Supplementation of Nicotinic Acid and Its Derivatives Up-Regulates Cellular NAD+ Level Rather than Nicotinamide Derivatives in Cultured Normal Human Epidermal Keratinocytes
Abstract
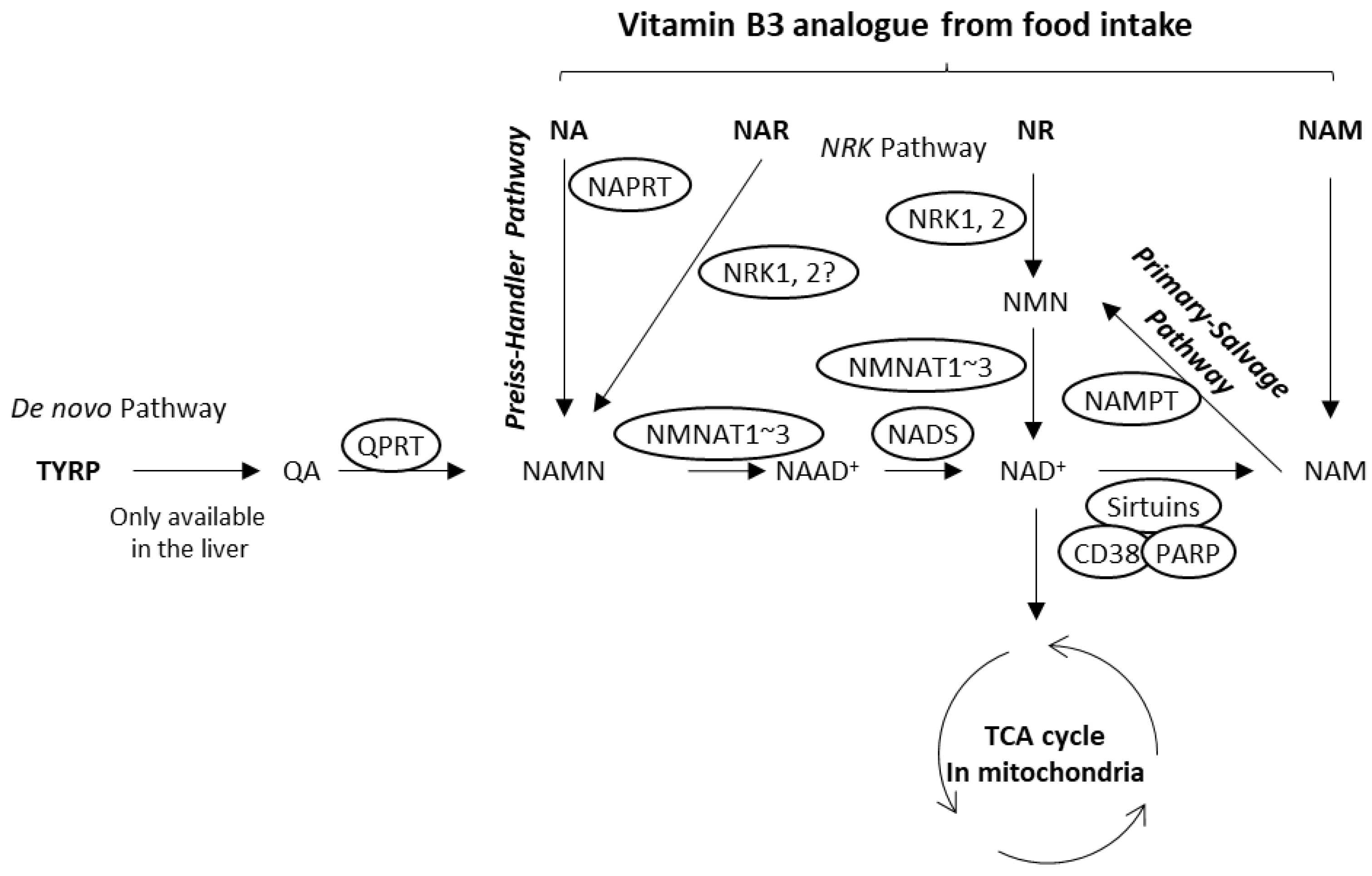
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Cells and Cell Culture
2.3. Measurement of Cellular NAD+ Levels
2.4. Immunoblot Analysis
2.5. Mitochondrial Functions
2.6. Statistical Analyses
2.7. Declaration of Generative AI in Scientific Writing
3. Results
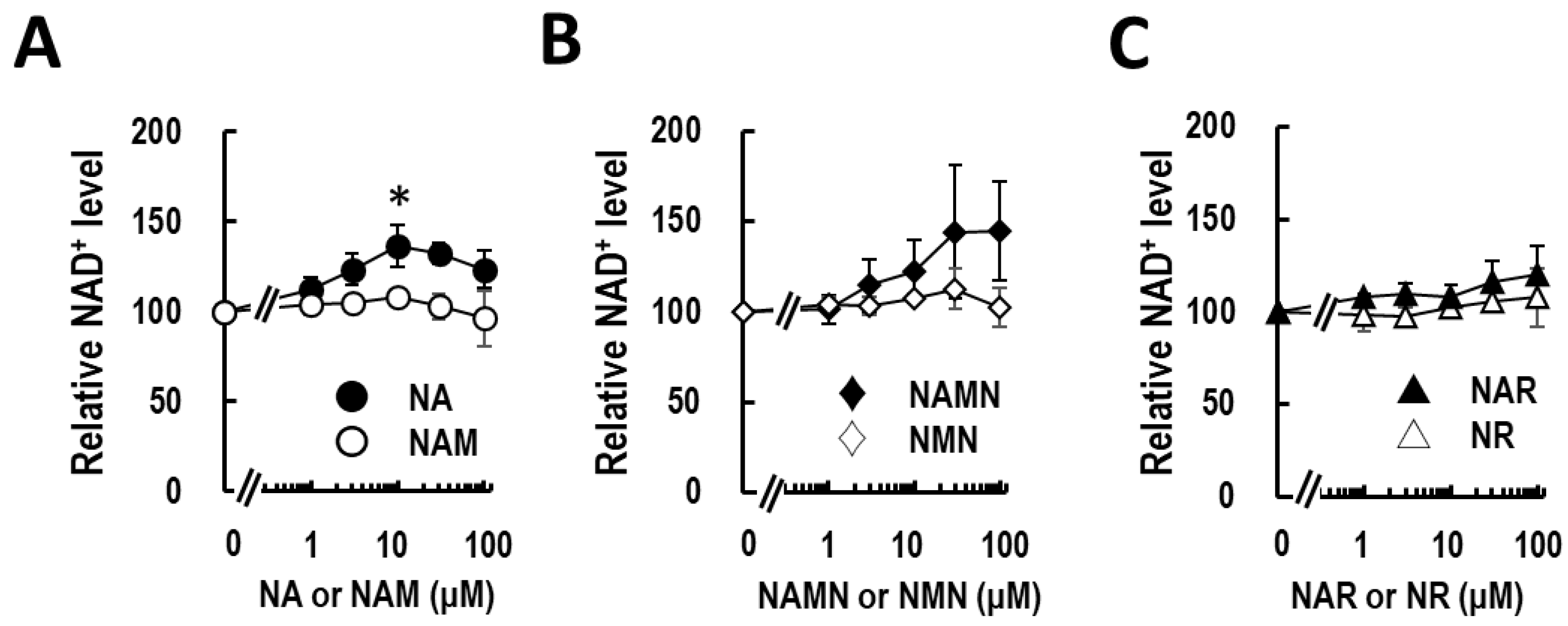
3.1. Nicotinic Acid Activates NAD+ Production in Normal Human Epidermal Keratinocytes
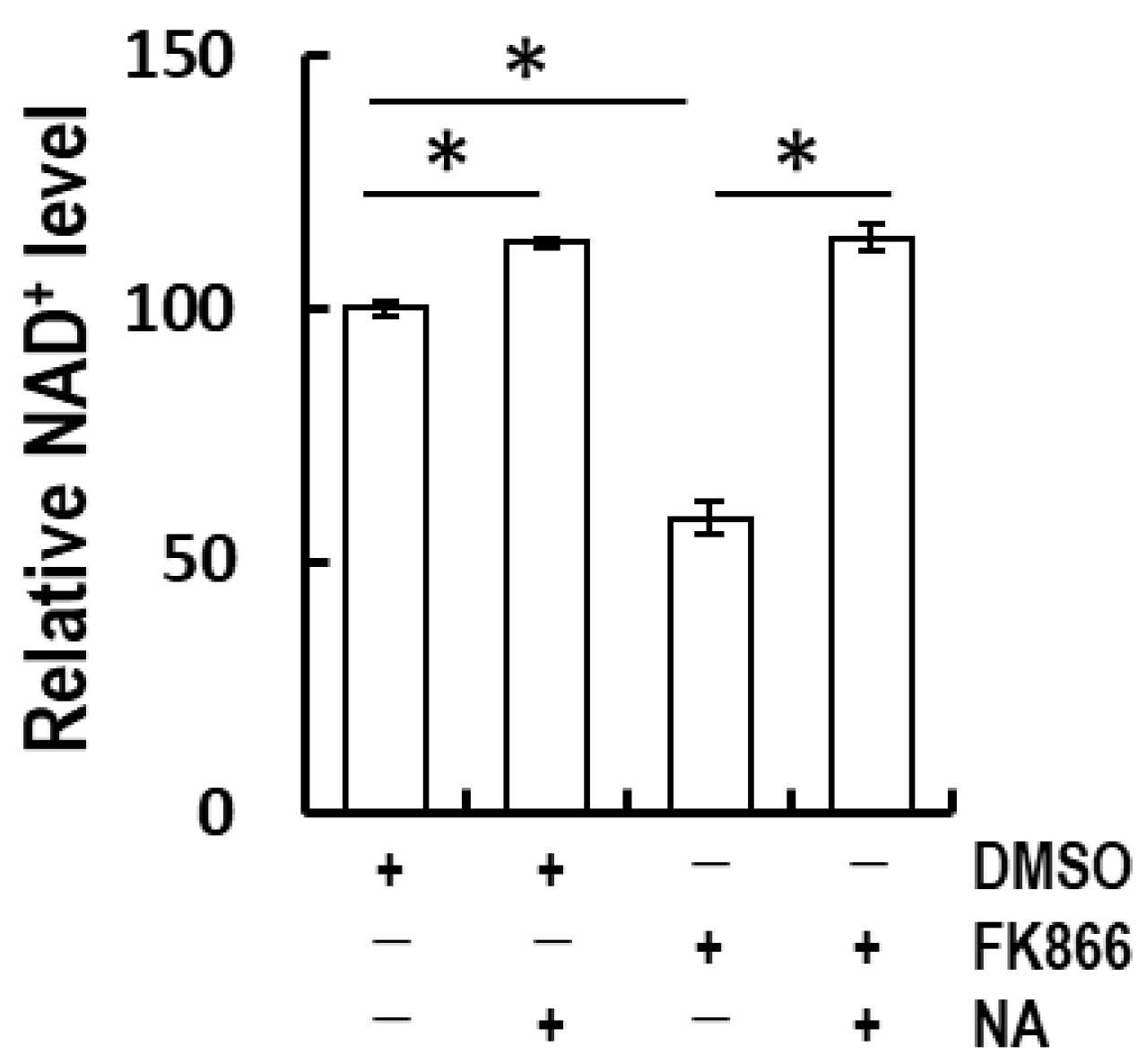
3.2. Nicotinic Acid-Recovered FK866-Induced NAD+ Depletion in Normal Human Epidermal Keratinocytes
3.3. Nicotinic Acid Supplementation Ablates Rotenone-Induced Mitochondrial ROS in Normal Human Epidermal Keratinocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Covarrubias, A.J.; Perrone, R.; Grozio, A.; Verdin, E. NAD+ Metabolism and Its Roles in Cellular Processes during Ageing. Nat. Rev. Mol. Cell Biol. 2021, 22, 119–141. [Google Scholar] [CrossRef]
- Bratic, A.; Larsson, N.-G. The Role of Mitochondria in Aging. J. Clin. Investig. 2013, 123, 951–957. [Google Scholar] [CrossRef]
- Pilkington, S.M.; Bulfone-Paus, S.; Griffiths, C.E.M.; Watson, R.E.B. Inflammaging and the Skin. J. Investig. Dermatol. 2021, 141, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Chini, C.C.S.; Zeidler, J.D.; Kashyap, S.; Warner, G.; Chini, E.N. Evolving Concepts in NAD+ Metabolism. Cell Metab. 2021, 33, 1076–1087. [Google Scholar] [CrossRef] [PubMed]
- Soma, M.; Lalam, S.K. The Role of Nicotinamide Mononucleotide (NMN) in Anti-Aging, Longevity, and Its Potential for Treating Chronic Conditions. Mol. Biol. Rep. 2022, 49, 9737–9748. [Google Scholar] [CrossRef] [PubMed]
- Bogan, K.L.; Brenner, C. Nicotinic Acid, Nicotinamide, and Nicotinamide Riboside: A Molecular Evaluation of NAD+ Precursor Vitamins in Human Nutrition. Annu. Rev. Nutr. 2008, 28, 115–130. [Google Scholar] [CrossRef]
- Kupis, W.; Pałyga, J.; Tomal, E.; Niewiadomska, E. The Role of Sirtuins in Cellular Homeostasis. J. Physiol. Biochem. 2016, 72, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Bieganowski, P.; Brenner, C. Discoveries of Nicotinamide Riboside as a Nutrient and Conserved NRK Genes Establish a Preiss-Handler Independent Route to NAD+ in Fungi and Humans. Cell 2004, 117, 495–502. [Google Scholar] [CrossRef]
- Belenky, P.; Racette, F.G.; Bogan, K.L.; McClure, J.M.; Smith, J.S.; Brenner, C. Nicotinamide Riboside Promotes Sir2 Silencing and Extends Lifespan via Nrk and Urh1/Pnp1/Meu1 Pathways to NAD+. Cell 2007, 129, 473–484. [Google Scholar] [CrossRef]
- Mehmel, M.; Jovanović, N.; Spitz, U. Nicotinamide Riboside-The Current State of Research and Therapeutic Uses. Nutrients 2020, 12, 1616. [Google Scholar] [CrossRef]
- Bogan, K.L.; Evans, C.; Belenky, P.; Song, P.; Burant, C.F.; Kennedy, R.; Brenner, C. Identification of Isn1 and Sdt1 as Glucose- and Vitamin-Regulated Nicotinamide Mononucleotide and Nicotinic Acid Mononucleotide 5′-Nucleotidases Responsible for Production of Nicotinamide Riboside and Nicotinic Acid Riboside. J. Biol. Chem. 2009, 284, 34861–34869. [Google Scholar] [CrossRef]
- Kropotov, A.; Kulikova, V.; Nerinovski, K.; Yakimov, A.; Svetlova, M.; Solovjeva, L.; Sudnitsyna, J.; Migaud, M.E.; Khodorkovskiy, M.; Ziegler, M.; et al. Equilibrative Nucleoside Transporters Mediate the Import of Nicotinamide Riboside and Nicotinic Acid Riboside into Human Cells. Int. J. Mol. Sci. 2021, 22, 1391. [Google Scholar] [CrossRef]
- Gao, J.; Feng, Z.; Wang, X.; Zeng, M.; Liu, J.; Han, S.; Xu, J.; Chen, L.; Cao, K.; Long, J.; et al. SIRT3/SOD2 Maintains Osteoblast Differentiation and Bone Formation by Regulating Mitochondrial Stress. Cell Death Differ. 2018, 25, 229–240. [Google Scholar] [CrossRef]
- Li, Q.; Liao, J.; Chen, W.; Zhang, K.; Li, H.; Ma, F.; Zhang, H.; Han, Q.; Guo, J.; Li, Y.; et al. NAC Alleviative Ferroptosis in Diabetic Nephropathy via Maintaining Mitochondrial Redox Homeostasis through Activating SIRT3-SOD2/Gpx4 Pathway. Free Radic. Biol. Med. 2022, 187, 158–170. [Google Scholar] [CrossRef]
- Miwa, S.; Kashyap, S.; Chini, E.; von Zglinicki, T. Mitochondrial Dysfunction in Cell Senescence and Aging. J. Clin. Investig. 2022, 132, e158447. [Google Scholar] [CrossRef]
- Chen, C.-L.; Zhang, L.; Jin, Z.; Kasumov, T.; Chen, Y.-R. Mitochondrial Redox Regulation and Myocardial Ischemia-Reperfusion Injury. Am. J. Physiol. Cell Physiol. 2022, 322, C12–C23. [Google Scholar] [CrossRef]
- Flynn, J.M.; Melov, S. SOD2 in Mitochondrial Dysfunction and Neurodegeneration. Free Radic. Biol. Med. 2013, 62, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Xiang, H.; Zhang, W. Review of Various NAMPT Inhibitors for the Treatment of Cancer. Front. Pharmacol. 2022, 13, 970553. [Google Scholar] [CrossRef] [PubMed]
- Oyama, T.; Ogawa, H.; Shirai, Y.; Abe, H.; Kamiya, T.; Abe, T.; Tanuma, S.-I. Hinokitiol-Induced Decreases of Tyrosinase and Microphthalmia-Associated Transcription Factor Are Mediated by the Endoplasmic Reticulum-Associated Degradation Pathway in Human Melanoma Cells. Biochimie 2022, 192, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Hara, N.; Yamada, K.; Shibata, T.; Osago, H.; Hashimoto, T.; Tsuchiya, M. Elevation of Cellular NAD Levels by Nicotinic Acid and Involvement of Nicotinic Acid Phosphoribosyltransferase in Human Cells. J. Biol. Chem. 2007, 282, 24574–24582. [Google Scholar] [CrossRef]
- Tanuma, S.-I.; Katsuragi, K.; Oyama, T.; Yoshimori, A.; Shibasaki, Y.; Asawa, Y.; Yamazaki, H.; Makino, K.; Okazawa, M.; Ogino, Y.; et al. Structural Basis of Beneficial Design for Effective Nicotinamide Phosphoribosyltransferase Inhibitors. Molecules 2020, 25, 3633. [Google Scholar] [CrossRef]
- Trnka, J.; Blaikie, F.H.; Logan, A.; Smith, R.A.J.; Murphy, M.P. Antioxidant Properties of MitoTEMPOL and Its Hydroxylamine. Free Radic. Res. 2009, 43, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Harden, A.; Young, W.J. The Alcoholic Ferment of Yeast-Juice. Proc. R. Soc. Lond. Ser. B Contain. Pap. A Biol. Character 1906, 77, 405–420. [Google Scholar]
- Elvehjem, C.A.; Madden, R.J.; Strong, F.M.; Woolley, D.W. Relation of Nicotinic Acid and Nicotinic Acid Amide to Canine Black Tongue. J. Am. Chem. Soc. 1937, 59, 1767–1768. [Google Scholar] [CrossRef]
- Jacobson, M.K.; Jacobson, E.L. Vitamin B3 in Health and Disease: Toward the Second Century of Discovery. Methods Mol. Biol. 2018, 1813, 3–8. [Google Scholar] [CrossRef]
- Abdellatif, M.; Baur, J.A. NAD+ Metabolism and Cardiometabolic Health: The Human Evidence. Cardiovasc. Res. 2021, 117, e106–e109. [Google Scholar] [CrossRef]
- Gensler, H.L.; Williams, T.; Huang, A.C.; Jacobson, E.L. Oral Niacin Prevents Photocarcinogenesis and Photoimmunosuppression in Mice. Nutr. Cancer 1999, 34, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, K.M.; Yoshino, J.; Brace, C.S.; Abrassart, D.; Kobayashi, Y.; Marcheva, B.; Hong, H.-K.; Chong, J.L.; Buhr, E.D.; Lee, C.; et al. Circadian Clock Feedback Cycle through NAMPT-Mediated NAD+ Biosynthesis. Science 2009, 324, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.S.; Song, S.B. Nicotinamide Is an Inhibitor of SIRT1 in Vitro, but Can Be a Stimulator in Cells. Cell. Mol. Life Sci. 2017, 74, 3347–3362. [Google Scholar] [CrossRef]
- Nakahata, Y.; Bessho, Y. The Circadian NAD+ Metabolism: Impact on Chromatin Remodeling and Aging. BioMed Res. Int. 2016, 2016, e3208429. [Google Scholar] [CrossRef]
- Fujiwara, R.; Takenaka, S.; Hashimoto, M.; Narawa, T.; Itoh, T. Expression of Human Solute Carrier Family Transporters in Skin: Possible Contributor to Drug-Induced Skin Disorders. Sci. Rep. 2014, 4, 5251. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Du, H.-H.; Long, X.; Pan, Y.; Hu, J.; Yu, J.; Zhao, X. β-Nicotinamide Mononucleotide (NMN) Administrated by Intraperitoneal Injection Mediates Protection Against UVB-Induced Skin Damage in Mice. J. Inflamm. Res. 2021, 14, 5165–5182. [Google Scholar] [CrossRef] [PubMed]
- Gehring, W. Nicotinic Acid/Niacinamide and the Skin. J. Cosmet. Dermatol. 2004, 3, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Kamanna, V.S.; Ganji, S.H.; Kashyap, M.L. The Mechanism and Mitigation of Niacin-Induced Flushing. Int. J. Clin. Pract. 2009, 63, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.H. Niacin Use and Cutaneous Flushing: Mechanisms and Strategies for Prevention. Am. J. Cardiol. 2008, 101, 14B–19B. [Google Scholar] [CrossRef] [PubMed]
- Benyó, Z.; Gille, A.; Kero, J.; Csiky, M.; Suchánková, M.C.; Nüsing, R.M.; Moers, A.; Pfeffer, K.; Offermanns, S. GPR109A (PUMA-G/HM74A) Mediates Nicotinic Acid-Induced Flushing. J. Clin. Investig. 2005, 115, 3634–3640. [Google Scholar] [CrossRef]
- Hanson, J.; Gille, A.; Zwykiel, S.; Lukasova, M.; Clausen, B.E.; Ahmed, K.; Tunaru, S.; Wirth, A.; Offermanns, S. Nicotinic Acid- and Monomethyl Fumarate-Induced Flushing Involves GPR109A Expressed by Keratinocytes and COX-2-Dependent Prostanoid Formation in Mice. J. Clin. Investig. 2010, 120, 2910–2919. [Google Scholar] [CrossRef]
- Cosmetic Ingredient Review Expert Panel. Final Report of the Safety Assessment of Niacinamide and Niacin. Int. J. Toxicol. 2005, 24 (Suppl. S5), 1–31. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oyama, T.; Yamamoto, T.; Kameda, T.; Kamiya, T.; Abe, H.; Abe, T.; Tanuma, S.-i. Supplementation of Nicotinic Acid and Its Derivatives Up-Regulates Cellular NAD+ Level Rather than Nicotinamide Derivatives in Cultured Normal Human Epidermal Keratinocytes. Life 2024, 14, 413. https://doi.org/10.3390/life14030413
Oyama T, Yamamoto T, Kameda T, Kamiya T, Abe H, Abe T, Tanuma S-i. Supplementation of Nicotinic Acid and Its Derivatives Up-Regulates Cellular NAD+ Level Rather than Nicotinamide Derivatives in Cultured Normal Human Epidermal Keratinocytes. Life. 2024; 14(3):413. https://doi.org/10.3390/life14030413
Chicago/Turabian StyleOyama, Takahiro, Takumi Yamamoto, Takeshi Kameda, Takanori Kamiya, Hideaki Abe, Takehiko Abe, and Sei-ichi Tanuma. 2024. "Supplementation of Nicotinic Acid and Its Derivatives Up-Regulates Cellular NAD+ Level Rather than Nicotinamide Derivatives in Cultured Normal Human Epidermal Keratinocytes" Life 14, no. 3: 413. https://doi.org/10.3390/life14030413
APA StyleOyama, T., Yamamoto, T., Kameda, T., Kamiya, T., Abe, H., Abe, T., & Tanuma, S.-i. (2024). Supplementation of Nicotinic Acid and Its Derivatives Up-Regulates Cellular NAD+ Level Rather than Nicotinamide Derivatives in Cultured Normal Human Epidermal Keratinocytes. Life, 14(3), 413. https://doi.org/10.3390/life14030413






