Angioleiomyoma: An Update with a 142-Case Series
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Inclusion and Exclusion Criteria
2.4. Data Analysis
3. Results
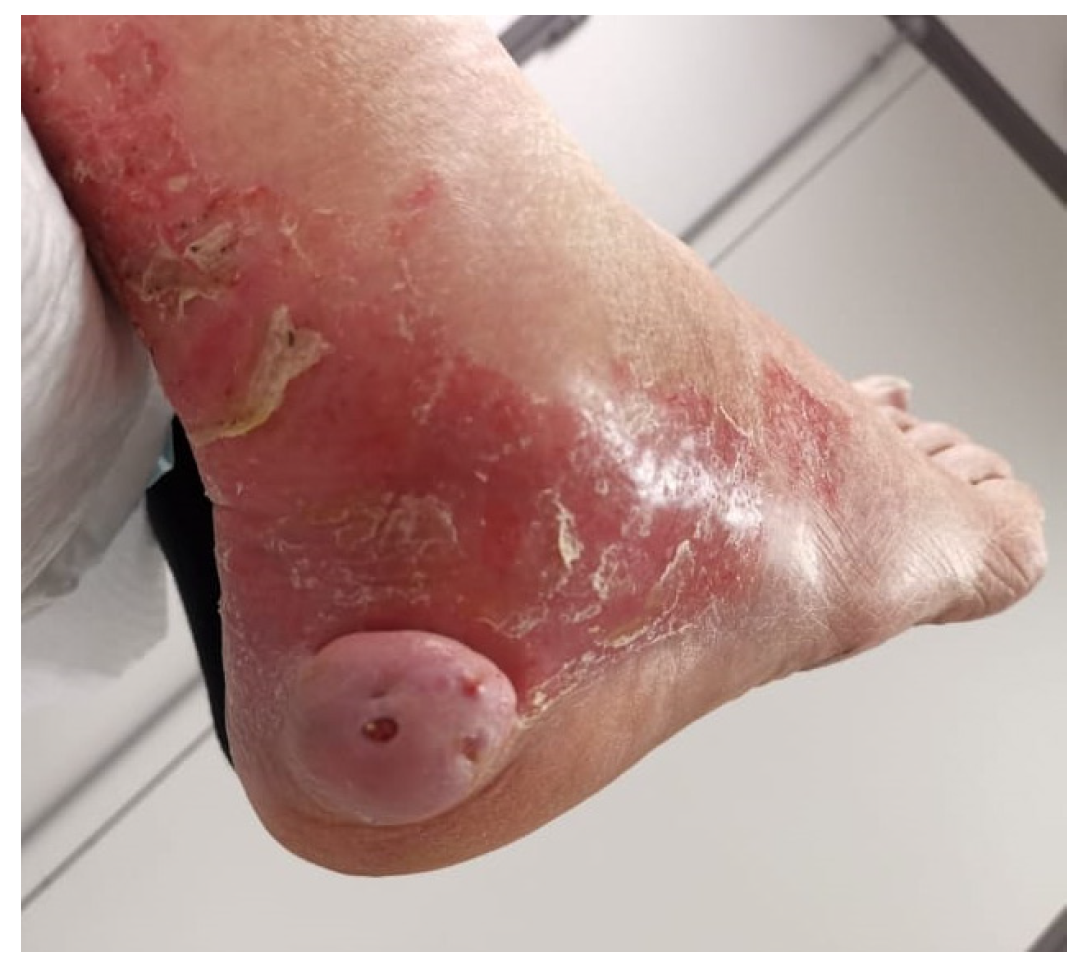
3.1. Clinical Data
3.2. Radiological Data
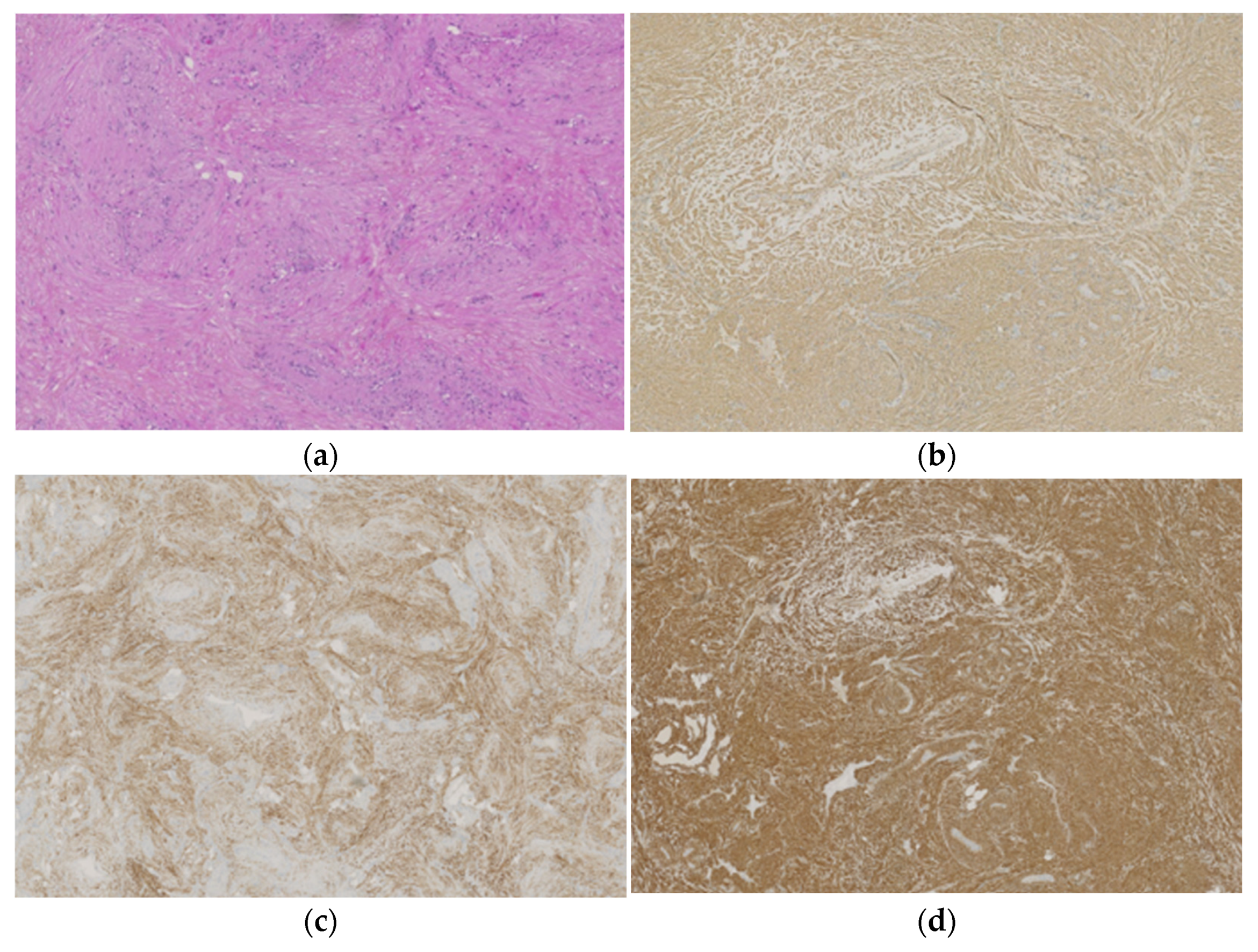
3.3. Histopathological Data
4. Discussion
4.1. Cross-Analysis
4.2. Imaging Features
4.3. Pathological Features
4.4. Strength and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koga, M.; Nishio, J.; Koga, T.; Koga, K.; Nakayama, S.; Yamamoto, T. An Update on Clinicopathological, Imaging, and Genetic Features of Angioleiomyoma. Cancer Diagn. Progn. 2023, 3, 145–150. [Google Scholar] [CrossRef]
- Kulkarni, M.S.; Vijayan, S.; Naik, M.; Rao, S.K. A Rare Tumour of Hand: Angioleiomyoma. BMJ Case Rep. 2017, 2017, bcr-2017-220005. [Google Scholar] [CrossRef]
- Houdek, M.T.; Rose, P.S.; Shon, W.; Kakar, S. Angioleiomyoma of the Upper Extremity. J. Hand Surg. 2013, 38, 1579–1583. [Google Scholar] [CrossRef]
- Yeung, C.M.; Moore, L.; Lans, J.; Lozano-Calderón, L. Angioleiomyoma of the Hand: A Case Series and Review of the Literature. Arch. Bone Jt. Surg. 2020, 8, 373–377. [Google Scholar] [CrossRef]
- Lawson, G.M.; Salter, D.M.; Hooper, G. Angioleiomyomas of the Hand: A Report of 14 Cases. J. Hand Surg. 1995, 20, 479–483. [Google Scholar] [CrossRef]
- Uchida, M.; Kojima, T.; Hirase, Y.; Lizuka, T. Clinical Characteristics of Vascular Leiomyoma of the Upper Extremity: Report of 11 Cases. Br. J. Plast. Surg. 1992, 45, 547–549. [Google Scholar] [CrossRef] [PubMed]
- Moncef, E.; Abdessamad, K.; Najib, A.; Najib, A.; Yacoubi, H. Léiomyome Vasculaire de l’avant Bras: Présentation d’un Cas Clinique et Revue de La Literature. Pan. Afr. Med. J. 2014, 19, 222. [Google Scholar] [CrossRef] [PubMed]
- Hanft, J.; Carbonell, J.; Do, H. Angioleiomyoma of the Lower Extremity. J. Am. Podiatr. Med. Assoc. 1997, 87, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Szolomayer, L.K.; Talusan, P.G.; Chan, W.F.; Lindskog, D.M. Leiomyoma of the Foot and Ankle: A Case Series. Foot Ankle Spec. 2017, 10, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Department of Radiology, Pamela Youde Nethersole Eastern Hospital, Chai Wan, Hong Kong; Lai, A.Y.; Tam, C.; Shum, J.S.; Khoo, J.L.; Tang, W. Magnetic Resonance Imaging Features of Vascular Leiomyoma of the Ankle. Hong Kong Med. J. 2015, 21, 73–76. [Google Scholar] [CrossRef]
- Liu, Y.; Li, B.; Li, L.; Liu, Y.; Wang, C.; Zha, L. Angioleiomyomas in the Head and Neck: A Retrospective Clinical and Immunohistochemical Analysis. Oncol. Lett. 2014, 8, 241–247. [Google Scholar] [CrossRef]
- Alam, M.S.; Subramanian, N.; Koka, K.; Subramanian, K. Orbital Angioleiomyoma: A Rare Orbital Neoplasm. Orbit 2016, 35, 113–116. [Google Scholar] [CrossRef]
- Giambusso, M.; Caprino, P.; Sacchetti, F.; Potenza, A.E.; Pastena, D.; Sofo, L. Multiloculated Omental Cystic Tumor Hiding an Angioleiomyoma: Case Report of a Rare and Atypical Presentation and Literature Review. J. Surg. Case Rep. 2023, 2023, rjad231. [Google Scholar] [CrossRef]
- Hachisuga, T.; Hashimoto, H.; Enjoji, M. Angioleiomyoma. A Clinicopathologic Reappraisal of 562 Cases. Cancer 1984, 54, 126–130. [Google Scholar] [CrossRef]
- Woo, K.S.; Kim, S.H.; Kim, H.S.; Cho, P.D. Clinical Experience with Treatment of Angioleiomyoma. Arch. Plast. Surg. 2014, 41, 374–378. [Google Scholar] [CrossRef]
- Edo, H.; Matsunaga, A.; Matsukuma, S.; Mikoshi, A.; Susa, M.; Horiuchi, K.; Shinmoto, H. Angioleiomyoma of the Extremities: Correlation of Magnetic Resonance Imaging with Histopathological Findings in 25 Cases. Skelet. Radiol. 2022, 51, 837–848. [Google Scholar] [CrossRef]
- Morimoto, N. Angiomyoma. A Clinicopathologic Study. Med. J. Kagoshima Univ. 1973, 24, 663–687. [Google Scholar]
- Yoo, H.J.; Choi, J.-A.; Chung, J.-H.; Oh, J.H.; Lee, G.-K.; Choi, J.-Y.; Hong, S.H.; Kang, H.S. Angioleiomyoma in Soft Tissue of Extremities: MRI Findings. Am. J. Roentgenol. 2009, 192, W291–W294. [Google Scholar] [CrossRef] [PubMed]
- Bodapati, V.S.; Sunderamoorthy, D. Angioleiomyoma—Rare Soft Tissue Tumor of the Foot and Ankle, Review of Two Patients and Review of the Literature. J. Surg. Case Rep. 2021, 2021, rjab535. [Google Scholar] [CrossRef]
- Yuk Kwan Tang, C.; Fung, B.; Fok, M.; Zhu, J. Schwannoma in the Upper Limbs. BioMed Res. Int. 2013, 2013, 167196. [Google Scholar] [CrossRef] [PubMed]
- Adani, R.; Tarallo, L.; Mugnai, R.; Colopi, S. Schwannomas of the Upper Extremity: Analysis of 34 Cases. Acta Neurochir. 2014, 156, 2325–2330. [Google Scholar] [CrossRef]
- Morey, V.M.; Garg, B.; Kotwal, P.P. Glomus Tumours of the Hand: Review of Literature. J. Clin. Orthop. Trauma 2016, 7, 286–291. [Google Scholar] [CrossRef]
- Cox, J.; Bartlett, E.; Lee, E. Vascular Malformations: A Review. Semin. Plast. Surg. 2014, 28, 058–063. [Google Scholar] [CrossRef]
- Wang, C.; Song, R.-R.; Kuang, P.-D.; Wang, L.-H.; Zhang, M.-M. Giant Cell Tumor of the Tendon Sheath: Magnetic Resonance Imaging Findings in 38 Patients. Oncol. Lett. 2017, 13, 4459–4462. [Google Scholar] [CrossRef]
- Spinnato, P.; Sambri, A.; Fujiwara, T.; Ceccarelli, L.; Clinca, R.; Medellin, M.R.; Paolis, M.D.; Donati, D.M.; Bianchi, G. Myxofibrosarcoma: Clinical and Prognostic Value of MRI Features. Curr. Med. Imaging 2021, 17, 217–224. [Google Scholar] [CrossRef]
- Vanni, S.; De Vita, A.; Gurrieri, L.; Fausti, V.; Miserocchi, G.; Spadazzi, C.; Liverani, C.; Cocchi, C.; Calabrese, C.; Bongiovanni, A.; et al. Myxofibrosarcoma Landscape: Diagnostic Pitfalls, Clinical Management and Future Perspectives. Ther. Adv. Med. Oncol. 2022, 14, 17588359221093973. [Google Scholar] [CrossRef]
- Gazendam, A.M.; Popovic, S.; Munir, S.; Parasu, N.; Wilson, D.; Ghert, M. Synovial Sarcoma: A Clinical Review. Curr. Oncol. 2021, 28, 1909–1920. [Google Scholar] [CrossRef]
- Mahajan, H.; Lorigan, J.G.; Shirkhoda, A. Synovial Sarcoma: MR Imaging. Magn. Reson. Imaging 1989, 7, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Varela-Duran, J.; Enzinger, F.M. Calcifying Synovial Sarcoma. Cancer 1982, 50, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Wilkerson, B.; Crim, J.; Hung, M.; Layfield, L. Characterization of Synovial Sarcoma Calcification. AJR Am. J. Roentgenol. 2012, 199, W730–W734. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Bernal, S.; Rodríguez-Pazos, L.; Concheiro, J.; Ginarte, M.; Toribio, J. Calcified Acral Angioleiomyoma. J. Cutan. Pathol. 2010, 37, 710–711. [Google Scholar] [CrossRef] [PubMed]
- Herren, D.B.; Zimmermann, A.; Büchler, U. Vascular Leiomyoma in an Index Finger Undergoing Malignant Transformation. J. Hand Surg. 1995, 20, 484–487. Available online: https://journals.sagepub.com/doi/epdf/10.1016/S0266-7681%2805%2980158-5 (accessed on 21 July 2023). [CrossRef] [PubMed]
- Vetterkind, S.; Morgan, K.G. Regulation of Smooth Muscle Contraction. In Muscle; Elsevier: Amsterdam, The Netherlands, 2012; pp. 1173–1180. ISBN 978-0-12-381510-1. [Google Scholar]



| Women n = 75 (53%) | Men n = 67 (47%) | Total n = 142 (100%) | p Value | |
|---|---|---|---|---|
| Upper Limb | 13 (43%) | 17(57%) | 30(21%) | 0.24 |
| Hand | 9 | 13 | 22 | 0.22 |
| Elbow | 3 | 3 | 6 | 1.00 |
| Arm | 1 | 1 | 2 | 1.00 |
| Lower Limb | 59 (55%) | 49 (45%) | 108 (76%) | 0.44 |
| Toes | 1 | 1 | 2 | 1.0 |
| Foot | 10 | 8 | 18 | 0.80 |
| Heel | 8 | 6 | 14 | 0.73 |
| Ankle | 9 | 8 | 17 | 0.99 |
| Leg | 15 | 8 | 23 | 0.19 |
| Knee | 14 | 16 | 30 | 0.44 |
| Thigh | 2 | 2 | 4 | 1.00 |
| Head and neck | 3 (75%) | 1 (15%) | 4 (3%) | 0.62 |
| Helix | 0 | 1 | 1 | 0.47 |
| Cheek | 1 | 0 | 1 | 1.00 |
| Lip | 2 | 0 | 2 | 0.49 |
| n | % | |
| Gender | ||
| Female | 75 | 53 |
| Male | 67 | 47 |
| Age | Mean (min–max) | - |
| Total | 59 (1–87) | - |
| Women | 61 (32–87) | - |
| Men | 57 (1–83) | - |
| Mode of discovery | n | % |
| Volume increase | 45 | 32 |
| Pain | 46 | 32 |
| Incidental finding on imaging | 4 | 3 |
| Discomfort | 18 | 13 |
| Inflammation | 1 | 1 |
| Presence of mass | 18 | 13 |
| No information | 10 | 7 |
| Type of pain * | ||
| No pain | 68 | 48 |
| Tenderness | 50 | 35 |
| Spontaneous | 13 | 9 |
| Paresthesia | 1 | 1 |
| No information | 14 | 10 |
| Laterality | ||
| Right | 73 | 51 |
| Left | 66 | 46 |
| No information | 3 | 2 |
| Depth (n = 138) | ||
| Subcutaneous | 120 | 85 |
| Subaponeurotic | 11 | 8 |
| Cutaneous | 7 | 5 |
| Cavernous n = 7 (5%) | Solid n = 86 (65%) | Venous n = 21 (17%) | NA n = 17 (13%) | Total n = 131 | p Value Fisher Test | |
|---|---|---|---|---|---|---|
| No pain | 4 | 42 | 16 | 5 | 67 | 0.06 |
| Palpation pain | 1 | 34 | 5 | 10 | 50 | 0.23 |
| Spontaneous pain | 2 | 9 | 0 | 2 | 13 | 0.07 |
| Paresthesia | 0 | 1 | 0 | 0 | 1 | 1 |
| Cavernous (n = 4) | Solid (n = 57) | Venous (n = 15) | p Value ** | |
|---|---|---|---|---|
| Reticular sign + (n = 42 *) | 1 | 32 | 9 | 0.52 |
| Reticular sign − (n = 34 *) | 3 | 25 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernard, M.; Le Nail, L.-R.; de Pinieux, G.; Samargandi, R. Angioleiomyoma: An Update with a 142-Case Series. Life 2024, 14, 338. https://doi.org/10.3390/life14030338
Bernard M, Le Nail L-R, de Pinieux G, Samargandi R. Angioleiomyoma: An Update with a 142-Case Series. Life. 2024; 14(3):338. https://doi.org/10.3390/life14030338
Chicago/Turabian StyleBernard, Mathilde, Louis-Romée Le Nail, Gonzague de Pinieux, and Ramy Samargandi. 2024. "Angioleiomyoma: An Update with a 142-Case Series" Life 14, no. 3: 338. https://doi.org/10.3390/life14030338
APA StyleBernard, M., Le Nail, L.-R., de Pinieux, G., & Samargandi, R. (2024). Angioleiomyoma: An Update with a 142-Case Series. Life, 14(3), 338. https://doi.org/10.3390/life14030338







