Investigation of Vitamin D Levels in Men with Suspected Infertility
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Design
2.2. Patient Selection and Sampling
2.3. Demographic Parameters
2.4. Semen Sample Collection and Analysis
2.5. Measurement of Laboratory Parameters
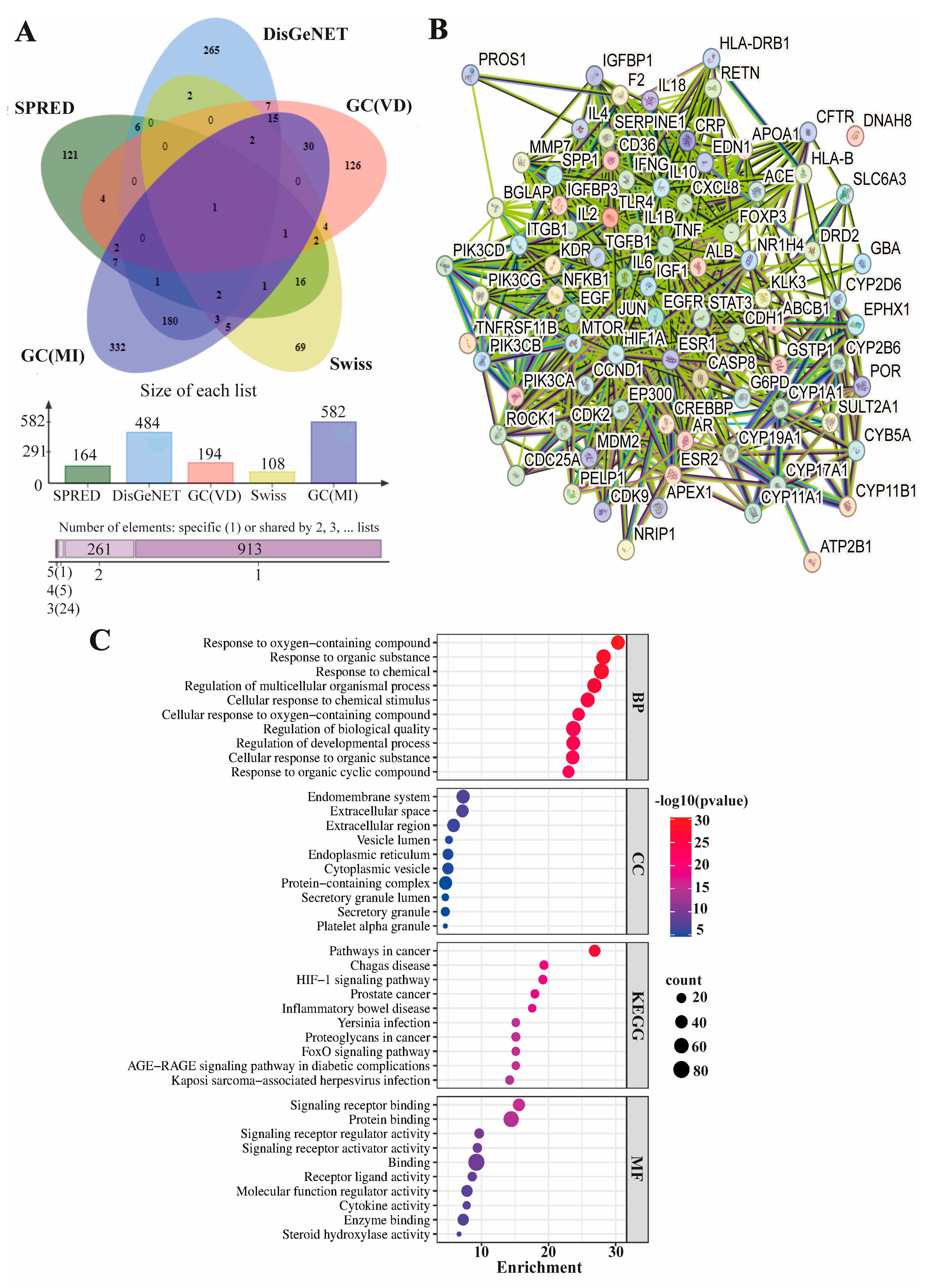
2.6. Identification of Male-Infertility-Associated Molecular Targets of Vitamin D and Functional Enrichment Analysis
2.7. Statistical Analysis
3. Results
3.1. General Characteristics of Participants
3.2. Semen Analysis of Patients
3.3. Hormonal Analysis of Patients
3.4. Blood and Essential Minerals of Patients
3.5. Correlation Analyses of Demographic and BMI Parameters
3.6. Correlation Analyses of Semen Parameters
3.7. Correlation Analyses of Hormonal Parameters
3.8. Correlation Analyses of Iron and Essential Mineral Parameters
3.9. Male-Infertility-Correlated Molecular Targets of Vitamin D and Their Functional Annotations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boivin, J.; Bunting, L.; Collins, J.A.; Nygren, K.G. International Estimates of Infertility Prevalence and Treatment-Seeking: Potential Need and Demand for Infertility Medical Care. Hum. Reprod. 2007, 22, 2800. [Google Scholar] [CrossRef]
- World Health Organization. Towards More Objectivity in Diagnosis and Management of Male Infertility. Int. J. Androl. 1987, 7, 1–53. [Google Scholar]
- Cavallini, G. Male Idiopathic Oligoasthenoteratozoospermia. Asian J. Androl. 2006, 8, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Ventimiglia, E.; Capogrosso, P.; Boeri, L.; Serino, A.; Colicchia, M.; Ippolito, S.; Scano, R.; Papaleo, E.; Damiano, R.; Montorsi, F.; et al. Infertility as a Proxy of General Male Health: Results of a Cross-Sectional Survey. Fertil. Steril. 2015, 104, 48–55. [Google Scholar] [CrossRef]
- Lotti, F.; Maggi, M. Sexual Dysfunction and Male Infertility. Nat. Rev. Urol. 2018, 15, 287–307. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Dusilová-Sulková, S. Vıtamın D metabolısm and Vıtamın D tradıtıonal and nontradıtıonal, target organs: Implıcatıons for kıdney patıents. J. Ren. Care 2009, 35, 39–44. [Google Scholar] [CrossRef]
- de Angelis, C.; Galdiero, M.; Pivonello, C.; Garifalos, F.; Menafra, D.; Cariati, F.; Salzano, C.; Galdiero, G.; Piscopo, M.; Vece, A.; et al. The Role of Vitamin D in Male Fertility: A Focus on the Testis. Rev. Endocr. Metab. Disord. 2017, 18, 285–305. [Google Scholar] [CrossRef]
- Chun, R.F.; Peercy, B.E.; Orwoll, E.S.; Nielson, C.M.; Adams, J.S.; Hewison, M. Vitamin D and DBP: The Free Hormone Hypothesis Revisited. J. Steroid Biochem. Mol. Biol. 2014, 144, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D Metabolism, Mechanism of Action, and Clinical Applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Cito, G.; Cocci, A.; Micelli, E.; Gabutti, A.; Russo, G.I.; Coccia, M.E.; Franco, G.; Serni, S.; Carini, M.; Natali, A. Vitamin D and Male Fertility: An Updated Review. World J. Men’s Health 2019, 37, 164–177. [Google Scholar] [CrossRef]
- Calagna, G.; Catinella, V.; Polito, S.; Schiattarella, A.; Franciscis, P.D.; D’Antonio, F.; Calì, G.; Perino, A.; Cucinella, G. Vitamin D and Male Reproduction: Updated Evidence Based on Literature Review. Nutrients 2022, 14, 3278. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Society, E. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Sood, S.; Marya, R.K.; Reghunandanan, R.; Singh, G.P.; Jaswal, T.S.; Gopinathan, K. Effect of Vitamin D Deficiency on Testicular Function in the Rat. Ann. Nutr. Metab. 1992, 36, 203–208. [Google Scholar] [CrossRef]
- Akhavizadegan, H.; Karbakhsh, M. Comparison of Serum Vitamin D between Fertile and Infertile Men in A Vitamin D Deficient Endemic Area: A Case-Control Study. Urol. J. 2017, 84, 218–220. [Google Scholar] [CrossRef]
- Banks, N.; Sun, F.; Krawetz, S.A.; Coward, R.M.; Masson, P.; Smith, J.F.; Trussell, J.C.; Santoro, N.; Zhang, H.; Steiner, A.Z. Male Vitamin D Status and Male Factor Infertility. Fertil. Steril. 2021, 116, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Bardou, P.; Mariette, J.; Escudié, F.; Djemiel, C.; Klopp, C. Jvenn: An Interactive Venn Diagram Viewer. BMC Bioinform. 2014, 15, 293. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A Free Online Platform for Data Visualization and Graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef] [PubMed]
- Kwiecinski, G.G.; Petrie, G.I.; DeLuca, H.F. Vitamin D Is Necessary for Reproductive Functions of the Male Rat. J. Nutr. 1989, 119, 741–744. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.B.; Bjerrum, P.J.; Jessen, T.E.; Nielsen, J.E.; Joensen, U.N.; Olesen, I.A.; Petersen, J.H.; Juul, A.; Dissing, S.; Jørgensen, N. Vitamin D Is Positively Associated With Sperm Motility and Increases Intracellular Calcium in Human Spermatozoa. Obstet. Gynecol. Surv. 2011, 66, 556–558. [Google Scholar] [CrossRef]
- Maghsoumi-Norouzabad, L.; Javid, A.Z.; Mansoori, A.; Dadfar, M.; Serajian, A. The Effects of Vitamin D3 Supplementation on Spermatogram and Endocrine Factors in Asthenozoospermia Infertile Men: A Randomized, Triple Blind, Placebo-Controlled Clinical Trial. Reprod. Biol. Endocrinol. 2021, 19, 102. [Google Scholar] [CrossRef]
- Rafiq, R.; van Schoor, N.M.; Sohl, E.; Zillikens, M.C.; Oosterwerff, M.M.; Schaap, L.; Lips, P.; de Jongh, R.T. Associations of Vitamin D Status and Vitamin D-Related Polymorphisms with Sex Hormones in Older Men. J. Steroid Biochem. Mol. Biol. 2016, 164, 11–17. [Google Scholar] [CrossRef]
- Wentz, L.M.; Berry-Cabán, C.S.; Wu, Q.; Eldred, J.D. Vitamin D Correlation with Testosterone Concentration in Male US Soldiers and Veterans. J. Mil. Veterans’ Health 2016, 24, 17–23. [Google Scholar]
- Amini, L.; Mohammadbeigi, R.; Vafa, M.; Haghani, H.; Vahedian-Azimi, A.; Karimi, L.; Jahanfar, S.; Jamialahmadi, T.; Talebi, A.; Sahebkar, A. Evaluation of the Effect of Vitamin D3 Supplementation on Quantitative and Qualitative Parameters of Spermograms and Hormones in Infertile Men: A Randomized Controlled Trial. Complement. Ther. Med. 2020, 53, 102529. [Google Scholar] [CrossRef] [PubMed]
- Snyder, P.J. Hypogonadism in Elderly Men—What to Do Until the Evidence Comes. N. Engl. J. Med. 2004, 350, 440–442. [Google Scholar] [CrossRef] [PubMed]
- Adamczewska, D.; Słowikowska-Hilczer, J.; Walczak-Jędrzejowska, R. The Association between Vitamin D and the Components of Male Fertility: A Systematic Review. Biomedicines 2022, 11, 90. [Google Scholar] [CrossRef]
- Mirnamniha, M.; Faroughi, F.; Tahmasbpour, E.; Ebrahimi, P.; Harchegani, A.B. An Overview on Role of Some Trace Elements in Human Reproductive Health, Sperm Function and Fertilization Process. Rev. Environ. Health 2019, 34, 339–348. [Google Scholar] [CrossRef]
- Özkorkmaz, E.G.; Başaran, S.Ö.; Afşin, M.; Aşir, F. Comparison of Testosterone, FSH, LH and E2 Hormone Levels in Infertility Suspected Males with COVID-19 Infection. Medicine 2023, 102, e35256. [Google Scholar] [CrossRef]
- Allouche-Fitoussi, D.; Breitbart, H. The Role of Zinc in Male Fertility. Int. J. Mol. Sci. 2020, 21, 7796. [Google Scholar] [CrossRef]
- Aljaser, F.; Tabassum, H.; Fatima, S.; Abudawood, M.; Banu, N. Effect of Trace Elements on the Seminal Oxidative Status and Correlation to Sperm Motility in Infertile Saudi Males. Saudi J. Biol. Sci. 2021, 28, 4455–4460. [Google Scholar] [CrossRef]
- Prien, S.D.; Lox, C.D.; Messer, R.H.; DeLeon, F.D. Seminal Concentrations of Total and Ionized Calcium from Men with Normal and Decreased Motility. Fertil. Steril. 1990, 54, 171–172. [Google Scholar] [CrossRef]
- Chung, J.-J.; Navarro, B.; Krapivinsky, G.; Krapivinsky, L.; Clapham, D.E. A Novel Gene Required for Male Fertility and Functional CATSPER Channel Formation in Spermatozoa. Nat. Commun. 2011, 2, 153. [Google Scholar] [CrossRef]
- Golpour, A.; Pšenička, M.; Niksirat, H. Subcellular Distribution of Calcium during Spermatogenesis of Zebrafish, Danio Rerio. J. Morphol. 2017, 278, 1149–1159. [Google Scholar] [CrossRef]
- Joseph, S.; Mahale, S.D. Male Infertility Knowledgebase: Decoding the Genetic and Disease Landscape. Database J. Biol. Databases Curation 2021, 2021, baab049. [Google Scholar] [CrossRef]
- Xue, G.; Gao, R.; Liu, Z.; Xu, N.; Cao, Y.; Zhao, B.; Du, J. Vitamin D/VDR Signaling Inhibits Colitis by Suppressing HIF-1α Activation in Colonic Epithelial Cells. Am. J. Physiol.-Gastrointest. Liver Physiol. 2021, 320, G837–G846. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, J.; Wang, D.; Wang, Y.; Zhang, F.; Jin, G.; Yuan, C.; Wang, X.; Qin, Q. Effect of Silencing HIF-1α Gene on Testicle Spermatogenesis Function in Varicocele Rats. Cell Tissue Res. 2019, 378, 543–554. [Google Scholar] [CrossRef]
- Zaidi, A.; Sheikh, Z.I.; Akbar, A.; Naz, S.; Ramzan, S.; Habib, F. Hypoxia Induced Infertility in Males: Role of Transcription Factor Hypoxia Inducible Factor -1 Alpha. Pak. J. Méd. Health Sci. 2022, 16, 165–168. [Google Scholar] [CrossRef]
- Huang, P.; Zhou, Z.; Shi, F.; Shao, G.; Wang, R.; Wang, J.; Wang, K.; Ding, W. Effects of the IGF-1/PTEN/Akt/FoxO Signaling Pathway on Male Reproduction in Rats Subjected to Water Immersion and Restraint Stress. Mol. Med. Rep. 2016, 14, 5116–5124. [Google Scholar] [CrossRef] [PubMed]
- Goertz, M.J.; Wu, Z.; Gallardo, T.D.; Hamra, F.K.; Castrillon, D.H. Foxo1 Is Required in Mouse Spermatogonial Stem Cells for Their Maintenance and the Initiation of Spermatogenesis. J. Clin. Investig. 2011, 121, 3456–3466. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, J.; Fang, Y.; Shen, Q.; Zhao, K.; Liu, C.; Zhang, H. Microbiology and Immune Mechanisms Associated with Male Infertility. Front. Immunol. 2023, 14, 1139450. [Google Scholar] [CrossRef] [PubMed]
| Parameters (n = 306) | Mean ± SD | Median (Q1–Q3) |
|---|---|---|
| Age (years) | 31.45 ± 6.96 | 30.6 (27–36) |
| BMI (kg/m2) | 24.95 ± 3.05 | 24.78 (23.4–27.23) |
| Marriage (years) | 4.58 ± 3.86 | 4 (2–6) |
| Years without children (n) | 3.17 ± 2.67 | 2 (1–4) |
| Number of children having (n) | 0.42 ± 0.74 | 2 (0–1) |
| Vitamin D (ng/mL) | 20.76 ± 8.09 | 20.5 (13.6–25.93) |
| Sperm concentration (million/mL) | 38.04 ± 38.32 | 29 (5.87–60) |
| Semen volume (mL) | 3.01 ± 1.46 | 2.7 (2–3.77) |
| Total sperm count (million/mL) | 105.08 ± 116.09 | 70 (13.9–156.25) |
| Total count progressive motile sperm (million/mL) | 57.47 ± 78.20 | 30 (3–84) |
| Type A and B motility (%) | 46.14 ± 23.89 | 50 (29.75–65) |
| Type C motility (%) | 8.49 ± 7.15 | 5 (5–11) |
| Type D motility (%) | 45.58 ± 24.34 | 41 (28.75–56) |
| Abstinence time (day) | 3.41 ± 0.53 | 3 (3–4) |
| TSH (mU/L) | 1.74 ± 0.93 | 1.59 (1.11–2.13) |
| FSH (U/L) | 7.86 ± 7.94 | 5.74 (3.8–8.53) |
| LH (U/L) | 6.88 ± 4.46 | 6.14 (4.61–7.85) |
| Prolactin (µg/L) | 16.17 ± 7.35 | 15.14 (11.23–18.66) |
| E2 (ng/L) | 24.45 ± 10.88 | 23.06 (17.96–29.82) |
| Testosterone (ng/mL) | 4.74 ± 2.09 | 4.34 (3.28–5.63) |
| Folic acid (ng/mL) | 7.34 ± 3.12 | 6.81 (5.23–8.62) |
| Iron (µg/dL) | 97.91 ± 34.91 | 87.5 (76–113) |
| Ferritin (µg/L) | 120.76 ± 70.09 | 112 (74–152) |
| Hemoglobin (g/dL) | 16.16 ± 0.84 | 16.3 (15.67–16.7) |
| Hematocrit (g/dL) | 47.37 ± 2.73 | 47.6 (45.9–48.9) |
| Calcium (mg/dL) | 9.57 ± 0.49 | 9.6 (9.37–9.8) |
| Magnesium (mg/dL) | 2.01 ± 0.37 | 1.97 (1.79–2.17) |
| Phosphorus (mg/dL) | 3.49 ± 0.43 | 3.42 (3.24–3.69) |
| Parameters | N (%) | Significance with Vitamin D | |
|---|---|---|---|
| Education | Primary education | 167 (54.57%) | 0.245 |
| Secondary education | 53 (17.32%) | ||
| Higher education | 86 (28.10%) | ||
| Job | Jobless | 33 (10.78%) | 0.969 |
| Worker | 211 (68.95%) | ||
| Civil servant | 62 (20.26%) | ||
| Location | Rural | 72 (23.52%) | 0.177 |
| Urban | 234 (76.47%) | ||
| Infertility type | Primary | 219 (71.56%) | 0.529 |
| Secondary | 87 (28.43%) | ||
| Consanguineous marriage | No | 239 (78.10%) | 0.099 |
| Yes | 67 (21.89%) | ||
| Number of children | 0 | 218 (71.24%) | 0.486 |
| 1 | 52 (16.99%) | ||
| 2 | 30 (9.80%) | ||
| 3 | 6 (1.96%) | ||
| Vitamin D | Age (years) | Marriage (years) | Years (Childless) | BMI (kg/m2) |
|---|---|---|---|---|
| Low (N = 146) | 30 (26–36) | 3 (2–5) | 2 (1–4) | 24.55 (22.71–27.11) |
| High (N = 160) | 30 (28–35) | 4 (2–6) | 2 (1–4) | 25.15 (23.77–27.67) |
| p value | 0.365 | 0.472 | 0.809 | 0.071 |
| Vitamin D | Sperm Concentration (million/mL) | Semen Volume (mL) | Total Sperm Count (million/mL) | Total Count Progressive Motile Sperm (million/mL) | AB Motility(%) | C Motility(%) | D Motility(%) | Abstinence Time (day) |
|---|---|---|---|---|---|---|---|---|
| Low (N = 146) | 28 (7–55) | 2.8 (2–4) | 70.5 (16–142.75) | 28.5 (2.35–76.25) | 50 (25–60.5) | 6 (5–13) | 43 (30–60) | 3 (3–4) |
| High (N = 160) | 33 (4.65–65.00) | 2.6 (2.0–3.5) | 70.0 (12.25–174) | 33 (3.22–93) | 50.5 (31.5–69.5) | 5 (5–10) | 38 (25–55) | 3 (3–4) |
| p value | 0.658 | 0.377 | 0.813 | 0.436 | 0.022 | 0.57 | 0.039 | 0.489 |
| Vitamin D | TSH (mU/L) | FSH (U/L) | LH (U/L) | Prolactin (µg/L) | E2 (ng/L) | Testosterone (ng/mL) | Folic Acid (ng/mL) |
|---|---|---|---|---|---|---|---|
| Low (N = 146) | 1.63 (1.10–2.17) | 5.81 (4.15–8.24) | 6.12 (4.12–7.59) | 14.51 (10.96–17.96) | 22.03 (18.03–29.72) | 4.15 (3.21–5.45) | 6.77 (5.47–8.36) |
| High (N = 160) | 1.53 (1.12–2.1) | 5.57 (3.58–8.55) | 6.16 (4.95–8.25) | 16.11 (11.85–19.68) | 23.16 (17.58–30.32) | 4.59 (3.36–6.13) | 6.84 (5.14–8.66) |
| p value | 0.439 | 0.557 | 0.277 | 0.023 | 0.642 | 0.104 | 0.896 |
| Vitamin D | Iron (µg/dL) | Ferritin (µg/L) | Hemoglobin (g/dL) | Hematocrit (g/dL) | Calcium (mg/dL) | Magnesium (mg/dL) | Phosphorus (mg/dL) |
|---|---|---|---|---|---|---|---|
| Low (N = 146) | 87 (78–108.25) | 114.5 (76.25–150) | 16.3 (15.77–16.7) | 47.6 (45.17–48.9) | 9.59 (9.24–9.8) | 1.96 (1.79–2.16) | 3.41 (3.25–3.6) |
| High (N = 160) | 89 (74–116) | 98 (74–155) | 16.3 (15.6–16.6) | 47.6 (46–49) | 9.635 (9.5–9.8) | 1.99 (1.84–2.17) | 3.45 (3.23–3.7) |
| p value | 0.753 | 0.457 | 0.310 | 0.597 | 0.091 | 0.382 | 0.797 |
| Age | Marriage | Years (Childless) | BMI | ||
|---|---|---|---|---|---|
| Vitamin D | rs | 0.068 | 0.104 | 0.048 | 0.107 |
| P | 0.234 | 0.070 | 0.403 | 0.063 | |
| N | 306 | 306 | 306 | 306 |
| Sperm Concentration (million/mL) | Semen Volume (mL) | Total Sperm Count (million) | AB Motility (%) | C Motility (%) | D Motility (%) | Progressive Motile Sperm Count (million) | Abstinence Time | ||
|---|---|---|---|---|---|---|---|---|---|
| Vitamin D | rs | 0.013 | −0.071 | −0.036 | 0.143 | −0.134 | −0.133 | 0.035 | 0.081 |
| P | 0.815 | 0.218 | 0.531 | 0.012 | 0.019 | 0.020 | 0.542 | 0.156 | |
| N | 306 | 306 | 306 | 306 | 306 | 306 | 306 | 306 |
| TSH (mU/L) | FSH (U/L) | LH (U/L) | Prolactin (µg/L) | E2 (ng/L) | Testosterone (ng/mL) | Folic Acid (ng/mL) | ||
|---|---|---|---|---|---|---|---|---|
| Vitamin D | rs | −0.083 | 0.011 | 0.101 | 0.088 | 0.061 | 0.135 | 0.048 |
| P | 0.147 | 0.847 | 0.078 | 0.123 | 0.285 | 0.018 | 0.407 | |
| N | 306 | 306 | 306 | 306 | 306 | 306 | 306 |
| Iron (µg/dL) | Ferritin (µg/L) | Hemoglobin (g/dL) | Hematocrit (g/dL) | Calcium (mg/dL) | Magnesium (mg/dL) | Phosphorus (mg/dL) | ||
|---|---|---|---|---|---|---|---|---|
| Vitamin D | rs | −0.022 | −0.070 | −0.027 | 0.097 | 0.111 | 0.065 | −0.002 |
| P | 0.707 | 0.224 | 0.643 | 0.092 | 0.052 | 0.254 | 0.974 | |
| N | 306 | 306 | 306 | 306 | 306 | 306 | 306 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aşır, F.; Duran, S.Ç.; Afşin, M.; Duran, E.; Korak, T.; Şahin, F. Investigation of Vitamin D Levels in Men with Suspected Infertility. Life 2024, 14, 273. https://doi.org/10.3390/life14020273
Aşır F, Duran SÇ, Afşin M, Duran E, Korak T, Şahin F. Investigation of Vitamin D Levels in Men with Suspected Infertility. Life. 2024; 14(2):273. https://doi.org/10.3390/life14020273
Chicago/Turabian StyleAşır, Fırat, Senem Çetin Duran, Muhammet Afşin, Enis Duran, Tuğcan Korak, and Fırat Şahin. 2024. "Investigation of Vitamin D Levels in Men with Suspected Infertility" Life 14, no. 2: 273. https://doi.org/10.3390/life14020273
APA StyleAşır, F., Duran, S. Ç., Afşin, M., Duran, E., Korak, T., & Şahin, F. (2024). Investigation of Vitamin D Levels in Men with Suspected Infertility. Life, 14(2), 273. https://doi.org/10.3390/life14020273






