Abstract
Background: Vasculitis diseases include Kawasaki disease (KD), Kawasaki disease shock syndrome (KDSS), Multisystem Inflammatory Syndrome (MIS), Henoch–Schönlein purpura (HS), or IgA vasculitis, and additional vasculitis diseases. These diseases are often preceded by infections or immunizations. Disease incidence rates are higher in children than in adults. These diseases have been extensively studied, but understanding of the disease etiology remains to be established. Objective: Many studies have failed to demonstrate an association between vasculitis diseases and vaccination; this study examines possible associations. Methods: Herein, the Vaccine Adverse Event Reporting System (VAERS) database is retrospectively examined for associations between vasculitis diseases and immunizations. Results: For some vaccines, the number of rare cases of KD, MIS, and HS are higher than the background rates. These rare cases are predicted to occur in individuals with (1) genetic risk factors with (2) antibody titer levels above the primary immune response level. Herein, the model of humoral immune response antibodies bound to antigens (pathogen or vaccine) creating immune complexes is proposed. These immune complexes are proposed to bind Fc receptors on immune cells and platelets, resulting in cell activation and the release of inflammatory molecules including histamine and serotonin. Immune complexes and inflammatory molecules including serotonin and histamine likely trigger vasculitis. Elevated serotonin and possibly histamine drive initial vasoconstrictions, disrupting blood flow. Increased blood flow pressure from cardiac capillary vasoconstrictions is predicted to trigger coronary artery aneurysms (CAA) or lesions (CAL) in some patients. For KDSS and MIS patients, these cardiac capillary vasoconstrictions are predicted to result in ischemia followed by ventricular dysfunction. Ongoing ischemia can result in long-term cardiac damage. Cases associated with pathogens are likely to have persistent infections triggering disease onset. Conclusion: The proposed model of immune complexes driving disease initial disease etiology by Fc receptor activation of immune cells and platelets, resulting in elevated histamine and serotonin levels, is testable and is consistent with disease symptoms and current treatments.
1. Introduction
Kawasaki disease (KD), or mucocutaneous lymph node syndrome, and Multisystem Inflammatory Syndromes (MIS) are associated with the inflammation of medium-sized blood vessels (vasculitis). Henoch–Schönlein purpura (HS), or IgA vasculitis, is an IgA immune complex systemic vasculitis (inflammation of blood vessels) disease with palpable purpura (small, raised areas of bleeding underneath the skin). Vasculitis diseases have been linked to antibodies, circulating immune complexes of antibodies bound to antigens, infections, some vaccines, and genetic variants.
KD is a rare vasculitis disease of unknown etiology. KD results in a fever that typically lasts for more than five days and is unresponsive to paracetamol (acetaminophen) or ibuprofen. Additional common patient symptoms include extreme irritability, large lymph nodes in the neck, red eyes, very red lips, characteristic “strawberry tongue”, coronary artery aneurysms (~25%) or lesions, myocarditis, swollen lips with vertical cracking and bleeding, painful joints, and rashes in the genital area, lips, palms, and soles of the feet. The skin from the hands and feet may peel after recovery. KD frequently affects younger children (KD-C), aged 0 to 5, but can also appear in adults (KD-A). Fever associated with KD does not respond to normal fever treatments but does respond to high-dose aspirin. KDSS occurs with sustained systolic hypotension (decrease in blood pressure). Current treatments include high-dose aspirin, intravenous immunoglobulin, and sometimes the addition of a corticosteroid. Note that aspirin is normally counter-indicated for children due to the possible risk of Reye’s syndrome [1]. An increased proportion of KD patients with coronary artery aneurysms also had plasma fibrinogen (FG) alpha genotype Thr312Ala [2]. Genetic studies of KD susceptibility variants support the role of alterations in immune cell activation and the involvement of immune complex activation of immune cells and platelets [3,4]. The risk level for developing KD is likely higher for individuals with these genetic risk variants. KD is not thought to be contagious, though it may be caused by an infectious agent.
Multiple clinical studies have attempted to identify a causative infectious agent for KD. Clusters of KD cases frequently occur with a 4-to-6-week onset delay following pathogen outbreaks. Associations between KD and multiple pathogens have been reported as follows:
- Adenovirus [5,6];
- Human bocavirus [7];
- Coronavirus [6];
- Human coronavirus 229E [8];
- Human coronavirus (HCoV-NH) NL63 [9];
- Cytomegalovirus [10];
- Dengue [11,12];
- Enterovirus [6,13];
- Epstein–Barr virus [14];
- Human herpesvirus 6 [15];
- Human lymphotropic virus [16];
- Human rhinovirus [6];
- Influenza [17];
- Measles [18];
- Parvovirus B19 [19,20];
- Parainfluenza virus type 2 [21];
- Respiratory syncytial virus (RSV) [22];
- Rotavirus [23];
- Varicella zoster (chicken pox) [24,25];
- Torque teno virus [26];
- Staphylococcus aureus [27], and;
- Streptococcus [14,28].
KD has also been reported as a rare adverse event associated with vaccinations and vaccine combinations as follows:
- Bacillus Calmette-Gue’rin (BCG) vaccination [29].
- COVID-19 vaccine Vaxzevria (nonreplicating viral vector) [30].
- Diphtheria, tetanus, and acellular pertussis (DTaP or DTAP) [31].
- Hepatitis B [32].
- Influenza [31,33,34,35].
- Lanzhou lamb rotavirus vaccine (LLR) and freeze-dried live attenuated hepatitis A vaccine (HAV or HEPA) [36].
- Measles, mumps, and rubella (MMR) [31].
- Polysaccharide pneumococcal vaccine (Pneumo 23) [34].
- Pneumococcal conjugate vaccine (PCV or PNC) [31].
- Rotavirus [31,37].
- SARS-CoV-2 [38].
- Yellow fever vaccine [39].
- DTaP/Poliovirus vaccine inactivated (IPV)/Hepatitis B virus vaccine (HepB or HEP), [40].
- Prevnar 13 (PNC13 or PCV13).
- Rotarix [40].
- DTaP/IPV/Haemophilus B conjugate vaccine (Hib or HIBV)/PCV [41].
- DTaP/IPV/Hib; meningitis C; PCV [41].
- DTaP/IPV; MMR [41].
- DTaP-IPV/Hib and PCV13 [42].
- Hib; meningitis C; PCV; MMR [41].
- Measles/rubella (MR), varicella, pneumococcal [43].
- One report of an adult with both KD and (MIS-A) following second dose of the Pfizer SARS-CoV-2 vaccine [38].
Associations have also been made between KD and air pollutants such as carbon monoxide (CO), nitric oxide (NO), nitric dioxide (NO2), and nitrogen oxide (NOx) during pregnancy and childhood exposure [44]. In healthy blood vessels, the endothelium constitutively expresses nitric oxide synthase (NOSIII), which produce the vasoactive hormone nitric oxide (NO); in diseased blood vessels, vascular smooth muscle cells express inducible NOSII, resulting in the release of large amounts of NO [45]. For acute phase KD patients, neutrophils were identified as the major source of NO which decreases after intravenous immunoglobulin (IVIG) treatment [46]. NO relaxes blood vessels and inhibits platelet activation [47]. During the acute phase of KD, plasma hydrogen sulfide (H2S) levels significantly decreased and NO levels significantly increased (p < 0.01) [48]. Elevated levels of NO are associated with the development of coronary artery abnormalities in KD [49]. Elevated inducible NO and decreased H2S levels can predict the risk of coronary artery ectasia in KD patients [50]. H2S is involved in the regulation of vascular tone, blood pressure, and protection of the myocardium from ischemia-reperfusion injury (review [51]). A study of endothelial cells (ECs) found normal microvascular function in controls and after acute KD with EC injury confined to the endothelium of medium-sized arteries [52]. In contrast, plasma nitrate levels may not be associated with a higher risk of coronary artery lesions (CAL) [53]. A study of genetic polymorphisms of endothelial constitutive NOS (ecNOS) and inducible NOS (iNOS) genes did not find significant associations between CAL and KD [54]. The role of NO in KD pathogenesis was reviewed by Tsuge et al. [55]. Increased platelet activation markers and decreased levels of asymmetric dimethylarginine (ADMA) were found in KD patients compared to normal controls [56]. It is possible that elevated NO levels could be a marker for CAL. The etiology for KD remains unknown. While possible associations with multiple pathogens and vaccines have been reported in the literature (summarized above), no associations have been established and accepted by the medical community, which continues in its search to isolate the causative agent.
With overlapping symptoms with KD, Multisystem Inflammatory Syndrome (MIS) can affect different populations, including children (MIS-C), adults (MIS-A), neonates (MIS-N), and vaccine recipients (MIS-V). KD and KDSS, have many similar symptoms, such as inflammation of the heart, lungs, kidneys, brain, skin, eyes, and/or gastrointestinal tract. Like KD, MIS-C, MIS-A, and MIS-N are rare diseases. MIS-C, -A, and -N are associated with or follow SARS-CoV-2 infections. MIS-V is a rare potential occurrence after SARS-CoV-2 immunization in some vaccinees. With significant overlap with KD and KDSS symptoms, MIS-X (-C, -A, or -V) are characterized by persistent fever and possible acute abdominal pain with diarrhea or vomiting, muscle pain and general tiredness, inflamed blood vessels, low blood pressure, red eyes, rashes, enlarged lymph nodes, swollen hands and feet, “strawberry tongue”, coronary artery dilation to aneurysm, and various mental disturbances are possible. MIS-N patients often present with respiratory and cardiac symptoms, with only 18–20% presenting with fever [43,44]. Like KD, clusters of new cases can appear two to six weeks after local surges in SARS-CoV-2 infections.
Analogous to KD and MIS, Henoch–Schönlein purpura (HS), or IgA vasculitis, are associated with the formation of large circulating immune complexes with deposition in small blood vessels. Additional HS symptoms can include joint pain, abdominal pain, hematuria (blood in urine), and proteinuria (protein in urine); rare kidney involvement can proceed to chronic kidney disease. HS primarily occurs in children, with a higher frequency in males. Cases of HS have been reported after Epstein–Barr virus [57,58,59], Helicobacter pylori [60], parainfluenza [61,62], parvovirus B19 [63,64,65], Staphylococcus [62], Streptococcus [62], and Varicella zoster [66] infections, cytomegalovirus reactivation [67], and BCG therapy [68]. Case reports of HS have been reported following hepatitis A [69], influenza [70,71,72,73], MMR [74], meningitis C [75], rabies [76], and SARS-CoV-2 mRNA vaccination [77,78,79,80,81,82,83]. In contrast to these case reports, a systematic literature review reported no causal association between vaccination and KD or HS [84].
During both infection and vaccination, foreign proteins are introduced into the body. Antibodies bind these antigens, forming immune complexes. Immune complexes bind and activate immune cells and platelets with Fc receptors. Activated platelets release serotonin, histamine, and additional inflammatory molecules. Activated mast cells and other granulocytes also release histamine and other inflammatory molecules. Elevated serotonin (5-hydroxytryptamine) is associated with coronary artery disease and some cardiac diseases [85]; serotonin has several effects on the vascular wall, proliferation of smooth muscle cells, promotes thrombogenesis and mitogenesis [85]. Serotonin released from activated platelets is associated with vasoconstriction [85,86] and also vasodilation in the absence of endothelium damage [86]. Serotonin-specific effects can be blocked by the serotonin receptor antagonist ketanserin [85]. Fluoxetine, a serotonin reuptake inhibitor (SSRI), is associated with urticaria and angioedema in one case report [87]. Cardiac adverse events of the β-imanazolylethylamine derivative of histamine include altered blood-pressure, constriction of coronary arterioles, constriction of pulmonary arterioles, altered heart rate, and heart failure varying according to dose and animal species [88]. Histamine is also involved in cardiac arrhythmias [89] and cardiac adverse events associated with histamine intolerance (HIT) [90]. Elevated histamine and/or serotonin are likely associated with cardiac vasoconstrictions associated with KD, KDSS, and MIS.
This study retrospectively analyzes reports of adverse events for KD, MIS, HS, and vasculitis in the Vaccine Adverse Event Reporting System (VAERS). The etiology of these diseases is considered in the context of associations with multiple infectious pathogens and also multiple possible vaccine associations. The hypothesis that KD, KDSS, MIS-C, MIS-A, MIS-N, and MIS-V are associated with adverse reactions to immune complexes (antibodies bound to pathogen or vaccine proteins) with associated Fc receptor activation of immune cells and platelets, releasing serotonin, histamine, and other inflammatory molecules, is advanced. Likewise, immune complexes likely drive the other vasculitis diseases.
2. Materials and Methods
This is a retrospective analysis of the VAERS database from 1 January 1990 to 27 October 2023. The names of VAERS adverse events searched for were Henoch-Schonlein purpura, Kawasaki’s disease, Multisystem Inflammatory Syndrome, Multisystem Inflammatory Syndrome in adults, Multisystem Inflammatory Syndrome in children, and vasculitis. No patients with these adverse events were excluded. The Python program vaers_reports.py was developed for retrospective analysis of the VAERS data files VAERSDATA, VAERSSYMPTOMS, and VAERSVAX for the years 1990 to 2023 and NonDomestic.
3. Results
Adverse events occur at background population frequencies with the addition of any vaccine-associated adverse events. For adverse event, X, the reported numbers are diminished by reporting bias, , as time (day) increases. Background population events (b) for individuals of the same age and a specific adverse event (X) are modeled as a constant, noted as . The reporting bias (r) for a specific adverse event (X) is modeled as a different constant dependent upon the number of days since immunization, noted as . For a population, P, and n days of data collection, the expected number of background adverse events can be modeled by the following Equation (1), where and are a series of constants for days and age, respectively:
The addition of any vaccine-specific, V, associated adverse events can be modeled with an additional age series of constants, , in Equation (2):
If the association of KD with vaccines only reflects the background population frequency rate, then the normalized frequencies for different vaccines should be similar with expected random variations. The normalized frequency of KD cases per 100,000 VAERS reports with symptoms is summarized in Table 1 for vaccines with five or more KD adverse event reports. The normalized frequencies per 100,000 VAERS reports varied widely from lower frequencies associated with the vaccines against Diphtheria and tetanus toxoids and the pertussis vaccine (DTP) (51 per 100,000) to the Meningococcal group b vaccine (MENB) (3262 per 100,000), with only seven VAERS vaccine codes having frequencies greater than 1000 per 100,000 (Table 1). The large differences in observed normalized frequencies in Table 1 are inconsistent with expected background variations, as modeled by Equation (1). Normalized frequencies of 93 and above were evaluated with a chi-squared test (p < 0.00001) compared to background KD frequency of (9 to) 20 per 100,000 per year [91]; the results are still significant after Bonferroni multiple testing correction (p < 0.00167) for 29 of 30 statistical tests (normalized frequencies of 72 and above). Note that the normalized frequencies in Table 1 represent underestimates after considerations for partial year for VAERS reports (120 days), reporting bias, etc.

Table 1.
Kawasaki disease adverse event reports with normalized frequency per 100,000 patients with reported symptoms in VAERS; data from 1990 to 27 October 2023.
The reported days of onset for KD cases post vaccination are summarized in Table 2 for nine vaccines. Day 0 and 1 had the highest numbers of KD reports (Table 2). The data in Table 2 likely reflect underreporting due to reporting bias as modeled in Equation (2).

Table 2.
Kawasaki disease onset post vaccination from VAERS; data from 1990 to 27 October 2023.
The clinical symptoms for multiple diseases, including KD, KDSS, MIS, mast cell activation syndrome (MCAS), and type III hypersensitivity share overlaps as summarized in Table 3. Many of the symptoms could be possibly associated with elevated histamine levels and also serotonin; activated platelets are likely source for both, and granulocytes including mast cells are a possible additional source of histamine.

Table 3.
Symptom overlaps between Kawasaki disease, KDSS, MIS, and mast cell activation syndrome (MCAS).
The age of onset for KD, MIS-C, and MIS are summarized in Table 4. The normalized frequencies of KD, MIS-C, MIS, HS, and vasculitis are summarized by age when receiving COVID-19 vaccines in Table 4. For KD, infants less than one year of age had the highest number of reports (689 reports), followed by one-year-old infants (169 reports) (Table 4). Likewise, vasculitis had the highest number of reports for infants less than one year of age (169 reports) and one-year-old infants (116 reports) (Table 4). Vasculitis reports in infants are driven by the following vaccines: 6VAX-F, DTAP, HEP, HEPA, HIBV, IPV, MMR, PNC, PNC13, RV1 (Rotavirus vaccine, live, oral), RV5 (Rotavirus vaccine, live, oral, pentavalent), and VARCEL. The Henoch–Schönlein purpura adverse events peak for infant vaccinations (104 and 80 for infants aged <1 and <2, respectively) and also preschool vaccinations at age 4 (81 reports) and 5 (96 reports); for HS, increases for children aged 4 and 5 years old are driven by MMR, DTAP, MMRV (measles, mumps, rubella and varicella vaccine live), DTAPIPV (Diphtheria and tetanus toxoids and acellular pertussis vaccine and inactivated poliovirus vaccine), and VARCEL reports in VAERS (Table 4).

Table 4.
Age of onset for Kawasaki disease, MIS-C, Henoch–Schönlein purpura, and vasculitis adverse events in VAERS; data from 1990 to 27 October 2023.
The age of onset and normalized frequency per 100,000 vaccinations with symptoms associated with adverse events for COVID-19 vaccination, namely KD, MIS, MIS-A, and MIS-C, are summarized in Table 5; the frequencies from age 1 to 13 are higher (mean 391.5, SD 204.9) with observed lower frequencies for ages 14 to 17 (mean 142.5, SD 19.1) followed by much lower rates for adults aged 18 to 30 (mean 19.9, SD 15.1).

Table 5.
COVID-19-associated adverse events for KD, MIS, MIS-A, or MIS-C normalized frequency per 100,000 cases with symptoms by age in VAERS; data from 1990 to 27 October 2023.
4. Discussion
Authors: multiple hypotheses can be proposed for adverse events for Kawasaki disease:
Hypothesis 1.
There is no association of Kawasaki disease with vaccination. This is the current general medical consensus.
Hypothesis 2.
Kawasaki disease occurs in a subset of individuals with immune complexes from persistent infections or immunization activating immune cells and platelets with the risk level increased by relevant genetic variants. The number of adverse events observed for each vaccine may be correlated with vaccine reactogenicity level or with other vaccine-specific attributes including possible manufacturing contaminations.
The large disparities between the normalized frequency of Kawasaki disease per 100,000 reports with any symptom across multiple vaccines in VAERS, summarized in Table 1, is inconsistent with these events representing only background adverse events, as modeled by Equation (1); hence, Hypothesis 1 is rejected. The data shown in Table 1 are consistent with modeling by Equation (2) and Hypothesis 2; other possible hypotheses may be consistent with the data observed in Table 1.
4.1. Kawasaki Disease and Multisystem Inflammatory Syndromes Etiology Model
It has been previously proposed that KD is associated with immune complexes [4,92]. KD has been characterized as a triphasic illness with the first phase (feverish phase) characterized by high fever, mucocutaneous manifestations, lymphadenopathy, and normal platelet count. The second phase (subacute phase) includes a dramatic rise in platelet count, may include immune complex vasculitis, and also involves desquamation of the hands and feet and sometimes coronary artery aneurysms (CAA). The third phase is the convalescent phase, where the platelet count decreases and immune complexes become undetectable [92]. Platelet counts are typically elevated by the second week of illness [93]. Immune-complex-induced platelet aggregation correlates with IgG and IgA titers [92]. In a murine KD model, platelets promoted vascular inflammation via the formation of monocyte–platelet aggregates (MPAs) and exacerbated the development of cardiovascular lesions [94]. In KD, platelets and activated monocytes can result in Kawasaki disease complicated with macrophage activation syndrome (KD-MAS) [95]. Immune complex activation of platelets is a key step in the proposed KD and MIS disease etiology.
The disease symptoms for KD and MIS combined with disease characteristics, associations with multiple pathogens, associations with multiple vaccines, and unusual treatments point towards a candidate etiology of immune complexes activating immune cells and platelets via Fc receptor binding; this results in the release of inflammatory molecules including histamine and serotonin (Figure 1). This model proposes that antibody levels that are higher than primary immune response levels binding to either infectious pathogen or vaccine proteins, creating immune complexes, are circulating and activating immune responses by binding to Fc receptors on immune cells and platelets, including granulocytes and mast cells. Vasculitis, rash, and fever are associated with Type III hypersensitivity-like responses to immune complexes. The activation of platelets and likely granulocytes, including mast cells, induces the release of high levels of histamine, serotonin, and other inflammatory molecules associated with mast cell activation syndrome (MCAS) symptoms, including fever, rash, diarrhea, nausea, vomiting, red eyes, headaches, migraines, palpitation, arrhythmia, etc. Elevated levels of serotonin are associated with vasoconstrictions [85]. Contractions of cardiac capillary pericyte cells in response to high levels of histamine have been previously proposed to cause (pressure-induced) coronary artery aneurysms, myocarditis, and pericarditis [96]. Pericytes, attached to the surface of capillaries, play an important role in local blood flow [97]. Pericytes are also involved in innate immune responses [98]. Pericytes also mediate coronary no-reflow after myocardial ischemia [99]. Pericyte loss has been correlated with microaneurysm size in diabetic retinopathy [100]. The SARS-CoV-2 Spike protein disrupts human cardiac pericytes function through CD147 receptor-mediated signaling [101] with possible relevance in MIS. Mast cells and eosinophils are known to create feedback loops. The eosinophil-to-lymphocyte ratio is a useful diagnostic for KD [102]. Immune responses to infectious pathogens are likely associated with overlapping symptoms.
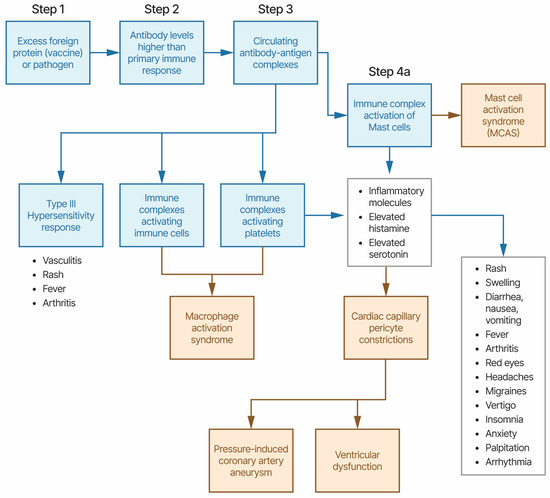
Figure 1.
Etiology model of Kawasaki disease and MIS-X. The symptoms present in boxes shaded blue are likely in most patients; those present in boxes shaded yellow are likely in a subset of patients.
Rare KD cases frequently appear roughly 4 to 6 weeks following pathogen outbreaks or SARS-CoV-2 infection for MIS-C, MIS-A, and MIS-N. This etiology model proposes that primary immune response antibody titer levels are insufficient to trigger KD and MIS due to low affinity for IgG1 antibodies by Fc receptors on immune cells and platelets. Ongoing infections will continue antibody responses above the primary immune response levels and may be a component in the delayed onset of KD and MIS following pathogen outbreaks. KD and MIS are not considered contagious. For these cases associated with pathogen outbreaks, this etiology model proposes that the patient has an ongoing pathogen infection, perhaps gastrointestinal (based upon MIS-C and MIS-A cases [103,104,105,106]). Prior exposure to the antibody antigen via previous infection or vaccination is an alternative to ongoing infection with secondary antibody response antibody titer levels that are significantly higher than primary antibody response levels. Rare KD and MIS cases appear in neonates (KD-N) [107,108,109] and MIS-N [110,111,112]. This etiology model proposes that these neonates likely have higher antibody titer levels due to a combination of neonate antibody responses combined with maternally acquired antibodies transferred during pregnancy [110].
4.2. KD and MIS Treatments and Etiology Model
The current KD and MIS treatments are high-dose aspirin, IVIG, and, sometimes, a corticosteroid. IVIG treatment within 7 days of illness onset lowers the risk of the patient developing coronary artery lesions and cardiac sequelae [113]. Treatment with aspirin also lowers the risk of developing coronary artery lesions [114]. KD patients presenting with fever did not respond to normal fever treatments. The proposed etiology model provides an explanation for mechanisms of actions for current treatments. The current treatments all target immune complex binding to antibody-heavy chains via Fc receptors (IVIG), mast cell stabilization (high-dose aspirin), or immune modulation (corticosteroid) (Figure 2). We propose that the efficacy of IVIG treatment is likely due to competitive binding to Fc receptors, reducing the binding of immune complexes to immune cells and platelets. Aspirin is also an inhibitor of the cyclooxygenase-1 (COX-1) and -2 (COX-2) pathways, with both pathways involved in inflammation immune responses. Plasma exchange, when used to treat KD patients [108], also reduces circulating immune complexes. Additional candidate treatments targeting mast cells, antibody binding to Fc receptors, and serotonin receptors may be worth evaluating in approved clinical studies.
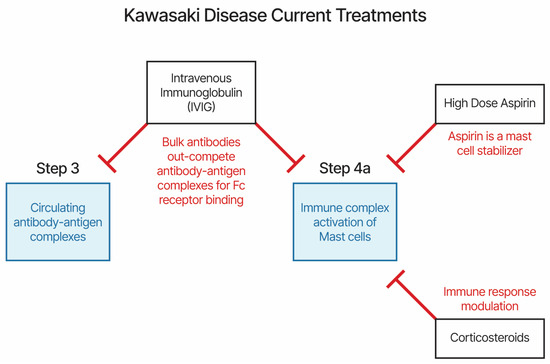
Figure 2.
Current treatments for Kawasaki disease and MIS-X.
4.3. Kawasaki Disease and Vaccinations (KD-V)
Calculating normalized frequencies of KD cases per 100,000 vaccinations with symptoms enables comparisons of frequencies across multiple vaccines. Some vaccines have a much higher normalized frequency (e.g., MENB [Meningococcal group b vaccine, rDNA absorbed], TYP [Typhoid vaccine], BCG, DTPIPV [Diphtheria and tetanus toxoids, pediatric and inactivated poliovirus vaccine], 6VAX-F [Diphtheria and tetanus toxoids and acellular pertussis adsorbed and inactivated poliovirus and hepatitis B and haemophilus B conjugate vaccine], and MEN [Meningococcal polysaccharide vaccine]) compared to vaccines with lower normalized frequencies (e.g., DTP, VARCEL [Varivax-varicella virus live], and FLU3 [Influenza virus vaccine, trivalent]), as shown in Table 1; these vaccines all include components from pathogenic bacteria. Prior exposure to pathogens, previous vaccine doses received, vaccine reactogenicity level, unknown characteristics of vaccines, excipients, or even possible contaminants might impact the observed differences. It is clear from Table 1, showing normalized frequencies, that higher normalized frequencies observed do not simply reflect background population occurrences. Likewise, genetic risk variants alone cannot account for the disparities observed between different vaccine normalized frequencies. The data reported to VAERS are negatively impacted by reporting bias with expected decreased probability of reporting as the number of days post immunization increases. Table 2 summarizes the day of KD onset post vaccination. Days 0 and 1 reflect the highest days for KD reports; this may simply reflect reporting bias. Alternatively, this may reflect activation of memory immune cells from previous antigen exposures.
4.4. Nitric Oxide (NO) and Coronary Artery Lesions (CAL)
This model predicts that coronary artery dilations and aneurysms are pressure-induced by cardiac capillary vasoconstrictions; hence, the risk level for CAL may be highest in KD-N [107,108,109] and MIS-N [111,112] patients. This simple model shows that artery resilience to increased pressure improves with age, with neonates having the lowest resilience to increased pressure levels. The risk level for CAL may decrease with increased age; ischemia from proposed cardiac capillary vasoconstrictions likely results in ventricular dysfunction observed in KDSS, MIS-C, and MIS-A patients. Cardiac capillary vasoconstrictions and resulting ischemia can also result in hypotension, as seen in KDSS patients. For patients developing CAL, this model predicts that the first step is pressure-induced dilation of the coronary artery, triggering cellular injury signaling that attracts the infiltration of immune response cells. At the sites of pressure-induced injuries, the induction of NO has been observed [46,49]. These same injury response signals were not observed in KD patient control internal mammary arteries [115]. Dilation and aneurysm injuries are specific to the coronary artery due to the closest associations to the predicted cardiac capillary vasoconstrictions elevating the risk of experiencing pressure-induced injuries [96]. Hence, elevated NO and, also, decreased H2S levels are likely indicators of CAL injuries.
4.5. Multisystem Inflammatory Syndromes (MIS-C, MIS-A, MIS-N, and MIS-V)
With parallels to KD and KDSS, Multisystem Inflammatory Syndrome appeared in children (MIS-C), adults (MIS-A), and neonates (MIS-N) associated with SARS-CoV-2 infections [105,106,111,112,116] and COVID-19 vaccines (MIS-V) [117,118]. The age of onset of MIS-C in children is older than for KD [104,105,106]. KD and MIS are currently considered to be distinct due to differences in patient age demographics and additional MIS-X symptoms (e.g., ventricle dysfunction, gastrointestinal, and neurological) [119]. Complement activation is seen in some MIS-C children with rapid improvement after IVIG treatment; however, this was not associated with detectable immune complexes [120]. Roughly half of MIS-C patients suddenly developed cardiogenic shock requiring intensive care unit (ICU) admission in 50% [116] to 80% [105,106] of patients. For MIS-C, sustained levels of inflammatory macrophage-activating, Fc receptor-binding antibodies were selectively maintained in severe disease [121]. In a review study, the majority of MIS-C patients are reported as SARS-CoV-2 IgG-positive [122]. In contrast, SARS-CoV-2-specific IgA antibody responses linked to neutrophil activation in severe MIS-A disease [121]. The age of KD, MIS-C, and combined ages are compared in Table 4. For KD-V and vasculitis, the majority of the cases are associated with infant vaccinations (Table 4). For vasculitis, reports associated with the vaccines COVID-19, HEP, HPV2, and HPV4 predominate for teenagers. In contrast, cases of MIS-V (recorded in VAERS as MIS-C) are primarily children aged 5 to 17 years, with fewer COVID-19 vaccinations administered to infants.
Here, we propose that KD, KDSS, and MIS-X all share the same etiology model driven by immune complexes activating immune cells and platelets. The main difference between MIS-C and KDSS is where SARS-CoV-2 was identified as the infectious agent [123]. KD is associated with CAL and long-term cardiovascular sequelae, while MIS-C presents as more intense Inflammatory Syndrome, myocardial dysfunction, and cardiogenic shock [124]. In a retrospective review of 395 MIS-C and 69 KD patients, MIS-C patients presented with gastrointestinal (80%), cardiovascular (74%), and respiratory (52%) symptoms, while KD patients presented with dermatological (99% vs. 68%) and mucosal changes (94% vs. 64%), and cervical lymph node swelling (51% vs. 34%) [125]. Additional MIS-X symptoms may derive from ongoing SARS-CoV-2 infections and differences in the age demographics for current cases. Deep immune profiling identified activated macrophages, neutrophils, B-plasmablasts, and CD8+ T cells in MIS-C patients with activation largely independent of anti-SARS-CoV-2 humoral immune response [120]; rapid improvement of MIS-C was driven by decreased activation of complement following IVIG treatment [120]. Table 5 suggests that the KD and MIS-X risks levels may start decreasing at age 14 with low risk levels for adults.
4.6. Henoch–Schönlein Purpura and Other Vasculitis Diseases
IgA immune complexes cause Henoch–Schönlein purpura, and immune complexes cause other vasculitis diseases. For diseases following infections, it is proposed that persistent infections provide antigens for the immune complexes. For immunization-associated onset of these vasculitis diseases, the vaccine protein provides the antigen. Many adverse events as a result of HS-C, KD, and vasculitis appear to be associated with childhood immunizations (Table 4).
4.7. Study Limitations
The VAERS database collects only a small subset of adverse events experienced by vaccinees. Any reporting biases or exclusion of adverse events would perturb the accuracy of VAERS representing the immunization population (note: reporting bias was anticipated and modeled in this study).
4.8. Study Recommendations
This study proposes that immune complexes binding to Fc receptors drive the etiology of both KD and MIS. Many of the disease symptoms are consistent with predicted elevated levels of histamine and/or serotonin. Evaluations of adjunctive treatments targeting elevated histamine or serotonin levels are candidates for evaluation in approved clinical studies. Early treatments may reduce the risk levels of CAL in KD patients and ventricular dysfunction in MIS patients. KD and MIS cases not associated with immunizations may have undiagnosed persistent infections for which appropriate treatments can be considered.
5. Conclusions
This retrospective study identified candidate associations between KD and 29 vaccines. This study, combined with other reported pathogen and vaccine associations, supports the hypothesis that KD is associated with multiple pathogens and vaccinations, with possibly implication of immune complexes. The etiology model of immune complexes binding Fc receptors and activating immune cells and platelets, driving both Kawasaki disease and Multisystem Inflammatory Syndrome, was advanced. Disease onset is conjectured to occur in the context of persistent infections or antibody titer levels higher than primary immune response levels. Histamine, serotonin, and inflammatory molecules are proposed to induce cardiac capillary vasoconstrictions resulting in pressure-induced coronary artery aneurysms, ventricular dysfunction, and other cardiac adverse events. Rare vaccine associations were observed for multiple vaccines with higher normalized frequencies for some bacterial vaccines; for Kawasaki disease, multiple of these associations are with live vaccines. Childhood immunizations are age-associated with Kawasaki disease onset. It can be inferred that the risk level of coronary artery dilations and aneurysms likely decreases with age from the model for pressure inducement from cardiac capillary vasoconstrictions. Persistent infections with higher antibody titer levels combined with genetic risk factors are likely causes of Kawasaki disease, Multisystem Inflammatory Syndromes, and Henoch–Schönlein purpura; vaccines are an alternate source of antigens or attenuated pathogens that can also drive the formation of immune complexes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/life14030353/s1. The Python program vaers_reports.py for generating reports for adverse events is available: https://github.com/mit-ll/Vaers_Reports (accessed on 1 January 2024).
Author Contributions
Conceptualization, D.O.R. and N.S.; methodology, D.O.R.; software, D.O.R.; validation, D.O.R.; formal analysis, D.O.R.; investigation, D.O.R.; data curation, D.O.R.; writing—original draft preparation, D.O.R.; writing—review and editing, D.O.R. and N.S.; visualization, D.O.R. and N.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Department of the Air Force under Air Force Contract No. FA8702-15-D-0001.
Data Availability Statement
Data are contained within the Supplemental Materials.
Acknowledgments
Distribution Statement: A. Approved for public release. Distribution is unlimited. Any opinions, findings, conclusions, or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the Department of the Air Force. The authors wish to acknowledge Tommy O’Connell for graphics art assistance.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Bayer. Aspirin Product Monograh. Available online: https://www.bayer.com/sites/default/files/2020-11/aspirin-pm-en.pdf (accessed on 1 January 2024).
- Liu, X.; Chen, Y.; Yang, Y.; Su, Z.; Wang, F.; Zhanghuang, C.; Wu, Y.; Zhang, X. Association between FGA gene polymorphisms and coronary artery lesion in Kawasaki disease. Front. Med. 2023, 10, 1193303. [Google Scholar] [CrossRef]
- Fossard, C.; Thompson, R.A. Mucocutaneous lymph-node syndrome (Kawasaki disease): Probable soluble-complex disorder. Br. Med. J. 1977, 1, 883. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Menikou, S.; Langford, P.R.; Levin, M. Kawasaki Disease: The Role of Immune Complexes Revisited. Front. Immunol. 2019, 10, 1156. [Google Scholar] [CrossRef] [PubMed]
- Embil, J.A.; McFarlane, E.S.; Murphy, D.M.; Krause, V.W.; Stewart, H.B. Adenovirus type 2 isolated from a patient with fatal Kawasaki disease. Can. Med. Assoc. J. 1985, 132, 1400. [Google Scholar] [PubMed]
- Chang, L.-Y.; Lu, C.-Y.; Shao, P.-L.; Lee, P.-I.; Lin, M.-T.; Fan, T.-Y.; Cheng, A.-L.; Lee, W.-L.; Hu, J.-J.; Yeh, S.-J.; et al. Viral infections associated with Kawasaki disease. J. Formos. Med. Assoc. 2014, 113, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Catalano-Pons, C.; Giraud, C.; Rozenberg, F.; Meritet, J.F.; Lebon, P.; Gendrel, D. Detection of human bocavirus in children with Kawasaki disease. Clin. Microbiol. Infect. 2007, 13, 1220–1222. [Google Scholar] [CrossRef] [PubMed]
- Shirato, K.; Imada, Y.; Kawase, M.; Nakagaki, K.; Matsuyama, S.; Taguchi, F. Possible involvement of infection with human coronavirus 229E, but not NL63, in Kawasaki disease. J. Med. Virol. 2014, 86, 2146–2153. [Google Scholar] [CrossRef]
- Esper, F.; Weibel, C.; Ferguson, D.; Landry, M.L.; Kahn, J.S. Evidence of a Novel Human Coronavirus That Is Associated with Respiratory Tract Disease in Infants and Young Children. J. Infect. Dis. 2005, 191, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Catalano-Pons, C.; Quartier, P.; Leruez-Ville, M.; Kaguelidou, F.; Gendrel, D.; Lenoir, G.; Casanova, J.-L.; Bonnet, D. Primary Cytomegalovirus Infection, Atypical Kawasaki Disease, and Coronary Aneurysms in 2 Infants. Clin. Infect. Dis. 2005, 41, e53–e56. [Google Scholar] [CrossRef][Green Version]
- Jagadeesh, A.; Krishnamurthy, S.; Mahadevan, S. Kawasaki Disease in a 2-year-old Child with Dengue Fever. Indian J. Pediatr. 2016, 83, 602–603. [Google Scholar] [CrossRef]
- Sopontammarak, S.; Promphan, W.; Roymanee, S.; Phetpisan, S. Positive Serology for Dengue Viral Infection in Pediatric Patients With Kawasaki Disease in Southern Thailand. Circ. J. 2008, 72, 1492–1494. [Google Scholar] [CrossRef]
- Weng, K.-P.; Cheng-Chung Wei, J.; Hung, Y.-M.; Huang, S.-H.; Chien, K.-J.; Lin, C.-C.; Huang, S.-M.; Lin, C.-L.; Cheng, M.-F. Enterovirus Infection and Subsequent Risk of Kawasaki Disease: A Population-based Cohort Study. Pediatr. Infect. Dis. J. 2018, 37, 310–315. [Google Scholar] [CrossRef]
- Kikuta, H.; Nakanishi, M.; Ishikawa, N.; Konno, M.; Matsumoto, S. Detection of Epstein-Barr virus sequences in patients with Kawasaki disease by means of the polymerase chain reaction. Intervirology 1992, 33, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Okano, M.; Luka, J.; Thiele, G.M.; Sakiyama, Y.; Matsumoto, S.; Purtilo, D.T. Human herpesvirus 6 infection and Kawasaki disease. J. Clin. Microbiol. 1989, 27, 2379–2380. [Google Scholar] [CrossRef] [PubMed]
- Okano, M. Kawasaki Disease and Human Lymphotropic Virus Infection. Curr. Med. Res. Opin. 1999, 15, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.V.; Jones, K.D.J.; Buckley, A.-M.; Coren, M.E.; Kampmann, B. Kawasaki disease coincident with influenza A H1N1/09 infection. Pediatr. Int. 2011, 53, e1–e2. [Google Scholar] [CrossRef] [PubMed]
- Whitby, D.; Hoad, J.G.; Tizard, E.J.; Dillon, M.J.; Weber, J.N.; Weiss, R.A.; Schulz, T.F. Isolation of measles virus from child with Kawasaki disease. Lancet 1991, 338, 1215. [Google Scholar] [CrossRef] [PubMed]
- Holm, J.M.; Hansen, L.K.; Oxhøj, H. Kawasaki disease associated with parvovirus B19 infection. Eur. J. Pediatr. 1995, 154, 633–634. [Google Scholar] [CrossRef]
- Nigro, G.; Krzysztofiak, A.; Porcaro, M.A.; Mango, T.; Zerbini, M.; Gentilomi, G.; Musiani, M. Active or recent parvovirus B19 infection in children with Kawasaki disease. Lancet 1994, 343, 1260–1261. [Google Scholar] [CrossRef] [PubMed]
- Keim, D.; Keller, E.; Hirsch, M. Mucocutaneous Lymph-Node Syndrome and Parainfluenza 2 Virus Infection. Lancet 1977, 310, 303. [Google Scholar] [CrossRef]
- Kim, G.B.; Park, S.; Kwon, B.S.; Han, J.W.; Park, Y.W.; Hong, Y.M. Evaluation of the Temporal Association between Kawasaki Disease and Viral Infections in South Korea. Korean Circ. J. 2014, 44, 250–254. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matsuno, S.; Utagawa, E.; Sugiura, A. Association of Rotavirus Infection with Kawasaki Syndrome. J. Infect. Dis. 1983, 148, 177. [Google Scholar] [CrossRef] [PubMed]
- Ogboli, M.I.; Parslew, R.; Verbov, J.; Smyth, R. Kawasaki disease associated with varicella: A rare association. Br. J. Dermatol. 1999, 141, 1136–1152. [Google Scholar] [CrossRef] [PubMed]
- Kossiva, L.; Papadopoulos, M.; Lagona, E.; Papadopoulos, G.; Athanassaki, C. Myocardial infarction in a 35-day-old infant with incomplete Kawasaki disease and chicken pox. Cardiol. Young 2010, 20, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Thissen, J.B.; Isshiki, M.; Jaing, C.; Nagao, Y.; Lebron Aldea, D.; Allen, J.E.; Izui, M.; Slezak, T.R.; Ishida, T.; Sano, T. A novel variant of torque teno virus 7 identified in patients with Kawasaki disease. PLoS ONE 2018, 13, e0209683. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.; Hoyt, L.; Ferrieri, P.; Schlievert, P.M.; Jenson, H.B. Kawasaki Syndrome-Like Illness Associated with Infection Caused by Enterotoxin B-Secreting Staphylococcus aureus. Clin. Infect. Dis. 1999, 29, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Shinomiya, N.; Takeda, T.; Kuratsuji, T.; Takagi, K.; Kosaka, T.; Tatsuzawa, O.; Tsurumizu, T.; Hashimoto, T.; Kobayashi, N. Variant Streptococcus sanguis as an etiological agent of Kawasaki disease. Prog. Clin. Biol. Res. 1987, 250, 571–572. [Google Scholar]
- Banday, A.Z.; Patra, P.K.; Jindal, A.K. Kawasaki disease—When Bacillus Calmette–Guérin (BCG) lymphadenitis blooms again and the vaccination site peels off! Int. J. Dermatol. 2021, 60, e233–e234. [Google Scholar] [CrossRef]
- Peralta-Amaro, A.L.; Tejada-Ruiz, M.I.; Rivera-Alvarado, K.L.; Cobos-Quevedo, O.D.; Romero-Hernández, P.; Macías-Arroyo, W.; Avendaño-Ponce, A.; Hurtado-Díaz, J.; Vera-Lastra, O.; Lucas-Hernández, A. Atypical Kawasaki Disease after COVID-19 Vaccination: A New Form of Adverse Event Following Immunization. Vaccines 2022, 10, 126. [Google Scholar] [CrossRef]
- Alsager, K.; Khatri Vadlamudi, N.; Jadavji, T.; Bettinger, J.A.; Constantinescu, C.; Vaudry, W.; Tan, B.; Sauvé, L.; Sadarangani, M.; Halperin, S.A.; et al. Kawasaki disease following immunization reported to the Canadian Immunization Monitoring Program ACTive (IMPACT) from 2013 to 2018. Hum. Vaccines Immunother. 2022, 18, 2088215. [Google Scholar] [CrossRef]
- Miron, D.; Fink, D.; Hashkes, P.J. Kawasaki disease in an infant following immunisation with hepatitis B vaccine. Clin. Rheumatol. 2003, 22, 461–463. [Google Scholar] [CrossRef]
- Jeong, S.W.; Kim, D.H.; Han, M.Y.; Cha, S.H.; Yoon, K.L. An infant presenting with Kawasaki disease following immunization for influenza: A case report. Biomed. Rep. 2018, 8, 301–303. [Google Scholar] [CrossRef]
- Kraszewska-Głomba, B.; Kuchar, E.; Szenborn, L. Three episodes of Kawasaki disease including one after the Pneumo 23 vaccine in a child with a family history of Kawasaki disease. J. Formos. Med. Assoc. 2016, 115, 885–886. [Google Scholar] [CrossRef]
- Shimada, S.; Watanabe, T.; Sato, S. A Patient with Kawasaki Disease Following Influenza Vaccinations. Pediatr. Infect. Dis. J. 2015, 34, 913. [Google Scholar] [CrossRef]
- Yin, S.; Liubao, P.; Chongqing, T.; Xiaomin, W. The first case of Kawasaki disease in a 20-month old baby following immunization with rotavirus vaccine and hepatitis A vaccine in China: A case report. Hum. Vaccines Immunother. 2015, 11, 2740–2743. [Google Scholar] [CrossRef]
- Huang, W.-T.; Juan, Y.-C.; Liu, C.-H.; Yang, Y.-Y.; Chan, K.A. Intussusception and Kawasaki disease after rotavirus vaccination in Taiwanese infants. Vaccine 2020, 38, 6299–6303. [Google Scholar] [CrossRef]
- Showers, C.R.; Maurer, J.M.; Khakshour, D.; Shukla, M. Case of adult-onset Kawasaki disease and Multisystem Inflammatory Syndrome following SARS-CoV-2 vaccination. BMJ Case Rep. 2022, 15, e249094. [Google Scholar] [CrossRef] [PubMed]
- Schmöeller, D.; Keiserman, M.W.; Staub, H.L.; Velho, F.P.; de Fátima Grohe, M. Yellow Fever Vaccination and Kawasaki Disease. Pediatr. Infect. Dis. J. 2009, 28, 1037–1038. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Islam, S. Kawasaki disease and vasculitis associated with immunization. Pediatr. Int. 2018, 60, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Hall, G.C.; Tulloh, R.M.R.; Tulloh, L.E. The incidence of Kawasaki disease after vaccination within the UK pre-school National Immunisation Programme: An observational THIN database study. Pharmacoepidemiol. Drug Saf. 2016, 25, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Ece, I.; Akbayram, S.; Demiroren, K.; Uner, A. Is Kawasaki Disease a Side Effect of Vaccination as Well? J. Vaccines Vaccin. 2014, 5, 234. [Google Scholar] [CrossRef]
- Matsubara, D.; Minami, T.; Seki, M.; Tamura, D.; Yamagata, T. Occurrence of Kawasaki disease after simultaneous immunization. Pediatr. Int. 2019, 61, 1171–1173. [Google Scholar] [CrossRef]
- Kuo, N.-C.; Lin, C.-H.; Lin, M.-C. Prenatal and early life exposure to air pollution and the incidence of Kawasaki disease. Sci. Rep. 2022, 12, 3415. [Google Scholar] [CrossRef]
- Mitchell, J.A.; Ali, F.; Bailey, L.; Moreno, L.; Harrington, L.S. Role of nitric oxide and prostacyclin as vasoactive hormones released by the endothelium. Exp. Physiol. 2008, 93, 141–147. [Google Scholar] [CrossRef]
- Yoshimura, K.; Tatsumi, K.; Iharada, A.; Tsuji, S.; Tateiwa, A.; Teraguchi, M.; Ogino, H.; Kaneko, K. Increased nitric oxide production by neutrophils in early stage of Kawasaki disease. Eur. J. Pediatr. 2009, 168, 1037–1041. [Google Scholar] [CrossRef]
- Nong, Z.; Hoylaerts, M.; Van Pelt, N.; Collen, D.; Janssens, S. Nitric Oxide Inhalation Inhibits Platelet Aggregation and Platelet-Mediated Pulmonary Thrombosis in Rats. Circ. Res. 1997, 81, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-h.; Zhang, C.-y.; Wu, J.-x.; Zhang, T. Changes in plasma hydrogen sulfide and nitric oxide levels and their clinical significance in children with Kawasaki disease. Chin. Med. J. 2011, 124, 3445–3449. [Google Scholar]
- Iizuka, T.; Oishi, K.; Sasaki, M.; Hatanaka, Y.; Minatogawa, Y.; Uemura, S.; Koike, M. Nitric oxide and aneurysm formation in Kawasaki disease. Acta Paediatr. 1997, 86, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Liu, G.; Li, X.; Xu, W.; Liu, J.; Jin, H. Elevated Inducible Nitric Oxide Levels and Decreased Hydrogen Sulfide Levels Can Predict the Risk of Coronary Artery Ectasia in Kawasaki Disease. Pediatr. Cardiol. 2016, 37, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-B.; Jin, H.-F.; Tang, C.-S.; Du, J.-B. Significance of endogenous sulphur-containing gases in the cardiovascular system. Clin. Exp. Pharmacol. Physiol. 2010, 37, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Kurio, G.H.; Zhiroff, K.A.; Jih, L.J.; Fronek, A.S.; Burns, J.C. Noninvasive Determination of Endothelial Cell Function in the Microcirculation in Kawasaki Syndrome. Pediatr. Cardiol. 2008, 29, 121–125. [Google Scholar] [CrossRef]
- Ikemoto, Y.; Teraguchi, M.; Ono, A.; Kino, M.; Yoshimura, K.; Kobayashi, Y. Serial changes of plasma nitrate in the acute phase of Kawasaki disease. Pediatr. Int. 2003, 45, 421–425. [Google Scholar] [CrossRef]
- Khajoee, V.; Kariyazono, H.; Ohno, T.; Ihara, K.; Mizuno, Y.; Kusuhara, K.; Hara, T. Inducible and endothelial constitutive nitric oxide synthase gene polymorphisms in Kawasaki disease. Pediatr. Int. 2003, 45, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, M.; Uda, K.; Eitoku, T.; Matsumoto, N.; Yorifuji, T.; Tsukahara, H. Roles of Oxidative Injury and Nitric Oxide System Derangements in Kawasaki Disease Pathogenesis: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 15450. [Google Scholar] [CrossRef]
- Straface, E.; Gambardella, L.; Metere, A.; Marchesi, A.; Palumbo, G.; Cortis, E.; Villani, A.; Pietraforte, D.; Viora, M.; Malorni, W.; et al. Oxidative stress and defective platelet apoptosis in naïve patients with Kawasaki disease. Biochem. Biophys. Res. Commun. 2010, 392, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, C.; Moretta, G.; Bersani, G.; Valentini, P.; Gatto, A.; Rigante, D. Epstein-Barr virus-related cutaneous necrotizing vasculitis in a girl heterozygous for factor V Leiden. J. Dermatol. Case Rep. 2017, 11, 25–28. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Burcu, K.; Sila, Y.; Deniz, Ç.; Pembe, G.G.; Sirin, G.; Ismail, I. Henoch-schonlein purpura associated with primary active epstein barr virus infection: A case report. PAMJ 2017, 27, 10481. [Google Scholar] [CrossRef]
- Hu, H.; Wu, J.; Cheng, Y.; Li, J.j. Epidemiology and clinical characteristics of Henoch-Schonlein purpura associated with Epstein-Barr virus infection. Mediterr. J. Hematol. Infect. Dis. 2021, 13, e2021064. [Google Scholar] [CrossRef]
- Mohamed, M.; Shariff, M.; Al Hillan, A.; Haj, R.A.; Kaunzinger, C.; Hossain, M.; Asif, A.; Pyrsopoulos, N.T. A Rare Case of Helicobacter pylori Infection Complicated by Henoch-Schonlein Purpura in an Adult Patient. J. Med. Cases 2020, 11, 160–165. [Google Scholar] [CrossRef]
- Chen, L.; Li, S.; Dong, L.; Feng, S.; Wang, Z. Parainfluenza infection is associated with Henoch-Schönlein purpura in children. Pediatr. Infect. Dis. 2016, 8, 110–114. [Google Scholar] [CrossRef]
- Pamela, F.W.; Andrew, J.K.; Xianqun, L.; Chris, F. Temporal Association of Streptococcus, Staphylococcus, and Parainfluenza Pediatric Hospitalizations and Hospitalized Cases of Henoch-Schönlein Purpura. J. Rheumatol. 2010, 37, 2587. [Google Scholar] [CrossRef]
- Veraldi, S.; Mancuso, R.; Rizzitelli, G.; Gianotti, R.; Ferrante, P. Henoch-Schönlein syndrome associated with human Parvovirus B19 primary infection. Eur. J. Dermatol. 1999, 9, 232–233. [Google Scholar] [PubMed]
- Veraldi, S.; Rizzitelli, G. Henoch-Schönlein Purpura and Human Parvovirus B19. Dermatology 2009, 189, 213–214. [Google Scholar] [CrossRef]
- Cioc, A.M.; Sedmak, D.D.; Nuovo, G.J.; Dawood, M.R.; Smart, G.; Magro, C.M. Parvovirus B19 associated adult Henoch Schönlein purpura. J. Cutan. Pathol. 2002, 29, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Kalman, S.; İbrahim Aydın, H.; Atay, A. Henoch-Schönlein Purpura in a Child Following Varicella. J. Trop. Pediatr. 2005, 51, 240–241. [Google Scholar] [CrossRef]
- Mariko, M.; Yoriaki, K.; Tomohiro, W.; Masatoshi, K. Purpura-free small intestinal IgA vasculitis complicated by cytomegalovirus reactivation. BMJ Case Rep. 2020, 13, e235042. [Google Scholar] [CrossRef]
- Tsukada, H.; Miyakawa, H. Henoch Schönlein Purpura Nephritis Associated with Intravesical Bacillus Calmette-Guerin (BCG) Therapy. Intern. Med. 2017, 56, 541–544. [Google Scholar] [CrossRef][Green Version]
- Jariwala, S.; Vernon, N.; Shliozberg, J. Henoch-Schönlein purpura after hepatitis A vaccination. Ann. Allergy Asthma Immunol. 2011, 107, 180–181. [Google Scholar] [CrossRef] [PubMed]
- McNally, A.; McGregor, D.; Searle, M.; Irvine, J.; Cross, N. Henoch–Schönlein purpura in a renal transplant recipient with prior IgA nephropathy following influenza vaccination. Clin. Kidney J. 2013, 6, 313–315. [Google Scholar] [CrossRef]
- Watanabe, T. Henoch-Schönlein purpura following influenza vaccinations during the pandemic of influenza A (H1N1). Pediatr. Nephrol. 2011, 26, 795–798. [Google Scholar] [CrossRef]
- Patel, U.; Bradley, J.R.; Hamilton, D.V. Henoch-Schönlein purpura after influenza vaccination. Br. Med. J. 1988, 296, 1800. [Google Scholar] [CrossRef]
- Kantor, R.; Galel, A.; Aviner, S. Henoch-Schönlein Purpura Post-Influenza Vaccination in a Pediatric Patient: A Rare but Possible Adverse Reaction to Vaccine. Isr. Med. Assoc. J. 2020, 22, 654–656. [Google Scholar] [PubMed]
- Da Dalt, L.; Zerbinati, C.; Strafella, M.S.; Renna, S.; Riceputi, L.; Di Pietro, P.; Barabino, P.; Scanferla, S.; Raucci, U.; Mores, N.; et al. Henoch-Schönlein purpura and drug and vaccine use in childhood: A case-control study. Ital. J. Pediatr. 2016, 42, 60. [Google Scholar] [CrossRef] [PubMed]
- Courtney, P.A.; Patterson, R.N.; Lee, R.J.E. Henoch–Schönlein purpura following meningitis C vaccination. Rheumatology 2001, 40, 345–346. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.-G.; Zheng, Y.; Lu, S.; Hu, Q.; Fang, Y. Rabies post-exposure prophylaxis for a male with severe Henoch Schönlein purpura following rabies vaccination. Hum. Vaccines Immunother. 2018, 14, 2666–2668. [Google Scholar] [CrossRef] [PubMed]
- Yanis, R.; Thomas, B.; Amel, B.; Léa, D.; Kladoum, N.; Kevin, D.; Carle, P.; Eric, L.; Ashley, T.; Gaëlle, R.-C.; et al. IgA Vasculitis Following COVID-19 Vaccination: A French Multicenter Case Series Including 12 Patients. J. Rheumatol. 2023, 50, 252. [Google Scholar] [CrossRef]
- Hines, A.M.; Murphy, N.; Mullin, C.; Barillas, J.; Barrientos, J.C. Henoch-Schönlein purpura presenting post COVID-19 vaccination. Vaccine 2021, 39, 4571–4572. [Google Scholar] [CrossRef] [PubMed]
- Grossman, M.E.; Appel, G.; Little, A.J.; Ko, C.J. Post-COVID-19 vaccination IgA vasculitis in an adult. J. Cutan. Pathol. 2022, 49, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Sirufo, M.M.; Raggiunti, M.; Magnanimi, L.M.; Ginaldi, L.; De Martinis, M. Henoch-Schönlein Purpura Following the First Dose of COVID-19 Viral Vector Vaccine: A Case Report. Vaccines 2021, 9, 1078. [Google Scholar] [CrossRef]
- Naitlho, A.; Lahlou, W.; Bourial, A.; Rais, H.; Ismaili, N.; Abousahfa, I.; Belyamani, L. A Rare Case of Henoch-Schönlein Purpura Following a COVID-19 Vaccine—Case Report. SN Compr. Clin. Med. 2021, 3, 2618–2621. [Google Scholar] [CrossRef]
- Casini, F.; Magenes, V.C.; De Sanctis, M.; Gattinara, M.; Pandolfi, M.; Cambiaghi, S.; Zuccotti, G.V.; Fabiano, V. Henoch-Schönlein purpura following COVID-19 vaccine in a child: A case report. Ital. J. Pediatr. 2022, 48, 158. [Google Scholar] [CrossRef]
- Yanis, R.; Jean Marc, G.; Jean François, A.; Eva, D.; Laurent, P.; Nicole, F.; Adrien, B.; Julie, M.; Stéphanie, J.; Elisabeth, D.; et al. Immunoglobulin A Vasculitis Following COVID-19: A French Multicenter Case Series. J. Rheumatol. 2022, 49, 1390. [Google Scholar] [CrossRef]
- Bonetto, C.; Trotta, F.; Felicetti, P.; Alarcón, G.S.; Santuccio, C.; Bachtiar, N.S.; Brauchli Pernus, Y.; Chandler, R.; Girolomoni, G.; Hadden, R.D.M.; et al. Vasculitis as an adverse event following immunization—Systematic literature review. Vaccine 2016, 34, 6641–6651. [Google Scholar] [CrossRef]
- Vikenes, K.; Farstad, M.; Nordrehaug, J.E. Serotonin Is Associated with Coronary Artery Disease and Cardiac Events. Circulation 1999, 100, 483–489. [Google Scholar] [CrossRef]
- Golino, P.; Piscione, F.; Willerson, J.T.; Cappelli-Bigazzi, M.; Focaccio, A.; Villari, B.; Indolfi, C.; Russolillo, E.; Condorelli, M.; Chiariello, M. Divergent Effects of Serotonin on Coronary-Artery Dimensions and Blood Flow in Patients with Coronary Atherosclerosis and Control Patients. N. Engl. J. Med. 1991, 324, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Tuman, T.C.; Tuman, B.; Polat, M.; Çakır, U. Urticaria and Angioedema Associated with Fluoxetine. Clin. Psychopharmacol. Neurosci. 2017, 15, 418–419. [Google Scholar] [CrossRef] [PubMed]
- Dale, H.H.; Laidlaw, P.P. The physiological action of β-iminazolylethylamine. J. Physiol. 1910, 41, 318–344. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.A.; Levi, R. Histamine and cardiac arrhythmias. Circ. Res. 1986, 58, 1–16. [Google Scholar] [CrossRef]
- Maintz, L.; Novak, N. Histamine and histamine intolerance2. Am. J. Clin. Nutr. 2007, 85, 1185–1196. [Google Scholar] [CrossRef]
- CDC. About Kawasaki Disease. Available online: https://www.cdc.gov/kawasaki/about.html (accessed on 20 February 2024).
- Levin, M.; Holland, P.C.; Nokes, T.J.; Novelli, V.; Mola, M.; Levinsky, R.J.; Dillon, M.J.; Barratt, T.M.; Marshall, W.C. Platelet immune complex interaction in pathogenesis of Kawasaki disease and childhood polyarteritis. Br. Med. J. 1985, 290, 1456. [Google Scholar] [CrossRef] [PubMed]
- Cannon, L.; Campbell, M.J.; Wu, E.Y. Multisystem Inflammatory Syndrome in Children and Kawasaki Disease: Parallels in Pathogenesis and Treatment. Curr. Allergy Asthma Rep. 2023, 23, 341–350. [Google Scholar] [CrossRef]
- Kocatürk, B.; Lee, Y.; Nosaka, N.; Abe, M.; Martinon, D.; Lane, M.E.; Moreira, D.; Chen, S.; Fishbein, M.C.; Porritt, R.A.; et al. Platelets exacerbate cardiovascular inflammation in a murine model of Kawasaki disease vasculitis. JCI Insight 2023, 8, 169855. [Google Scholar] [CrossRef]
- Zhang, H.-y.; Xiao, M.; Zhou, D.; Yan, F.; Zhang, Y. Platelet and ferritin as early predictive factors for the development of macrophage activation syndrome in children with Kawasaki disease: A retrospective case-control study. Front. Pediatr. 2023, 11, 1088525. [Google Scholar] [CrossRef]
- Ricke, D.O.; Gherlone, N.; Fremont-Smith, P.; Tisdall, P.; Fremont-Smith, M. Kawasaki Disease, Multisystem Inflammatory Syndrome in Children: Antibody-Induced Mast Cell Activation Hypothesis. J. Pediatr. Pediatr. Med. 2020, 4, 1–7. [Google Scholar] [CrossRef]
- Longden, T.A.; Zhao, G.; Hariharan, A.; Lederer, W.J. Pericytes and the Control of Blood Flow in Brain and Heart. Annu. Rev. Physiol. 2023, 85, 137–164. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Andreeva, E.R.; Eremin, I.I.; Markin, A.M.; Nadelyaeva, I.I.; Orekhov, A.N.; Melnichenko, A.A. The Role of Pericytes in Regulation of Innate and Adaptive Immunity. Biomedicines 2023, 11, 600. [Google Scholar] [CrossRef] [PubMed]
- O’Farrell, F.M.; Mastitskaya, S.; Hammond-Haley, M.; Freitas, F.; Wah, W.R.; Attwell, D. Capillary pericytes mediate coronary no-reflow after myocardial ischaemia. eLife 2017, 6, e29280. [Google Scholar] [CrossRef]
- An, D.; Tan, B.; Yu, D.-Y.; Balaratnasingam, C. Differentiating Microaneurysm Pathophysiology in Diabetic Retinopathy Through Objective Analysis of Capillary Nonperfusion, Inflammation, and Pericytes. Diabetes 2022, 71, 733–746. [Google Scholar] [CrossRef]
- Avolio, E.; Carrabba, M.; Milligan, R.; Kavanagh Williamson, M.; Beltrami, A.P.; Gupta, K.; Elvers, K.T.; Gamez, M.; Foster, R.R.; Gillespie, K.; et al. The SARS-CoV-2 Spike protein disrupts human cardiac pericytes function through CD147 receptor-mediated signalling: A potential non-infective mechanism of COVID-19 microvascular disease. Clin. Sci. 2021, 135, 2667–2689. [Google Scholar] [CrossRef]
- Guo, X.; Liao, J.; Fan, X.; Xu, M. Exploring the diagnostic value of eosinophil-to-lymphocyte ratio to differentiate Kawasaki disease from other febrile diseases based on clinical prediction model. Sci. Rep. 2023, 13, 3399. [Google Scholar] [CrossRef]
- Patel, P.; DeCuir, J.; Abrams, J.; Campbell, A.P.; Godfred-Cato, S.; Belay, E.D. Clinical Characteristics of Multisystem Inflammatory Syndrome in Adults: A Systematic Review. JAMA Netw. Open 2021, 4, e2126456. [Google Scholar] [CrossRef]
- Darby, J.B.; Jackson, J.M. Kawasaki Disease and Multisystem Inflammatory Syndrome in Children: An Overview and Comparison. Am. Fam. Physician 2021, 104, 244–252. [Google Scholar]
- Dufort, E.M.; Koumans, E.H.; Chow, E.J.; Rosenthal, E.M.; Muse, A.; Rowlands, J.; Barranco, M.A.; Maxted, A.M.; Rosenberg, E.S.; Easton, D.; et al. Multisystem Inflammatory Syndrome in Children in New York State. N. Engl. J. Med. 2020, 383, 347–358. [Google Scholar] [CrossRef]
- Feldstein, L.R.; Rose, E.B.; Horwitz, S.M.; Collins, J.P.; Newhams, M.M.; Son, M.B.F.; Newburger, J.W.; Kleinman, L.C.; Heidemann, S.M.; Martin, A.A.; et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N. Engl. J. Med. 2020, 383, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.; Wei, L.; Jiao, F.; Pjetraj, D.; Feng, J.; Wang, J.; Catassi, C.; Gatti, S. Very early onset of coronary artery aneurysm in a 3-month infant with Kawasaki disease: A case report and literature review. Ital. J. Pediatr. 2023, 49, 60. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, K.; Kiyomatsu, K.; Teramachi, Y.; Suda, K. A case of incomplete Kawasaki disease—A 2-month-old infant with 1 day of fever who developed multiple arterial aneurysms. Ann. Pediatr. Cardiol. 2022, 15, 536–538. [Google Scholar] [CrossRef]
- Hamwi, S.; Alebaji, M.B.; Mahboub, A.E.; Alkaabi, E.H.; Alkuwaiti, N.S. Multiple Systemic Arterial Aneurysms in Kawasaki Disease. Cureus 2023, 15, e42714. [Google Scholar] [CrossRef] [PubMed]
- Pawar, R.; Gavade, V.; Patil, N.; Mali, V.; Girwalkar, A.; Tarkasband, V.; Loya, S.; Chavan, A.; Nanivadekar, N.; Shinde, R.; et al. Neonatal Multisystem Inflammatory Syndrome (MIS-N) Associated with Prenatal Maternal SARS-CoV-2: A Case Series. Children 2021, 8, 572. [Google Scholar] [CrossRef]
- De Rose, D.U.; Pugnaloni, F.; Calì, M.; Ronci, S.; Caoci, S.; Maddaloni, C.; Martini, L.; Santisi, A.; Dotta, A.; Auriti, C. Multisystem Inflammatory Syndrome in Neonates Born to Mothers with SARS-CoV-2 Infection (MIS-N) and in Neonates and Infants Younger Than 6 Months with Acquired COVID-19 (MIS-C): A Systematic Review. Viruses 2022, 14, 750. [Google Scholar] [CrossRef]
- Mascarenhas, D.; Goyal, M.; Haribalakrishna, A.; Nanavati, R.; Ish, P.; Kunal, S. Multisystem inflammatory syndrome in neonates (MIS-N): A systematic review. Eur. J. Pediatr. 2023, 182, 2283–2298. [Google Scholar] [CrossRef]
- Li, Z.; Cai, J.; Lu, J.; Wang, M.; Yang, C.; Zeng, Z.; Tang, Q.; Li, J.; Tang, W.; Luo, H.; et al. The therapeutic window of intravenous immunoglobulin (IVIG) and its correlation with clinical outcomes in Kawasaki disease: A systematic review and meta-analysis. Ital. J. Pediatr. 2023, 49, 45. [Google Scholar] [CrossRef]
- Rife, E.; Gedalia, A. Kawasaki Disease: An Update. Curr. Rheumatol. Rep. 2020, 22, 75. [Google Scholar] [CrossRef]
- Fukazawa, R.; Ikegam, E.; Watanabe, M.; Hajikano, M.; Kamisago, M.; Katsube, Y.; Yamauchi, H.; Ochi, M.; Ogawa, S. Coronary Artery Aneurysm Induced by Kawasaki Disease in Children Show Features Typical Senescence. Circ. J. 2007, 71, 709–715. [Google Scholar] [CrossRef]
- Whittaker, E.; Bamford, A.; Kenny, J.; Kaforou, M.; Jones, C.E.; Shah, P.; Ramnarayan, P.; Fraisse, A.; Miller, O.; Davies, P.; et al. Clinical Characteristics of 58 Children with a Pediatric Inflammatory Multisystem Syndrome Temporally Associated with SARS-CoV-2. JAMA 2020, 324, 259–269. [Google Scholar] [CrossRef]
- Nune, A.; Iyengar, K.P.; Goddard, C.; Ahmed, A.E. Multisystem inflammatory syndrome in an adult following the SARS-CoV-2 vaccine (MIS-V). BMJ Case Rep. 2021, 14, e243888. [Google Scholar] [CrossRef]
- Iyengar, K.P.; Nune, A.; Ish, P.; Botchu, R.; Shashidhara, M.K.; Jain, V.K. Multisystem inflammatory syndrome after SARS-CoV-2 vaccination (MIS-V), to interpret with caution. Postgrad. Med. J. 2021, 98, e91. [Google Scholar] [CrossRef]
- Lee, S.; Kim, D.; Kim, B.J.; Rhim, J.W.; Lee, S.-Y.; Jeong, D.C. Comparison of COVID-19-associated Multisystem Inflammatory Syndrome in children (MIS-C) and Kawasaki disease shock syndrome: Case reports and literature review. J. Rheum. Dis. 2023, 30, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Sinkovits, G.; Schnur, J.; Hurler, L.; Kiszel, P.; Prohászka, Z.Z.; Sík, P.; Kajdácsi, E.; Cervenak, L.; Maráczi, V.; Dávid, M.; et al. Evidence, detailed characterization and clinical context of complement activation in acute Multisystem Inflammatory Syndrome in children. Sci. Rep. 2022, 12, 19759. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, Y.C.; Wang, C.; Zohar, T.; Fischinger, S.; Atyeo, C.; Burke, J.S.; Kang, J.; Edlow, A.G.; Fasano, A.; Baden, L.R.; et al. Humoral signatures of protective and pathological SARS-CoV-2 infection in children. Nat. Med. 2021, 27, 454–462. [Google Scholar] [CrossRef]
- Vella, L.A.; Rowley, A.H. Current Insights Into the Pathophysiology of Multisystem Inflammatory Syndrome in Children. Curr. Pediatr. Rep. 2021, 9, 83–92. [Google Scholar] [CrossRef]
- Lee, J.; Kim, B.J.; Cho, K.-S.; Rhim, J.W.; Lee, S.-Y.; Jeong, D.C. Similarities and Differences between Multisystem Inflammatory Syndrome in Children (MIS-C) and Kawasaki Disease Shock Syndrome. Children 2023, 10, 1527. [Google Scholar] [CrossRef] [PubMed]
- Noval Rivas, M.; Arditi, M. Kawasaki Disease and Multisystem Inflammatory Syndrome in Children: Common Inflammatory Pathways of Two Distinct Diseases. Rheum. Dis. Clin. N. Am. 2023, 49, 647–659. [Google Scholar] [CrossRef]
- Hufnagel, M.; Armann, J.; Jakob, A.; Doenhardt, M.; Diffloth, N.; Hospach, A.; Schneider, D.T.; Trotter, A.; Roessler, M.; Schmitt, J.; et al. A comparison of pediatric inflammatory multisystem syndrome temporarily-associated with SARS-CoV-2 and Kawasaki disease. Sci. Rep. 2023, 13, 1173. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).