COVID-19 Clinical Features and Outcome in Italian Patients Treated with Biological Drugs Targeting Type 2 Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Recruitment
2.3. Biological Therapy
2.4. Questionnaire
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics of SARS-CoV-2 Infection
3.2. Asthma Exacerbation
3.3. Hospitalization
3.4. Vaccination Status
3.5. Safety Data
4. Discussion
4.1. The Possible Link between Type 2 Inflammation, COVID-19, and mAbs
4.2. Asthma Patients
4.3. Patients with Chronic Rhinosinusitis with Nasal Polyposis (CRSwNP)
4.4. Atopic Dermatitis (AD) Patients
4.5. Chronic Spontaneous Urticaria (CSU) Patients
4.6. Vaccination Status
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Sun, Y.; Wang, Y.; Yazici, D.; Azkur, D.; Ogulur, I.; Azkur, A.K.; Yang, Z.; Chen, X.; Zhang, A.; et al. Recent developments in the immunopathology of COVID-19. Allergy 2023, 78, 369–388. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.D.; Ding, M.; Dong, X.; Zhang, J.-J.; Azkur, A.K.; Azkur, D.; Gan, H.; Sun, Y.-L.; Fu, W.; Li, W.; et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy 2021, 76, 428–455. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef] [PubMed]
- Sajuthi, S.P.; DeFord, P.; Li, Y.; Jackson, N.D.; Montgomery, M.T.; Everman, J.L.; Rios, C.L.; Pruesse, E.; Nolin, J.D.; Plender, E.G.; et al. Type 2 and interferon inflammation regulate SARS-CoV-2 entry factor expression in the airway epithelium. Nat. Commun. 2020, 11, 5139. [Google Scholar] [CrossRef] [PubMed]
- Carr, T.F.; Kraft, M. Asthma and atopy in COVID-19: 2021 updates. J. Allergy Clin. Immunol. 2022, 149, 562–564. [Google Scholar] [CrossRef] [PubMed]
- Camiolo, M.; Gauthier, M.; Kaminski, N.; Ray, A.; Wenzel, S.E. Expression of SARS-CoV-2 receptor ACE2 and coincident host response signature varies by asthma inflammatoryenotype. J. Allergy Clin. Immunol. 2020, 146, 315–324. [Google Scholar] [CrossRef]
- Yang, J.M.; Koh, H.Y.; Moon, S.Y.; Yoo, I.K.; Ha, E.K.; You, S.; Kim, S.Y.; Yon, D.K.; Lee, S.W. Allergic disorders and susceptibility to and severity of COVID-19: A nationwide cohort study. J. Allergy Clin. Immunol. 2020, 146, 790–798. [Google Scholar] [CrossRef]
- Palmon, P.A.; Jackson, D.J.; Denlinger, L.C. COVID-19 Infections and Asthma. J. Allergy Clin. Immunol. Pract. 2022, 10, 658–663. [Google Scholar] [CrossRef]
- Adir, Y.; Saliba, W.; Beurnier, A.; Humbert, M. Asthma and COVID-19: An update. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2021, 30, 210152. [Google Scholar] [CrossRef]
- Giménez-Arnau, A.M.; DeMontojoye, L.; Asero, R.; Cugno, M.; Kulthanan, K.; Yanase, Y.; Hide, M.; Kaplan, A.P. The Pathogenesis of Chronic Spontaneous Urticaria: The Role of Infiltrating Cells. J. Allergy Clin. Immunol. Pract. 2021, 9, 2195–2208. [Google Scholar] [CrossRef] [PubMed]
- Prosty, C.; Gabrielli, S.; Ben-Shoshan, M.; Le, M.; Giménez-Arnau, A.M.; Litvinov, I.V.; Lefrançois, P.; Netchiporouk, E. In silico Identification of Immune Cell-Types and Pathways Involved in Chronic Spontaneous Urticaria. Front. Med. 2022, 9, 926753. [Google Scholar] [CrossRef]
- Xolair|European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/xolair (accessed on 20 February 2024).
- Nucala|European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/nucala (accessed on 20 February 2024).
- Fasenra|European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/fasenra (accessed on 20 February 2024).
- Dupixent|European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/dupixent (accessed on 20 February 2024).
- DUPIXENT (Dupilumab) Injection. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761055lbl.pdf (accessed on 12 March 2024).
- Veiga, V.C.; Cavalcanti, A.B. Age, host response, and mortality in COVID-19. Eur. Respir. J. 2023, 62, 2300796. [Google Scholar] [CrossRef]
- Lucas, C.; Wong, P.; Klein, J.; Castro, T.B.R.; Silva, J.; Sundaram, M.; Ellingson, M.K.; Mao, T.; Oh, J.E.; Israelow, B.; et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 2020, 584, 463–469. [Google Scholar] [CrossRef]
- Donlan, A.N.; Sutherland, T.E.; Marie, C.; Preissner, S.; Bradley, B.T.; Carpenter, R.M.; Sturek, J.M.; Ma, J.Z.; Moreau, G.B.; Donowitz, J.R.; et al. IL-13 is a driver of COVID-19 severity. JCI Insight 2021, 6, e150107. [Google Scholar] [CrossRef]
- Murdaca, G.; Di Gioacchino, M.; Greco, M.; Borro, M.; Paladin, F.; Petrarca, C.; Gangemi, S. Basophils and Mast Cells in COVID-19 Pathogenesis. Cells 2021, 10, 2754. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.P.; Yang, X.; Liu, X. Eosinopenia is associated with greater severity in patients with coronavirus disease 2019. Allergy 2021, 76, 562–564. [Google Scholar] [CrossRef]
- Ghiglioni, D.G.; Cozzi, L.; Castagnoli, R.; Bruschi, G.; Maffeis, L.; Marchisio, P.G.; Marseglia, G.L.; Licari, A. Omalizumab may protect allergic patients against COVID-19: A systematic review. World Allergy Organ J. 2023, 16, 100741. [Google Scholar] [CrossRef]
- Sekine, T.; Perez-Potti, A.; Rivera-Ballesteros, O.; Strålin, K.; Gorin, J.-B.; Olsson, A.; Llewellyn-Lacey, S.; Kamal, H.; Bogdanovic, G.; Muschiol, S.; et al. Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19. Cell 2020, 183, 158–168.e14. [Google Scholar] [CrossRef]
- Anti-IgE Monoclonal Antibodies as Potential Treatment in COVID-19—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/34018464/ (accessed on 17 July 2023).
- Kocatürk, E.; Salman, A.; Cherrez-Ojeda, I.; Criado, P.R.; Peter, J.; Comert-Ozer, E.; Abuzakouk, M.; Agondi, R.C.; Al-Ahmad, M.; Altrichter, S.; et al. The global impact of the COVID-19 pandemic on the management and course of chronic urticaria. Allergy 2021, 76, 816–830. [Google Scholar] [CrossRef]
- Sundbaum, J.K.; Konradsen, J.R.; Vanfleteren, L.E.; Fisk, S.A.; Pedroletti, C.; Sjöö, Y.; Syk, J.; Sterner, T.; Lindberg, A.; Tunsäter, A.; et al. Uncontrolled asthma predicts severe COVID-19: A report from the Swedish National Airway Register. Ther. Adv. Respir. Dis. 2022, 16, 17534666221091183. [Google Scholar] [CrossRef]
- Kocatürk, E.; Abrams, E.M.; Maurer, M.; Mitri, J.; Oppenheimer, J.; Vestergaard, C.; Zein, J. COVID-19 and Its Impact on Common Diseases in the Allergy Clinics. J. Allergy Clin. Immunol. Pract. 2023, 11, 3289–3303. [Google Scholar] [CrossRef]
- Ramos-Martínez, A.; Parra-Ramírez, L.M.; Morrás, I.; Carnevali, M.; Jiménez-Ibañez, L.; Rubio-Rivas, M.; Arnalich, F.; Beato, J.L.; Monge, D.; Asín, U.; et al. Frequency, risk factors, and outcomes of hospital readmissions of COVID-19 patients. Sci. Rep. 2021, 11, 13733. [Google Scholar] [CrossRef]
- Nassoro, D.D.; Mujwahuzi, L.; Mwakyula, I.H.; Possi, M.K.; Lyantagaye, S.L. Asthma and COVID-19: Emphasis on Adequate Asthma Control. Can. Respir. J. 2021, 2021, 9621572. [Google Scholar] [CrossRef]
- Dolby, T.; Nafilyan, V.; Morgan, A.; Kallis, C.; Sheikh, A.; Quint, J.K. Relationship between asthma and severe COVID-19: A national cohort study. Thorax 2023, 78, 120–127. [Google Scholar] [CrossRef]
- Musters, A.H.; Broderick, C.; Prieto-Merino, D.; Chiricozzi, A.; Damiani, G.; Peris, K.; Dhar, S.; De, A.; Freeman, E.; Arents, B.W.M.; et al. The effects of systemic immunomodulatory treatments on COVID-19 outcomes in patients with atopic dermatitis: Results from the global SECURE-AD registry. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 365–381. [Google Scholar] [CrossRef]
- Heffler, E.; Detoraki, A.; Contoli, M.; Papi, A.; Paoletti, G.; Malipiero, G.; Brussino, L.; Crimi, C.; Morrone, D.; Padovani, M.; et al. COVID-19 in Severe Asthma Network in Italy (SANI) patients: Clinical features, impact of comorbidities and treatments. Allergy 2021, 76, 887. Available online: https://pubmed.ncbi.nlm.nih.gov/32738147/ (accessed on 22 September 2023). [CrossRef]
- Eger, K.; Hashimoto, S.; Braunstahl, G.J.; Brinke, A.T.; Patberg, K.W.; Beukert, A.; Smeenk, F.; van der Sar-van der Brugge, S.; Weersink, E.J.M.; Bel, E.H. Poor outcome of SARS-CoV-2 infection in patients with severe asthma on biologic therapy. Respir. Med. 2020, 177, 106287. [Google Scholar] [CrossRef]
- Le, M.; Khoury, L.; Lu, Y.; Prosty, C.; Cormier, M.; Cheng, M.P.; Fowler, R.; Murthy, S.; Tsang, J.L.Y.; Ben-Shoshan, M.; et al. COVID-19 Immunologic Antiviral therapy with Omalizumab (CIAO)—A Randomized-Controlled Clinical Trial. In Open Forum Infectious Diseases; Oxford University Press: Oxford, UK, 2024. [Google Scholar] [CrossRef]
- Hanon, S.; Brusselle, G.; Deschampheleire, M.; Louis, R.; Michils, A.; Peché, R.; Pilette, C.; Rummens, P.; Schuermans, D.; Simonis, H.; et al. COVID-19 and biologics in severe asthma: Data from the Belgian Severe Asthma Registry. Eur. Respir. J. 2020, 56, 2002857. [Google Scholar] [CrossRef]
- Poddighe, D.; Kovzel, E. Impact of Anti type 2 Inflammation Biologic Therapy on COVID-19 Clinical Course and Outcome. J. Inflamm. Res. 2021, 14, 6845–6853. [Google Scholar] [CrossRef]
- Förster-Ruhrmann, U.; Szczepek, A.J.; Bachert, C.; Olze, H. COVID-19 in a patient with severe chronic rhinosinusitis with nasal polyps during therapy with dupilumab. J. Allergy Clin. Immunol. 2020, 146, 218–220.e2. [Google Scholar] [CrossRef]
- Klimek, L.; Hagemann, J.; Huppertz, T.; Bärhold, F.; Albrecht, T.; Klimek, F.; Casper, I.; Cuevas, M.; Bergmann, C. COVID-19 and chronic rhinosinusitis: Management and comorbidity—What have we learned? Expert Rev. Clin. Immunol. 2023, 19, 1399–1406. [Google Scholar] [CrossRef]
- Ferrucci, S.; Romagnolo, M.; Angileri, L.; Berti, E.; Tavecchio, S. Safety of dupilumab in severe atopic dermatitis and infection of Covid-19: Two case reports. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e303–e304. Available online: https://pubmed.ncbi.nlm.nih.gov/32330323/ (accessed on 22 September 2023). [CrossRef] [PubMed]
- Chiricozzi, A.; Talamonti, M.; De Simone, C.; Galluzzo, M.; Gori, N.; Fabbrocini, G.; Marzano, A.V.; Girolomoni, G.; Offidani, A.; Rossi, M.T.; et al. Management of patients with atopic dermatitis undergoing systemic therapy during COVID-19 pandemic in Italy: Data from the DA-COVID-19 registry. Allergy 2021, 76, 1813–1824. Available online: https://pubmed.ncbi.nlm.nih.gov/34152613/ (accessed on 22 September 2023). [CrossRef] [PubMed]
- Passante, M.; Napolitano, M.; Dastoli, S.; Bennardo, L.; Fabbrocini, G.; Nisticò, S.P.; Patruno, C. Safety of omalizumab treatment in patient with chronic spontaneous urticaria and COVID-19. Dermatol Ther. 2021, 34, e15111. [Google Scholar] [CrossRef]
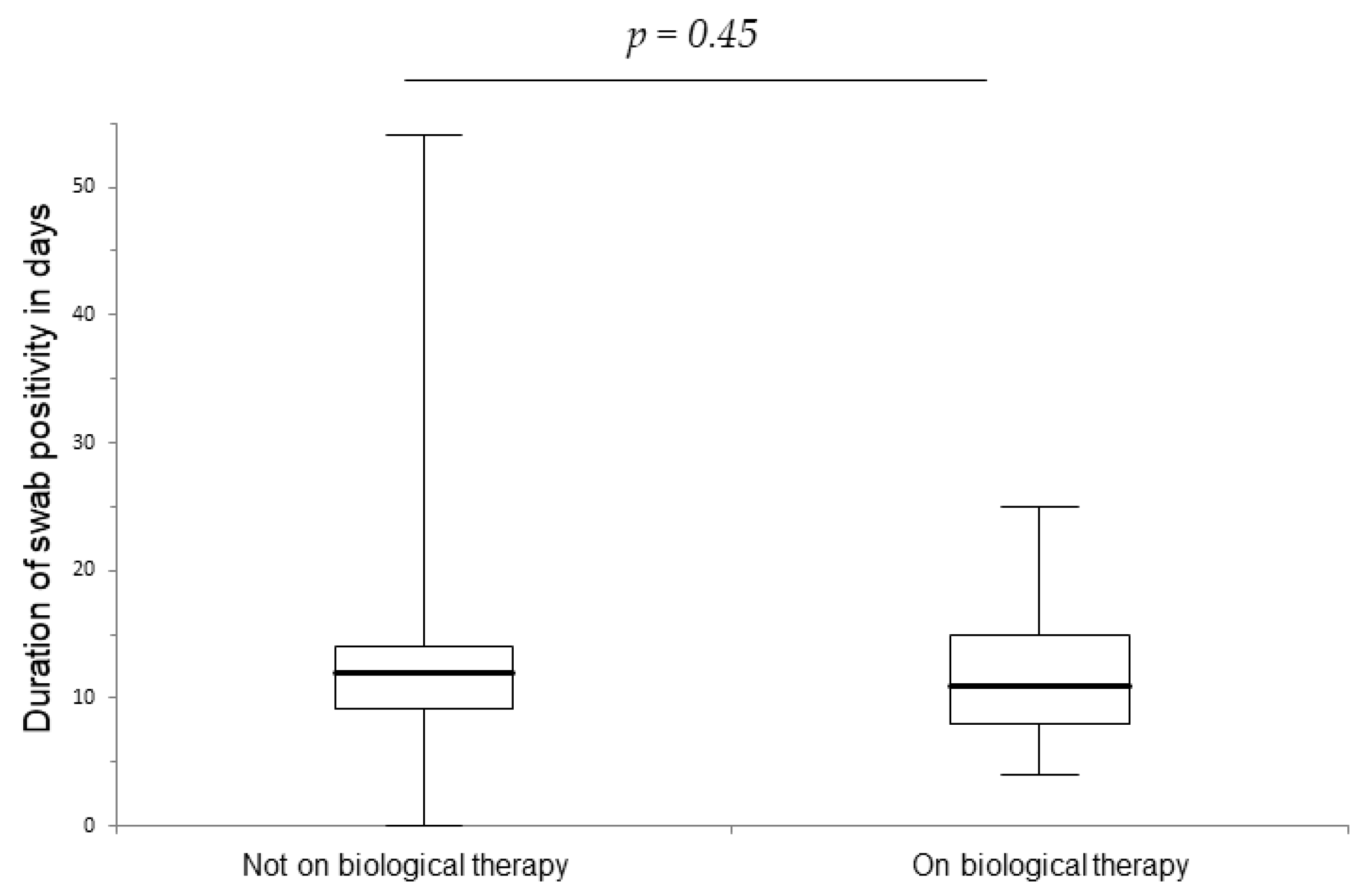
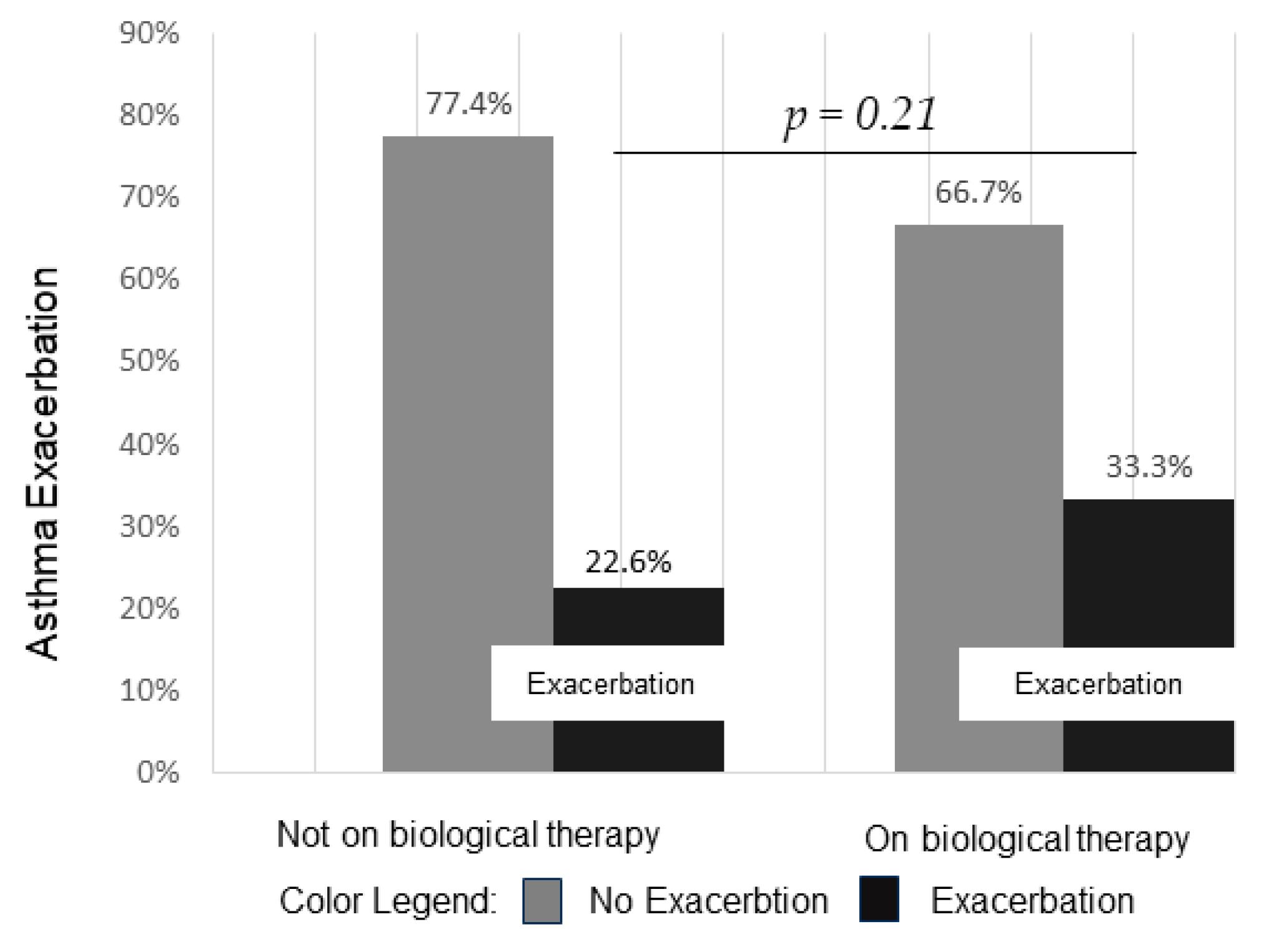
| Characteristic | On Biological n = 62 (47%) | Not on Biological n = 70 (53%) | p-Value |
|---|---|---|---|
| Age (years) Median ± IQR * | 56 ± 22 | 48 ± 26 | 0.028 |
| Female (%) | 47 | 53 | 0.93 |
| Never smoker (%) | 40 | 44 | 0.15 |
| BMI Median ± IQR † | 24 ± 5.93 | 24.10 ± 4.49 | 0.66 |
| OCS ‡ mg/day Median ± IQR * | 7.5 ± 6.46 | 5 ± 16.25 | 0.92 |
| Current on OCS ‡ (%) | 17.7 | 18.5 | / |
| Cohort of Patients | On Biological n = 62 (47%) | Not on Biological n = 70 (53%) |
|---|---|---|
| Asthma (%) | 87 | 93 |
| Chronic Rinosinusites with Nasal Polyposis (%) | 52 | 17 |
| Chronic Spontaneous Urticaria (%) | 10 | 10 |
| Atopic Dermatitis (%) | 6 | 4 |
| Omalizumab (%) | 30.6 | / |
| Mepolizumab (%) | 25.8 | / |
| Benralizumab (%) | 19.4 | / |
| Dupilumab (%) | 24.2 | / |
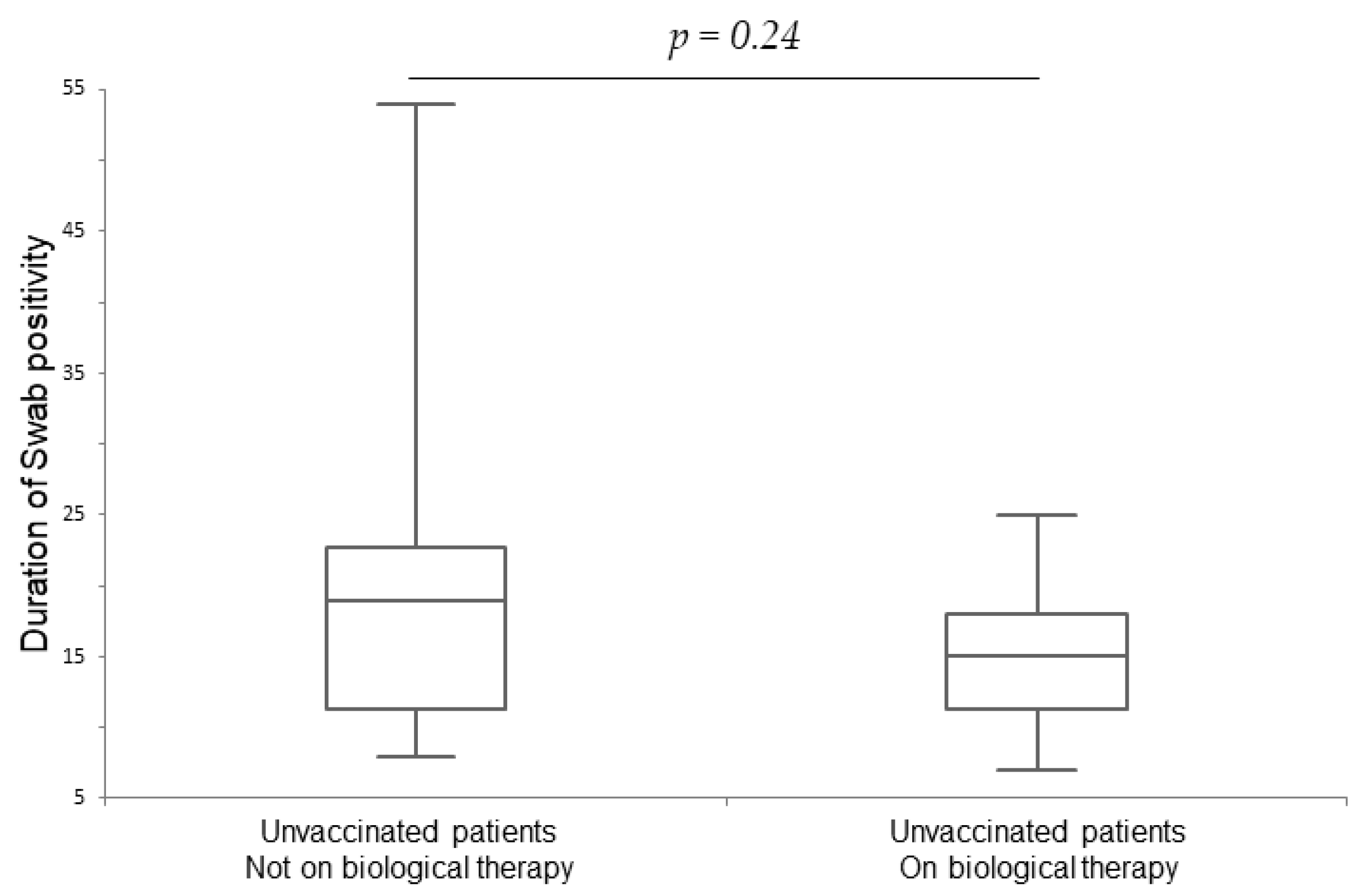
| Vaccinated Patients on Biologicals (n = 47) | Vaccinated Patients Not on Biologicals (n = 49) | p-Value | Unvaccinated Patients on Biologicals (n = 15) | Unvaccinated Patients Not on Biologicals (n = 21) | p-Value | |
|---|---|---|---|---|---|---|
| Duration of swab positivity in days | 10 ± 6.8 * | 10 ± 7 * | 0.94 | 15 ± 5.8 * | 19 ± 12.3 * | 0.24 |
| Duration of symptoms in days | 5 ± 6.5 * | 5 ± 6.3 * | 0.77 | 10 ± 5.8 * | 10 ± 11 * | 0.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sambugaro, G.; Brambilla, E.; Costanzo, G.; Bonato, V.; Ledda, A.G.; Del Giacco, S.; Scarpa, R.; Rattazzi, M.; Favero, E.; Cinetto, F.; et al. COVID-19 Clinical Features and Outcome in Italian Patients Treated with Biological Drugs Targeting Type 2 Inflammation. Life 2024, 14, 378. https://doi.org/10.3390/life14030378
Sambugaro G, Brambilla E, Costanzo G, Bonato V, Ledda AG, Del Giacco S, Scarpa R, Rattazzi M, Favero E, Cinetto F, et al. COVID-19 Clinical Features and Outcome in Italian Patients Treated with Biological Drugs Targeting Type 2 Inflammation. Life. 2024; 14(3):378. https://doi.org/10.3390/life14030378
Chicago/Turabian StyleSambugaro, Giada, Elena Brambilla, Giulia Costanzo, Vera Bonato, Andrea Giovanni Ledda, Stefano Del Giacco, Riccardo Scarpa, Marcello Rattazzi, Elisabetta Favero, Francesco Cinetto, and et al. 2024. "COVID-19 Clinical Features and Outcome in Italian Patients Treated with Biological Drugs Targeting Type 2 Inflammation" Life 14, no. 3: 378. https://doi.org/10.3390/life14030378
APA StyleSambugaro, G., Brambilla, E., Costanzo, G., Bonato, V., Ledda, A. G., Del Giacco, S., Scarpa, R., Rattazzi, M., Favero, E., Cinetto, F., & Firinu, D. (2024). COVID-19 Clinical Features and Outcome in Italian Patients Treated with Biological Drugs Targeting Type 2 Inflammation. Life, 14(3), 378. https://doi.org/10.3390/life14030378








