Consumption of Citric Acid by Bees Promotes the Gland Development and Enhances Royal Jelly Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Subjects
2.2. Reagents and Equipment
2.3. Experimental Procedure
2.3.1. Feed Preparation
2.3.2. Colony Rearing and Sampling Time
2.3.3. Gland Isolation and Measurement
2.3.4. Colony Feeding and Royal Jelly Production
2.3.5. Determination of the Major Components of Royal Jelly
2.4. Data Analysis
3. Results
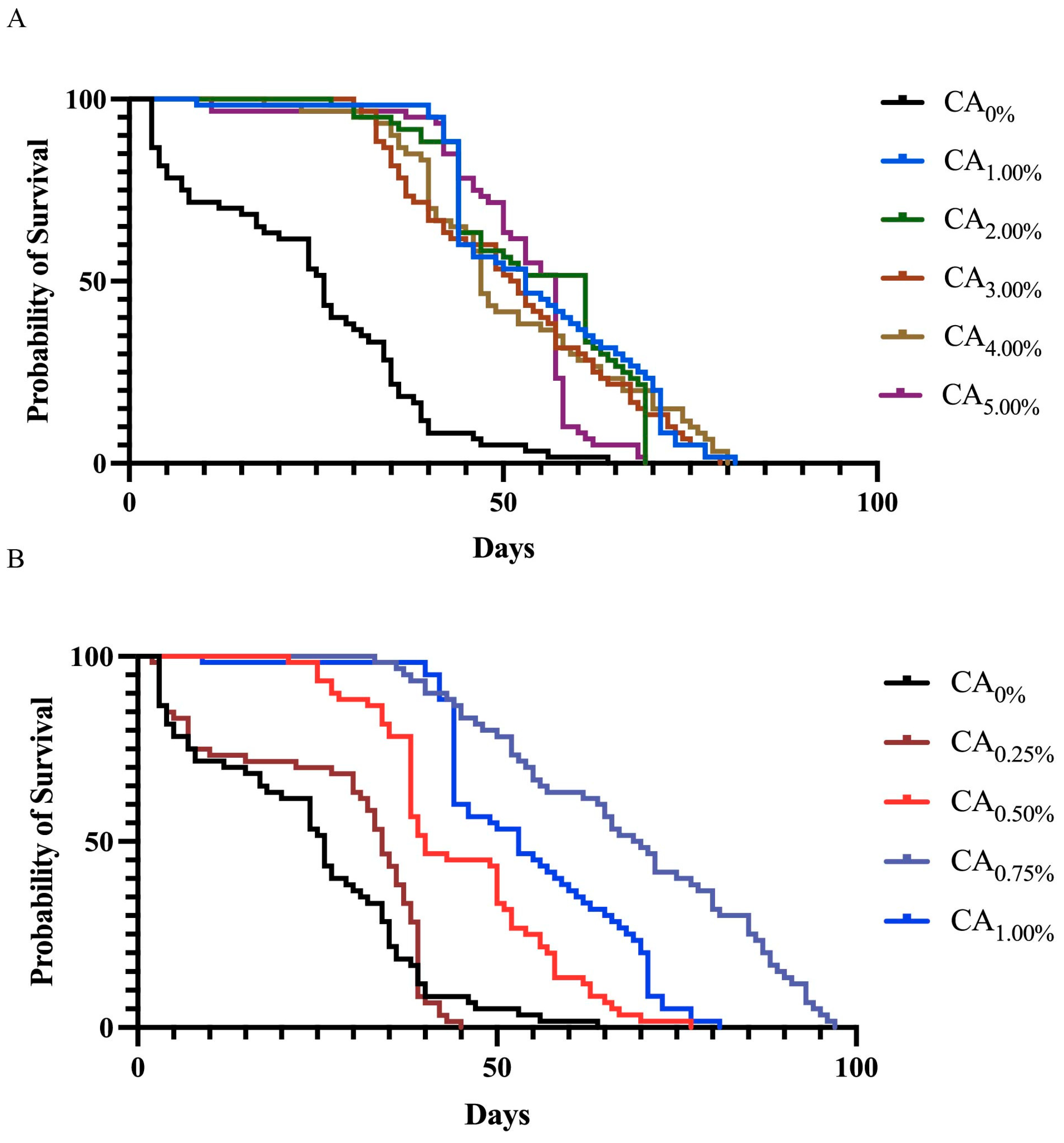
3.1. Survival Curve Analysis
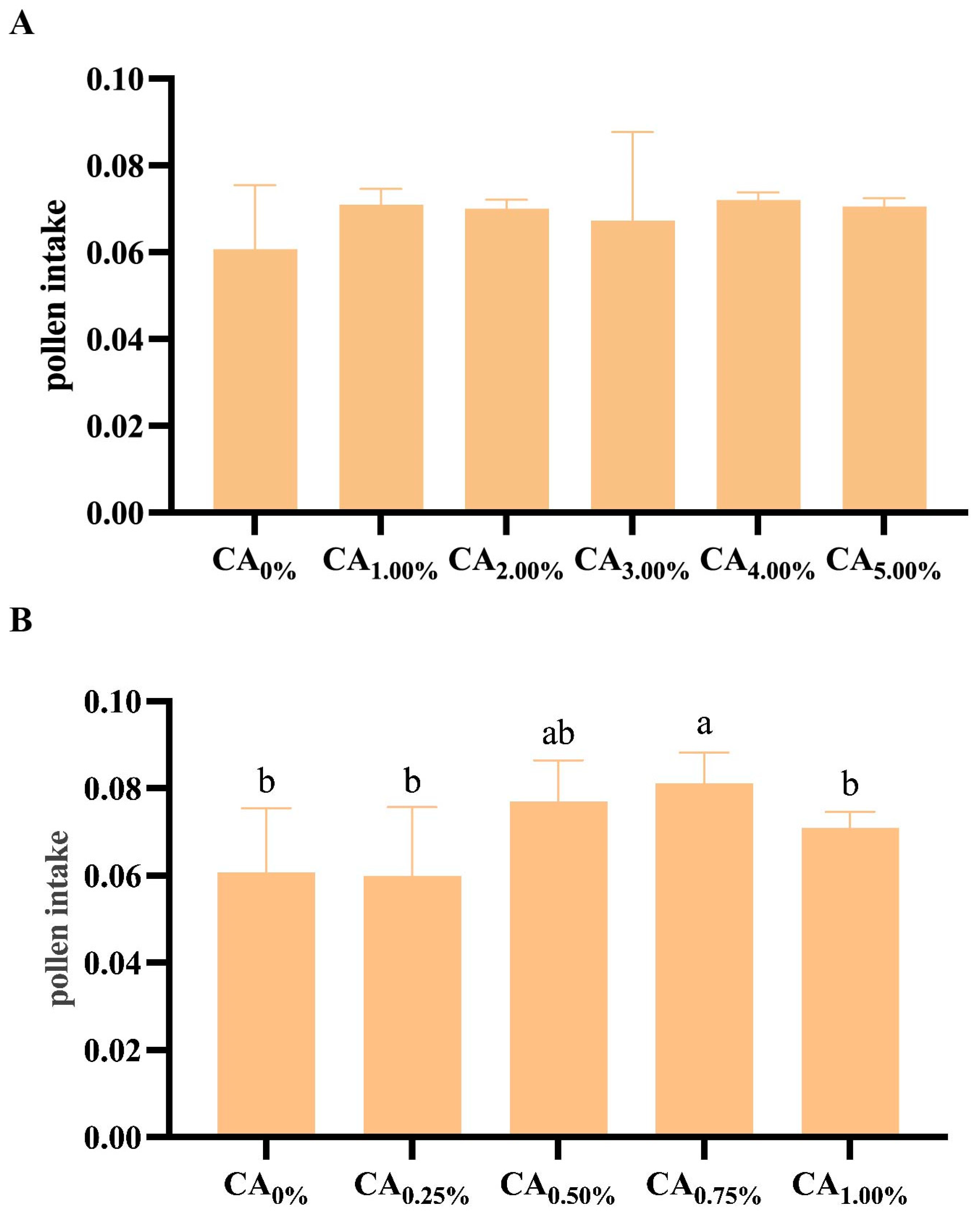
3.2. The Impact of Adding Citric Acid to Bee Feed on Pollen Consumption
3.3. Effect of Citric Acid Addition to Feed on Bee Gland Development
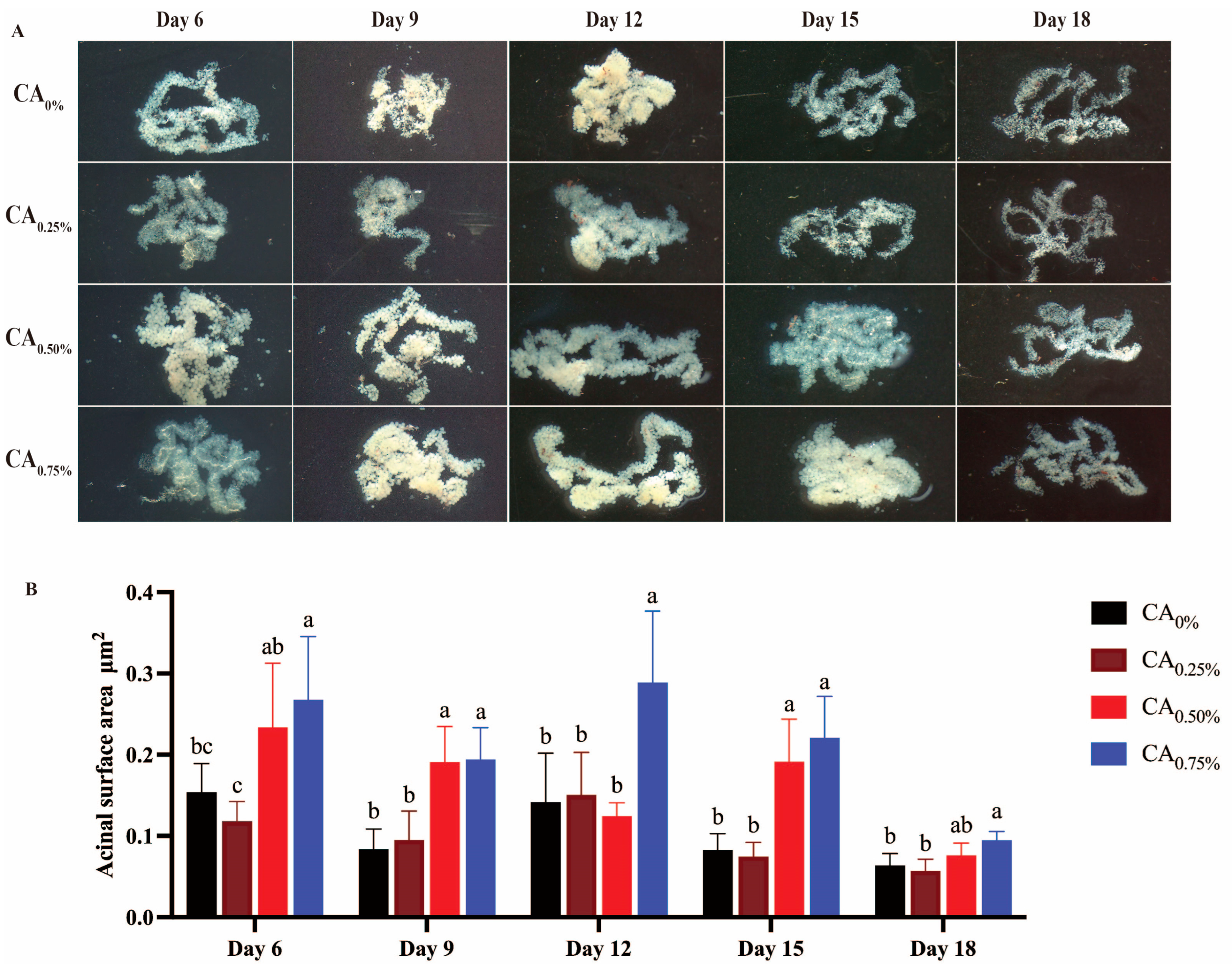
3.3.1. Mandibular Glands
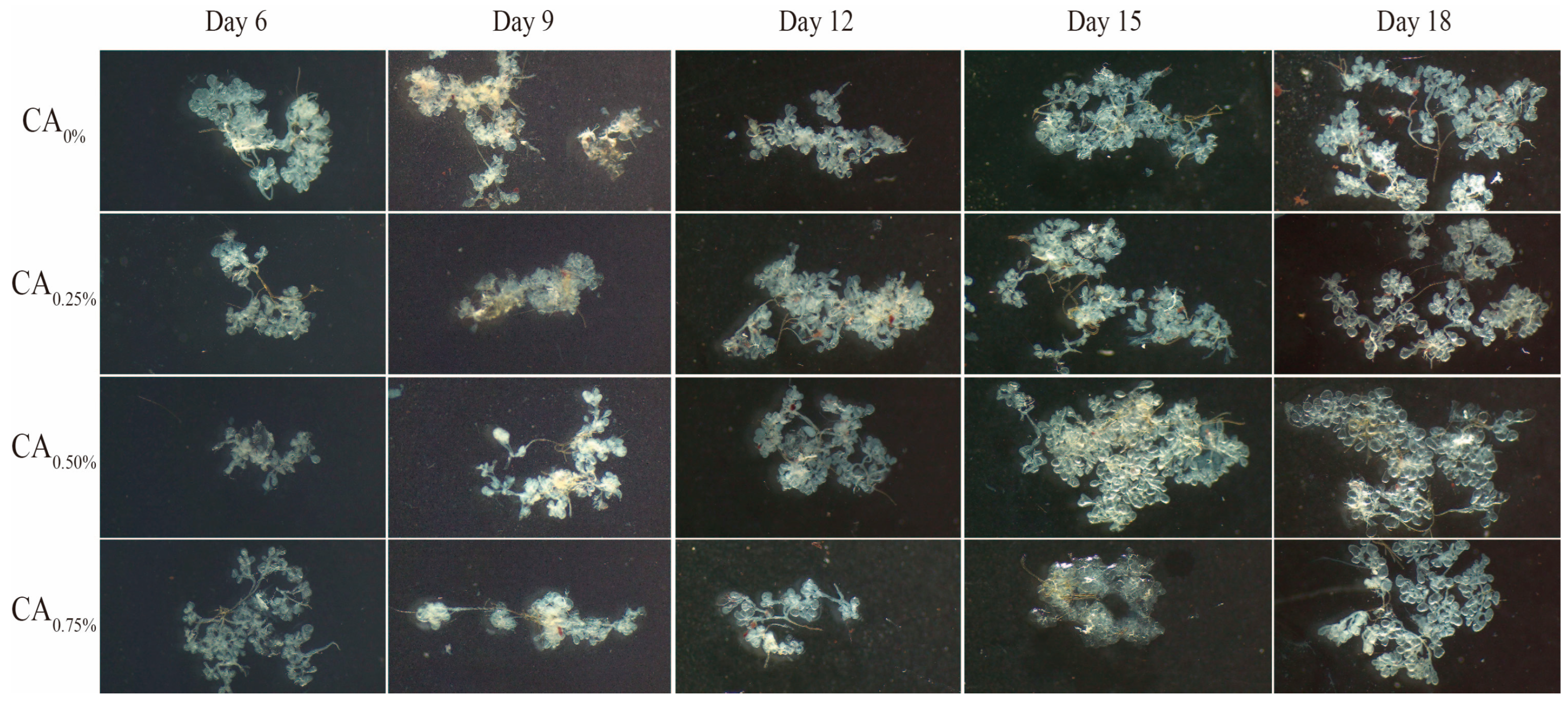
3.3.2. Hypopharyngeal Glands
3.3.3. Cephalic Salivary Glands
3.4. Effect of Citric Acid Addition to Feed on Royal Jelly Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fabrice, R.; Nestor, P.; Andersson, G.K.S.; Elsa, B.; Isabelle, M.; Garibaldi, L.A. Bee and non-bee pollinator importance for local food security. Trends Ecol. Evol. 2022, 38, 196–205. [Google Scholar]
- Khalifa, S.A.M.; Elshafiey, E.H.; Shetaia, A.A.; El-Wahed, A.A.A.; Algethami, A.F.; Musharraf, S.G.; AlAjmi, M.F.; Zhao, C.; Masry, S.H.D.; Abdel-Daim, M.M.; et al. Overview of Bee Pollination and Its Economic Value for Crop Production. Insects 2021, 12, 688. [Google Scholar] [CrossRef] [PubMed]
- Papini, R.; Mancianti, F.; Canovai, R.; Cosci, F.; Rocchigiani, G.; Benelli, G.; Canale, A. Prevalence of the microsporidian Nosema ceranae in honeybee (Apis mellifera) apiaries in Central Italy. Saudi J. Biol. Sci. 2017, 24, 979–982. [Google Scholar] [CrossRef] [PubMed]
- Simon, U.E.; Moritz, R.F.A.; Crewe, R.M. The ontogenetic pattern of mandibular gland components in queenless worker bees (Apis mellifera capensis Esch.). J. Insect Physiol. 2001, 47, 735–738. [Google Scholar] [CrossRef]
- Wu, Y.Q.; Zheng, H.Q.; Corona, M.; Pirk, C.; Meng, F.; Zheng, Y.F.; Hu, F.L. Comparative transcriptome analysis on the synthesis pathway of honey bee (Apis mellifera) mandibular gland secretions. Sci. Rep. 2017, 7, 4530. [Google Scholar] [CrossRef] [PubMed]
- Langlands, Z.; Du Rand, E.E.; Yusuf, A.A.; Pirk, C.W.W. Functional response of the hypopharyngeal glands to a social parasitism challenge in Southern African honey bee subspecies. Parasitol. Res. 2022, 121, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, Z.L.; Tian, L.Q.; Qin, Q.H.; Wu, X.B.; Yan, W.-Y.; Zeng, Z.-J. Transcriptome differences in the hypopharyngeal gland between Western Honeybees (Apis mellifera) and Eastern Honeybees (Apis cerana). BMC Genom. 2014, 15, 744. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Correia-Oliveira, M.; Shemilt, S.; Drijfhout, F. Is the Salivary Gland Associated with Honey Bee Recognition Compounds in Worker Honey Bees (Apis mellifera)? J. Chem. Ecol. 2018, 44, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Billen, J.; Sobotnik, J. Insect exocrine glands. Arthropod Struct. Dev. 2015, 44, 399–400. [Google Scholar] [CrossRef]
- Al-Sherif, A.A.; Mazeed, A.M.; Ewis, M.A.; Nafea, E.A.; Hagag, E.S.E.; Kamel, A.A. Activity of salivary glands in secreting honey-elaborating enzymes in two subspecies of honeybee (Apis mellifera L.). Physiol. Entomol. 2017, 42, 397–403. [Google Scholar] [CrossRef]
- Noll, F.B.; Justino, C.E.L.; Almeida, E.A.B.; Mateus, S.; Billen, J. Would wax glands help us to understand the relationships among corbiculate bees? Insectes Sociaux 2021, 68, 191–197. [Google Scholar] [CrossRef]
- Herbert, E.W.; Shimanuki, H. Effect of Fat Soluble Vitamins on the Brood Rearing Capabilities of Honey Bees Fed a Synthetic Diet. Ann. Entomol. Soc. Am. 1978, 71, 689–691. [Google Scholar] [CrossRef]
- Hyeonjeong, J.; Sampat, G.; Sukjun, S.; Hyeon-Woo, N.; Jun, C.K.; Sungmin, J.; Chuleui, J. Influence of Chlorella supplemented diet on honey bee (Apis mellifera) colony health. J. Asia-Pac. Entomol. 2023, 26, 102096. [Google Scholar]
- Wu, X.; Tovilla-Coutio, D.B.; Eiteman, M.A. Engineered citrate synthase improves citramalic acid generation in Escherichia coli. Biotechnol. Bioeng. 2020, 117, 2781–2790. [Google Scholar] [CrossRef] [PubMed]
- Beier, R.; Harvey, R.; Hernandez, C.; Hume, M.; Andrews, K.; Droleskey, R.; Davidson, M.; Bodeis-Jones, S.; Young, S.; Duke, S.; et al. Interactions of organic acids with Campylobacter coli from swine. PLoS ONE 2018, 13, e0202100. [Google Scholar] [CrossRef] [PubMed]
- Sahni, O.; Didzbalis, J.; Munafo, J.P., Jr. Saltiness Enhancement through the Synergism of Pyroglutamyl Peptides and Organic Acids. J. Agric. Food Chem. 2023, 72, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, A.; Tugnoli, B.; Rossi, B.; Giovagnoni, G.; Grilli, E. Nature-Identical Compounds and Organic Acids Reduce E. coli K88 Growth and Virulence Gene Expression In Vitro. Toxins 2020, 12, 468. [Google Scholar] [CrossRef]
- Hisano, H.; Sanchez, M.S.S.; Nascimento, M.S. Citric acid as a feed additive in pacu Piaractus mesopotamicus (Holmberg, 1887) diets. J. Appl. Ichthyol. 2017, 33, 478–484. [Google Scholar] [CrossRef]
- Elbaz, A.M.; Ibrahim, N.S.; Shehata, A.M.; Mohamed, N.G.; Abdel-Moneim, A.M.E. Impact of multi-strain probiotic, citric acid, garlic powder or their combinations on performance, ileal histomorphometry, microbial enumeration and humoral immunity of broiler chickens. Trop. Anim. Health Prod. 2021, 53, 115. [Google Scholar] [CrossRef]
- Ghayour-Najafabadi, P.; Khosravinia, H.; Gheisari, A.; Azarfar, A.; Khanahmadi, M. Productive performance, nutrient digestibility and intestinal morphometry in broiler chickens fed corn or wheat-based diets supplemented with bacterial- or fungal-originated xylanase. Ital. J. Anim. Sci. 2018, 17, 165–174. [Google Scholar] [CrossRef]
- Plettner, E.; Slessor, K.N.; Winston, M.L. Biosynthesis of Mandibular Acids in Honey Bees (Apis mellifera): De novo Synthesis, Route of Fatty Acid Hydroxylation and Caste Selective β-Oxidation. Insect Biochem. Mol. Biol. 1998, 28, 31–42. [Google Scholar] [CrossRef]
- Hasan, A.; Qazi, J.; Tabssum, F.; Hussain, A. Feeding probiotics and organic acids to honeybees enhances acinal surface area of their hypopharyngeal glands. Res. Vet. Sci. 2022, 149, 47–50. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, Y.; Wu, Y.; Wang, S.; Zheng, H.; Hu, F. The effect of nutritional status on the synthesis ability, protein content and gene expression of mandibular glands in honey bee (Apis mellifera) workers. J. Apic. Res. 2022. [Google Scholar] [CrossRef]
- Ararso, Z.; Ma, C.; Qi, Y.; Feng, M.; Han, B.; Hu, H.; Meng, L.; Li, J. Proteome Comparisons between Hemolymph of Two Honeybee Strains (Apis mellifera ligustica) Reveal Divergent Molecular Basis in Driving Hemolymph Function and High Royal Jelly Secretion. J. Proteome Res. 2018, 17, 402–419. [Google Scholar] [CrossRef] [PubMed]
- Ricigliano, V.A.; Cank, K.B.; Todd, D.A. Metabolomics-Guided Comparison of Pollen and Microalgae-Based Artificial Diets in Honey Bees. J. Agric. Food Chem. 2022, 70, 9790–9801. [Google Scholar] [CrossRef]
- Carreck, N.L.; Andree, M.; Brent, C.S.; Cox-Foster, D.; Dade, H.A.; Ellis, J.D.; Hatjina, F.; Vanenglesdorp, D. Standard methods for Apis mellifera anatomy and dissection. J. Apic. Res. 2013, 52, 1–40. [Google Scholar] [CrossRef]
- Jeroen, D.; Johan, B. Age-dependent morphology and ultrastructure of the hypopharyngeal gland of Apis mellifera workers (Hymenoptera, Apidae). Apidologie 2005, 36, 49–57. [Google Scholar]
- Grandi-Hoffman, D.; Chen, Y.; Huang, E.; Huang, M.H. The effect of diet on protein concentration, hypopharyngeal gland development and virus load in worker honey bees (Apis mellifera L.). J. Insect Physiol. 2010, 56, 1184–1191. [Google Scholar] [CrossRef]
- Bovi, T.d.S.; Caeiro, A.; Santos, S.A.A.d.; Zaluski, R.; Shinohara, A.J.; Lima, G.P.P.; Campos, M.D.G.R.; Junior, L.A.J.; Orsi, R.D.O. Seasonal variation of flavonoid content in bee bread: Potential impact on hypopharyngeal gland development in Apis mellifera honey bees. J. Apic. Res. 2020, 59, 170–177. [Google Scholar] [CrossRef]
- Bouzar, C.; Bankhead Dronnet, S.; Gévar, J.; Darrouzet, E. Chemical and genetic evidences that multiple hornet colonies attack honeybee colonies. Insectes Sociaux 2022, 69, 159–168. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, L.; Wang, H.; Liu, Z.; Chi, X.; Xu, B. Effects of Sucrose Feeding on the Quality of Royal Jelly Produced by Honeybee Apis mellifera L. Insects 2023, 14, 742. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, L.; Zhang, W.; Cui, X.; Wang, H.; Xu, B. Comparison of the nutrient composition of royal jelly and worker jelly of honey bees (Apis mellifera). Apidologie 2016, 47, 48–56. [Google Scholar] [CrossRef]
- Bayram, N.E.; Cebi, N.; Elik, S.; Gercek, Y.C.; Zkk, A. Turkish royal jelly: Amino acid, physicochemical, antioxidant, multi-elemental, antibacterial and fingerprint profiles by analytical techniques combined with chemometrics. J. Apic. Res. 2021, 60, 751–764. [Google Scholar] [CrossRef]
- Beykaya, M.; Inkaya, N.; Yorulmaz Onder, E.; Arici, Y.; Sahin, H. Comprehensive Study of the Physicochemical Properties of Royal Jelly from Various Regions of Türkiye. Chem. Biodivers. 2023, 20, e202300881. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.R.; Yang, Y.C.; Shi, L.S.; Peng, C.C. Antioxidant Properties of Royal Jelly Associated with Larval Age and Time of Harvest. J. Agric. Food Chem. 2008, 56, 11447–11452. [Google Scholar] [CrossRef] [PubMed]
- Usui, S.; Soda, M.; Iguchi, K.; Abe, N.; Kitaichi, K. Down-regulation of aquaporin 9 gene transcription by 10-ydroxyecenoic acid: A major fatty acid in royal jelly. Food Sci. Nutr. 2019, 7, 3819–3826. [Google Scholar] [CrossRef] [PubMed]
- Andyshe, R.; Nazemi-Rafie, J.; Maleki, M.; Fatehi, F. Comparative proteomics analysis of the head proteins of worker honey bees (Apis mellifera) in production stage of royal jelly. J. Apic. Res. 2022, 113, 253–270. [Google Scholar] [CrossRef]
- Brighenti, D.M.; Brighenti, C.R.G.; Carvalho, C.F. Life spans of Africanized honey bees fed sucrose diets enhanced with citric acid or lemon juice. J. Apic. Res. 2017, 56, 91–99. [Google Scholar] [CrossRef]
- Ling-Hsiu, L.; Wen-Yen, W.; May, B. Impacts of Dietary Phytochemicals in the Presence and Absence of Pesticides on Longevity of Honey Bees (Apis mellifera). Insects 2017, 8, 22. [Google Scholar]
- Sun, K.; Liu, H.; Fan, H.; Liu, T.; Zheng, C. Research progress on the application of feed additives in ruminal methane emission reduction: A review. PeerJ 2021, 9, e11151. [Google Scholar] [CrossRef]
- García Beltrán, J.; Esteban, M. Nature-identical compounds as feed additives in aquaculture. Fish Shellfish Immunol. 2022, 123, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, S.H.; Dong, F.M.; Hardy, R.W. Effects of dietary supplements on the availability of minerals in fish meal; preliminary observations. Aquaculture 1998, 160, 283–303. [Google Scholar] [CrossRef]
- Falkowski, J.F.; Aherne, F.X. Fumaric and Citric Acid as Feed Additives in Starter Pig Nutrition. J. Anim. Sci. 1988, 66, 2598–2605. [Google Scholar] [CrossRef]
- Peng, M.L.; Han, J.; Li, L.L.; Ma, H.T. Metabolomics reveals the mechanism of (-)-hydroxycitric acid promotion of protein synthesis and inhibition of fatty acid synthesis in broiler chickens. Animal 2018, 12, 774–783. [Google Scholar] [CrossRef]
- Han, N.; Li, L.; Peng, M.; Ma, H. (-)-Hydroxycitric Acid Nourishes Protein Synthesis via Altering Metabolic Directions of Amino Acids in Male Rats. Phytother. Res. PTR 2016, 30, 1316–1329. [Google Scholar] [CrossRef]
- Yadikar, N.; Ahmet, A.; Zhu, J.; Bao, X.; Yang, X.; Han, H.; Rozi, P. Exploring the mechanism of citric acid for treating glucose metabolism disorder induced by hyperlipidemia. J. Food Biochem. 2022, 46, e14404. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Haiyan, H.; Ying, M.; Shudi, L.; Zhimin, L. Determiners of cell fates: The tricarboxylic acid cycle versus the citrate-malate shuttle. Protein Cell 2022, 14, 162–164. [Google Scholar]
- Cui, M.; Zhang, Y.; Si, N.; Zhou, Y.; Wang, K.; Wang, H.; Li, G.; Ni, L.; Zhao, H. Mechanism of Xiaoer Chiqiao Qingre Granules in clearing heat and removing food stagnation in suckling rats with fever and food accumulation based on metabolomics. China J. Chin. Mater. Medica 2023, 48, 811–822. [Google Scholar] [CrossRef]
- Cui, E.; Tang, P.; Zhu, X.; Lv, M.; Wang, S.; Xue, Y.; Li, C.; Zhao, S. Network Pharmacology Combined with an Experimental Validation Study to Reveal the Effect and Mechanism of Eucommia ulmoides Leaf Polysaccharide against Immunomodulation. Foods 2023, 12, 1062. [Google Scholar] [CrossRef]
- Xue, J.; Huang, X.; Liu, Z.; Chen, Y.; Zhang, Y.; Luo, Y.; Wang, B.; Wang, Q.; Wang, C. Effects of citric acid supplementation on growth performance, intestinal morphology and microbiota, and blood parameters of geese from 1 to 28 days of age. Poult. Sci. 2023, 102, 102343. [Google Scholar] [CrossRef]
- Ferreira, J.L.W.; Watanabe, P.H.; Mendonca, I.B.; Nogueira, B.D.; Ferreira, A.C.S.; Nepomuceno, R.C.; Pascoal, L.A.F.; Almeida, J.M.S.; Guerra, R.R.; Trevisan, M.T.S.; et al. Calcium anacardate and citric acid as growth promoters for weaned piglets. Livest. Sci. 2020, 238. [Google Scholar] [CrossRef]
- Jianke, L.; Mao, F.; Begna, D.; Yu, F.; Aijuan, Z. Proteome comparison of hypopharyngeal gland development between Italian and royal jelly producing worker honeybees (Apis mellifera L.). J. Proteome Res. 2010, 9, 6578–6594. [Google Scholar] [PubMed]


| Groups | Moisture Content/% | 10-HDA/% | Total Protein/% | Water-Soluble Protein g/100 g | Total Sugar (as Glucose)/% | Acidity (1 mol/L NaOH)/(mL/100 g) | Total Phenolic Acid mg/g |
|---|---|---|---|---|---|---|---|
| CA0% | 66.80 ± 0.20 a | 1.725 ± 0.005 a | 12.65 ± 0.25 ab | 9.309 ± 0.501 a | 10.28 ± 0.32 a | 32.25 ± 0.25 a | 20.949 ± 2.522 a |
| CA0.50% | 66.30 ± 0.10 b | 1.995 ± 0.005 b | 12.30 ± 0.30 a | 9.969 ± 0.347 a | 9.78 ± 0.08 b | 34.70 ± 0.10 b | 22.272 ± 2.522 a |
| CA0.75% | 63.45 ± 0.15 c | 1.755 ± 0.005 a | 13.10 ± 0.20 b | 9.672 ± 0.379 a | 10.31 ± 0.09 a | 31.65 ± 0.25 a | 37.728 ± 3.007 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Ji, Q.; Zheng, X.; Zhang, J.; Wang, R.; Wang, X.; Peng, W.; Guo, J.; Zhao, Y. Consumption of Citric Acid by Bees Promotes the Gland Development and Enhances Royal Jelly Quality. Life 2024, 14, 340. https://doi.org/10.3390/life14030340
Wang X, Ji Q, Zheng X, Zhang J, Wang R, Wang X, Peng W, Guo J, Zhao Y. Consumption of Citric Acid by Bees Promotes the Gland Development and Enhances Royal Jelly Quality. Life. 2024; 14(3):340. https://doi.org/10.3390/life14030340
Chicago/Turabian StyleWang, Xue, Quanzhi Ji, Xing Zheng, Jun Zhang, Rongshen Wang, Xinyu Wang, Wenjun Peng, Jun Guo, and Yazhou Zhao. 2024. "Consumption of Citric Acid by Bees Promotes the Gland Development and Enhances Royal Jelly Quality" Life 14, no. 3: 340. https://doi.org/10.3390/life14030340
APA StyleWang, X., Ji, Q., Zheng, X., Zhang, J., Wang, R., Wang, X., Peng, W., Guo, J., & Zhao, Y. (2024). Consumption of Citric Acid by Bees Promotes the Gland Development and Enhances Royal Jelly Quality. Life, 14(3), 340. https://doi.org/10.3390/life14030340







