median sternotomy, lobectomy | |
| Significant cardiac co-morbidities like heart failure (LVEF < 45%), unstable cardiac arrhythmia, MI, CVA |
| Major Criteria for Lung Transplantation |
|---|
| Advanced lung disease despite maximal medical management, including pulmonary rehabilitation and oxygen therapy if indicated |
| Lack of candidacy for lung volume reduction surgery (LVRS) |
| Post-bronchodilator FEV1 < 25% of predicted |
| Resting hypercapnia with PaCO2 > 50 mm Hg or hypoxemia with PaO2 < 60 mm Hg |
| Body mass index (BMI), airflow obstruction, dyspnea, and exercise capacity (BODE) index score ≥ 5 |
| Relative Contraindications |
| Advanced age (>70 years old) |
| Active tobacco use |
| Poor functional status, unable to participated in pulmonary rehabilitation |
| Frailty, lack of social support at home |
| Severe osteopenia or osteoporosis |
| Severe co-morbidities like cirrhosis or advanced chronic kidney disease |
| Class II obesity and higher (BMI > 35) or underweight (BMI < 16) |
| Clinical Trial | Type of Study | Intervention Group | Control Group | Primary Outcomes | Adverse Events |
|---|---|---|---|---|---|
| NCT04072887 | Interventional randomized phase 2 trial | Oral QBW251 (icenticaftor) at varying dosing in patients with COPD on triple therapy | COPD patients on triple therapy | No change in FEV1 after 12 weeks in the intervention group but had reduced cough, sputum, and rescue inhaler use | All treatments were well tolerated |
| NCT04535986 | Interventional randomized phase 3 trial | Nebulized ensifentrine twice daily for 24 or 48 weeks in patients with moderate to severe COPD | Placebo twice daily | Intervention group had more improvement in FEV1 and dyspnea scores | No difference in adverse events |
| NCT03937479 | Interventional randomized parallel group phase 2b trial | Varying doses of nebulized ensifentrine twice daily in addition to tiotropium in moderate to severe COPD patients | Placebo twice daily in addition to tiotropium in moderate to severe COPD patients | Intervention group at all doses superior in terms of improvement in FEV1 and dyspnea scores | No difference in adverse events |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahi, M.S.; Mudgal, M.; Asokar, B.K.; Yella, P.R.; Gunasekaran, K. Management of Refractory Chronic Obstructive Pulmonary Disease: A Review. Life 2024, 14, 542. https://doi.org/10.3390/life14050542
Rahi MS, Mudgal M, Asokar BK, Yella PR, Gunasekaran K. Management of Refractory Chronic Obstructive Pulmonary Disease: A Review. Life. 2024; 14(5):542. https://doi.org/10.3390/life14050542
Chicago/Turabian StyleRahi, Mandeep Singh, Mayuri Mudgal, Bharat Kumar Asokar, Prashanth Reddy Yella, and Kulothungan Gunasekaran. 2024. "Management of Refractory Chronic Obstructive Pulmonary Disease: A Review" Life 14, no. 5: 542. https://doi.org/10.3390/life14050542
APA StyleRahi, M. S., Mudgal, M., Asokar, B. K., Yella, P. R., & Gunasekaran, K. (2024). Management of Refractory Chronic Obstructive Pulmonary Disease: A Review. Life, 14(5), 542. https://doi.org/10.3390/life14050542







