Applied Cardio-Oncology in Hematological Malignancies: A Narrative Review
Abstract
1. Introduction
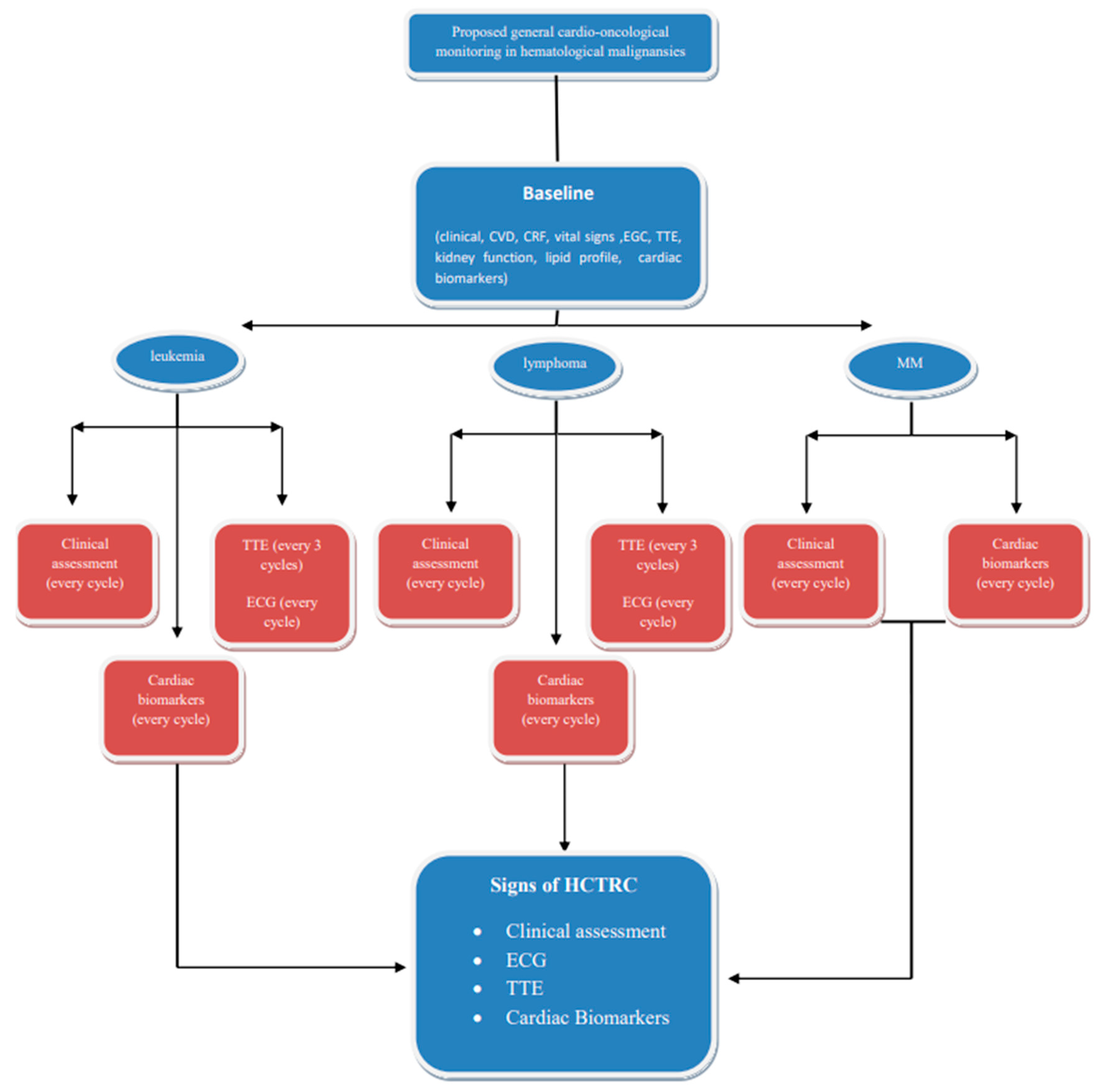
2. Risk Stratification-Hematological Cancer Therapy-Related Cardiotoxicity Definition
3. Cardiac Imaging Modalities and Cardiac Biomarkers in Patients with Hematologic Malignances
4. Main Anticancer Drugs in Hematological Malignances and Cardiotoxic Profiles
5. Conventional Chemotherapy’s Related Cardiovascular Toxicities
5.1. Anthracyclines
5.2. Bleomycin
5.3. Vincristine
5.4. Cyclophosphamide
6. Targeted Therapy’s Related Cardiovascular Toxicities
6.1. Rituximab
6.2. Breakpoint Cluster Region–Abelson Oncogene Locus Tyrosine Kinase Inhibitors
6.3. Bruton Tyrosine Kinase (BTK) Inhibitors
7. Multiple Myeloma Therapeutic Regimes
8. Multiple Myeloma Drug-Related Cardiovascular Toxicities
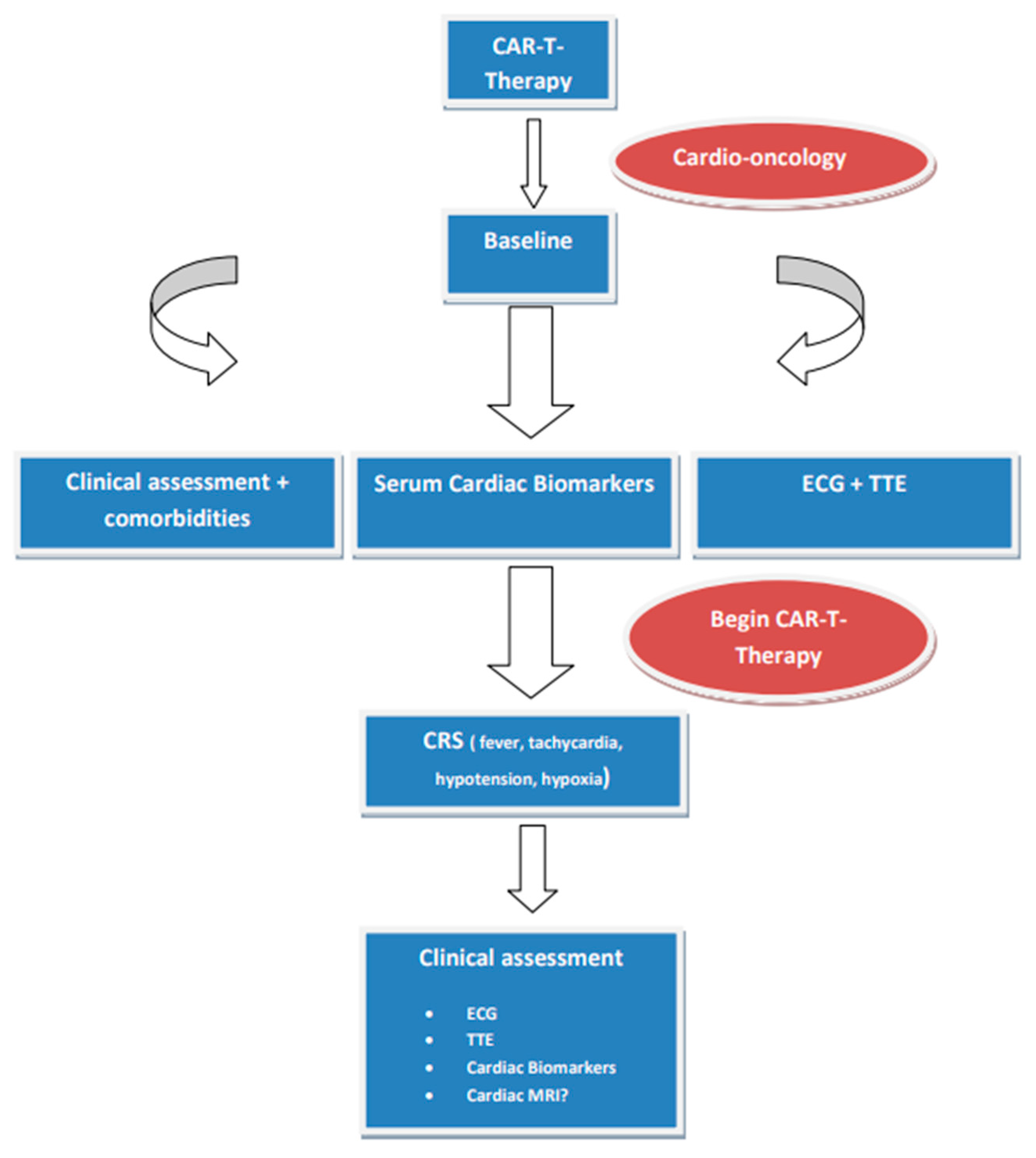
9. Chimeric Antigen Receptor T (CAR-T) Cell and Tumor-Infiltrating Lymphocytes Therapies
10. Bispecific Antibodies
11. Hematopoietic Stem Cell Transplantation
12. Radiotherapy
13. Conclusions and Future Directions
Funding
Conflicts of Interest
References
- Gent, D.G.; Rebecca, D. The 2022 European Society of Cardiology Cardio-oncology Guidelines in Focus. Eur Cardiol. 2023, 18, e16. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J.-Cardiovasc. Imaging 2022, 23, e333–e465. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; Dent, S.; Stanway, S.; Earl, H.; Brezden-Masley, C.; Cohen-Solal, A.; Tocchetti, C.G.; Moslehi, J.J.; Groarke, J.D.; Bergler-Klein, J.; et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: A position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur. J. Heart Fail. 2020, 22, 1945–1960. [Google Scholar] [CrossRef]
- Costanzo, V.; Ratre, Y.K.; Andretta, E.; Acharya, R.; Bhaskar, L.V.K.S.; Verma, H.K. A Comprehensive Review of Cancer Drug–Induced Cardiotoxicity in Blood Cancer Patients: Current Perspectives and Therapeutic Strategies. Curr. Treat. Options Oncol. 2024, 25, 465–495. [Google Scholar] [CrossRef]
- Madonna, R. Multi-Target Drugs for Blood Cancer in the Elderly: Implications of Damage and Repair in the Cardiovascular Toxicity. Front. Physiol. 2021, 12, 792751. [Google Scholar] [CrossRef]
- Bojan, A.; Torok-Vistai, T.; Parvu, A. Assessment and Management of Cardiotoxicity in Hematologic Malignancies. Dis. Markers 2021, 2021, 6616265. [Google Scholar] [CrossRef]
- Cheng, K.H.; Wu, Y.W.; Hou, C.J.; Hung, C.M. An Overview of Cardio-Oncology, a New Frontier to Be Explored. Acta Cardiol. Sin. 2021, 37, 457–463. [Google Scholar] [PubMed]
- Di Lisi, D.; Manno, G.; Novo, G. Subclinical Cardiotoxicity: The Emerging Role of Myocardial Work and Other Imaging Techniques. Curr. Probl. Cardiol. 2021, 46, 100818. [Google Scholar] [CrossRef] [PubMed]
- Sanadgol, G.; Samimi, S.; Shirini, D.; Nakhaei, P.; Mohseni, M.; Alizadehasl, A. Right ventricle toxicity in cancer treatment: A focused review on cardiac imaging. Futur. Cardiol. 2023, 19, 537–545. [Google Scholar] [CrossRef]
- Madanat, L.; Gupta, R.; Weber, P.; Kumar, N.; Chandra, R.; Ahaneku, H.; Bansal, Y.; Anderson, J.; Bilolikar, A.; Jaiyesimi, I. Cardiotoxicity of Biological Therapies in Cancer Patients: An In-depth Review. Curr. Cardiol. Rev. 2023, 19, 8–18. [Google Scholar] [CrossRef]
- Totzeck, M.; Aide, N.; Bauersachs, J.; Bucerius, J.; Georgoulias, P.; Herrmann, K.; Hyafil, F.; Kunikowska, J.; Lubberink, M.; Nappi, C.; et al. Nuclear medicine in the assessment and prevention of cancer therapy-related cardiotoxicity: Prospects and proposal of use by the European Association of Nuclear Medicine (EANM). Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 792–812. [Google Scholar] [CrossRef] [PubMed]
- Printezi, M.I.; Yousif, L.I.E.; Kamphuis, J.A.M.; van Laake, L.W.; Cramer, M.J.; Hobbelink, M.G.G.; Asselbergs, F.W.; Teske, A.J. LVEF by Multigated Acquisition Scan Compared to Other Imaging Modalities in Cardio-Oncology: A Systematic Review. Curr. Heart Fail. Rep. 2022, 19, 136–145. [Google Scholar] [CrossRef]
- Cannizzaro, M.T.; Inserra, M.C.; Passaniti, G.; Celona, A.; D‘Angelo, T.; Romeo, P.; Basile, A. Role of advanced cardiovascular imaging in chemotherapy-induced cardiotoxicity. Heliyon 2023, 9, e15226. [Google Scholar] [CrossRef]
- Kelly, J.M.; Babich, J.W. PET Tracers for Imaging Cardiac Function in Cardio-oncology. Curr. Cardiol. Rep. 2022, 24, 247–260. [Google Scholar] [CrossRef]
- Seraphim, A.; Westwood, M.; Bhuva, A.N.; Crake, T.; Moon, J.C.; Menezes, L.J.; Lloyd, G.; Ghosh, A.K.; Slater, S.; Oakervee, H.; et al. Advanced Imaging Modalities to Monitor for Cardiotoxicity. Curr. Treat. Options Oncol. 2019, 20, 73. [Google Scholar] [CrossRef]
- Biersmith, M.A.; Tong, M.S.; Guha, A.; Simonetti, O.P.; Addison, D. Multimodality Cardiac Imaging in the Era of Emerging Cancer Therapies. J. Am. Heart Assoc. 2020, 9, e013755. [Google Scholar] [CrossRef] [PubMed]
- Ananthan, K.; Lyon, A.R. The Role of Biomarkers in Cardio-Oncology. J. Cardiovasc. Transl. Res. 2020, 13, 431–450. [Google Scholar] [CrossRef]
- Joolharzadeh, P.; Rodriguez, M.; Zaghlol, R.; Pedersen, L.N.; Jimenez, J.; Bergom, C.; Mitchell, J.D. Recent Advances in Serum Biomarkers for Risk Stratification and Patient Management in Cardio-Oncology. Curr. Cardiol. Rep. 2023, 25, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Pudil, R.; Mueller, C.; Čelutkienė, J.; Henriksen, P.A.; Lenihan, D.; Dent, S.; Barac, A.; Stanway, S.; Moslehi, J.; Suter, T.M.; et al. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: A position statement from the Cardio-Oncology Study Group of the Heart Failure Association and the Cardio-Oncology Council of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 1966–1983. [Google Scholar] [CrossRef]
- Johnson, P.W.M. Are we reaching the maximum cure rate for Hodgkin lymphoma? Hematol Oncol. 2023, 41 (Suppl. S1), 57–61. [Google Scholar] [CrossRef]
- Cardinale, D.M.; Zaninotto, M.; Cipolla, C.M.; Passino, C.; Plebani, M.; Clerico, A. Cardiotoxic effects and myocardial injury: The search for a more precise definition of drug cardiotoxicity. Clin. Chem. Lab. Med. (CCLM) 2021, 59, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Iacopo, F.; Cipolla, C.M. Cardiotoxicity of anthracyclines. Front. Cardiovasc. Med. 2020, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Colombo, A.; Bacchiani, G.; Tedeschi, I.; Meroni, C.A.; Civelli, M.; Lamantia, G.; Colombo, N.; Cipolla, C.M.; Veglia, F.; et al. Early Detection of Anthracycline Cardiotoxicity and Improvement with Heart Failure Therapy. Circulation 2015, 131, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, P.A.; Hall, P.; Oikonomidou, O.; MacPherson, I.R.; Maclean, M.; Lewis, S.; McVicars, H.; Broom, A.; Scott, F.; McKay, P.; et al. Rationale and Design of the Cardiac CARE Trial: A Randomized Trial of Troponin-Guided Neurohormonal Blockade for the Prevention of Anthracycline Cardiotoxicity. Circ. Heart Fail. 2022, 15, e009445. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Weng, Y.; Jiang, T.; Ou, W.; Zhang, N.; Dong, Q.; Tang, X. Influencing factors of anthracycline-induced subclinical cardiotoxicity in acute leukemia patients. BMC Cancer 2023, 23, 976. [Google Scholar] [CrossRef] [PubMed]
- Lewis, W.D.; Lilly, S.; Jones, K.L. Lymphoma: Diagnosis and Treatment. Am. Fam. Physician 2020, 101, 34–41. [Google Scholar] [PubMed]
- Manavi, M.A.; Nasab, M.H.F.; Jafari, R.M.; Dehpour, A.R. Mechanisms underlying dose-limiting toxicities of conventional chemotherapeutic agents. J. Chemother. 2024, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Iqubal, A.; Iqubal, M.K.; Sharma, S.; Ansari, M.A.; Najmi, A.K.; Ali, S.M.; Ali, J.; Haque, S.E. Molecular mechanism involved in cyclophosphamide-induced cardiotoxicity: Old drug with a new vision. Life Sci. 2019, 218, 112–131. [Google Scholar] [CrossRef] [PubMed]
- Iqubal, A.; Wasim, M.; Ashraf, M.; Najmi, A.K.; Syed, M.A.; Ali, J.; Haque, S.E. Natural Bioactive as a Potential Therapeutic Approach for the Management of Cyclophosphamide-induced Cardiotoxicity. Curr. Top. Med. Chem. 2021, 21, 2647–2670. [Google Scholar] [CrossRef]
- Schmittlutz, K.; Marks, R. Current treatment options for aggressive non-Hodgkin lymphoma in elderly and frail patients: Practical considerations for the hematologist. Ther. Adv. Hematol. 2021, 12. [Google Scholar] [CrossRef]
- Molica, M.; Scalzulli, E.; Colafigli, G.; Foà, R.; Breccia, M. Insights into the optimal use of ponatinib in patients with chronic phase chronic myeloid leukaemia. Ther. Adv. Hematol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Binzaid, A.A.; Baqal, O.J.; Soheib, M.; Al Nahedh, M.; Samarkandi, H.H.; Aljurf, M. Cardiovascular Toxicity Associated with Tyrosine Kinase Inhibitor Therapy In Chronic Myeloid Leukemia. Gulf J. Oncol. 2021, 1, 79–84. [Google Scholar]
- Singh, A.P.; Umbarkar, P.; Tousif, S.; Lal, H. Cardiotoxicity of the BCR-ABL1 tyrosine kinase inhibitors: Emphasis on ponatinib. Int. J. Cardiol. 2020, 316, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Quartermaine, C.; Ghazi, S.M.; Yasin, A.; Awan, F.T.; Fradley, M.; Wiczer, T.; Kalathoor, S.; Ferdousi, M.; Krishan, S.; Habib, A.; et al. Cardiovascular Toxicities of BTK Inhibitors in Chronic Lymphocytic Leukemia: JACC: CardioOncology State-of-the-Art Review. Cardio Oncol. 2023, 5, 570–590. [Google Scholar]
- Christensen, B.W.; Zaha, V.G.; Awan, F.T. Cardiotoxicity of BTK inhibitors: Ibrutinib and beyond. Expert Rev. Hematol. 2022, 15, 321–331. [Google Scholar] [CrossRef]
- Seymour, J.F.; Byrd, J.C.; Ghia, P.; Kater, A.P.; Chanan-Khan, A.; Furman, R.R.; O‘Brien, S.; Brown, J.R.; Munir, T.; Mato, A.; et al. Detailed safety profile of acalabrutinib vs ibrutinib in previously treated chronic lymphocytic leukemia in the ELEVATE-RR trial. Blood 2023, 142, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Boriani, G.; Menna, P.; Morgagni, R.; Minotti, G.; Vitolo, M. Ibrutinib and Bruton’s Tyrosine Kinase Inhibitors in Chronic Lymphocytic Leukemia: Focus on Atrial Fibrillation and Ventricular Tachyarrhythmias/Sudden Cardiac Death. Chemotherapy 2023, 68, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Yan, Y.; Zeng, X.; Lin, N.; Tan, B. Ibrutinib-Associated Cardiotoxicity: From the Pharmaceutical to the Clinical. Drug Des. Dev. Ther. 2022, 16, 3225–3239. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.G.; Cornell, R.F. Cardiovascular Complications Associated with Multiple Myeloma Therapies: Incidence, Pathophysiology, and Management. Curr. Oncol. Rep. 2019, 21, 29. [Google Scholar] [CrossRef]
- Das, A.; Dasgupta, S.; Gong, Y.; Shah, U.A.; Fradley, M.G.; Cheng, R.K.; Roy, B.; Guha, A. Cardiotoxicity as an adverse effect of immunomodulatory drugs and proteasome inhibitors in multiple myeloma: A network meta-analysis of randomized clinical trials. Hematol. Oncol. 2022, 40, 233–242. [Google Scholar] [CrossRef]
- Montefusco, V.; Mussetti, A.; Salas, M.Q.; Martinelli, G.; Cerchione, C. Old and new generation proteasome inhibitors in multiple myeloma. Panminerva Medica 2021, 62, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.F.; Naaktgeboren, W.R.; Hua, A.; Ghosh, A.K.; Oakervee, H.; Hallam, S.; Manisty, C. Optimising cardiovascular care of patients with multiple myeloma. Heart 2021, 107, 1774–1782. [Google Scholar] [CrossRef]
- Jarchowsky, O.; Avnery, O.; Ellis, M.H. Thrombosis in multiple myeloma: Mechanisms, risk assessment and management. Leuk. Lymphoma 2023, 64, 1905–1913. [Google Scholar] [CrossRef]
- Costa, T.A.; Felix, N.; Costa, B.A.; Godoi, A.; Nogueira, A.; Rossi, A. Direct oral anticoagulants versus aspirin for primary thromboprophylaxis in patients with multiple myeloma undergoing outpatient therapy: A systematic review and updated meta-analysis. Br. J. Haematol. 2023, 203, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Palladini, G.; Milani, P. Diagnosis and Treatment of AL Amyloidosis. Drugs 2023, 83, 203–216. [Google Scholar] [CrossRef]
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio, A.L.P.; Damy, T.; Eriksson, U.; et al. Diagnosis and treatment of cardiac amyloidosis: A position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2021, 42, 1554–1568. [Google Scholar] [CrossRef]
- Al Hamed, R.; Bazarbachi, A.H.; Bazarbachi, A.; Malard, F.; Harousseau, J.-L.; Mohty, M. Comprehensive Review of AL amyloidosis: Some practical recommendations. Blood Cancer J. 2021, 11, 97. [Google Scholar] [CrossRef]
- Milone, M.C.; Bhoj, V.G. The Pharmacology of T Cell Therapies. Mol. Ther.-Methods Clin. Dev. 2018, 8, 210–221. [Google Scholar] [CrossRef]
- Haslauer, T.; Greil, R.; Zaborsky, N.; Geisberger, R. CAR T-Cell Therapy in Hematological Malignancies. Int. J. Mol. Sci. 2021, 22, 8996. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Zhang, Z.; Ren, Z.; Li, Y. Reactions Related to CAR-T Cell Therapy. Front. Immunol. 2021, 12, 663201. [Google Scholar] [CrossRef]
- Sterner, R.C.; Sterner, R.M. CAR-T cell therapy: Current limitations and potential strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Chen, D.H.; Guha, A.; Mackenzie, S.; Walker, J.M.; Roddie, C. CAR T Cell Therapy-Related Cardiovascular Outcomes and Management: Systemic Disease or Direct Cardiotoxicity? Cardio Oncol. 2020, 2, 97–109. [Google Scholar]
- Bock, A.M.; Nowakowski, G.S.; Wang, Y. Bispecific Antibodies for Non-Hodgkin Lymphoma Treatment. Curr. Treat. Options Oncol. 2022, 23, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Goebeler, M.-E.; Bargou, R.C. T cell-engaging therapies—BiTEs and beyond. Nat. Rev. Clin. Oncol. 2020, 17, 418–434. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Garfall, A.L.; van de Donk, N.W.; Nahi, H.; San-Miguel, J.F.; Oriol, A.; Nooka, A.K.; Martin, T.; Rosinol, L.; Chari, A.; et al. Teclistamab in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2022, 387, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Martino, E.A.; Bruzzese, A.; Labanca, C.; Mendicino, F.; Lucia, E.; Olivito, V.; Neri, A.; Morabito, F.; Vigna, E.; Gentile, M. Teclistamab-cqyv in multiple myeloma. Eur. J. Haematol. 2024, 112, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Alizadehasl, A.; Ghadimi, N.; Hosseinifard, H.; Roudini, K.; Emami, A.H.; Ghavamzadeh, A.; Khoda-Amorzideh, D. Cardiovascular diseases in patients after hematopoietic stem cell transplantation: Systematic review and Meta-analysis. Curr. Res. Transl. Med. 2023, 71, 103363. [Google Scholar] [CrossRef]
- Tuzovic, M.; Mead, M.; Young, P.A.; Schiller, G.; Yang, E.H. Cardiac Complications in the Adult Bone Marrow Transplant Patient. Curr. Oncol. Rep. 2019, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Bansal, N.; Joshi, C.; Adams, M.J.; Hutchins, K.; Ray, A.; Lipshultz, S.E. Cardiotoxicity in pediatric lymphoma survivors. Expert Rev. Cardiovasc. Ther. 2021, 19, 957–974. [Google Scholar] [CrossRef]
- Ratosa, I.; Pantar, M.I. Cardiotoxicity of mediastinal radiotherapy. Rep. Pract. Oncol. Radiother. 2019, 24, 629–643. [Google Scholar] [CrossRef]
- Siaravas, K.C.; Katsouras, C.S.; Sioka, C. Radiation Treatment Mechanisms of Cardiotoxicity: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 6272. [Google Scholar] [CrossRef] [PubMed]
| Therapeutic Interventions | Drug Class | Drugs | Risk Factors | Indications | Mechanisms of Cardiotoxicity | HCTRC |
|---|---|---|---|---|---|---|
| Conventional chemotherapy | ||||||
| Anthracyclines | Doxorubicin | Age (<5 or >65) | Leukemias | ROS | Subclinical myocardial dysfunction | |
| Epirubicin | Female sex | Lymphomas | DNA damage | HF | ||
| Daunorubicin | Hypertension | Apoptosis | Arrythmias | |||
| Idarubicin | DM | miRNAs | ||||
| AF | Fibrosis | |||||
| CAD | ||||||
| Prior mediasternal RT | ||||||
| Prior cardiotoxic cancer treatment | ||||||
| Cumulative dose doxorubicin ≥ 250 mg/m2 or equivalent | ||||||
| Genetic factors | ||||||
| Antibiotics | ||||||
| Bleomycin | ||||||
| Age | Hodgkin and non-Hodgkin Lymphomas | Oxidative stress | Hypotension | |||
| Cardiac disease | Mitochondrial dysfunction | Pericarditis | ||||
| Dose | Impaired energy metabolism | Myocardial ischemia | ||||
| Stroke | ||||||
| Vinca Alkaloids | ||||||
| Vincristine | ||||||
| ALL | Coronary artery vasospasm | Myocardial ischemia and infarction | ||||
| Burkitt Lymphomas | Cellular hypoxia | Arrythmias | ||||
| Hodgkin and non-Hodgkin Lymphomas | Endothelial dysfunction | Myocardial dysfunction | ||||
| MM | ||||||
| Alkylating agents | ||||||
| Cyclophosphamide | Age | Lymphomas | Oxidative and nitrosative stress | HF | ||
| Prior mediastinal RT | MM | Cardiac calcium overload | Myopericarditis | |||
| Anthracyclines | Leukemias | Mitochondrial damage | Arrythmias | |||
| Endothelial dysfunction | Tamponade | |||||
| Myocardial inflammation | ||||||
| Targeted therapy | ||||||
| BCR-ABL1 TKIs | ||||||
| Omatinib | Genetic predisposition | CML | On- and off-target effect | Hypertension | ||
| Dasatinib | CRF | Ph+ ALL | Mitochondrial damage and dysfunction | Hyperglycemia | ||
| Nilotinib | ROS | Arrythmias/QTc prolongation | ||||
| Bosutinib | Caspase activation | Vascular Toxicity | ||||
| Ponatinib | Apoptosis | Dyslipidemia | ||||
| Pleural and pericardial effusion | ||||||
| Myocarditis/Pericarditis | ||||||
| HF | ||||||
| Stroke | ||||||
| ACS | ||||||
| VTEs | ||||||
| BTKIs | ||||||
| Ibrutinib | Age | CLL | Off-target effect | Arrythmias/AF | ||
| Acalabrutinib | Hypetension | Lymphomas | Early and delayed afterdepolarizations | Hypertension | ||
| Zanubrutinib | Hyperlipidemia | WM | Platelet dysfuntion | SCD | ||
| Pirtobrutinib | DM | MM | CNS hemorrhage | |||
| Structural heart disease | Stroke | |||||
| Myocardial dysfunction | ||||||
| Proteasome inhibitors | ||||||
| Bortezomib | Age | MM | Mitochondrial dysfunction | Hypertension | ||
| Carfilzomib | Coexisting cardiac disease | MCL | Reduction of ATP synthesis | Myocardial dysfunction | ||
| Renal failure | Myocardial damage | HF | ||||
| MM-associated comorbidities | Thrombosis | |||||
| Arrythmias | ||||||
| Immunomodulatory drugs | ||||||
| Thalidomide | MM-associated comorbidities | MM | Anti-TNF | Arrythmias | ||
| Lenalidomide | Concommitant PI | Anti-angiogenic effects | VTEs | |||
| Pomalidomide | Endothelial dysfunction | MI | ||||
| Proteasome mediated protein degradation | Stroke | |||||
| Cereblon activation | HF | |||||
| Immunotherapy | ||||||
| Rituximab | Age | NHL | CRS | Angina | ||
| Coexisting cardiac disease | CLL | Platelet dysfuntion | ACS | |||
| Prior radiation therapy | Takotsubo | |||||
| Tumor lysis syndrome | Hypotension/Hypertension | |||||
| Cumulative dose | HF | |||||
| Hypersensitivity | Arrythmias | |||||
| CAR-T cell therapy | Age | ALL | CRS | Myocardial dysfunction | ||
| Tumor burden | Aggressive B-cell lymphomas | Off-target effect | HF | |||
| Coexisting cardiac disease | Refractory MM | Cross-reactivity between T-cells and titin | Takotsubo | |||
| Autoimmune disease | Arrythmias | |||||
| Genetic factors | Pericardial effusion | |||||
| SCD | ||||||
| Leak syndrome | ||||||
| Bispecific antibodies | Mosunetuzumab | Age | DLBCL | CRS | Hypotension/Hypertension | |
| Glofitamab | Coexisting cardiac disease | Refractory MM | Off-target effect | Arrythmias/AF | ||
| Odronextamab | Dose | AML | Immunogenicity | |||
| Teclistamab-cqyv | Prior cardiotoxic cancer treatment | |||||
| Hematopoietic stem cell transplantation | Allogenic HSCT | Leukemias | GVHD | Arrythmias/AF | ||
| Coexisting cardiac disease | Lymphomas | Endothelial dysfunction | HF | |||
| Cardiotoxic cancer treatment | MM | |||||
| RT | ||||||
| Radiotherapy | Younger age | Hodgkin and non-Hodgkin Lymphomas | Oxidatve stress | CAD | ||
| Cumulative radiation dose | ROS | VHD | ||||
| Smoking | Mitochondrial dysfunction | Pericardial disease | ||||
| Prior cardiotoxic cancer treatment | Cytoplasmic calcium overload | HF | ||||
| Dose per fraction (>2 Gy/day) | ncRNAs | Myocardial fibrosis | ||||
| Techniques of RT | Endothelial dysfunction | Conduction system disease | ||||
| Inflammation | ||||||
| Fibrosis | ||||||
| Autonomic dysfuntion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandala, E.; Lafara, K.; Kokkinovasilis, D.; Kalafatis, I.; Koukoulitsa, V.; Katodritou, E.; Lafaras, C. Applied Cardio-Oncology in Hematological Malignancies: A Narrative Review. Life 2024, 14, 524. https://doi.org/10.3390/life14040524
Mandala E, Lafara K, Kokkinovasilis D, Kalafatis I, Koukoulitsa V, Katodritou E, Lafaras C. Applied Cardio-Oncology in Hematological Malignancies: A Narrative Review. Life. 2024; 14(4):524. https://doi.org/10.3390/life14040524
Chicago/Turabian StyleMandala, Evdokia, Kyranna Lafara, Dimitrios Kokkinovasilis, Ioannis Kalafatis, Vasiliki Koukoulitsa, Eirini Katodritou, and Christos Lafaras. 2024. "Applied Cardio-Oncology in Hematological Malignancies: A Narrative Review" Life 14, no. 4: 524. https://doi.org/10.3390/life14040524
APA StyleMandala, E., Lafara, K., Kokkinovasilis, D., Kalafatis, I., Koukoulitsa, V., Katodritou, E., & Lafaras, C. (2024). Applied Cardio-Oncology in Hematological Malignancies: A Narrative Review. Life, 14(4), 524. https://doi.org/10.3390/life14040524








