The Use of Transcranial Magnetic Stimulation in Attention Optimization Research: A Review from Basic Theory to Findings in Attention-Deficit/Hyperactivity Disorder and Depression
Abstract
1. Introduction
1.1. The Importance of Attention in Everyday Life
1.2. The Importance of Brain Neuroimaging and TMS in Attention Research
2. Attention Optimization Theory
2.1. Neural Basis of Attention: Brain Regions and Neural Circuits Related to Attention
2.2. Neuroplasticity: Explores How the Brain Adapts and Changes to Optimize Attention
3. Basic Theory of Transcranial Magnetic Stimulation
3.1. Principles and Applications: Explain How TMS Affects Brain Activity through Magnetic Field Stimulation
3.2. Application of TMS in Attention Research: Discuss How TMS Can Be Applied to Regulate Attention Networks
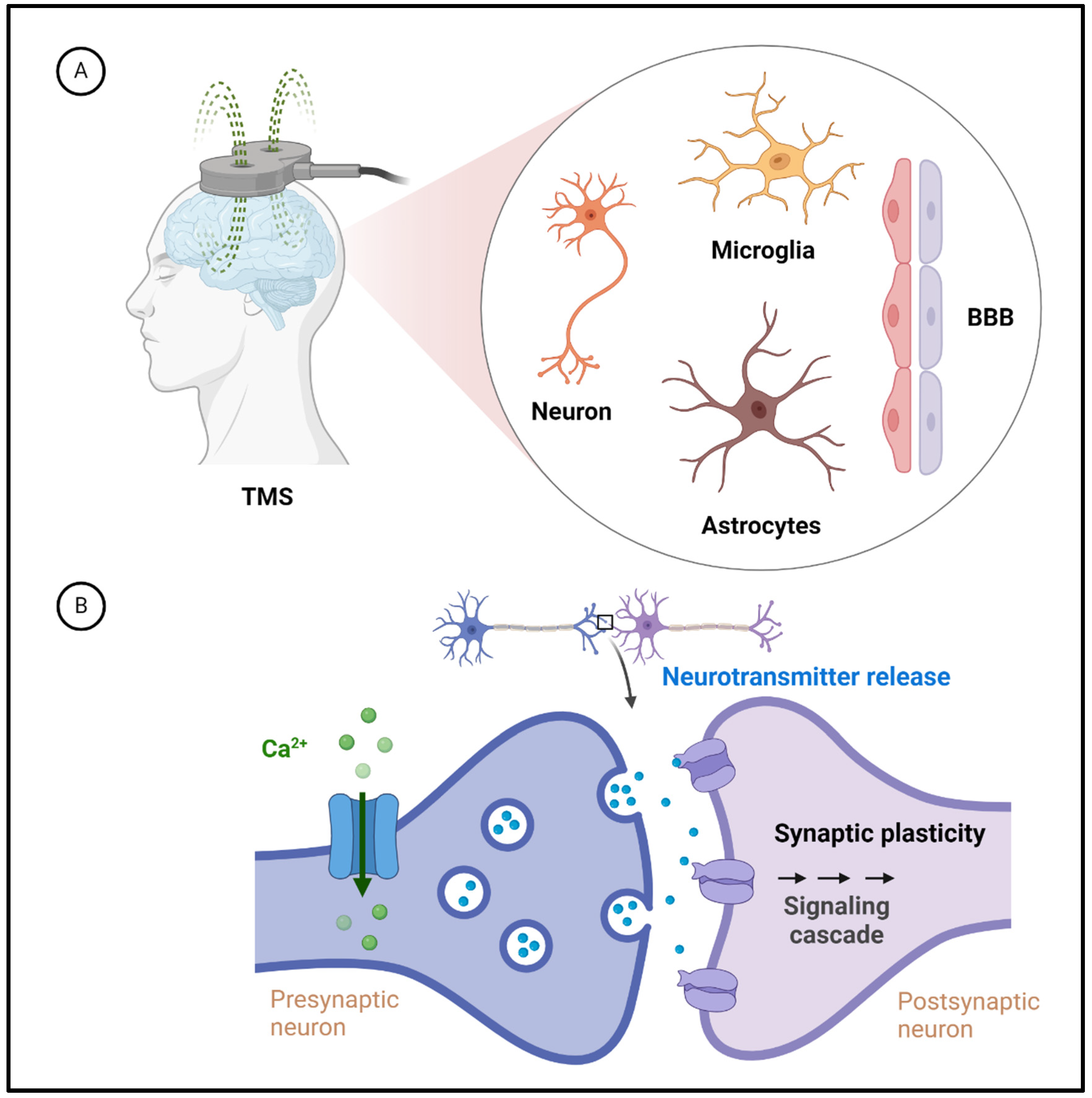
3.3. Molecular and Cellular Mechanisms Underlying TMS Effects on the Brain
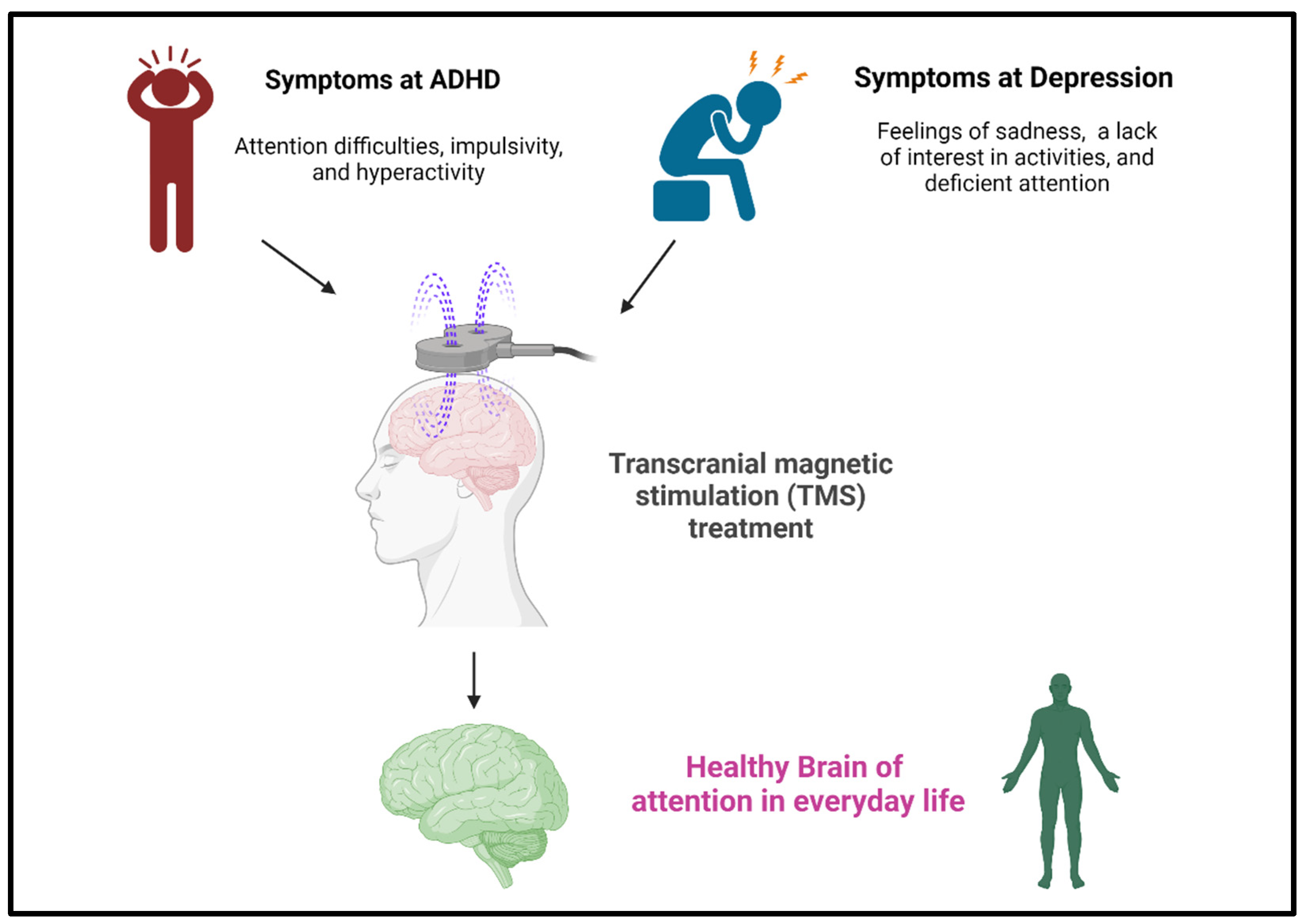
4. Clinical Applications: TMS for ADHD and Depression
4.1. TMS Is an Effective Clinical Treatment for ADHD
4.2. TMS Technology Is an Effective Method for Regulating Depression
4.3. Linking Synaptopathies to Synaptic Plasticity in Neurological Disorders and TMS: A Focus on ADHD and Depression
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oberauer, K. Working Memory and Attention—A Conceptual Analysis and Review. J. Cogn. 2019, 2, 36. [Google Scholar] [CrossRef] [PubMed]
- Lodge, J.M.; Harrison, W.J. The Role of Attention in Learning in the Digital Age. Yale J. Biol. Med. 2019, 92, 21–28. [Google Scholar] [PubMed]
- Draheim, C.; Pak, R.; Draheim, A.A.; Engle, R.W. The role of attention control in complex real-world tasks. Psychon. Bull. Rev. 2022, 29, 1143–1197. [Google Scholar] [CrossRef] [PubMed]
- Tyng, C.M.; Amin, H.U.; Saad, M.N.M.; Malik, A.S. The Influences of Emotion on Learning and Memory. Front. Psychol. 2017, 8, 1454. [Google Scholar] [CrossRef] [PubMed]
- Viviani, R. Emotion regulation, attention to emotion, and the ventral attentional network. Front. Hum. Neurosci. 2013, 7, 746. [Google Scholar] [CrossRef] [PubMed]
- Amso, D.; Scerif, G. The attentive brain: Insights from developmental cognitive neuroscience. Nat. Rev. Neurosci. 2015, 16, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Callan, D.E.; Tsytsarev, V.; Hanakawa, T.; Callan, A.M.; Katsuhara, M.; Fukuyama, H.; Turner, R. Song and speech: Brain regions involved with perception and covert production. Neuroimage 2006, 31, 1327–1342. [Google Scholar] [CrossRef] [PubMed]
- Kiepe, F.; Kraus, N.; Hesselmann, G. Sensory Attenuation in the Auditory Modality as a Window Into Predictive Processing. Front. Hum. Neurosci. 2021, 15, 704668. [Google Scholar] [CrossRef]
- Turoman, N.; Tivadar, R.I.; Retsa, C.; Murray, M.M.; Matusz, P.J. Towards understanding how we pay attention in naturalistic visual search settings. Neuroimage 2021, 244, 118556. [Google Scholar] [CrossRef]
- Harricharan, S.; McKinnon, M.C.; Lanius, R.A. How Processing of Sensory Information From the Internal and External Worlds Shape the Perception and Engagement With the World in the Aftermath of Trauma: Implications for PTSD. Front. Neurosci. 2021, 15, 625490. [Google Scholar] [CrossRef]
- Diniz, C.; Crestani, A.P. The times they are a-changin’: A proposal on how brain flexibility goes beyond the obvious to include the concepts of "upward" and "downward" to neuroplasticity. Mol. Psychiatry 2023, 28, 977–992. [Google Scholar] [CrossRef] [PubMed]
- Siebner, H.R.; Funke, K.; Aberra, A.S.; Antal, A.; Bestmann, S.; Chen, R.; Classen, J.; Davare, M.; Di Lazzaro, V.; Fox, P.T.; et al. Transcranial magnetic stimulation of the brain: What is stimulated?—A consensus and critical position paper. Clin. Neurophysiol. 2022, 140, 59–97. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.; Hanning, N.M.; Carrasco, M. Transcranial magnetic stimulation to frontal but not occipital cortex disrupts endogenous attention. Proc. Natl. Acad. Sci. USA 2023, 120, e2219635120. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.; Chiang, M.C. Examining the effect of online advertisement cues on human responses using eye-tracking, EEG, and MRI. Behav. Brain. Res. 2021, 402, 113128. [Google Scholar] [CrossRef]
- Cuypers, K.; Marsman, A. Transcranial magnetic stimulation and magnetic resonance spectroscopy: Opportunities for a bimodal approach in human neuroscience. Neuroimage 2021, 224, 117394. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Moradei, C.; Cecchelli, C. Will Transcranial Magnetic Stimulation Improve the Treatment of Obsessive-Compulsive Disorder? A Systematic Review and Meta-Analysis of Current Targets and Clinical Evidence. Life 2023, 13, 1494. [Google Scholar] [CrossRef] [PubMed]
- Yeager, B.E.; Dougher, C.C.; Cook, R.H.; Medaglia, J.D. The role of transcranial magnetic stimulation in understanding attention-related networks in single subjects. Curr. Res. Neurobiol. 2021, 2, 100017. [Google Scholar] [CrossRef]
- Yen, C.; Lin, C.L.; Chiang, M.C. Exploring the Frontiers of Neuroimaging: A Review of Recent Advances in Understanding Brain Functioning and Disorders. Life 2023, 13, 1472. [Google Scholar] [CrossRef]
- Sun, W.; Wu, Q.; Gao, L.; Zheng, Z.; Xiang, H.; Yang, K.; Yu, B.; Yao, J. Advancements in Transcranial Magnetic Stimulation Research and the Path to Precision. Neuropsychiatr. Dis. Treat. 2023, 19, 1841–1851. [Google Scholar] [CrossRef]
- Oathes, D.J.; Balderston, N.L.; Kording, K.P.; DeLuisi, J.A.; Perez, G.M.; Medaglia, J.D.; Fan, Y.; Duprat, R.J.; Satterthwaite, T.D.; Sheline, Y.I.; et al. Combining transcranial magnetic stimulation with functional magnetic resonance imaging for probing and modulating neural circuits relevant to affective disorders. Wiley Interdiscip. Rev. Cogn. Sci. 2021, 12, e1553. [Google Scholar] [CrossRef]
- Greene, A.S.; Horien, C.; Barson, D.; Scheinost, D.; Constable, R.T. Why is everyone talking about brain state? Trends Neurosci. 2023, 46, 508–524. [Google Scholar] [CrossRef]
- Curtin, A.; Tong, S.; Sun, J.; Wang, J.; Onaral, B.; Ayaz, H. A Systematic Review of Integrated Functional Near-Infrared Spectroscopy (fNIRS) and Transcranial Magnetic Stimulation (TMS) Studies. Front. Neurosci. 2019, 13, 84. [Google Scholar] [CrossRef]
- Karthikeyan, P.; Moradi, S.; Ferdinando, H.; Zhao, Z.; Myllylä, T. Optics Based Label-Free Techniques and Applications in Brain Monitoring. Appl. Sci. 2020, 10, 2196. [Google Scholar] [CrossRef]
- Chen, W.L.; Wagner, J.; Heugel, N.; Sugar, J.; Lee, Y.W.; Conant, L.; Malloy, M.; Heffernan, J.; Quirk, B.; Zinos, A.; et al. Functional Near-Infrared Spectroscopy and Its Clinical Application in the Field of Neuroscience: Advances and Future Directions. Front. Neurosci. 2020, 14, 724. [Google Scholar] [CrossRef] [PubMed]
- Idlett-Ali, S.L.; Salazar, C.A.; Bell, M.S.; Short, E.B.; Rowland, N.C. Neuromodulation for treatment-resistant depression: Functional network targets contributing to antidepressive outcomes. Front. Hum. Neurosci. 2023, 17, 1125074. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, G.W. Attention in Psychology, Neuroscience, and Machine Learning. Front. Comput. Neurosci. 2020, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Dolcos, F.; Katsumi, Y.; Moore, M.; Berggren, N.; de Gelder, B.; Derakshan, N.; Hamm, A.O.; Koster, E.H.W.; Ladouceur, C.D.; Okon-Singer, H.; et al. Neural correlates of emotion-attention interactions: From perception, learning, and memory to social cognition, individual differences, and training interventions. Neurosci. Biobehav. Rev. 2020, 108, 559–601. [Google Scholar] [CrossRef] [PubMed]
- Knight, H.C.; Smith, D.T.; Ellison, A. The role of the left dorsolateral prefrontal cortex in attentional bias. Neuropsychologia 2020, 148, 107631. [Google Scholar] [CrossRef] [PubMed]
- Nummenmaa, L.; Calder, A.J. Neural mechanisms of social attention. Trends Cogn. Sci. 2009, 13, 135–143. [Google Scholar] [CrossRef]
- Sheth, B.R.; Young, R. Two Visual Pathways in Primates Based on Sampling of Space: Exploitation and Exploration of Visual Information. Front. Integr. Neurosci. 2016, 10, 37. [Google Scholar] [CrossRef]
- Westendorff, S.; Kaping, D.; Everling, S.; Womelsdorf, T. Prefrontal and anterior cingulate cortex neurons encode attentional targets even when they do not apparently bias behavior. J. Neurophysiol. 2016, 116, 796–811. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, E.I. Neural Circuits That Mediate Selective Attention: A Comparative Perspective. Trends Neurosci. 2018, 41, 789–805. [Google Scholar] [CrossRef] [PubMed]
- Pagnotta, M.F.; Pascucci, D.; Plomp, G. Selective attention involves a feature-specific sequential release from inhibitory gating. Neuroimage 2022, 246, 118782. [Google Scholar] [CrossRef] [PubMed]
- da Silva, B.S.; Grevet, E.H.; Silva, L.C.F.; Ramos, J.K.N.; Rovaris, D.L.; Bau, C.H.D. An overview on neurobiology and therapeutics of attention-deficit/hyperactivity disorder. Discov. Ment. Health 2023, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Katzman, M.A.; Bilkey, T.S.; Chokka, P.R.; Fallu, A.; Klassen, L.J. Adult ADHD and comorbid disorders: Clinical implications of a dimensional approach. BMC Psychiatry 2017, 17, 302. [Google Scholar] [CrossRef]
- Voss, P.; Thomas, M.E.; Cisneros-Franco, J.M.; de Villers-Sidani, E. Dynamic Brains and the Changing Rules of Neuroplasticity: Implications for Learning and Recovery. Front. Psychol. 2017, 8, 1657. [Google Scholar] [CrossRef]
- Bauer, C.C.C.; Rozenkrantz, L.; Caballero, C.; Nieto-Castanon, A.; Scherer, E.; West, M.R.; Mrazek, M.; Phillips, D.T.; Gabrieli, J.D.E.; Whitfield-Gabrieli, S. Mindfulness training preserves sustained attention and resting state anticorrelation between default-mode network and dorsolateral prefrontal cortex: A randomized controlled trial. Hum. Brain Mapp. 2020, 41, 5356–5369. [Google Scholar] [CrossRef]
- Han, Y.; Yuan, M.; Guo, Y.S.; Shen, X.Y.; Gao, Z.K.; Bi, X. The role of enriched environment in neural development and repair. Front. Cell Neurosci. 2022, 16, 890666. [Google Scholar] [CrossRef]
- Kumar, J.; Patel, T.; Sugandh, F.; Dev, J.; Kumar, U.; Adeeb, M.; Kachhadia, M.P.; Puri, P.; Prachi, F.; Zaman, M.U.; et al. Innovative Approaches and Therapies to Enhance Neuroplasticity and Promote Recovery in Patients With Neurological Disorders: A Narrative Review. Cureus 2023, 15, e41914. [Google Scholar] [CrossRef]
- Aderinto, N.; AbdulBasit, M.O.; Olatunji, G.; Adejumo, T. Exploring the transformative influence of neuroplasticity on stroke rehabilitation: A narrative review of current evidence. Ann. Med. Surg. 2023, 85, 4425–4432. [Google Scholar] [CrossRef]
- Lefaucheur, J.P. Transcranial magnetic stimulation. Handb. Clin. Neurol. 2019, 160, 559–580. [Google Scholar]
- Chail, A.; Saini, R.K.; Bhat, P.S.; Srivastava, K.; Chauhan, V. Transcranial magnetic stimulation: A review of its evolution and current applications. Ind. Psychiatry J. 2018, 27, 172–180. [Google Scholar]
- Hernandez-Pavon, J.C.; Veniero, D.; Bergmann, T.O.; Belardinelli, P.; Bortoletto, M.; Casarotto, S.; Casula, E.P.; Farzan, F.; Fecchio, M.; Julkunen, P.; et al. TMS combined with EEG: Recommendations and open issues for data collection and analysis. Brain Stimul. 2023, 16, 567–593. [Google Scholar] [CrossRef]
- Li, B.; Virtanen, J.P.; Oeltermann, A.; Schwarz, C.; Giese, M.A.; Ziemann, U.; Benali, A. Lifting the veil on the dynamics of neuronal activities evoked by transcranial magnetic stimulation. Elife 2017, 6, e30552. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Hendee, J.; Ruan, J.; Zhirova, A.; Ye, J.; Dima, M. Neuron matters: Neuromodulation with electromagnetic stimulation must consider neurons as dynamic identities. J. Neuroeng. Rehabil. 2022, 19, 116. [Google Scholar] [CrossRef] [PubMed]
- Rienecker, K.D.A.; Poston, R.G.; Segales, J.S.; Finholm, I.W.; Sono, M.H.; Munteanu, S.J.; Ghaninejad-Esfahani, M.; Rejepova, A.; Tejeda-Garibay, S.; Wickman, K.; et al. Mild membrane depolarization in neurons induces immediate early gene transcription and acutely subdues responses to a successive stimulus. J. Biol. Chem. 2022, 298, 102278. [Google Scholar] [CrossRef]
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A.; Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009, 120, 2008–2039. [Google Scholar] [CrossRef]
- Magnuson, J.; Ozdemir, M.A.; Mathieson, E.; Kirkman, S.; Passera, B.; Rampersad, S.; Dufour, A.B.; Brooks, D.; Pascual-Leone, A.; Fried, P.J.; et al. Neuromodulatory effects and reproducibility of the most widely used repetitive transcranial magnetic stimulation protocols. PLoS ONE 2023, 18, e0286465. [Google Scholar] [CrossRef]
- Kweon, J.; Vigne, M.M.; Jones, R.N.; Carpenter, L.L.; Brown, J.C. Practice makes plasticity: 10-Hz rTMS enhances LTP-like plasticity in musicians and athletes. Front. Neural. Circuits 2023, 17, 1124221. [Google Scholar] [CrossRef]
- Jiang, S.; Carpenter, L.L.; Jiang, H. Optical neuroimaging: Advancing transcranial magnetic stimulation treatments of psychiatric disorders. Vis Comput. Ind. Biomed. Art 2022, 5, 22. [Google Scholar] [CrossRef]
- Somaa, F.A.; de Graaf, T.A.; Sack, A.T. Transcranial Magnetic Stimulation in the Treatment of Neurological Diseases. Front. Neurol. 2022, 13, 793253. [Google Scholar] [CrossRef]
- Zhou, L.; Jin, Y.; Wu, D.; Cun, Y.; Zhang, C.; Peng, Y.; Chen, N.; Yang, X.; Zhang, S.; Ning, R.; et al. Current evidence, clinical applications, and future directions of transcranial magnetic stimulation as a treatment for ischemic stroke. Front. Neurosci. 2023, 17, 1177283. [Google Scholar] [CrossRef]
- Rossi, S.; Antal, A.; Bestmann, S.; Bikson, M.; Brewer, C.; Brockmoller, J.; Carpenter, L.L.; Cincotta, M.; Chen, R.; Daskalakis, J.D.; et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert Guidelines. Clin. Neurophysiol. 2021, 132, 269–306. [Google Scholar] [CrossRef]
- Keller, A.S.; Leikauf, J.E.; Holt-Gosselin, B.; Staveland, B.R.; Williams, L.M. Paying attention to attention in depression. Transl. Psychiatry 2019, 9, 279. [Google Scholar] [CrossRef]
- Liu, A.; Voroslakos, M.; Kronberg, G.; Henin, S.; Krause, M.R.; Huang, Y.; Opitz, A.; Mehta, A.; Pack, C.C.; Krekelberg, B.; et al. Immediate neurophysiological effects of transcranial electrical stimulation. Nat. Commun. 2018, 9, 5092. [Google Scholar] [CrossRef]
- Ozturk, H.; Venugopal, S. Transcranial Magnetic Stimulation as a Therapeutic Option for Neurologic Diseases and Psychiatric Disorders: A Systematic Review. Cureus 2022, 14, e28259. [Google Scholar] [CrossRef]
- Moretti, J.; Rodger, J. A little goes a long way: Neurobiological effects of low intensity rTMS and implications for mechanisms of rTMS. Curr. Res. Neurobiol. 2022, 3, 100033. [Google Scholar] [CrossRef]
- Bleich-Cohen, M.; Gurevitch, G.; Carmi, N.; Medvedovsky, M.; Bregman, N.; Nevler, N.; Elman, K.; Ginou, A.; Zangen, A.; Ash, E.L. A functional magnetic resonance imaging investigation of prefrontal cortex deep transcranial magnetic stimulation efficacy in adults with attention deficit/hyperactive disorder: A double blind, randomized clinical trial. Neuroimage Clin. 2021, 30, 102670. [Google Scholar] [CrossRef]
- Song, P.; Lin, H.; Liu, C.; Jiang, Y.; Lin, Y.; Xue, Q.; Xu, P.; Wang, Y. Transcranial Magnetic Stimulation to the Middle Frontal Gyrus During Attention Modes Induced Dynamic Module Reconfiguration in Brain Networks. Front. Neuroinform. 2019, 13, 22. [Google Scholar] [CrossRef]
- Luber, B.; Lisanby, S.H. Enhancement of human cognitive performance using transcranial magnetic stimulation (TMS). Neuroimage 2014, 85 Pt 3, 961–970. [Google Scholar] [CrossRef]
- Li, Y.; Pang, J.; Wang, J.; Wang, W.; Bo, Q.; Lei, L.; Wang, X.; Wang, M. High-frequency rTMS over the left DLPFC improves the response inhibition control of young healthy participants: An ERP combined (1)H-MRS study. Front. Psychol. 2023, 14, 1144757. [Google Scholar] [CrossRef]
- Bagattini, C.; Zanni, M.; Barocco, F.; Caffarra, P.; Brignani, D.; Miniussi, C.; Defanti, C.A. Enhancing cognitive training effects in Alzheimer’s disease: rTMS as an add-on treatment. Brain Stimul. 2020, 13, 1655–1664. [Google Scholar] [CrossRef]
- Mizutani-Tiebel, Y.; Tik, M.; Chang, K.Y.; Padberg, F.; Soldini, A.; Wilkinson, Z.; Voon, C.C.; Bulubas, L.; Windischberger, C.; Keeser, D. Concurrent TMS-fMRI: Technical Challenges, Developments, and Overview of Previous Studies. Front. Psychiatry 2022, 13, 825205. [Google Scholar] [CrossRef] [PubMed]
- Pitcher, D.; Parkin, B.; Walsh, V. Transcranial Magnetic Stimulation and the Understanding of Behavior. Annu. Rev. Psychol. 2021, 72, 97–121. [Google Scholar] [CrossRef]
- Dufor, T.; Lohof, A.M.; Sherrard, R.M. Magnetic Stimulation as a Therapeutic Approach for Brain Modulation and Repair: Underlying Molecular and Cellular Mechanisms. Int. J. Mol. Sci. 2023, 24, 16456. [Google Scholar] [CrossRef] [PubMed]
- Eldaief, M.C.; Dickerson, B.C.; Camprodon, J.A. Transcranial Magnetic Stimulation for the Neurological Patient: Scientific Principles and Applications. Semin. Neurol. 2022, 42, 149–157. [Google Scholar] [CrossRef]
- Uzair, M.; Abualait, T.; Arshad, M.; Yoo, W.K.; Mir, A.; Bunyan, R.F.; Bashir, S. Transcranial magnetic stimulation in animal models of neurodegeneration. Neural. Regen. Res. 2022, 17, 251–265. [Google Scholar]
- Banerjee, J.; Sorrell, M.E.; Celnik, P.A.; Pelled, G. Immediate Effects of Repetitive Magnetic Stimulation on Single Cortical Pyramidal Neurons. PLoS ONE 2017, 12, e0170528. [Google Scholar] [CrossRef]
- Belardinelli, P.; Konig, F.; Liang, C.; Premoli, I.; Desideri, D.; Muller-Dahlhaus, F.; Gordon, P.C.; Zipser, C.; Zrenner, C.; Ziemann, U. TMS-EEG signatures of glutamatergic neurotransmission in human cortex. Sci. Rep. 2021, 11, 8159. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Bungert, A.; Bowtell, R.; Jackson, S.R. Modulating Brain Networks With Transcranial Magnetic Stimulation Over the Primary Motor Cortex: A Concurrent TMS/fMRI Study. Front. Hum. Neurosci. 2020, 14, 31. [Google Scholar] [CrossRef]
- Vazana, U.; Schori, L.; Monsonego, U.; Swissa, E.; Pell, G.S.; Roth, Y.; Brodt, P.; Friedman, A.; Prager, O. TMS-Induced Controlled BBB Opening: Preclinical Characterization and Implications for Treatment of Brain Cancer. Pharmaceutics 2020, 12, 946. [Google Scholar] [CrossRef]
- Kricheldorff, J.; Goke, K.; Kiebs, M.; Kasten, F.H.; Herrmann, C.S.; Witt, K.; Hurlemann, R. Evidence of Neuroplastic Changes after Transcranial Magnetic, Electric, and Deep Brain Stimulation. Brain Sci. 2022, 12, 929. [Google Scholar] [CrossRef]
- Thomson, A.C.; Kenis, G.; Tielens, S.; de Graaf, T.A.; Schuhmann, T.; Rutten, B.P.F.; Sack, A.T. Transcranial Magnetic Stimulation-Induced Plasticity Mechanisms: TMS-Related Gene Expression and Morphology Changes in a Human Neuron-Like Cell Model. Front. Mol. Neurosci. 2020, 13, 528396. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Izumi, S.I. Transcranial Magnetic Stimulation and Neocortical Neurons: The Micro-Macro Connection. Front. Neurosci. 2022, 16, 866245. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.; Beros, J.; Bates, K.A.; Harvey, A.R.; Tang, A.D.; Rodger, J. Low intensity repetitive magnetic stimulation reduces expression of genes related to inflammation and calcium signalling in cultured mouse cortical astrocytes. Brain Stimul. 2021, 14, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Li, C.T.; Huang, Y.Z.; Bai, Y.M.; Tsai, S.J.; Su, T.P.; Cheng, C.M. Critical role of glutamatergic and GABAergic neurotransmission in the central mechanisms of theta-burst stimulation. Hum. Brain Mapp. 2019, 40, 2001–2009. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Bao, L.; Han, N.; Hou, Y.; Feng, F. rTMS alleviates AD-induced cognitive impairment by inhibitng apoptosis in SAMP8 mouse. Aging 2021, 13, 26034–26045. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Lee, G.Y.; Kim, S.K.; Kwon, Y.J.; Seo, E.B.; Lee, H.; Lee, S.H.; Kim, S.J.; Lee, S.; Ye, S.K. Protective Effects of Repetitive Transcranial Magnetic Stimulation Against Streptozotocin-Induced Alzheimer’s Disease. Mol. Neurobiol. 2023, 61, 1687–1703. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, X.; Bao, J.; Chen, Z.; Tang, J.; Gong, X.; Ni, J.; Fang, Q.; Liu, Y.; Su, M. High-Frequency Repetitive Transcranial Magnetic Stimulation Mediates Autophagy Flux in Human Bone Mesenchymal Stromal Cells via NMDA Receptor-Ca(2+)-Extracellular Signal-Regulated Kinase-Mammalian Target of Rapamycin Signaling. Front. Neurosci. 2019, 13, 1225. [Google Scholar] [CrossRef]
- Zuo, C.; Cao, H.; Feng, F.; Li, G.; Huang, Y.; Zhu, L.; Gu, Z.; Yang, Y.; Chen, J.; Jiang, Y.; et al. Repetitive transcranial magnetic stimulation exerts anti-inflammatory effects via modulating glial activation in mice with chronic unpredictable mild stress-induced depression. Int. Immunopharmacol. 2022, 109, 108788. [Google Scholar] [CrossRef]
- Thapar, A.; Cooper, M. Attention deficit hyperactivity disorder. Lancet 2016, 387, 1240–1250. [Google Scholar] [CrossRef]
- Osborne, J.B.; Zhang, H.; Carlson, M.; Shah, P.; Jonides, J. The association between different sources of distraction and symptoms of attention deficit hyperactivity disorder. Front. Psychiatry 2023, 14, 1173989. [Google Scholar] [CrossRef]
- Wang, J.; Wu, X.; Lai, W.; Long, E.; Zhang, X.; Li, W.; Zhu, Y.; Chen, C.; Zhong, X.; Liu, Z.; et al. Prevalence of depression and depressive symptoms among outpatients: A systematic review and meta-analysis. BMJ Open 2017, 7, e017173. [Google Scholar] [CrossRef]
- Zuckerman, H.; Pan, Z.; Park, C.; Brietzke, E.; Musial, N.; Shariq, A.S.; Iacobucci, M.; Yim, S.J.; Lui, L.M.W.; Rong, C.; et al. Recognition and Treatment of Cognitive Dysfunction in Major Depressive Disorder. Front. Psychiatry 2018, 9, 655. [Google Scholar] [CrossRef]
- Kim, T.D.; Hong, G.; Kim, J.; Yoon, S. Cognitive Enhancement in Neurological and Psychiatric Disorders Using Transcranial Magnetic Stimulation (TMS): A Review of Modalities, Potential Mechanisms and Future Implications. Exp. Neurobiol. 2019, 28, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Oberman, L.M.; Benussi, A. Transcranial Magnetic Stimulation Across the Lifespan: Impact of Developmental and Degenerative Processes. Biol. Psychiatry 2023, 95, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.G.; Hu, X.Y.; Zhu, D.D.; Li, L.; Bao, F.; Ren, L.; Mao, P.X.; Ma, X.; Ren, Y.P.; Tang, Y.L. The cognitive effects of adjunctive repetitive transcranial magnetic stimulation for late-onset depression: A randomized controlled trial with 4 week follow-up. Front. Psychiatry 2023, 14, 1240261. [Google Scholar] [CrossRef] [PubMed]
- Kurkin, S.; Gordleeva, S.; Savosenkov, A.; Grigorev, N.; Smirnov, N.; Grubov, V.V.; Udoratina, A.; Maksimenko, V.; Kazantsev, V.; Hramov, A.E. Transcranial Magnetic Stimulation of the Dorsolateral Prefrontal Cortex Increases Posterior Theta Rhythm and Reduces Latency of Motor Imagery. Sensors 2023, 23, 4661. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Liu, J.; Tang, Z.; Su, W.; Liu, Y.; Lu, H.; Zhang, H. Effects of excitatory transcranial magnetic stimulation over the different cerebral hemispheres dorsolateral prefrontal cortex for post-stroke cognitive impairment: A systematic review and meta-analysis. Front. Neurosci. 2023, 17, 1102311. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J.S.; Bernhard, A.; Fann, N.; Boxhoorn, S.; Hartman, C.A.; Reif, A.; Freitag, C.M. Cognitive mechanisms underlying depressive disorders in ADHD: A systematic review. Neurosci. Biobehav. Rev. 2021, 121, 307–345. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.; Sahakian, B.J.; Chen, S.; Sallie, S.N.; Walker, C.; White, S.R.; Weber, J.; Skandali, N.; Robbins, T.W.; Murray, G.K. Impact and centrality of attention dysregulation on cognition, anxiety, and low mood in adolescents. Sci. Rep. 2023, 13, 9106. [Google Scholar] [CrossRef]
- Yadav, S.K.; Bhat, A.A.; Hashem, S.; Nisar, S.; Kamal, M.; Syed, N.; Temanni, M.R.; Gupta, R.K.; Kamran, S.; Azeem, M.W.; et al. Genetic variations influence brain changes in patients with attention-deficit hyperactivity disorder. Transl. Psychiatry 2021, 11, 349. [Google Scholar] [CrossRef]
- Moncrieff, J.; Cooper, R.E.; Stockmann, T.; Amendola, S.; Hengartner, M.P.; Horowitz, M.A. The serotonin theory of depression: A systematic umbrella review of the evidence. Mol. Psychiatry 2023, 28, 3243–3256. [Google Scholar] [CrossRef]
- Hauer, L.; Sellner, J.; Brigo, F.; Trinka, E.; Sebastianelli, L.; Saltuari, L.; Versace, V.; Holler, Y.; Nardone, R. Effects of Repetitive Transcranial Magnetic Stimulation over Prefrontal Cortex on Attention in Psychiatric Disorders: A Systematic Review. J. Clin. Med. 2019, 8, 416. [Google Scholar] [CrossRef]
- Deng, Y.; Li, W.; Zhang, B. Functional Activity in the Effect of Transcranial Magnetic Stimulation Therapy for Patients with Depression: A Meta-Analysis. J. Pers. Med. 2023, 13, 405. [Google Scholar] [CrossRef]
- Dubin, M. Imaging TMS: Antidepressant mechanisms and treatment optimization. Int. Rev. Psychiatry 2017, 29, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Memon, A.M. Transcranial Magnetic Stimulation in Treatment of Adolescent Attention Deficit/Hyperactivity Disorder: A Narrative Review of Literature. Innov. Clin. Neurosci. 2021, 18, 43–46. [Google Scholar] [PubMed]
- Hanoglu, L.; Toplutas, E.; Saricaoglu, M.; Velioglu, H.A.; Yildiz, S.; Yulug, B. Therapeutic Role of Repetitive Transcranial Magnetic Stimulation in Alzheimer’s and Parkinson’s Disease: Electroencephalography Microstate Correlates. Front. Neurosci. 2022, 16, 798558. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Lu, W.; Qiu, J.; Shi, L. Identifying individuals with attention-deficit/hyperactivity disorder based on multisite resting-state functional magnetic resonance imaging: A radiomics analysis. Hum. Brain Mapp. 2023, 44, 3433–3445. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, O.S.; Mehta, M.A.; O’Daly, O.G.; Criaud, M. Task-Based Functional Connectivity in Attention-Deficit/Hyperactivity Disorder: A Systematic Review. Biol. Psychiatry Glob. Open Sci. 2022, 2, 350–367. [Google Scholar] [CrossRef] [PubMed]
- Rubia, K.; Westwood, S.; Aggensteiner, P.M.; Brandeis, D. Neurotherapeutics for Attention Deficit/Hyperactivity Disorder (ADHD): A Review. Cells 2021, 10, 2156. [Google Scholar] [CrossRef]
- Firouzabadi, F.D.; Ramezanpour, S.; Firouzabadi, M.D.; Yousem, I.J.; Puts, N.A.J.; Yousem, D.M. Neuroimaging in Attention-Deficit/Hyperactivity Disorder: Recent Advances. AJR Am. J. Roentgenol. 2022, 218, 321–332. [Google Scholar] [CrossRef]
- Cao, P.; Xing, J.; Cao, Y.; Cheng, Q.; Sun, X.; Kang, Q.; Dai, L.; Zhou, X.; Song, Z. Clinical effects of repetitive transcranial magnetic stimulation combined with atomoxetine in the treatment of attention-deficit hyperactivity disorder. Neuropsychiatr. Dis. Treat. 2018, 14, 3231–3240. [Google Scholar] [CrossRef]
- Kahl, C.K.; Swansburg, R.; Hai, T.; Wrightson, J.G.; Bell, T.; Lemay, J.F.; Kirton, A.; MacMaster, F.P. Differences in neurometabolites and transcranial magnetic stimulation motor maps in children with attention-deficit/hyperactivity disorder. J. Psychiatry Neurosci. 2022, 47, E239–E249. [Google Scholar] [CrossRef]
- Detrick, J.A.; Zink, C.; Rosch, K.S.; Horn, P.S.; Huddleston, D.A.; Crocetti, D.; Wu, S.W.; Pedapati, E.V.; Wassermann, E.M.; Mostofsky, S.H.; et al. Motor cortex modulation and reward in children with attention-deficit/hyperactivity disorder. Brain Commun. 2021, 3, fcab093. [Google Scholar] [CrossRef]
- Chen, Y.H.; Liang, S.C.; Sun, C.K.; Cheng, Y.S.; Tzang, R.F.; Chiu, H.J.; Wang, M.Y.; Cheng, Y.C.; Hung, K.C. A meta-analysis on the therapeutic efficacy of repetitive transcranial magnetic stimulation for cognitive functions in attention-deficit/hyperactivity disorders. BMC Psychiatry 2023, 23, 756. [Google Scholar] [CrossRef]
- Weaver, L.; Rostain, A.L.; Mace, W.; Akhtar, U.; Moss, E.; O’Reardon, J.P. Transcranial magnetic stimulation (TMS) in the treatment of attention-deficit/hyperactivity disorder in adolescents and young adults: A pilot study. J ECT 2012, 28, 98–103. [Google Scholar] [CrossRef]
- Luber, B.M.; Davis, S.; Bernhardt, E.; Neacsiu, A.; Kwapil, L.; Lisanby, S.H.; Strauman, T.J. Using neuroimaging to individualize TMS treatment for depression: Toward a new paradigm for imaging-guided intervention. Neuroimage 2017, 148, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Philip, N.S.; Barredo, J.; Aiken, E.; Carpenter, L.L. Neuroimaging Mechanisms of Therapeutic Transcranial Magnetic Stimulation for Major Depressive Disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2018, 3, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Li, B.J.; Friston, K.; Mody, M.; Wang, H.N.; Lu, H.B.; Hu, D.W. A brain network model for depression: From symptom understanding to disease intervention. CNS Neurosci. Ther. 2018, 24, 1004–1019. [Google Scholar] [CrossRef] [PubMed]
- Hilland, E.; Landro, N.I.; Harmer, C.J.; Browning, M.; Maglanoc, L.A.; Jonassen, R. Attentional bias modification is associated with fMRI response toward negative stimuli in individuals with residual depression: A randomized controlled trial. J. Psychiatry Neurosci. 2020, 45, 23–33. [Google Scholar] [CrossRef]
- Dehghani, A.; Soltanian-Zadeh, H.; Hossein-Zadeh, G.A. Probing fMRI brain connectivity and activity changes during emotion regulation by EEG neurofeedback. Front. Hum. Neurosci. 2022, 16, 988890. [Google Scholar] [CrossRef]
- Sacco, L.; Ceroni, M.; Pacifico, D.; Zerboni, G.; Rossi, S.; Galati, S.; Caverzasio, S.; Kaelin-Lang, A.; Riccitelli, G.C. Transcranial Magnetic Stimulation Improves Executive Functioning through Modulation of Social Cognitive Networks in Patients with Mild Cognitive Impairment: Preliminary Results. Diagnostics 2023, 13, 415. [Google Scholar] [CrossRef]
- Qiu, X.; He, Z.; Cao, X.; Zhang, D. Transcranial magnetic stimulation and transcranial direct current stimulation affect explicit but not implicit emotion regulation: A meta-analysis. Behav. Brain Funct. 2023, 19, 15. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.M.; Coman, J.T.; Stetz, P.C.; Walker, N.C.; Kozel, F.A.; George, M.S.; Yoon, J.; Hack, L.M.; Madore, M.R.; Lim, K.O.; et al. Identifying response and predictive biomarkers for Transcranial magnetic stimulation outcomes: Protocol and rationale for a mechanistic study of functional neuroimaging and behavioral biomarkers in veterans with Pharmacoresistant depression. BMC Psychiatry 2021, 21, 35. [Google Scholar] [CrossRef] [PubMed]
- Blumberger, D.M.; Vila-Rodriguez, F.; Thorpe, K.E.; Feffer, K.; Noda, Y.; Giacobbe, P.; Knyahnytska, Y.; Kennedy, S.H.; Lam, R.W.; Daskalakis, Z.J.; et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): A randomised non-inferiority trial. Lancet 2018, 391, 1683–1692. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Huang, Y.; Chen, T.; Wang, X.; Guo, Y.; Fang, Y.; He, K.; Zhu, C.; Wang, K.; Zhang, L. Repetitive transcranial magnetic stimulation promotes response inhibition in patients with major depression during the stop-signal task. J. Psychiatr. Res. 2022, 151, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, P.B. Targeting repetitive transcranial magnetic stimulation in depression: Do we really know what we are stimulating and how best to do it? Brain Stimul. 2021, 14, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Kaster, T.S.; Downar, J.; Vila-Rodriguez, F.; Baribeau, D.A.; Thorpe, K.E.; Daskalakis, Z.J.; Blumberger, D.M. Differential symptom cluster responses to repetitive transcranial magnetic stimulation treatment in depression. EClinicalMedicine 2023, 55, 101765. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, D.R.; Okhravi, H.R.; Neumann, S.A. Low-Frequency Transcranial Magnetic Stimulation (LF-TMS) in Treating Depression in Patients With Impaired Cognitive Functioning. Arch. Clin. Neuropsychol. 2021, 36, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Yıldız, T.; Oğuzhanoğlu, N.K.; Topak, O.Z. Cognitive outcomes of transcranial magnetic stimulation in treatment-resistant depression: A randomized controlled study. Turk. J. Med. Sci. 2023, 53, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, A.; Arbuckle, M.R. Synaptic Plasticity: The Role of Learning and Unlearning in Addiction and Beyond. Biol. Psychiatry 2016, 80, e73–e75. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.E.; Bligh, T.W.B.; Garden, J.F. The Hebb Synapse Before Hebb: Theories of Synaptic Function in Learning and Memory Before, With a Discussion of the Long-Lost Synaptic Theory of William McDougall. Front. Behav. Neurosci. 2021, 15, 732195. [Google Scholar] [CrossRef] [PubMed]
- Lazari, A.; Salvan, P.; Cottaar, M.; Papp, D.; Rushworth, M.F.S.; Johansen-Berg, H. Hebbian activity-dependent plasticity in white matter. Cell Rep. 2022, 39, 110951. [Google Scholar] [CrossRef] [PubMed]
- Zenke, F.; Gerstner, W. Hebbian plasticity requires compensatory processes on multiple timescales. Philos. Trans. R Soc. Lond. B Biol. Sci. 2017, 372, 20160259. [Google Scholar] [CrossRef]
- Rajani, V.; Sengar, A.S.; Salter, M.W. Tripartite signalling by NMDA receptors. Mol. Brain 2020, 13, 23. [Google Scholar] [CrossRef]
- Washbourne, P. Synapse assembly and neurodevelopmental disorders. Neuropsychopharmacology 2015, 40, 4–15. [Google Scholar] [CrossRef]
- Calabrese, F.; Riva, M.A.; Molteni, R. Synaptic alterations associated with depression and schizophrenia: Potential as a therapeutic target. Expert. Opin. Ther. Targets 2016, 20, 1195–1207. [Google Scholar] [CrossRef]
- Kessi, M.; Duan, H.; Xiong, J.; Chen, B.; He, F.; Yang, L.; Ma, Y.; Bamgbade, O.A.; Peng, J.; Yin, F. Attention-deficit/hyperactive disorder updates. Front. Mol. Neurosci. 2022, 15, 925049. [Google Scholar] [CrossRef]
- Levy, M.J.F.; Boulle, F.; Steinbusch, H.W.; van den Hove, D.L.A.; Kenis, G.; Lanfumey, L. Neurotrophic factors and neuroplasticity pathways in the pathophysiology and treatment of depression. Psychopharmacology 2018, 235, 2195–2220. [Google Scholar] [CrossRef]
- Appelbaum, L.G.; Shenasa, M.A.; Stolz, L.; Daskalakis, Z. Synaptic plasticity and mental health: Methods, challenges and opportunities. Neuropsychopharmacology 2023, 48, 113–120. [Google Scholar] [CrossRef]
- Rubia, K. Cognitive Neuroscience of Attention Deficit Hyperactivity Disorder (ADHD) and Its Clinical Translation. Front. Hum. Neurosci. 2018, 12, 100. [Google Scholar] [CrossRef]
- Zaman, R. Transcranial magnetic stimulation (TMS) in Attention Deficit Hyperactivity Disorder (ADHD). Psychiatr. Danub. 2015, 27 (Suppl. S1), S530–S532. [Google Scholar] [PubMed]
- Fitzsimmons, S.M.D.D.; Oostra, E.; Postma, T.S.; van der Werf, Y.D.; van den Heuvel, O.A. Repetitive Transcranial Magnetic Stimulation–Induced Neuroplasticity and the Treatment of Psychiatric Disorders: State of the Evidence and Future Opportunities. Biological. Psychiatry 2024, 95, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Jangwan, N.S.; Ashraf, G.M.; Ram, V.; Singh, V.; Alghamdi, B.S.; Abuzenadah, A.M.; Singh, M.F. Brain augmentation and neuroscience technologies: Current applications, challenges, ethics and future prospects. Front. Syst. Neurosci. 2022, 16, 1000495. [Google Scholar] [CrossRef] [PubMed]
- Hill-Bowen, L.D.; Riedel, M.C.; Poudel, R.; Salo, T.; Flannery, J.S.; Camilleri, J.A.; Eickhoff, S.B.; Laird, A.R.; Sutherland, M.T. The cue-reactivity paradigm: An ensemble of networks driving attention and cognition when viewing drug and natural reward-related stimuli. Neurosci. Biobehav. Rev. 2021, 130, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimzadeh, E.; Saharkhiz, S.; Rajabion, L.; Oskouei, H.B.; Seraji, M.; Fayaz, F.; Saliminia, S.; Sadjadi, S.M.; Soltanian-Zadeh, H. Simultaneous electroencephalography-functional magnetic resonance imaging for assessment of human brain function. Front. Syst. Neurosci. 2022, 16, 934266. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.-C.; Yen, C.; Chen, H.-L. Does Age Matter? Using Neuroscience Approaches to Understand Consumers’ Behavior towards Purchasing the Sustainable Product Online. Sustainability 2022, 14, 11352. [Google Scholar]
- Yen, C.; Chiang, M.-C. Trust me, if you can: A study on the factors that influence consumers’ purchase intention triggered by chatbots based on brain image evidence and self-reported assessments. Behav. Inf. Technol. 2021, 40, 1177–1194. [Google Scholar] [CrossRef]
- Phillips, J.M.; Kambi, N.A.; Redinbaugh, M.J.; Mohanta, S.; Saalmann, Y.B. Disentangling the influences of multiple thalamic nuclei on prefrontal cortex and cognitive control. Neurosci. Biobehav. Rev. 2021, 128, 487–510. [Google Scholar] [CrossRef]
- Rizvi, S.; Khan, A.M. Use of Transcranial Magnetic Stimulation for Depression. Cureus 2019, 11, e4736. [Google Scholar] [CrossRef]
- Peng, Z.; Zhou, C.; Xue, S.; Bai, J.; Yu, S.; Li, X.; Wang, H.; Tan, Q. Mechanism of Repetitive Transcranial Magnetic Stimulation for Depression. Shanghai Arch. Psychiatry 2018, 30, 84–92. [Google Scholar] [PubMed]
- Ma, Q.; Geng, Y.; Wang, H.L.; Han, B.; Wang, Y.Y.; Li, X.L.; Wang, L.; Wang, M.W. High Frequency Repetitive Transcranial Magnetic Stimulation Alleviates Cognitive Impairment and Modulates Hippocampal Synaptic Structural Plasticity in Aged Mice. Front. Aging Neurosci. 2019, 11, 235. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, S.; Rogasch, N.C.; Premoli, I.; Blumberger, D.M.; Casarotto, S.; Chen, R.; Di Lazzaro, V.; Farzan, F.; Ferrarelli, F.; Fitzgerald, P.B.; et al. Clinical utility and prospective of TMS-EEG. Clin. Neurophysiol. 2019, 130, 802–844. [Google Scholar] [CrossRef] [PubMed]
- Sanches, C.; Stengel, C.; Godard, J.; Mertz, J.; Teichmann, M.; Migliaccio, R.; Valero-Cabre, A. Past, Present, and Future of Non-invasive Brain Stimulation Approaches to Treat Cognitive Impairment in Neurodegenerative Diseases: Time for a Comprehensive Critical Review. Front. Aging Neurosci. 2020, 12, 578339. [Google Scholar] [CrossRef] [PubMed]
- van Rooij, S.J.H.; Arulpragasam, A.R.; McDonald, W.M.; Philip, N.S. Accelerated TMS—Moving quickly into the future of depression treatment. Neuropsychopharmacology 2023, 49, 128–137. [Google Scholar] [CrossRef]
- Nani, A.; Manuello, J.; Mancuso, L.; Liloia, D.; Costa, T.; Cauda, F. The Neural Correlates of Consciousness and Attention: Two Sister Processes of the Brain. Front. Neurosci. 2019, 13, 1169. [Google Scholar] [CrossRef] [PubMed]
- Craske, M.G.; Herzallah, M.M.; Nusslock, R.; Patel, V. From neural circuits to communities: An integrative multidisciplinary roadmap for global mental health. Nat. Ment. Health 2023, 1, 12–24. [Google Scholar] [CrossRef]
- Ziegler, D.A.; Anguera, J.A.; Gallen, C.L.; Hsu, W.Y.; Wais, P.E.; Gazzaley, A. Leveraging technology to personalize cognitive enhancement methods in aging. Nat. Aging 2022, 2, 475–483. [Google Scholar] [CrossRef]
- Singh, V.; Kumar, A.; Gupta, S. Mental Health Prevention and Promotion-A Narrative Review. Front. Psychiatry 2022, 13, 898009. [Google Scholar] [CrossRef]

| Study | Effects | Reference |
|---|---|---|
| Kahl, C. K. et al. (2022) | This study analyzed differences in neurometabolic and cortical motor representations obtained through TMS in 26 children with ADHD and 25 typically developing children. Furthermore, detected changes in neurometabolite levels (glutamate, glutamine, and GABA) correlated with TMS-derived measurements. Research has found that the neurochemistry and neurophysiology of critical nodes in the motor network may be altered in children diagnosed with ADHD. | [104] |
| Detrick, J. A. et al. (2021) | Fifty-five children diagnosed with ADHD, of whom thirty-seven were male, and fifty typically developing control children between the ages of 8 and 12 years, of whom thirty-two were male, took part in the research—a child-friendly reward-motivated task evaluated cortical disinhibition with noninvasive TMS. The significant results revealed modifications in short-interval cortical inhibition and increased the amplitudes of motor-evoked potentials obtained during the reward task. Overall, this research endorses the potential utility of transcranial magnetic stimulation-induced cortical inhibition and task-evoked excitability as biomarkers for areas of clinically significant dysfunction in childhood ADHD. | [105] |
| Chen, Y. H. et al. (2023) | This meta-analysis of 189 participants (average age in the adult and child/adolescent groups was 32.78 and 8.53 years, respectively) showed that rTMS was more effective than a control group in improving persistence. Results support the therapeutic efficacy of rTMS in enhancing sustained attention and processing speed in ADHD patients. | [106] |
| Bleich-Cohen, M. et al. (2021) | This study investigated the neural effects of high-frequency repetitive deep TMS applied to the right or left PFC in 62 adults with ADHD. Increased activation in the right dorsolateral prefrontal cortex (rDLPFC), right parietal cortex, and right insula/inferior frontal gyrus (IFG) were found after stimulation treatment. This study shows that dTMS can effectively modulate attention-related brain networks, suggesting it is a viable technique that can improve attention symptoms in adults with ADHD. | [58] |
| Weaver, L. et al. (2012) | TMS was applied to the right prefrontal cortex at 10 Hz (100% of the observed motor threshold) for 2000 pulses each. The study used a sham-controlled crossover design with nine participants. The ten sessions of TMS lasted over two weeks, with a one-week interval between active and sham phases. In summary, TMS is safe and effective in treating ADHD in adolescents and young adults. | [107] |
| Study | Effects | Reference |
|---|---|---|
| Blumberger, D. M. et al. (2018) | Efficacy, safety, and tolerability of standard high-frequency (10 Hz) rTMS in adults with treatment-resistant depression. Participants were aged 18–65 years and diagnosed with treatment-resistant depression and major depressive disorder. Two hundred five participants were assigned to the 10 Hz rTMS group. The 10 Hz rTMS treatment approach was safe and tolerable. | [116] |
| Yu, F. et al. (2022) | People with major depressive disorder (MDD) often exhibit cognitive impairment. This study investigated the effects of individualized rTMS targeting the left dorsolateral prefrontal cortex (lDLPFC)–nucleus accumbens (NAcc) network in patients with major depressive disorder (MDD). In a double-blind, sham-controlled trial, 44 patients diagnosed with MDD were randomly assigned to receive active rTMS (10 Hz, 100% of resting motor threshold) or sham rTMS. Research shows that individualized rTMS treatment may be a practical approach for patients with depression. | [117] |
| Fitzgerald, P. B. et al. (2021) | The use of rTMS in treating depression highlights the importance of improved local stimulation methods. It is recommended that optimal results from rTMS be achieved by stimulating relatively anterior parts of the left DLPFC. rTMS is an established treatment for patients with depression who do not respond well to antidepressant medications. Although effective, there is room for improvement in clinical outcomes. | [118] |
| Kaster, T. S. et al. (2023) | A study to examine differential responses of symptom clusters to rTMS in patients with treatment-resistant depression (TRD). rTMS is a treatment for depression that targets specific neural circuits, and these trials involved delivering rTMS to the left DLPFC. The study included 596 participants with TRD; preliminary analysis of the THREE-D treatment trial shows that symptom clusters differ in response to rTMS treatment. The anxiety symptom cluster was significantly less responsive to treatment than the other symptom clusters. In summary, this study highlights the potential to tailor rTMS treatment to the specific symptom clusters experienced by patients with treatment-resistant depression, with a focus on addressing the differential responses of these symptom clusters to rTMS treatment. | [119] |
| Schaffer, D. R. et al. (2021) | A study exploring the use of low-frequency TMS (LF-TMS) in the treatment of depression in cognitively impaired individuals. Fifty-three participants received LF-TMS treatment. LF-TMS resulted in significant reductions in individual depressive symptoms in both cognitive function groups. The significant group-by-time interaction showed differential effects between the two cognitive function groups. In summary, this study suggests that LF-TMS may be a promising treatment for depressive symptoms in individuals with impaired cognitive function, with potential additional benefits on neurocognitive function. | [120] |
| Yıldız, T. et al. (2023) | This study included 30 patients with depression (aged 18–50 years) to explore the potential benefits of TMS on cognitive function in patients with treatment-resistant depression. TMS may be beneficial in improving cognitive function in patients with treatment-resistant depression and may provide early cognitive improvements during treatment. | [121] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, C.; Valentine, E.P.; Chiang, M.-C. The Use of Transcranial Magnetic Stimulation in Attention Optimization Research: A Review from Basic Theory to Findings in Attention-Deficit/Hyperactivity Disorder and Depression. Life 2024, 14, 329. https://doi.org/10.3390/life14030329
Yen C, Valentine EP, Chiang M-C. The Use of Transcranial Magnetic Stimulation in Attention Optimization Research: A Review from Basic Theory to Findings in Attention-Deficit/Hyperactivity Disorder and Depression. Life. 2024; 14(3):329. https://doi.org/10.3390/life14030329
Chicago/Turabian StyleYen, Chiahui, Ethan P. Valentine, and Ming-Chang Chiang. 2024. "The Use of Transcranial Magnetic Stimulation in Attention Optimization Research: A Review from Basic Theory to Findings in Attention-Deficit/Hyperactivity Disorder and Depression" Life 14, no. 3: 329. https://doi.org/10.3390/life14030329
APA StyleYen, C., Valentine, E. P., & Chiang, M.-C. (2024). The Use of Transcranial Magnetic Stimulation in Attention Optimization Research: A Review from Basic Theory to Findings in Attention-Deficit/Hyperactivity Disorder and Depression. Life, 14(3), 329. https://doi.org/10.3390/life14030329








