Abstract
Applied cardio-oncology in hematological malignancies refers to the integration of cardiovascular care and management for patients with blood cancer, particularly leukemia, lymphoma, and multiple myeloma. Hematological cancer therapy-related cardiotoxicity deals with the most common cardiovascular complications of conventional chemotherapy, targeted therapy, immunotherapy, chimeric antigen receptor T (CAR-T) cell and tumor-infiltrating lymphocyte therapies, bispecific antibodies, and hematopoietic stem cell transplantation. This narrative review focuses on hematological cancer-therapy-related cardiotoxicity’s definition, risk stratification, multimodality imaging, and use of cardiac biomarkers to detect clinical and/or subclinical myocardial dysfunction and electrical instability. Moreover, the most common cardiotoxic profiles of the main drugs and/or therapeutic interventions in patients with hematological malignancies are described thoroughly.
1. Introduction
Applied cardio-oncology in hematological malignancies refers to the integration of cardiovascular care and oncology management for patients with blood cancers. This specialized field focuses on assessing and managing the potential cardiovascular complications that may arise during cancer treatment, particularly in hematological malignancies such as leukemia, lymphoma, and multiple myeloma.
Cardiovascular complications can occur due to the direct effects of cancer on the heart and blood vessels, as well as the side effects of cancer treatments like chemotherapy and radiation therapy. These complications may include cardiomyopathy, arrhythmias, thromboembolic events and heart failure. In the context of applied cardio-oncology, healthcare professionals collaborate to monitor and mitigate these risks. This involves comprehensive cardiovascular assessment before and during cancer treatment, close monitoring of cardiac function, and the implementation of preventive strategies to minimize cardiovascular damage. By integrating cardio-oncology principles into the management of hematological malignancies, healthcare providers aim to optimize cancer treatment outcomes while minimizing the risk of cardiovascular complications. This multidisciplinary approach ensures that patients receive comprehensive care that addresses both their cancer and their cardiovascular health needs [1,2,3].
2. Risk Stratification-Hematological Cancer Therapy-Related Cardiotoxicity Definition
Risk stratification is of great value and is multifactorial, involving advanced age, pre-existing cardiovascular conditions, type and stage of hematologic malignancy, and treatment regimen. Older age is associated with an increased risk of cardiovascular complications. Patients with pre-existing heart disease, hypertension, diabetes, or other cardiovascular risk factors may be at higher risk [1,2]. Additionally, certain types and stages of hematologic malignancies may have a higher likelihood of cardiovascular complications, as the specific chemotherapeutic drugs, radiation therapy, or targeted therapies used in the treatment can influence the total risk. When refining the definition of risk stratification for hematological cancer therapy-related cardiotoxicity, it is beneficial to specify the types of hematological cancers being considered. By categorizing them according to disease entities such as leukemia, lymphoma, myeloma, and others, the risk assessment can be more targeted and tailored to the specific characteristics and treatment regimens associated with each type of cancer. Each type of hematological cancer has distinct features in terms of pathophysiology, prognosis, treatment options, and potential cardiotoxic effects from therapy. In the treatment of hematologic malignancies, several classes of drugs are commonly used to target cancer cells and inhibit their growth. These drugs include conventional chemotherapy agents such as anthracyclines (e.g., doxorubicin), alkylating agents (e.g., cyclophosphamide), and antimetabolites (e.g., cytarabine). Tyrosine kinase inhibitors (e.g., imatinib, dasatinib, nilotinib) and monoclonal antibodies (e.g., rituximab, daratumumab) are examples of targeted therapies used in certain hematologic malignancies. Finally, types of immunotherapy such as immune checkpoint inhibitors (e.g., pembrolizumab, nivolumab), chimeric antigen receptor (CAR)-T cell therapy and hemopoietic stem cell transplantation are emerging treatments for hematologic malignancies [4,5]. Healthcare providers need to consider these risk factors and cardiotoxic profiles when designing treatment plans for patients with hematologic malignancies. Regular monitoring of cardiac function and close collaboration between hematologists and cardiologists are crucial in order to minimize the risk of cardiovascular complications and ensure optimal patient care.
Hematologic cancer therapy-related cardiotoxicity refers to the adverse effects on the cardiovascular system that can occur as a result of treatment for hematologic malignancies, such as leukemia, lymphoma, and multiple myeloma. Various treatment modalities used in hematologic cancer therapy, including conventional chemotherapy, targeted therapies, immunotherapies and radiation therapy, can potentially lead to a range of cardiovascular complications. The development of cardiotoxicity during hematologic cancer therapy can have significant implications for patient outcomes, as it may necessitate dose reductions or the discontinuation of potentially life-saving treatments. Therefore, it is crucial to identify and monitor cardiotoxicity early on, allowing for timely intervention and management; the implementation of strategies to mitigate further damage; the adjustment of treatment plans; and the provision of appropriate supportive care to minimize the impact on the patient’s cardiovascular health [2,5,6].
Fortunately, there has been a universal agreement on hematological-related cardiovascular toxicities’ definition, which may have important implications in the diagnosis, treatment, and follow-up of these patients, as well as for future research and clinical trials. These include the following: A. Symptomatic hematological cancer therapy-related cardiac dysfunction (HCTRCD), characterized by heart failure syndrome, classified as very severe (HF requiring inotropic and/or mechanical circulatory support), severe (HF needing hospitalization), moderate (outpatient intensification of HF therapy) or mild (stable HF treatment).
B. Asymptomatic HCTRCD, based on threshold changes of LVEF detected through echocardiographic study during cancer treatment or as an incidental finding during surveillance, including more sensitive methods to detect and confirm cardiac dysfunction, such as the new relative decline in global longitudinal strain (GLS) and the concomitant use of cardiac serum biomarkers-cardiac troponin (cTn) I or T and natriuretic peptides (NP) [2,3].
Additionally, the most common clinical manifestations of cardiovascular toxicity include myocarditis (immune-related, especially after extensive use of checkpoint inhibitors); vascular toxicity (either asymptomatic or symptomatic, as venous or arterial thrombosis, stroke, ACS, PAD, vasospastic and/or microvascular angina, or carotid artery disease); arterial hypertension; and cardiac arrhythmias (bradycardias, supraventricular tachycardias, ventricular arrhythmias, atrial fibrillation, torsade de pointes) [1,3,7].
3. Cardiac Imaging Modalities and Cardiac Biomarkers in Patients with Hematologic Malignances
Echocardiography is the cornerstone low-cost modality for the screening, surveillance, and detection of hematological cancer therapy-related cardiac dysfunction (HCTRCD) including two-dimensional (2D) and three-dimensional (3D) volumes, 2D and 3D LV ejection fraction (LVEF), global longitudinal strain (GLS), right ventricular (RV) size and systolic function assessment. 2D volumetric Simpson’s is recommended, preferably from measurements in apical four- and two-chamber views. As diastolic dysfunction can precede systolic dysfunction, full diastolic assessment should be done at the baseline and all sequential imaging. When two adjacent LV segments from any apical view are not adequately seen, contrast is recommended, and should then be used in all sequential testing [2,3,8,9].
It should be emphasized that 3D LVEF reportedly has superior reproducibility compared to 2D LVEF. However, it remains very sensitive to image quality and has low availability in real-world clinical practice. GLS through speckle-tracking echocardiography detects myocardial deformation from base to apex, as it refers to the change in length of the myocardium between relaxation and contraction, quantified by dedicated speckle tracking software which tracks different regions of the myocardium based on their unique speckle patterns, with more negative values indicating greater deformation [2,9,10].
CMR, as a second-line imaging modality, should be considered for the assessment of biventricular cardiac function when echocardiography is unavailable or non-diagnostic, due to poor-quality echocardiographic windows. Moreover, CMR can depict structural changes in the myocardium, including signs of edema and inflammation, before LV dysfunction. On the other hand, the higher cost, adverse reactions to gadolinium contrast agents, contraindications related to renal failure, the presence of metal devices and longer scanning times causing claustrophobia are the main advantages of the above imaging approach [2,3,11].
Multigated acquisition nuclear imaging (MUGA) should be considered as a third-line modality due to radiation exposure when TTE and CMR are both unavailable or non-diagnostic. Nuclear medicine can provide important insights into the early detection of impending cardiotoxicity, assist in the monitoring of cardiotoxic therapy, and may also be used as a tracking tool in the investigation of cardiotoxicity in novel therapies. SPECT MPI has a very low intra- and inter-observer variability; it allows accurate assessment of regional and global wall motion, phase analysis, and ventricular volumes in a single examination without the examination protocol having to be extended; and its value is validated by studies involving thousands of patients [11,12].
PET allows us to image changes in cellular metabolism with high sensitivity and therefore, appears well suited to identifying earlier stages of cardiomyocyte toxicity before irreversible myocardial damage develops. It should be established that cardiotoxic therapy may cause abnormalities in myocardial glucose metabolism. Additionally, myocardial inflammatory reactions may occur as a result of cancer therapy detected by PET, when the patient is adequately prepared. Also, 2-[18F]FDG PET is useful in detecting altered metabolism of the myocardium or in detecting cardiac inflammatory effects. Finally, cardiac perfusion PET may be applied for the effective detection of myocardial ischemia in patients who are candidates for potentially cardiotoxic cancer treatments or as a late sequelae after therapy, to monitor myocardial blood flow (MBF) or myocardial flow reserve (MFR) and cardiotoxic therapy or to explore mechanisms of cardiotoxicity [11,13,14,15,16].
The application of serum cardiac biomarkers to detect cardiotoxicity at a stage before it becomes irreversible is well known. The most important markers of cardiac injury are cardiac troponins and natriuretic peptides, whilst markers of inflammation such as interleukin-6, C-reactive protein, myeloperoxidase, Galectin-3 and growth differentiation factor-15, micro-ribonucleic acids, and immunoglobulin E are under investigation. Cardiac troponins (cTn) (particularly troponin I) are the gold standard biomarkers for the detection of cardiac injury and cardiomyocyte necrosis, and the most extensively used biomarkers to detect cardiac toxicity. Moreover, brain natriuretic peptide (BNP) and N-terminal pro-B type natriuretic peptide (NT-proBNP) are simulated to be secreted by cardiomyocytes from increased transmural tension and neurohormonal stimulation operating as sensitive indicators of pressure overload, myocardial stretch, and eventually cancer therapy-related cardiac dysfunction. Cardiac serum biomarkers are widely used for risk stratification at the baseline and during therapy, although recommendations concerning therapeutic strategies are mostly based on expert opinion. Baseline cardiac serum biomarker measurements are required if the degree of change in the biomarkers is to be used to identify subclinical cardiac injury during cancer treatment or to identify high-risk patients. There is a need for larger prospective studies to validate the clinical utility of the emerging serum biomarkers in the risk stratification and modification of treatment strategies to mitigate adverse cardiovascular events in patients with hematological malignancies [17,18,19].
4. Main Anticancer Drugs in Hematological Malignances and Cardiotoxic Profiles
The treatment of lymphomas consists of chemotherapy either alone or in combination with radiotherapy. It is well known that the standard treatment for Hodgkin lymphoma is ABVD (Adriamycin, Bleomycin, Vinblastine, and Dacarbazine), but other regimens such as the Stanford V (doxorubicin, vinblastine, mechlorethamine, etoposide, vincristine, bleomycin, and prednisone) and escalated-BEACOPP (Bleomycin, Etoposide, Adriamycin, Cyclophosphamide, Vincristine, Procarbazine, and Prednisone) treatments can be used. Additionally, R-CHOP (Rituximab, Hydroxydoxorubicin, Vincristine [Oncovin], Prednisolone), administered every 3 weeks for up to six cycles with curative intent, is the main chemotherapeutic regimen for most types of non-Hodgkin lymphomas [20].
5. Conventional Chemotherapy’s Related Cardiovascular Toxicities
5.1. Anthracyclines
It is well known that anthracyclines represent the cornerstone conventional chemotherapeutic agents in hematologic malignancies. Anthracycline-induced cardiotoxicity is potentially a continuous dose-dependent phenomenon causing immediate myocardial cell injury, myocardial deformation, asymptomatic cardiotoxicity, and finally, overt cardiotoxicity and consequently heart failure syndrome [21,22,23]. Identification of both symptomatic and asymptomatic HCTRCD should be achieved by careful clinical assessment, estimation of serum cardiac biomarkers, and cardiac imaging using the classic and modern modalities of transthoracic echocardiography. It is crucial to emphasize the importance of patients’ risk stratification, as high-risk oncologic patients should undergo, in addition to thorough baseline screening, echocardiography every second cycle of chemotherapy, as well as estimation of biomarkers, troponin, and/or natriuretic peptides during each visit [2,3,6]. Primary prevention strategies include CV factors’ control, limitation of AC doses, use of less cardiotoxic AC analogs (liposomal and/or pegylated AC), Dexrazoxane, and concomitant use of b-blockers, RAS inhibitors, and statins in high-risk patients. There is an anthracycline equivalence dose when doxorubicin is considered as a reference of a cumulative dose above 400 mg/m2, epirubicin above 900 mg/m2, daunorubicin > 900 mg/m2 and idarubicin > 150 mg/m2, as the incidence of HF rises > 5% [2,23,24,25].
5.2. Bleomycin
Cardiovascular toxicity is a rare adverse effect of bleomycin and may be expressed clinically as hypotension, pericarditis, acute substernal chest pain, coronary artery disease, myocardial ischemia, myocardial infarction, and cerebral vascular accident, with an incidence of 1–3% [26,27].
5.3. Vincristine
The main cardiovascular side effects provoked by vincristine are myocardial ischemia and infarction, which tend to occur during or shortly after therapy and might, therefore, be related to coronary artery vasospasm as a result of cellular hypoxia, causing significant transitory impairment in the vasodilator and the contractile function, as well as temporary vascular damage [27].
5.4. Cyclophosphamide
Cyclophosphamide (CP) is a nitrogen mustard alkylating agent with potent antineoplastic, immunosuppressive, and immunomodulatory properties. It and its metabolites aldophosphamide, 4-hydroxy cyclophosphamide, and acrolein are identified as cardiotoxic agents, with the last being the most toxic metabolite. The proposed molecular mechanisms of CP cardiotoxicity include increased oxidative and nitrosative stress; cardiac calcium overload; reduced mitochondrial fatty acid oxidation; myocardial inflammation; apoptosis; and endothelial damage, leading to myocardial systolic and diastolic dysfunction. The incidence of fulminant congestive heart failure is reported to be 5% to 19%. Symptoms may include arrhythmias, acute fulminant heart failure, myopericarditis, pericardial effusion, and cardiac tamponade, usually occurring within 1 to 3 weeks. Older age, previous or concomitant use of anthracyclines, and prior mediastinal irradiation are the main risk factors for drug-induced cardiotoxicity [28,29].
6. Targeted Therapy’s Related Cardiovascular Toxicities
6.1. Rituximab
Rituximab is a monoclonal antibody against the CD20 antigen on malignant B lymphocytes, widely used to treat a variety of hematologic malignancies. It may cause angina pectoris, acute coronary syndromes, cardiac arrhythmias, hypertension and/or hypotension, heart failure, and Takotsubo cardiomyopathy, particularly as acute reactions related to the manifestation of cytokine release syndrome. These acute infusion-related effects have been reported during less than 1 percent of infusions [26,30].
6.2. Breakpoint Cluster Region–Abelson Oncogene Locus Tyrosine Kinase Inhibitors
It is well known that chronic myeloid leukemia (CML) is associated with chromosomal translocation (i.e., Philadelphia chromosome), which encodes the BCR: ABL1 oncoprotein through the fusion of the BCR and ABL1 genes with active tyrosine kinase activity, so tyrosine kinase inhibitors (TKIs) are currently the cornerstone treatment of CML. The cardiovascular toxicities of small-molecule TKIs targeting BCR-ABL, imatinib, dasatinib, nilotinib, bosutinib, and ponatinib are due to their ‘on- and off-target’ effects. Furthermore, there is individualized variability in cardiotoxicity as a result of TKI targets, genetic predisposition, and cardiovascular risk factor interactions. The 1st generation BCR-ABL TKI imatinib causes in 0.1–1% of cases hypertension, heart failure, hyperglycemia, and pleural effusion, while 2nd generation nolitinib causes hypertension, QTc prolongation, atrial fibrillation, dyslipidemia, and vascular toxicity (1–10%). Dasatinib is commonly responsible for pleural effusion (10%), while less common adverse drug reactions include hypertension, heart failure, pericardial effusion, and pulmonary hypertension (1–10%). The last-generation BCR-ABL TKI bosutinib may cause hypertension, as well as pleural and pericardial effusion, while ponatinib, 3rd generation BCR-ABL TKI may cause hypertension (10%), atrial fibrillation, heart failure, hyperglycemia, dyslipidemia, pericardial or pleural effusion, and vascular toxicity (1–10%). Baseline risk assessment and monitoring and baseline echocardiography during the usage of first- and third-generation tyrosine kinase inhibitors, and then every 3 months during the first year and every 6 months thereafter, is recommended. Moreover, QTc estimation should be considered at the baseline, 2–4 weeks after TKI’s administration, and 2 weeks after any dose increase (IIa, C), while echocardiography should be performed every 3 months for high-risk patients (IIa, C) [31,32,33].
6.3. Bruton Tyrosine Kinase (BTK) Inhibitors
BTK inhibitors (Ibrutinib, Acalabrutinib, Zanubrutinib, Pirtobrutinib) represent a promising therapeutic target for various B cell malignancies (chronic lymphocytic leukemia, small lymphocytic lymphoma, marginal zone lymphoma, diffuse large B cell lymphoma, and follicular lymphoma), Waldenstrom macroglobulinemia, and multiple myeloma. Their main cardiotoxic effects include atrial fibrillation and flutter, hypertension, ventricular arrhythmias, and sudden cardiac death [34,35]. The mechanism of arrhythmia from BTK inhibitors involves off-target inhibition of Tec protein tyrosine kinase (TEC) and downstream phosphoinositide 3-kinase (Akt) signaling, leading to enhanced automaticity, prolonging cardiac action potential which increases vulnerability to early and delayed afterdepolarizations. The cardiotoxicity linked to BTK inhibitors stems from their interference with signaling pathways that are essential for cardiac function. While the primary target of BTK inhibitors is the B-cell receptor signaling pathway in cancer cells, these drugs can also affect other kinases and signaling molecules in the heart, leading to adverse cardiac events. In conclusion, hematological cancer therapy-related cardiotoxicity associated with BTK inhibitors is a class effect driven by the drugs’ mechanisms of action. Second-generation BTK inhibitors like acalabrutinib and zanubrutinib offer improved cardiotoxicity profiles due to their enhanced selectivity and reduced off-target effects on cardiac tissues [36,37]. Particularly, the administration of Ibrutinib is associated with AF (13–16%) initiated in the first 3 months, which may persist despite stopping or reducing the dose. Moreover, BTK inhibitors especially Ibrutinib are responsible for an approximately 50% risk of bleeding, as a result of the inhibition of multiple pathways that regulate platelet function. Although anticoagulation is still recommended for CHA2DS2-VASc>2 if there is not a high risk of bleeding, optimal management involves multidisciplinary collaboration between cardio-oncologists and hematologists [35,38].
7. Multiple Myeloma Therapeutic Regimes
Therapeutic regimes for multiple myeloma include alkylating agents (cyclophosphamide, melphalan), proteasome inhibitors (bortezomib, carfilzomib, and ixazomib), immunomodulatory drugs (thalidomide, lenalidomide, pomalidomide) and monoclonal antibodies (daratumumab, elotuzumab, and isatuximab). The most popular regiments are bortezomib, lenalidomide, dexamethasone (“VRd”) and daratumumab, lenalidomide, dexamethasone (“DRd”). Evaluation of patients’ eligibility for stem cell transplantation is crucial, as it offers a significant and prolonged response. If they are candidates for autologous stem cell transplantation, they are often treated with a four-drug regimen such as DVRd or DVTd, followed by lenalidomide in combination with a proteasome inhibitor, or by daratumumab as maintenance therapy. Bispecific antibodies (teclistamab, elranatamab, and talquetamab) are designed to simultaneously bind to a target moiety on tumor cells and to CD3 on T cells, then activate the patient’s T cells to kill their tumor cells, and have shown impressive results in relapsed refractory myeloma. Additionally, the nuclear export inhibitor, selinexor, can be used for the treatment of multiply relapsed multiple myeloma. A modern therapeutic intervention is CAR-T cell therapy, a form of immunotherapy including the patient’s immune cells (T cells) which are collected, genetically modified, and re-induced, directly targeting the cancer cells of the patient [39,40,41].
8. Multiple Myeloma Drug-Related Cardiovascular Toxicities
Alkylating agents may cause heart failure and atrial fibrillation (1–10%), and immunomodulatory drugs may cause venous and arterial thromboembolic events (0.1–10%), hypertension (1–10%), hyperglycemia (1–10%), and atrial fibrillation (0.1–10%). Additionally, the main adverse cardiovascular events of proteasome inhibitors, particularly carfilzomib, including heart failure, hypertension, ischemia, and atrial fibrillation (>18%) usually occur during the first three months of therapy and are more common among patients with elevated baseline levels of brain natriuretic peptide (BNP) or NT-proBNP [37,39]. As the increase in venous thromboembolism (VTE) takes place, thromboprophylaxis is advised when carfilzomib is administered in combination with lenalidomide and dexamethasone. Monoclonal antibodies are associated with an increased risk of hypertension, diabetes mellitus, heart failure, atrial fibrillation, and venous thrombotic events (1–10%). Cardiovascular monitoring in patients with multiple myeloma receiving all the therapeutic regimens is of great value both at the baseline and during treatment. In particular, all myeloma patients should undergo thorough clinical assessment, BP, ECG, TTE, NT-Pro-BNP, and cTn at the baseline and clinical evaluation; BP during every clinical visit, NP and cTn every cycle during the first six cycles; and TTE every three cycles under carfilzomib or bortezomib, particularly in high-risk patients [2,3,42].
It is well known that thrombogenicity in MM is multifactorial, as a result of a combination of patient, disease, and treatment-related factors. Patient-related factors include advanced age; history of venous thromboembolism; obesity; immobility; central venous catheter; acute infection or hospitalization; comorbidities; history of inherited thrombophilia; recent surgery; and ongoing hormone therapy. Disease-related factors include the stage of active multiple myeloma; evidence of hyperviscosity; pathological fractures conditioning immobilization; and the requirement of surgery. Finally, treatment-related factors include immunomodulatory drugs in combination with high-dose dexamethasone; multi-agent chemotherapeutic regimens; and/or exposure to erythropoietin-stimulating agents. Thus, thromboprophylaxis with LMWH is recommended for patients with MM, at least during the first 6 months of therapy (Class I, Level A), and therapeutic doses of LMWH are recommended for MM patients with previous VTE (Class I, Level B) [2,43].
In the modern era of DOACs, thromboprophylaxis with low doses of xabans (apixaban, edoxaban, rivaroxaban) should be considered as an alternative to LMWH (standard of care), at least during the first 6 months of therapy. The drug-drug interactions of antithrombotic agents and antimyeloma drugs, as well as patients’ compliance and preferences, should be taken into account in the choice of thromboprophylaxis. Severe thrombocytopenia (platelet count < 20 × 109/L), active bleeding, congenital bleeding disorders (hemophilia, von Willebrand disease, severe deficiency of coagulation factors), and acquired coagulopathy are absolute contraindications to thromboprophylaxis [2,3,43,44].
Historically, AL amyloidosis was considered a rare metabolic disorder due to challenges in its diagnosis and limited awareness among healthcare professionals. However, with the advent of systematic screening programs and the establishment of specialized cardiac amyloid clinics, the perception of AL amyloidosis as a rare disease has shifted. Insoluble fibrils are deposited in various tissues, causing organ dysfunction and eventually death; therefore, early detection is crucial. AL amyloidosis is most commonly diagnosed when the affected patient has less than 10% bone marrow plasma cells the quantity required to make a diagnosis of myeloma. Patients with a higher burden of disease, as manifested by high levels of free light chains, increased numbers of bone marrow plasma cells, and more severe end-organ involvement, are more likely to experience treatment failure and worse prognosis. Amyloid deposits may result in a wide range of clinical manifestations depending upon their type, location, and amount in various organs. Thus, the most common clinical manifestations include gastrointestinal disease (hepatomegaly with or without splenomegaly, bleeding, gastroparesis, constipation, bacterial overgrowth, malabsorption, and intestinal pseudo-obstruction), renal involvement (asymptomatic proteinuria or clinically apparent nephrotic syndrome), peripheral and autonomic neuropathy, central nervous system disease (cerebral amyloid angiopathy, cortical and subcortical intracranial bleeding, ischemic embolic stroke), cardiac involvement (infiltrative cardiomyopathy), skin manifestations (waxy thickening, ecchymoses, subcutaneous nodules or plaques, raccoon eyes), and pulmonary disease (tracheobronchial infiltration, pleural effusions, amyloidomas, and pulmonary hypertension) [45,46]. Finally, the diagnostic evaluation is based on the clinical presentation of amyloidosis. Cardiac amyloidosis is presented as heart failure, angina, syncope/presyncope, unexplained “thickening of left and/or right walls”, ECG abnormalities (low voltage, conduction disease), pericardial disease, and thromboembolism/stroke. Therapy is individualized and should be risk-adapted and response-tailored cardiac biomarkers (NT-Pro-BNP, cTn). Chemotherapeutic regimens are the cornerstone therapy including alkylating agents (melphalan, cyclophosphamide), proteasome inhibitors (bortezomib, ixazomib), monoclonal antibodies (daratumumab), and anti-myeloid antibodies (NEOD001). The aim of therapy is a clonal hematologic response, a cardiac response, and improved survival. Treatment should be guided by the ASCT eligibility (~20%) of patients, given the robust and durable response, and preceded by induction chemotherapy with bortezomib-based regimens [2,45,47].
9. Chimeric Antigen Receptor T (CAR-T) Cell and Tumor-Infiltrating Lymphocytes Therapies
CAR-T cells, used as a form of genetically modified autologous immunotherapy, are genetically modified ex vivo and infused back into the patient, encoding a chimeric antigen receptor to direct the patient’s T cells against the leukemic cells. This modern immunotherapy is currently used for the treatment of acute lymphocytic leukemia and aggressive B-cell lymphomas when they are refractory or in second or later relapse, as well as for the treatment of relapsed and refractory multiple myeloma [48,49,50]. Cardiovascular toxicities are associated with the occurrence of cytokine release syndrome (CRS), manifesting as left ventricular dysfunction, heart failure, cardiac arrhythmias, pericardial effusion, Takotsubo cardiomyopathy, and cardiac arrest (Figure 1). Additional adverse events are hypersensitivity reactions, infections, prolonged cytopenias, hypogammaglobulinemia, and second malignancies. The degrees of CAR-T cell activation and activation kinetics are influenced by the level of tumor antigen expressed on malignant cells, tumor burden, the antigen binding domain’s affinity to its target epitope, and the CAR’s costimulatory elements [51]. It should be emphasized that CRS usually responds to treatment with the Interleukin-6 receptor antagonist tocilizumab, administered with or without glucocorticoids [49,50,52].
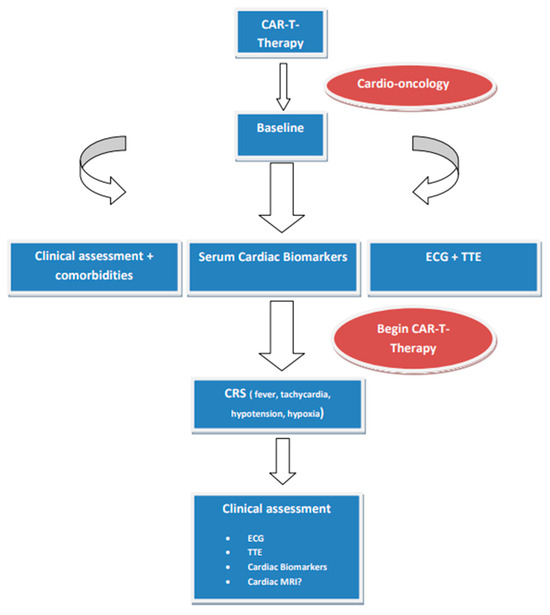
Figure 1.
Proposed protocol in patients treated with CAR-T cells. Abbreviations: CRS, cytokine release syndrome; ECG, electrocardiogram; MRI, magnetic resonance imaging; TTE, transthoracic echocardiogram.
10. Bispecific Antibodies
Bispecific T-cell engagers represent a new class of immunotherapy that induces the patients’ immune cells to attack tumors by retargeting T-cells to tumor cells. These bispecific antibodies are currently being investigated in DLBCL (mosunetuzumab, glofitamab, epcoritamab, and odronextamab) in intensively treated patients after CAR-T cell therapy, particularly in patients who will not achieve a durable remission. Bispecifics have lower rates of CRS compared with CAR-T, although there are limited data in patients with underlying heart disease. Cardiovascular side effects include hypotension (24–31%), tachycardia (24–31%), and atrial fibrillation (5%) [53,54]. Teclistamab-cqyv is a humanized antibody and a bispecific BCMA-directed CD3 T-cell engager used for the treatment of patients with relapsed or refractory multiple myeloma. BCMA is a protein expressed by late-stage B cells and plasma cells. Treatment-related adverse events have occurred, particularly CRS and ICANS (immune effector cell-associated neurotoxicity syndrome), but less frequently and less severely compared with CAR T-cell therapy. Cardiovascular complications are due to CRS: hypertension (18%), hypotension (12%), and cardiac arrhythmias (16%) [55,56].
11. Hematopoietic Stem Cell Transplantation
Patients with hematological relapsed and/or refractory malignancies are suitable for hematopoietic stem cell transplantation. A graft of hematopoietic stem cells is infused to restore hematopoiesis following the administration of conventional therapy (intensive chemotherapy and/or radiation). The graft can be the patient’s cells (autologous HCT), or the donor can be another individual (allogeneic HCT). Cardiovascular surveillance in patients in patients referred for hematopoietic stem cell transplantation (HSCT) includes baseline CV assessment, blood pressure measurement, ECG, lipid profile, HbA1C, and echocardiography. Risk stratification is of great value, as high-risk patients (allogenic HSCT, pre-existing heart disease and co-morbidities, and oncologic treatment history) should undergo a thorough clinical evaluation, TTE, NP and ECG 3 and 12 months after HSCT if new cardiac symptoms occur at any time and annually thereafter. It should be emphasized that HSCT is associated with excess cardiovascular risk partially due to exposure to cardiotoxic chemotherapy and radiation, as well as indirect and direct detrimental effects on cardiovascular reserve [2,57,58].
12. Radiotherapy
The toxic effects of mediastinal radiotherapy on heart substructures, such as the pericardium, myocardium, conducting system, coronary arteries, and heart valves, are well documented. Most of our knowledge regarding cardiotoxicity in long-term Hodgkin and non-Hodgkin lymphoma survivors comes from radiation therapy delivered in the past using mantle field and prescribed radiation doses of ≥40 Gy [57,58,59]. It is well known that the cardiotoxicity of radiotherapy is multifactorial, as cumulative radiation dose, irradiated heart volume, young age at the time of radiation exposure (especially doses > 30–50 Gy), fractional dosage (>2 Gy), the time elapsed since exposure, concomitant use of other chemotherapeutic agents, and classical cardiovascular risk factors play a pivotal role. Fortunately, the development of radioprotective oncology has led to the minimization of mean heart dose, and more targeted application of dosage, which reduces the amount of irradiated heart tissue. Additionally, modern heart-sparing RT strategies such as the optimal use of modern intensity, modulated photon RT technologies, the use of deep inspiration breath-hold or respiratory-gated techniques, image-guided RT, and personalized management prevent and attenuate CV complications [2,60,61]. Table 1 summarizes the main therapeutic interventions, risk factors, indications, mechanisms of cardiotoxicity, and HCTRC in hematological malignancies.

Table 1.
The main therapeutic interventions, risk factors, indications, mechanisms of cardiotoxicity, and HCTRC in hematological malignancies.
13. Conclusions and Future Directions
Patients with hematological malignancies are vulnerable to cardiovascular complications due to the type and stage of the disease, as well as patient and/or therapy-related risk factors that adversely impact survival. Comprehensive cardiac evaluation before, during, and after treatment is crucial, and the incorporation of surveillance strategies leads to early detection and management, resulting in improved survival via the multidisciplinary interactions of hematologists and cardio-oncologists for optimal medical care. Risk stratification is of great value as close monitoring is suggested for high-risk hematological cancer patients who are recommended to receive severe cardiotoxic therapy. Adopting a disease-specific approach to risk stratification for hematological cancer therapy-related cardiotoxicity aligns with the principles of precision medicine. This personalized approach allows for more accurate risk assessment and proactive management strategies that are tailored to each patient’s unique clinical profile and treatment plan (Figure 2). The early detection, diagnosis, and treatment of therapy-related CV complications are carried out according to ESC guidelines on cardio-oncology, developed in collaboration with the European Hematology Association (EHA); the European Society for Therapeutic Radiology and Oncology (ESTRO); and the International Cardio-Oncology Society (IC-OS) developed by the task force on cardio-oncology of the European Society of Cardiology. Furthermore, it is essential to record cardio-hematology registries in order to collect ‘big data’, a prerequisite for artificial intelligence to identify and predict the risk of HCTR-CVT and responses to specific cardioprotective interventions.
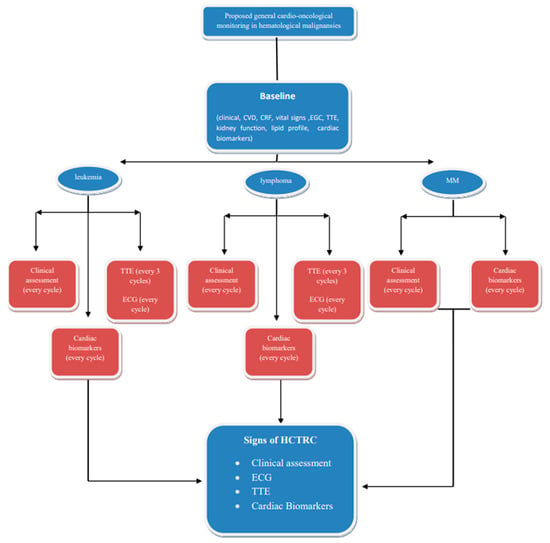
Figure 2.
Proposed simplified cardio-oncological monitoring/surveillance algorithm in hematological malignancies. This protocol should be modified according the possible HCTRC effects of therapeutic regimens. Abbreviations: CVD, cardiovascular disease; CRF, cardiac risk factors; ECG, electrocardiogram; HCTRC, hematological cancer therapy-related cardiotoxicity; MM, multiple myeloma; TTE, transthoracic echocardiogram.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gent, D.G.; Rebecca, D. The 2022 European Society of Cardiology Cardio-oncology Guidelines in Focus. Eur Cardiol. 2023, 18, e16. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J.-Cardiovasc. Imaging 2022, 23, e333–e465. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; Dent, S.; Stanway, S.; Earl, H.; Brezden-Masley, C.; Cohen-Solal, A.; Tocchetti, C.G.; Moslehi, J.J.; Groarke, J.D.; Bergler-Klein, J.; et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: A position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur. J. Heart Fail. 2020, 22, 1945–1960. [Google Scholar] [CrossRef]
- Costanzo, V.; Ratre, Y.K.; Andretta, E.; Acharya, R.; Bhaskar, L.V.K.S.; Verma, H.K. A Comprehensive Review of Cancer Drug–Induced Cardiotoxicity in Blood Cancer Patients: Current Perspectives and Therapeutic Strategies. Curr. Treat. Options Oncol. 2024, 25, 465–495. [Google Scholar] [CrossRef]
- Madonna, R. Multi-Target Drugs for Blood Cancer in the Elderly: Implications of Damage and Repair in the Cardiovascular Toxicity. Front. Physiol. 2021, 12, 792751. [Google Scholar] [CrossRef]
- Bojan, A.; Torok-Vistai, T.; Parvu, A. Assessment and Management of Cardiotoxicity in Hematologic Malignancies. Dis. Markers 2021, 2021, 6616265. [Google Scholar] [CrossRef]
- Cheng, K.H.; Wu, Y.W.; Hou, C.J.; Hung, C.M. An Overview of Cardio-Oncology, a New Frontier to Be Explored. Acta Cardiol. Sin. 2021, 37, 457–463. [Google Scholar] [PubMed]
- Di Lisi, D.; Manno, G.; Novo, G. Subclinical Cardiotoxicity: The Emerging Role of Myocardial Work and Other Imaging Techniques. Curr. Probl. Cardiol. 2021, 46, 100818. [Google Scholar] [CrossRef] [PubMed]
- Sanadgol, G.; Samimi, S.; Shirini, D.; Nakhaei, P.; Mohseni, M.; Alizadehasl, A. Right ventricle toxicity in cancer treatment: A focused review on cardiac imaging. Futur. Cardiol. 2023, 19, 537–545. [Google Scholar] [CrossRef]
- Madanat, L.; Gupta, R.; Weber, P.; Kumar, N.; Chandra, R.; Ahaneku, H.; Bansal, Y.; Anderson, J.; Bilolikar, A.; Jaiyesimi, I. Cardiotoxicity of Biological Therapies in Cancer Patients: An In-depth Review. Curr. Cardiol. Rev. 2023, 19, 8–18. [Google Scholar] [CrossRef]
- Totzeck, M.; Aide, N.; Bauersachs, J.; Bucerius, J.; Georgoulias, P.; Herrmann, K.; Hyafil, F.; Kunikowska, J.; Lubberink, M.; Nappi, C.; et al. Nuclear medicine in the assessment and prevention of cancer therapy-related cardiotoxicity: Prospects and proposal of use by the European Association of Nuclear Medicine (EANM). Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 792–812. [Google Scholar] [CrossRef] [PubMed]
- Printezi, M.I.; Yousif, L.I.E.; Kamphuis, J.A.M.; van Laake, L.W.; Cramer, M.J.; Hobbelink, M.G.G.; Asselbergs, F.W.; Teske, A.J. LVEF by Multigated Acquisition Scan Compared to Other Imaging Modalities in Cardio-Oncology: A Systematic Review. Curr. Heart Fail. Rep. 2022, 19, 136–145. [Google Scholar] [CrossRef]
- Cannizzaro, M.T.; Inserra, M.C.; Passaniti, G.; Celona, A.; D‘Angelo, T.; Romeo, P.; Basile, A. Role of advanced cardiovascular imaging in chemotherapy-induced cardiotoxicity. Heliyon 2023, 9, e15226. [Google Scholar] [CrossRef]
- Kelly, J.M.; Babich, J.W. PET Tracers for Imaging Cardiac Function in Cardio-oncology. Curr. Cardiol. Rep. 2022, 24, 247–260. [Google Scholar] [CrossRef]
- Seraphim, A.; Westwood, M.; Bhuva, A.N.; Crake, T.; Moon, J.C.; Menezes, L.J.; Lloyd, G.; Ghosh, A.K.; Slater, S.; Oakervee, H.; et al. Advanced Imaging Modalities to Monitor for Cardiotoxicity. Curr. Treat. Options Oncol. 2019, 20, 73. [Google Scholar] [CrossRef]
- Biersmith, M.A.; Tong, M.S.; Guha, A.; Simonetti, O.P.; Addison, D. Multimodality Cardiac Imaging in the Era of Emerging Cancer Therapies. J. Am. Heart Assoc. 2020, 9, e013755. [Google Scholar] [CrossRef] [PubMed]
- Ananthan, K.; Lyon, A.R. The Role of Biomarkers in Cardio-Oncology. J. Cardiovasc. Transl. Res. 2020, 13, 431–450. [Google Scholar] [CrossRef]
- Joolharzadeh, P.; Rodriguez, M.; Zaghlol, R.; Pedersen, L.N.; Jimenez, J.; Bergom, C.; Mitchell, J.D. Recent Advances in Serum Biomarkers for Risk Stratification and Patient Management in Cardio-Oncology. Curr. Cardiol. Rep. 2023, 25, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Pudil, R.; Mueller, C.; Čelutkienė, J.; Henriksen, P.A.; Lenihan, D.; Dent, S.; Barac, A.; Stanway, S.; Moslehi, J.; Suter, T.M.; et al. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: A position statement from the Cardio-Oncology Study Group of the Heart Failure Association and the Cardio-Oncology Council of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 1966–1983. [Google Scholar] [CrossRef]
- Johnson, P.W.M. Are we reaching the maximum cure rate for Hodgkin lymphoma? Hematol Oncol. 2023, 41 (Suppl. S1), 57–61. [Google Scholar] [CrossRef]
- Cardinale, D.M.; Zaninotto, M.; Cipolla, C.M.; Passino, C.; Plebani, M.; Clerico, A. Cardiotoxic effects and myocardial injury: The search for a more precise definition of drug cardiotoxicity. Clin. Chem. Lab. Med. (CCLM) 2021, 59, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Iacopo, F.; Cipolla, C.M. Cardiotoxicity of anthracyclines. Front. Cardiovasc. Med. 2020, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Colombo, A.; Bacchiani, G.; Tedeschi, I.; Meroni, C.A.; Civelli, M.; Lamantia, G.; Colombo, N.; Cipolla, C.M.; Veglia, F.; et al. Early Detection of Anthracycline Cardiotoxicity and Improvement with Heart Failure Therapy. Circulation 2015, 131, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, P.A.; Hall, P.; Oikonomidou, O.; MacPherson, I.R.; Maclean, M.; Lewis, S.; McVicars, H.; Broom, A.; Scott, F.; McKay, P.; et al. Rationale and Design of the Cardiac CARE Trial: A Randomized Trial of Troponin-Guided Neurohormonal Blockade for the Prevention of Anthracycline Cardiotoxicity. Circ. Heart Fail. 2022, 15, e009445. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Weng, Y.; Jiang, T.; Ou, W.; Zhang, N.; Dong, Q.; Tang, X. Influencing factors of anthracycline-induced subclinical cardiotoxicity in acute leukemia patients. BMC Cancer 2023, 23, 976. [Google Scholar] [CrossRef] [PubMed]
- Lewis, W.D.; Lilly, S.; Jones, K.L. Lymphoma: Diagnosis and Treatment. Am. Fam. Physician 2020, 101, 34–41. [Google Scholar] [PubMed]
- Manavi, M.A.; Nasab, M.H.F.; Jafari, R.M.; Dehpour, A.R. Mechanisms underlying dose-limiting toxicities of conventional chemotherapeutic agents. J. Chemother. 2024, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Iqubal, A.; Iqubal, M.K.; Sharma, S.; Ansari, M.A.; Najmi, A.K.; Ali, S.M.; Ali, J.; Haque, S.E. Molecular mechanism involved in cyclophosphamide-induced cardiotoxicity: Old drug with a new vision. Life Sci. 2019, 218, 112–131. [Google Scholar] [CrossRef] [PubMed]
- Iqubal, A.; Wasim, M.; Ashraf, M.; Najmi, A.K.; Syed, M.A.; Ali, J.; Haque, S.E. Natural Bioactive as a Potential Therapeutic Approach for the Management of Cyclophosphamide-induced Cardiotoxicity. Curr. Top. Med. Chem. 2021, 21, 2647–2670. [Google Scholar] [CrossRef]
- Schmittlutz, K.; Marks, R. Current treatment options for aggressive non-Hodgkin lymphoma in elderly and frail patients: Practical considerations for the hematologist. Ther. Adv. Hematol. 2021, 12. [Google Scholar] [CrossRef]
- Molica, M.; Scalzulli, E.; Colafigli, G.; Foà, R.; Breccia, M. Insights into the optimal use of ponatinib in patients with chronic phase chronic myeloid leukaemia. Ther. Adv. Hematol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Binzaid, A.A.; Baqal, O.J.; Soheib, M.; Al Nahedh, M.; Samarkandi, H.H.; Aljurf, M. Cardiovascular Toxicity Associated with Tyrosine Kinase Inhibitor Therapy In Chronic Myeloid Leukemia. Gulf J. Oncol. 2021, 1, 79–84. [Google Scholar]
- Singh, A.P.; Umbarkar, P.; Tousif, S.; Lal, H. Cardiotoxicity of the BCR-ABL1 tyrosine kinase inhibitors: Emphasis on ponatinib. Int. J. Cardiol. 2020, 316, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Quartermaine, C.; Ghazi, S.M.; Yasin, A.; Awan, F.T.; Fradley, M.; Wiczer, T.; Kalathoor, S.; Ferdousi, M.; Krishan, S.; Habib, A.; et al. Cardiovascular Toxicities of BTK Inhibitors in Chronic Lymphocytic Leukemia: JACC: CardioOncology State-of-the-Art Review. Cardio Oncol. 2023, 5, 570–590. [Google Scholar]
- Christensen, B.W.; Zaha, V.G.; Awan, F.T. Cardiotoxicity of BTK inhibitors: Ibrutinib and beyond. Expert Rev. Hematol. 2022, 15, 321–331. [Google Scholar] [CrossRef]
- Seymour, J.F.; Byrd, J.C.; Ghia, P.; Kater, A.P.; Chanan-Khan, A.; Furman, R.R.; O‘Brien, S.; Brown, J.R.; Munir, T.; Mato, A.; et al. Detailed safety profile of acalabrutinib vs ibrutinib in previously treated chronic lymphocytic leukemia in the ELEVATE-RR trial. Blood 2023, 142, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Boriani, G.; Menna, P.; Morgagni, R.; Minotti, G.; Vitolo, M. Ibrutinib and Bruton’s Tyrosine Kinase Inhibitors in Chronic Lymphocytic Leukemia: Focus on Atrial Fibrillation and Ventricular Tachyarrhythmias/Sudden Cardiac Death. Chemotherapy 2023, 68, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Yan, Y.; Zeng, X.; Lin, N.; Tan, B. Ibrutinib-Associated Cardiotoxicity: From the Pharmaceutical to the Clinical. Drug Des. Dev. Ther. 2022, 16, 3225–3239. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.G.; Cornell, R.F. Cardiovascular Complications Associated with Multiple Myeloma Therapies: Incidence, Pathophysiology, and Management. Curr. Oncol. Rep. 2019, 21, 29. [Google Scholar] [CrossRef]
- Das, A.; Dasgupta, S.; Gong, Y.; Shah, U.A.; Fradley, M.G.; Cheng, R.K.; Roy, B.; Guha, A. Cardiotoxicity as an adverse effect of immunomodulatory drugs and proteasome inhibitors in multiple myeloma: A network meta-analysis of randomized clinical trials. Hematol. Oncol. 2022, 40, 233–242. [Google Scholar] [CrossRef]
- Montefusco, V.; Mussetti, A.; Salas, M.Q.; Martinelli, G.; Cerchione, C. Old and new generation proteasome inhibitors in multiple myeloma. Panminerva Medica 2021, 62, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.F.; Naaktgeboren, W.R.; Hua, A.; Ghosh, A.K.; Oakervee, H.; Hallam, S.; Manisty, C. Optimising cardiovascular care of patients with multiple myeloma. Heart 2021, 107, 1774–1782. [Google Scholar] [CrossRef]
- Jarchowsky, O.; Avnery, O.; Ellis, M.H. Thrombosis in multiple myeloma: Mechanisms, risk assessment and management. Leuk. Lymphoma 2023, 64, 1905–1913. [Google Scholar] [CrossRef]
- Costa, T.A.; Felix, N.; Costa, B.A.; Godoi, A.; Nogueira, A.; Rossi, A. Direct oral anticoagulants versus aspirin for primary thromboprophylaxis in patients with multiple myeloma undergoing outpatient therapy: A systematic review and updated meta-analysis. Br. J. Haematol. 2023, 203, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Palladini, G.; Milani, P. Diagnosis and Treatment of AL Amyloidosis. Drugs 2023, 83, 203–216. [Google Scholar] [CrossRef]
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio, A.L.P.; Damy, T.; Eriksson, U.; et al. Diagnosis and treatment of cardiac amyloidosis: A position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2021, 42, 1554–1568. [Google Scholar] [CrossRef]
- Al Hamed, R.; Bazarbachi, A.H.; Bazarbachi, A.; Malard, F.; Harousseau, J.-L.; Mohty, M. Comprehensive Review of AL amyloidosis: Some practical recommendations. Blood Cancer J. 2021, 11, 97. [Google Scholar] [CrossRef]
- Milone, M.C.; Bhoj, V.G. The Pharmacology of T Cell Therapies. Mol. Ther.-Methods Clin. Dev. 2018, 8, 210–221. [Google Scholar] [CrossRef]
- Haslauer, T.; Greil, R.; Zaborsky, N.; Geisberger, R. CAR T-Cell Therapy in Hematological Malignancies. Int. J. Mol. Sci. 2021, 22, 8996. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Zhang, Z.; Ren, Z.; Li, Y. Reactions Related to CAR-T Cell Therapy. Front. Immunol. 2021, 12, 663201. [Google Scholar] [CrossRef]
- Sterner, R.C.; Sterner, R.M. CAR-T cell therapy: Current limitations and potential strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Chen, D.H.; Guha, A.; Mackenzie, S.; Walker, J.M.; Roddie, C. CAR T Cell Therapy-Related Cardiovascular Outcomes and Management: Systemic Disease or Direct Cardiotoxicity? Cardio Oncol. 2020, 2, 97–109. [Google Scholar]
- Bock, A.M.; Nowakowski, G.S.; Wang, Y. Bispecific Antibodies for Non-Hodgkin Lymphoma Treatment. Curr. Treat. Options Oncol. 2022, 23, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Goebeler, M.-E.; Bargou, R.C. T cell-engaging therapies—BiTEs and beyond. Nat. Rev. Clin. Oncol. 2020, 17, 418–434. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Garfall, A.L.; van de Donk, N.W.; Nahi, H.; San-Miguel, J.F.; Oriol, A.; Nooka, A.K.; Martin, T.; Rosinol, L.; Chari, A.; et al. Teclistamab in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2022, 387, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Martino, E.A.; Bruzzese, A.; Labanca, C.; Mendicino, F.; Lucia, E.; Olivito, V.; Neri, A.; Morabito, F.; Vigna, E.; Gentile, M. Teclistamab-cqyv in multiple myeloma. Eur. J. Haematol. 2024, 112, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Alizadehasl, A.; Ghadimi, N.; Hosseinifard, H.; Roudini, K.; Emami, A.H.; Ghavamzadeh, A.; Khoda-Amorzideh, D. Cardiovascular diseases in patients after hematopoietic stem cell transplantation: Systematic review and Meta-analysis. Curr. Res. Transl. Med. 2023, 71, 103363. [Google Scholar] [CrossRef]
- Tuzovic, M.; Mead, M.; Young, P.A.; Schiller, G.; Yang, E.H. Cardiac Complications in the Adult Bone Marrow Transplant Patient. Curr. Oncol. Rep. 2019, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Bansal, N.; Joshi, C.; Adams, M.J.; Hutchins, K.; Ray, A.; Lipshultz, S.E. Cardiotoxicity in pediatric lymphoma survivors. Expert Rev. Cardiovasc. Ther. 2021, 19, 957–974. [Google Scholar] [CrossRef]
- Ratosa, I.; Pantar, M.I. Cardiotoxicity of mediastinal radiotherapy. Rep. Pract. Oncol. Radiother. 2019, 24, 629–643. [Google Scholar] [CrossRef]
- Siaravas, K.C.; Katsouras, C.S.; Sioka, C. Radiation Treatment Mechanisms of Cardiotoxicity: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 6272. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).