Role of Tumor-Associated Macrophages in Cervical Cancer: Integrating Classical Perspectives with Recent Technological Advances
Abstract
1. Introduction
2. Origin and Polarization of TAMs
2.1. Origin of TAMs
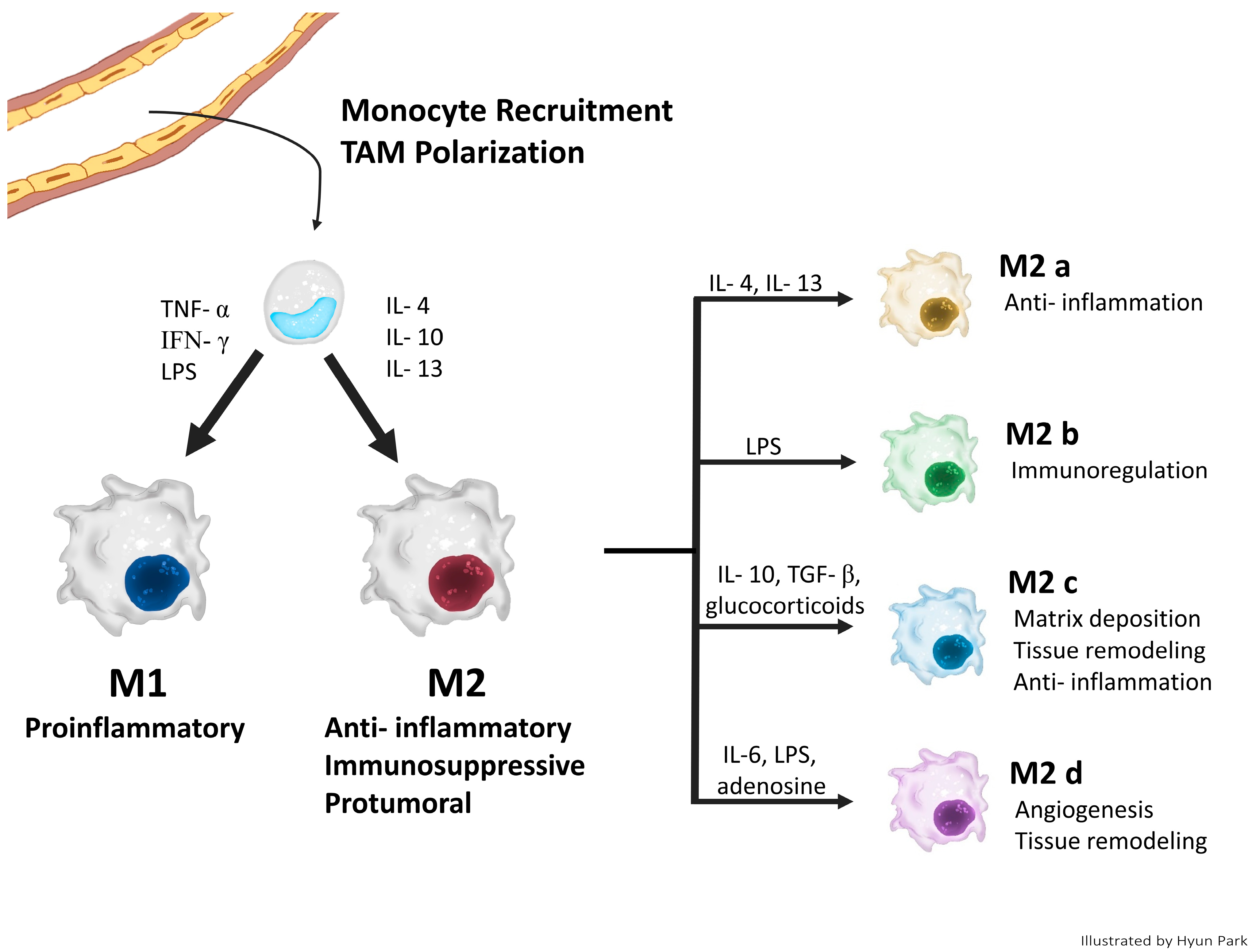
2.2. Classical Concept of TAM Polarization
3. The Role of TAMs in CC Progression
3.1. Inflammation/Initiation
3.2. Angiogenesis
3.3. Invasion/Migration/Metastasis
3.4. Immunosuppression
3.5. Cancer Stem Cells
3.6. Prognosis
4. TAM-Targeting Therapy
4.1. Depletion of TAMs
4.2. Inhibiting Monocyte/Macrophage Recruitment
4.3. Reprogramming/Re-Education of TAMs into M1-like Phenotype
4.4. Other Methods
5. Novel Findings of TAMs in the TME with Recent Technologies: Multi-Omics, SC RNA-Seq, and SP RNA-Seq
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sica, A.; Saccani, A.; Mantovani, A. Tumor-Associated Macrophages: A Molecular Perspective. Int. Immunopharmacol. 2002, 2, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Bae, J.S. Tumor-Associated Macrophages and Neutrophils in Tumor Microenvironment. Mediat. Inflamm. 2016, 2016, 6058147. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Steger, A.; Mahner, S.; Jeschke, U.; Heidegger, H. The Formation and Therapeutic Update of Tumor-Associated Macrophages in Cervical Cancer. Int. J. Mol. Sci. 2019, 20, 3310. [Google Scholar] [CrossRef] [PubMed]
- Petty, A.J.; Yang, Y. Tumor-Associated Macrophages: Implications in Cancer Immunotherapy. Immunotherapy 2017, 9, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Sioud, M. Tumor-Associated Macrophage Subsets: Shaping Polarization and Targeting. Int. J. Mol. Sci. 2023, 24, 7493. [Google Scholar] [CrossRef]
- Mantovani, A.; Schioppa, T.; Porta, C.; Allavena, P.; Sica, A. Role of Tumor-Associated Macrophages in Tumor Progression and Invasion. Cancer Metastasis Rev. 2006, 25, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Locati, M. Tumor-Associated Macrophages as a Paradigm of Macrophage Plasticity, Diversity, and Polarization Lessons and Open Questions. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1478–1483. [Google Scholar] [CrossRef] [PubMed]
- Chanmee, T.; Ontong, P.; Konno, K.; Itano, N. Tumor-Associated Macrophages as Major Players in the Tumor Microenvironment. Cancers 2014, 6, 1670–1690. [Google Scholar] [CrossRef]
- Krishnan, V.; Schaar, B.; Tallapragada, S.; Dorigo, O. Tumor Associated Macrophages in Gynecologic Cancers. Gynecol. Oncol. 2018, 149, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zeng, F.; Liao, S.; Cao, L.; Zhou, Y. Effects of Glycolysis on the Polarization and Function of Tumor-Associated Macrophages (Review). Int. J. Oncol. 2023, 62, 70. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Allavena, P.; Mantovani, A. Tumor-Associated Macrophages: Functional Diversity, Clinical Significance, and Open Questions. Semin. Immunopathol. 2013, 35, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Hourani, T.; Holden, J.A.; Li, W.; Lenzo, J.C.; Hadjigol, S.; O’Brien-Simpson, N.M. Tumor Associated Macrophages: Origin, Recruitment, Phenotypic Diversity, and Targeting. Front. Oncol. 2021, 11, 788365. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage Plasticity, Polarization, and Function in Health and Disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, G.; Maciejewski, T.M.; Szukiewicz, D. Current Progress in the Inflammatory Background of Angiogenesis in Gynecological Cancers. Inflamm. Res. 2019, 68, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Yu, S.; Zhang, J.; Wu, S. Dysregulated Tumor-Associated Macrophages in Carcinogenesis, Progression and Targeted Therapy of Gynecological and Breast Cancers. J. Hematol. Oncol. 2021, 14, 181. [Google Scholar] [CrossRef]
- Tan, J.; Yang, L.; Zhao, H.; Ai, Y.; Ren, L.; Zhang, F.; Dong, W.; Shi, R.; Sun, D.; Feng, Y. The Role of NFATc1/c-Myc/PKM2/IL-10 Axis in Activating Cervical Cancer Tumor-Associated M2 Macrophage Polarization to Promote Cervical Cancer Progression. Exp. Cell Res. 2022, 413, 113052. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Reyes, K.; Bravo-Cuellar, A.; Hernández-Flores, G.; Lerma-Díaz, J.M.; Jave-Suárez, L.F.; Gómez-Lomelí, P.; De Celis, R.; Aguilar-Lemarroy, A.; Domínguez-Rodríguez, J.R.; Ortiz-Lazareno, P.C. Cervical Cancer Cell Supernatants Induce a Phenotypic Switch from U937-Derived Macrophage-Activated M1 State into M2-like Suppressor Phenotype with Change in Toll-like Receptor Profile. Biomed Res. Int. 2014, 2014, 683068. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, G.; Zhang, S. Associations between Intratumoral and Peritumoral M2 Macrophage Counts and Cervical Squamous Cell Carcinoma Invasion Patterns. Int. J. Gynecol. Obstet. 2017, 139, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, L.; Li, Y.; Zhao, X. Research Progress on Tumor-Associated Macrophages and Inflammation in Cervical Cancer. Biomed Res. Int. 2020, 2020, 6842963. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Song, Y.; Du, W.; Gong, L.; Chang, H.; Zou, Z. Tumor-Associated Macrophages: An Accomplice in Solid Tumor Progression. J. Biomed. Sci. 2019, 26, 78. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Yi, M.; Wu, Y.; Dong, B.; Wu, K. Roles of Tumor-Associated Macrophages in Tumor Progression: Implications on Therapeutic Strategies. Exp. Hematol. Oncol. 2021, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Allavena, P.; Sica, A.; Solinas, G.; Porta, C.; Mantovani, A. The Inflammatory Micro-Environment in Tumor Progression: The Role of Tumor-Associated Macrophages. Crit. Rev. Oncol. Hematol. 2008, 66, 1–9. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Malekghasemi, S.; Majidi, J.; Baghbanzadeh, A.; Abdolalizadeh, J.; Baradaran, B.; Aghebati-Maleki, L. Tumor-Associated Macrophages: Protumoral Macrophages in Inflammatory Tumor Microenvironment. Adv. Pharm. Bull. 2020, 10, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Utrera-Barillas, D.; Castro-Manrreza, M.; Castellanos, E.; Gutiérrez-Rodríguez, M.; Arciniega-Ruíz de Esparza, O.; García-Cebada, J.; Velazquez, J.R.; Flores-Reséndiz, D.; Hernández-Hernández, D.; Benítez-Bribiesca, L. The Role of Macrophages and Mast Cells in Lymphangiogenesis and Angiogenesis in Cervical Carcinogenesis. Exp. Mol. Pathol. 2010, 89, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Steinberger, K.J.; Eubank, T.D. The Underexplored Landscape of Hypoxia-Inducible Factor 2 Alpha and Potential Roles in Tumor Macrophages: A Review. Oxygen 2023, 3, 45–76. [Google Scholar] [CrossRef] [PubMed]
- Henze, A.; Mazzone, M.; Henze, A.; Mazzone, M. The Impact of Hypoxia on Tumor-Associated Macrophages Find the Latest Version: The Impact of Hypoxia on Tumor-Associated Macrophages. J. Clin. Investig. 2016, 126, 3672–3679. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Li, Y.; Jian, L.; Yang, Y.; Zhao, L.; Wei, M. The Hypoxia-Driven Crosstalk between Tumor and Tumor-Associated Macrophages: Mechanisms and Clinical Treatment Strategies. Mol. Cancer 2022, 21, 177. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhang, S. Tumor-Associated Macrophages and Their Functional Transformation in the Hypoxic Tumor Microenvironment. Front. Immunol. 2021, 12, 741305. [Google Scholar] [CrossRef] [PubMed]
- Baradaran, A.; Asadzadeh, Z.; Hemmat, N.; Baghbanzadeh, A.; Shadbad, M.A.; Khosravi, N.; Derakhshani, A.; Alemohammad, H.; Afrashteh Nour, M.; Safarpour, H.; et al. The Cross-Talk between Tumor-Associated Macrophages and Tumor Endothelium: Recent Advances in Macrophage-Based Cancer Immunotherapy. Biomed. Pharmacother. 2022, 146, 112588. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Wu, S.; Yan, R.M.; Fan, L.S.; Yu, L.; Zhang, Y.M.; Wei, W.F.; Zhou, C.F.; Wu, X.G.; Zhong, M.; et al. The Role of the Hypoxia-Nrp-1 Axis in the Activation of M2-like Tumor-Associated Macrophages in the Tumor Microenvironment of Cervical Cancer. Mol. Carcinog. 2019, 58, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Mazibrada, J.; Rittà, M.; Mondini, M.; De Andrea, M.; Azzimonti, B.; Borgogna, C.; Ciotti, M.; Orlando, A.; Surico, N.; Chiusa, L.; et al. Interaction between Inflammation and Angiogenesis during Different Stages of Cervical Carcinogenesis. Gynecol. Oncol. 2008, 108, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Schoppmann, S.F.; Birner, P.; Stöckl, J.; Kalt, R.; Ullrich, R.; Caucig, C.; Nagy, K.; Alitalo, K.; Kerjaschki, D. Tumor-Associated Macrophages Express Lymphatic Endothelial Growth Factors and Are Related to Peritumoral Lymphangiogenesis. Am. J. Pathol. 2002, 161, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Cai, J.; Mao, M.; Fang, Y.; Huang, Z.; Jia, J.; Li, T.; Xu, L.; Wang, J.; Zhou, J.; et al. Tumor-Associated Macrophages Induce Lymphangiogenesis in Cervical Cancer via Interaction with Tumor Cells. Apmis 2014, 122, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Wei, W.F.; Wang, Z.C.; Wang, N.; Guo, C.H.; Zhou, C.F.; Liang, L.J.; Wu, S.; Liang, L.; Wang, W. A Novel Lymphatic Pattern Promotes Metastasis of Cervical Cancer in a Hypoxic Tumour-Associated Macrophage-Dependent Manner. Angiogenesis 2021, 24, 549–565. [Google Scholar] [CrossRef] [PubMed]
- Aras, S.; Raza Zaidi, M. TAMeless Traitors: Macrophages in Cancer Progression and Metastasis. Br. J. Cancer 2017, 117, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.Z.; Chan, M.K.K.; Chan, A.S.W.; Leung, K.T.; Jiang, X.; To, K.F.; Wu, Y.; Tang, P.M.K. Tumour-Associated Macrophages: Versatile Players in the Tumour Microenvironment. Front. Cell Dev. Biol. 2023, 11, 1261749. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, L.; Peng, X.; Zhan, X. Progress of Tumor-Associated Macrophages in the Epithelial-Mesenchymal Transition of Tumor. Front. Oncol. 2022, 12, 911410. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Ye, M.; Zhang, W. E6/E7 Oncoproteins of High Risk HPV-16 Upregulate MT1-MMP, MMP-2 and MMP-9 and Promote the Migration of Cervical Cancer Cells. Int. J. Clin. Exp. Pathol. 2015, 8, 4981–4989. [Google Scholar] [PubMed]
- Hu, W.; Li, X.; Zhang, C.; Yang, Y.; Jiang, J.; Wu, C. Tumor-Associated Macrophages in Cancers. Clin. Transl. Oncol. 2016, 18, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Sun, P.L.; He, Y.; Yao, M.; Gao, H. Immune Stromal Features in Cervical Squamous Cell Carcinoma Are Prognostic Factors for Distant Metastasis: A Retrospective Study. Pathol. Res. Pract. 2020, 216, 152751. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Yang, Y.; Fang, M.; Li, X.; Yuan, X.; Yuan, J. Co-Evolution of Tumor-Associated Macrophages and Tumor Neo-Vessels during Cervical Cancer Invasion. Oncol. Lett. 2016, 12, 2625–2631. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Deng, Y.R.; Wang, Z.C.; Wei, W.F.; Zhou, C.F.; Zhang, Y.M.; Yan, R.M.; Liang, L.J.; Zhong, M.; Liang, L.; et al. Hypoxia-Induced ZEB1 Promotes Cervical Cancer Progression via CCL8-Dependent Tumour-Associated Macrophage Recruitment. Cell Death Dis. 2019, 10, 508. [Google Scholar] [CrossRef] [PubMed]
- Ruffell, B.; Affara, N.I.; Coussens, L.M. Differential Macrophage Programming in the Tumor Microenvironment Macrophages in Solid Malignancies. Trends Immunol. 2012, 33, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Takeya, M.; Komohara, Y. Role of Tumor-Associated Macrophages in Human Malignancies: Friend or Foe? Pathol. Int. 2016, 66, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Petty, A.J.; Li, A.; Wang, X.; Dai, R.; Heyman, B.; Hsu, D.; Huang, X.; Yang, Y. Hedgehog Signaling Promotes Tumor-Associated Macrophage Polarization to Suppress Intratumoral CD8+ T Cell Recruitment. J. Clin. Investig. 2019, 129, 5151–5162. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Feng, Y.C.; Zhao, G.; Zhang, R.; Cheng, Z.Z.; Kong, W.N.; Wu, H.L.; Xu, B.; Lv, X.; Ma, X.M. Tumor-Associated CD163+ M2 Macrophage Infiltration Is Highly Associated with PD-L1 Expression in Cervical Cancer. Cancer Manag. Res. 2020, 12, 5831–5843. [Google Scholar] [CrossRef] [PubMed]
- Ring, K.L.; Yemelyanova, A.V.; Soliman, P.T.; Frumovitz, M.M.; Jazaeri, A.A. Potential Immunotherapy Targets in Recurrent Cervical Cancer. Gynecol. Oncol. 2017, 145, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Morales, V.A.; Chávez-Sánchez, L.; Rocha-Zavaleta, L.; Espíndola-Garibay, S.; Monroy-García, A.; Castro-Manrreza, M.E.; Fajardo-Orduña, G.R.; Apresa-García, T.; Gutiérrez-de la Barrera, M.; Mayani, H.; et al. Mesenchymal Stem/Stromal Cells Derived from Cervical Cancer Promote M2 Macrophage Polarization. Cells 2023, 12, 1047. [Google Scholar] [CrossRef] [PubMed]
- Heeren, A.M.; Punt, S.; Bleeker, M.C.; Gaarenstroom, K.N.; Van Der Velden, J.; Kenter, G.G.; De Gruijl, T.D.; Jordanova, E.S. Prognostic Effect of Different PD-L1 Expression Patterns in Squamous Cell Carcinoma and Adenocarcinoma of the Cervix. Mod. Pathol. 2016, 29, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Yang, G.; Ye, P.; Cao, N.; Chi, X.; Yang, W.H.; Yan, X. Macrophages Are a Double-Edged Sword: Molecular Crosstalk between Tumor-Associated Macrophages and Cancer Stem Cells. Biomolecules 2022, 12, 850. [Google Scholar] [CrossRef] [PubMed]
- Jinushi, M.; Baghdadi, M.; Chiba, S.; Yoshiyama, H. Regulation of Cancer Stem Cell Activities by Tumor-Associated Macrophages. Am. J. Cancer Res. 2012, 2, 529–539. [Google Scholar] [PubMed]
- Chen, Y.; Tan, W.; Wang, C. Tumor-Associated Macrophage-Derived Cytokines Enhance Cancer Stem-like Characteristics through Epithelial–Mesenchymal Transition. Onco Targets Ther. 2018, 11, 3817–3826. [Google Scholar] [CrossRef] [PubMed]
- Sainz, B.; Carron, E.; Vallespinós, M.; Machado, H.L. Cancer Stem Cells and Macrophages: Implications in Tumor Biology and Therapeutic Strategies. Mediat. Inflamm. 2016, 2016, 9012369. [Google Scholar] [CrossRef] [PubMed]
- Radharani, N.N.V.; Yadav, A.S.; Nimma, R.; Kumar, T.V.S.; Bulbule, A.; Chanukuppa, V.; Kumar, D.; Patnaik, S.; Rapole, S.; Kundu, G.C. Tumor-Associated Macrophage Derived IL-6 Enriches Cancer Stem Cell Population and Promotes Breast Tumor Progression via Stat-3 Pathway. Cancer Cell Int. 2022, 22, 122. [Google Scholar] [CrossRef] [PubMed]
- Raonic, J.; Lopicic, M.; Vuckovic, L.; Vucinic, J. Immunohistochemical Analysis of CD68, CD4, CD8 and CD20 Expression in Cervical Dysplasia and Its Relationship with HR-HPV Infection. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 7598–7606. [Google Scholar] [CrossRef]
- Davidson, B.; Goldberg, I.; Kopolovic, J. Inflammatory Response in Cervical Intraepithelial Neoplasia and Squamous Cell Carcinoma of the Uterine Cervix. Pathol. Res. Pract. 1997, 193, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Zitvogel, L.; Sautès-Fridman, C.; Kroemer, G. The Immune Contexture in Cancer Prognosis and Treatment. Nat. Rev. Clin. Oncol. 2017, 14, 717–734. [Google Scholar] [CrossRef] [PubMed]
- Horta, B.; Pereira, T.; Medeiros, R.; Cerqueira, F. Cervical Cancer Outcome and Tumor-Associated Macrophages: Research Evidence. Immuno 2022, 2, 460–468. [Google Scholar] [CrossRef]
- Hammes, L.S.; Tekmal, R.R.; Naud, P.; Edelweiss, M.I.; Kirma, N.; Valente, P.T.; Syrjänen, K.J.; Cunha-Filho, J.S. Macrophages, Inflammation and Risk of Cervical Intraepithelial Neoplasia (CIN) Progression-Clinicopathological Correlation. Gynecol. Oncol. 2007, 105, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Z.; Gao, A.; Wen, Q.; Sun, Y. The Prognostic Landscape of Tumor-Infiltrating Immune Cells in Cervical Cancer. Biomed. Pharmacother. 2019, 120, 109444. [Google Scholar] [CrossRef] [PubMed]
- Den Boon, J.A.; Pyeon, D.; Wang, S.S.; Horswill, M.; Schiffman, M.; Sherman, M.; Zuna, R.E.; Wang, Z.; Hewitt, S.M.; Pearson, R.; et al. Molecular Transitions from Papillomavirus Infection to Cervical Precancer and Cancer: Role of Stromal Estrogen Receptor Signaling. Proc. Natl. Acad. Sci. USA 2015, 112, E3255–E3264. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, M.; Zannoni, G.F.; Martinelli, E.; Anchora, L.P.; Ferrandina, G.; Tropeano, G.; Fagotti, A.; Scambia, G. Polarisation of Tumor-Associated Macrophages toward M2 Phenotype Correlates with Poor Response to Chemoradiation and Reduced Survival in Patients with Locally Advanced Cervical Cancer. PLoS ONE 2015, 10, e0136654. [Google Scholar] [CrossRef] [PubMed]
- Kawachi, A.; Yoshida, H.; Kitano, S.; Ino, Y.; Kato, T.; Hiraoka, N. Tumor-Associated CD204+ M2 Macrophages Are Unfavorable Prognostic Indicators in Uterine Cervical Adenocarcinoma. Cancer Sci. 2018, 109, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Carus, A.; Ladekarl, M.; Hager, H.; Nedergaard, B.S.; Donskov, F. Tumour-Associated CD66b + Neutrophil Count Is an Independent Prognostic Factor for Recurrence in Localised Cervical Cancer. Br. J. Cancer 2013, 108, 2116–2122. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Han, L.F.; Wu, X.G.; Wei, W.F.; Wu, L.F.; Yi, H.Y.; Yan, R.M.; Bai, X.Y.; Zhong, M.; Yu, Y.H.; et al. Clinical Significance of CD163+ and CD68+ Tumor-Associated Macrophages in High-Risk HPV-Related Cervical Cancer. J. Cancer 2017, 8, 3868–3875. [Google Scholar] [CrossRef]
- Guo, F.; Kong, W.; Zhao, G.; Cheng, Z.; Ai, L.; Lv, J.; Feng, Y.; Ma, X. The Correlation between Tumor-Associated Macrophage Infiltration and Progression in Cervical Carcinoma. Biosci. Rep. 2021, 41, BSR20203145. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, P.; Vasileva-Slaveva, M.; Shivarov, V.; Hasan, I.; Yordanov, A. Infiltration by Intratumor and Stromal CD8 and CD68 in Cervical Cancer. Medicina 2023, 59, 728. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Luo, Y. Targeting Macrophages in Cancer Immunotherapy. Signal Transduct. Target. Ther. 2021, 6, 127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Y.; Song, X.Y.; Li, Y.; Ye, L.L.; Zhou, Q.; Yang, W.B. Tumor-Associated Macrophages: A Promising Target for a Cancer Immunotherapeutic Strategy. Pharmacol. Res. 2020, 161, 105111. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, R.; Gao, Q. The Roles and Targeting of Tumor-Associated Macrophages. Front. Biosci. Landmark 2023, 28, 207. [Google Scholar] [CrossRef] [PubMed]
- Baci, D.; Bosi, A.; Gallazzi, M.; Rizzi, M.; Noonan, D.M.; Poggi, A.; Bruno, A.; Mortara, L. The Ovarian Cancer Tumor Immune Microenvironment (Time) as Target for Therapy: A Focus on Innate Immunity Cells as Therapeutic Effectors. Int. J. Mol. Sci. 2020, 21, 3125. [Google Scholar] [CrossRef] [PubMed]
- Truxova, I.; Cibula, D.; Spisek, R.; Fucikova, J. Targeting Tumor-Associated Macrophages for Successful Immunotherapy of Ovarian Carcinoma. J. Immunother. Cancer 2023, 11, e005968. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Yang, Q. Tumor-Associated Macrophage-Targeted Therapeutics in Ovarian Cancer. Int. J. Cancer 2021, 149, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, Y.; Ma, P.; Huang, H.; Tang, Q.; Miao, H.; Fang, Y.; Jiang, N.; Li, Y.; Zhu, Q.; et al. Landscape and Perspectives of Macrophage -Targeted Cancer Therapy in Clinical Trials. Mol. Ther. Oncolytics 2022, 24, 799–813. [Google Scholar] [CrossRef]
- Cao, Y.; Qiao, B.; Chen, Q.; Xie, Z.; Dou, X.; Xu, L.; Ran, H.; Zhang, L.; Wang, Z. Tumor Microenvironment Remodeling via Targeted Depletion of M2-like Tumor-Associated Macrophages for Cancer Immunotherapy. Acta Biomater. 2023, 160, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Jia, L.; Niu, Y.; Qi, H.; Chen, X.; Zhang, Q.; Zhang, J.; Wang, Y.; Dong, L.; Wang, C. Targeted Depletion of Tumour-Associated Macrophages by an Alendronate-Glucomannan Conjugate for Cancer Immunotherapy. Biomaterials 2014, 35, 10046–10057. [Google Scholar] [CrossRef]
- De Nola, R.; Menga, A.; Castegna, A.; Loizzi, V.; Ranieri, G.; Cicinelli, E.; Cormio, G. The Crowded Crosstalk between Cancer Cells and Stromal Microenvironment in Gynecological Malignancies: Biological Pathways and Therapeutic Implication. Int. J. Mol. Sci. 2019, 20, 2401. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Yin, H. Engeletin Suppresses Cervical Carcinogenesis in Vitro and in Vivo by Reducing NF-ΚB-Dependent Signaling. Biochem. Biophys. Res. Commun. 2020, 526, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Almahariq, M.F.; Quinn, T.J.; Kesarwani, P.; Kant, S.; Miller, C.R.; Chinnaiyan, P. Inhibition of Colony-Stimulating Factor-1 Receptor Enhances the Efficacy of Radiotherapy and Reduces Immune Suppression in Glioblastoma. In Vivo 2021, 35, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, Q.; Xu, M.; Wang, L.; Chen, X.; Feng, Y.; Li, Y.; Zhang, X.; Cui, W.; Jia, X. CCL2-CCR2 Axis Recruits Tumor Associated Macrophages to Induce Immune Evasion through PD-1 Signaling in Esophageal Carcinogenesis. Mol. Cancer 2020, 19, 41. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Luo, Z.; Li, X.; Han, X.; Shi, S.; Zhang, T. Tumor-Associated Macrophages: Role in Tumorigenesis and Immunotherapy Implications. J. Cancer 2021, 12, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Guo, M.; Zhou, Q. A Clinical Study of Pegylated Recombinant Human Granulocyte Colony Stimulating Factor (PEG-RhG-CSF) in Preventing Neutropenia during Concurrent Chemoradiotherapy of Cervical Cancer. BMC Cancer 2021, 21, 661. [Google Scholar] [CrossRef] [PubMed]
- Wanderley, C.W.; Colón, D.F.; Luiz, J.P.M.; Oliveira, F.F.; Viacava, P.R.; Leite, C.A.; Pereira, J.A.; Silva, C.M.; Silva, C.R.; Silva, R.L.; et al. Paclitaxel Reduces Tumor Growth by Reprogramming Tumor-Associated Macrophages to an M1 Profile in a TLR4-Dependent Manner. Cancer Res. 2018, 78, 5891–5900. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Marchesi, F.; Garlanda, C. Macrophages as Tools and Targets in Cancer Therapy. Nat. Rev. Drug Discov. 2022, 21, 799–820. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Wei, S.; Hurt, E.M.; Green, M.D.; Zhao, L.; Vatan, L.; Szeliga, W.; Herbst, R.; Harms, P.W.; Fecher, L.A.; et al. Host Expression of PD-L1 Determines Efficacy of PD-L1 Pathway Blockade–Mediated Tumor Regression. J. Clin. Investig. 2018, 128, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Mittman, S.; Rodriguez, R.; Moskalenko, M.; Pacheco-Sanchez, P.; Yang, Y.; Nickles, D.; Cubas, R. Anti-PD-L1 Treatment Results in Functional Remodeling of the Macrophage Compartment. Cancer Res. 2019, 79, 1493–1506. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Ni, T.; Wang, J.; Liu, Y.; Fan, Q.; Wang, Y.; Huang, T.; Chu, Y.; Sun, X.; Wang, Y. CD47 Blockade Inhibits Tumor Progression through Promoting Phagocytosis of Tumor Cells by M2 Polarized Macrophages in Endometrial Cancer. J. Immunol. Res. 2018, 2018, 6156757. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Saito, Y.; Kotani, T.; Matozaki, T. CD47-Signal Regulatory Protein α Signaling System and Its Application to Cancer Immunotherapy. Cancer Sci. 2018, 109, 2349–2357. [Google Scholar] [CrossRef]
- Pan, Y.; Lu, F.; Fei, Q.; Yu, X.; Xiong, P.; Yu, X.; Dang, Y.; Hou, Z.; Lin, W.; Lin, X.; et al. Single-Cell RNA Sequencing Reveals Compartmental Remodeling of Tumor-Infiltrating Immune Cells Induced by Anti-CD47 Targeting in Pancreatic Cancer. J. Hematol. Oncol. 2019, 12, 124. [Google Scholar] [CrossRef]
- Hayat, S.M.G.; Bianconi, V.; Pirro, M.; Jaafari, M.R.; Hatamipour, M.; Sahebkar, A. CD47: Role in the Immune System and Application to Cancer Therapy. Cell. Oncol. 2020, 43, 19–30. [Google Scholar] [CrossRef]
- Locati, M.; Curtale, G.; Mantovani, A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 123–147. [Google Scholar] [CrossRef] [PubMed]
- Newick, K.; Moon, E.; Albelda, S.M. Chimeric Antigen Receptor T-Cell Therapy for Solid Tumors. Mol. Ther. Oncolytics 2016, 3, 16006. [Google Scholar] [CrossRef] [PubMed]
- Kankeu Fonkoua, L.A.; Sirpilla, O.; Sakemura, R.; Siegler, E.L.; Kenderian, S.S. CAR T Cell Therapy and the Tumor Microenvironment: Current Challenges and Opportunities. Mol. Ther. Oncolytics 2022, 25, 69–77. [Google Scholar] [CrossRef]
- Klichinsky, M.; Ruella, M.; Shestova, O.; Lu, X.M.; Best, A.; Zeeman, M.; Schmierer, M.; Gabrusiewicz, K.; Anderson, N.R.; Petty, N.E.; et al. Human Chimeric Antigen Receptor Macrophages for Cancer Immunotherapy. Nat. Biotechnol. 2020, 38, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Guo, M.; Xu, J.; Wu, F.; Fan, J.; Huang, Q.; Yang, G.; Lv, Z.; Wang, X.; Jin, Y. Nanoparticles Targeting Macrophages as Potential Clinical Therapeutic Agents against Cancer and Inflammation. Front. Immunol. 2019, 10, 1998. [Google Scholar] [CrossRef] [PubMed]
- Penn, C.A.; Yang, K.; Zong, H.; Lim, J.Y.; Cole, A.; Yang, D.; Baker, J.; Goonewardena, S.N.; Buckanovich, R.J. Therapeutic Impact of Nanoparticle Therapy Targeting Tumor-Associated Macrophages. Mol. Cancer Ther. 2018, 17, 96–106. [Google Scholar] [CrossRef]
- He, Y.; de Araújo Júnior, R.F.; Cruz, L.J.; Eich, C. Functionalized Nanoparticles Targeting Tumor-Associated Macrophages as Cancer Therapy. Pharmaceutics 2021, 13, 1670. [Google Scholar] [CrossRef]
- Andersen, M.H. The Targeting of Tumor-Associated Macrophages by Vaccination. Cell Stress 2019, 3, 139–140. [Google Scholar] [CrossRef]
- Che, Y.; Yang, Y.; Suo, J.; An, Y.; Wang, X. Induction of Systemic Immune Responses and Reversion of Immunosuppression in the Tumor Microenvironment by a Therapeutic Vaccine for Cervical Cancer. Cancer Immunol. Immunother. 2020, 69, 2651–2664. [Google Scholar] [CrossRef] [PubMed]
- Hiremath, S.B.; Devendrappa, S.L. Safety and Efficacy of Tirapazamine as Anti-Cancer Drug: A Meta-Analysis of Randomized Controlled Trials. Int. J. Basic Clin. Pharmacol. 2018, 7, 783. [Google Scholar] [CrossRef]
- Ou, Z.; Lin, S.; Qiu, J.; Ding, W.; Ren, P.; Chen, D.; Wang, J.; Tong, Y.; Wu, D.; Chen, A.; et al. Single-Nucleus RNA Sequencing and Spatial Transcriptomics Reveal the Immunological Microenvironment of Cervical Squamous Cell Carcinoma. Adv. Sci. 2022, 9, 2203040. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.Y.; Ng, C.W.; Rajapakse, M.P.; Ang, N.; Yeong, J.P.S.; Lau, M.C. The Promise and Challenge of Spatial Omics in Dissecting Tumour Microenvironment and the Role of AI. Front. Oncol. 2023, 13, 1172314. [Google Scholar] [CrossRef] [PubMed]
- Ptacek, J.; Locke, D.; Finck, R.; Cvijic, M.E.; Li, Z.; Tarolli, J.G.; Aksoy, M.; Sigal, Y.; Zhang, Y.; Newgren, M.; et al. Multiplexed Ion Beam Imaging (MIBI) for Characterization of the Tumor Microenvironment across Tumor Types. Lab. Investig. 2020, 100, 1111–1123. [Google Scholar] [CrossRef] [PubMed]
- Lambrechts, D.; Wauters, E.; Boeckx, B.; Aibar, S.; Nittner, D.; Burton, O.; Bassez, A.; Decaluwé, H.; Pircher, A.; Van den Eynde, K.; et al. Phenotype Molding of Stromal Cells in the Lung Tumor Microenvironment. Nat. Med. 2018, 24, 1277–1289. [Google Scholar] [CrossRef] [PubMed]
- Laviron, M.; Petit, M.; Weber-Delacroix, E.; Combes, A.J.; Arkal, A.R.; Barthélémy, S.; Courau, T.; Hume, D.A.; Combadière, C.; Krummel, M.F.; et al. Tumor-Associated Macrophage Heterogeneity Is Driven by Tissue Territories in Breast Cancer. Cell Rep. 2022, 39, 110865. [Google Scholar] [CrossRef]
- Yin, W.; Ping, Y.F.; Li, F.; Lv, S.Q.; Zhang, X.N.; Li, X.G.; Guo, Y.; Liu, Q.; Li, T.R.; Yang, L.Q.; et al. A Map of the Spatial Distribution and Tumour-Associated Macrophage States in Glioblastoma and Grade 4 IDH-Mutant Astrocytoma. J. Pathol. 2022, 258, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Peng, C.W.; Yang, G.F.; Hu, W.Q.; Yang, X.J.; Huang, C.Q.; Xiong, B.; Li, Y. Distribution Pattern of Tumor Associated Macrophages Predicts the Prognosis of Gastric Cancer. Oncotarget 2017, 8, 92757–92769. [Google Scholar] [CrossRef] [PubMed]
- Artemova, D.; Vishnyakova, P.; Khashchenko, E.; Elchaninov, A.; Sukhikh, G.; Fatkhudinov, T. Endometriosis and Cancer: Exploring the Role of Macrophages. Int. J. Mol. Sci. 2021, 22, 5196. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.S.M.R.; Moriyama, M.; Kubota, K.; Ishiguro, N.; Sakamoto, M.; Chinju, A.; Mochizuki, K.; Sakamoto, T.; Kaneko, N.; Munemura, R.; et al. CD206+ Tumor-Associated Macrophages Promote Proliferation and Invasion in Oral Squamous Cell Carcinoma via EGF Production. Sci. Rep. 2019, 9, 14611. [Google Scholar] [CrossRef] [PubMed]
- De Logu, F.; Ugolini, F.; Iannone, L.F.; Simi, S.; Maio, V.; de Giorgi, V.; Maria di Giacomo, A.; Miracco, C.; Cossu, A.; Palmieri, G.; et al. Spatial Proximity and Relative Distribution of Tumor-Infiltrating Lymphocytes and Macrophages Predict Survival in Melanoma. Lab. Investig. 2023, 103, 100259. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, X.; Huang, Q.; Zhang, M.; Lei, T.; Wang, F.; Zou, W.; Huang, R.; Hu, X.; Wang, C.; et al. Single-Cell RNA-Sequencing Reveals Radiochemotherapy-Induced Innate Immune Activation and MHC-II Upregulation in Cervical Cancer. Signal Transduct. Target. Ther. 2023, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Qu, X.; Tang, X.; Song, Y.; Wang, J.; Hua, K.; Qiu, J. Spatiotemporally Deciphering the Mysterious Mechanism of Persistent HPV-induced Malignant Transition and Immune Remodelling from HPV-infected Normal Cervix, Precancer to Cervical Cancer: Integrating Single-cell RNA-sequencing and Spatial Transcriptome. Clin. Transl. Med. 2023, 13, e1219. [Google Scholar] [CrossRef]
- Cheng, S.; Li, Z.; Gao, R.; Xing, B.; Gao, Y.; Yang, Y.; Qin, S.; Zhang, L.; Ouyang, H.; Du, P.; et al. A Pan-Cancer Single-Cell Transcriptional Atlas of Tumor Infiltrating Myeloid Cells. Cell 2021, 184, 792–809.e23. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.Y.; Black, A.; Qian, B.Z. Macrophage Diversity in Cancer Revisited in the Era of Single-Cell Omics. Trends Immunol. 2022, 43, 546–563. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hua, K. Dissecting the Single-Cell Transcriptome Network of Immune Environment Underlying Cervical Premalignant Lesion, Cervical Cancer and Metastatic Lymph Nodes. Front. Immunol. 2022, 13, 897366. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.; Wang, Q.; Zhang, J.; Hu, Q.; Liu, C. Understanding Cervical Cancer at Single-Cell Resolution. Cancer Lett. 2023, 576, 216408. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Q.; Chen, G.; Luo, D. Multi-Omics Analysis Showed the Clinical Value of Gene Signatures of C1QC+ and SPP1+ TAMs in Cervical Cancer. Front. Immunol. 2021, 12, 694801. [Google Scholar] [CrossRef] [PubMed]
- Khanduri, I.; Maru, D.M.; Parra, E.R. Exploratory Study of Macrophage Polarization and Spatial Distribution in Colorectal Cancer Liver Metastasis: A Pilot Study. Front. Immunol. 2023, 14, 1223864. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lin, J.; Wang, G.; Xu, D. Targeting Proliferating Tumor-Infiltrating Macrophages Facilitates Spatial Redistribution of CD8+ T Cells in Pancreatic Cancer. Cancers 2022, 14, 1474. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Guo, Z.; Wei, X.; Zhao, G.; Han, D.; Zhang, T.; Chen, X.; Cao, F.; Dong, J.; Zhao, L.; et al. Spatial Distribution and Predictive Significance of Dendritic Cells and Macrophages in Esophageal Cancer Treated With Combined Chemoradiotherapy and PD-1 Blockade. Front. Immunol. 2022, 12, 786429. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Yang, Y.C.; An, Z.J.; Zhang, M.H.; Fu, X.H.; Huang, Z.F.; Yuan, Y.; Hou, J. Advances in Spatial Transcriptomics and Related Data Analysis Strategies. J. Transl. Med. 2023, 21, 330. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.G.; Lee, H.J.; Asatsuma, T.; Vento-Tormo, R.; Haque, A. An Introduction to Spatial Transcriptomics for Biomedical Research. Genome Med. 2022, 14, 68. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Lin, K.; Li, X.; Yuan, X.; Xu, P.; Ni, P.; Xu, D. Redefining Tumor-Associated Macrophage Subpopulations and Functions in the Tumor Microenvironment. Front. Immunol. 2020, 11, 1731. [Google Scholar] [CrossRef] [PubMed]
- Gorvel, L.; Panouillot, M.; Rouvière, M.-S.; Sonongbua, J.; Fattori, S.; Boucherit, N.; Amara, A.B.; Quilichini, O.; Granjeaud, S.; Degos, C.; et al. Tertiary Lymphoid Structures Are Associated with Enhanced Macrophage and Dendritic Cell Activation and Proximity to CD8+ T Cells, Which Better Predict the Clinical Outcome of Cervical Cancer Patients. bioRxiv 2023. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.; Lee, D.; Kim, N.Y.; Seo, I.; Park, N.J.-Y.; Chong, G.O. Role of Tumor-Associated Macrophages in Cervical Cancer: Integrating Classical Perspectives with Recent Technological Advances. Life 2024, 14, 443. https://doi.org/10.3390/life14040443
Choi Y, Lee D, Kim NY, Seo I, Park NJ-Y, Chong GO. Role of Tumor-Associated Macrophages in Cervical Cancer: Integrating Classical Perspectives with Recent Technological Advances. Life. 2024; 14(4):443. https://doi.org/10.3390/life14040443
Chicago/Turabian StyleChoi, Yeseul, Donghyeon Lee, Na Young Kim, Incheol Seo, Nora Jee-Young Park, and Gun Oh Chong. 2024. "Role of Tumor-Associated Macrophages in Cervical Cancer: Integrating Classical Perspectives with Recent Technological Advances" Life 14, no. 4: 443. https://doi.org/10.3390/life14040443
APA StyleChoi, Y., Lee, D., Kim, N. Y., Seo, I., Park, N. J.-Y., & Chong, G. O. (2024). Role of Tumor-Associated Macrophages in Cervical Cancer: Integrating Classical Perspectives with Recent Technological Advances. Life, 14(4), 443. https://doi.org/10.3390/life14040443





