Epidemiology and Risk Factors for Nosocomial Infections in Left Ventricular Assist Device Recipients
Abstract
1. Introduction
2. Materials and Methods
2.1. Definitions
2.2. Statistical Analysis
2.3. Microbiological Data Collection
2.4. Antibiotic Prophylaxis
3. Results
3.1. Nosocomial Infections
3.2. Risk Factors for Nosocomial Infections
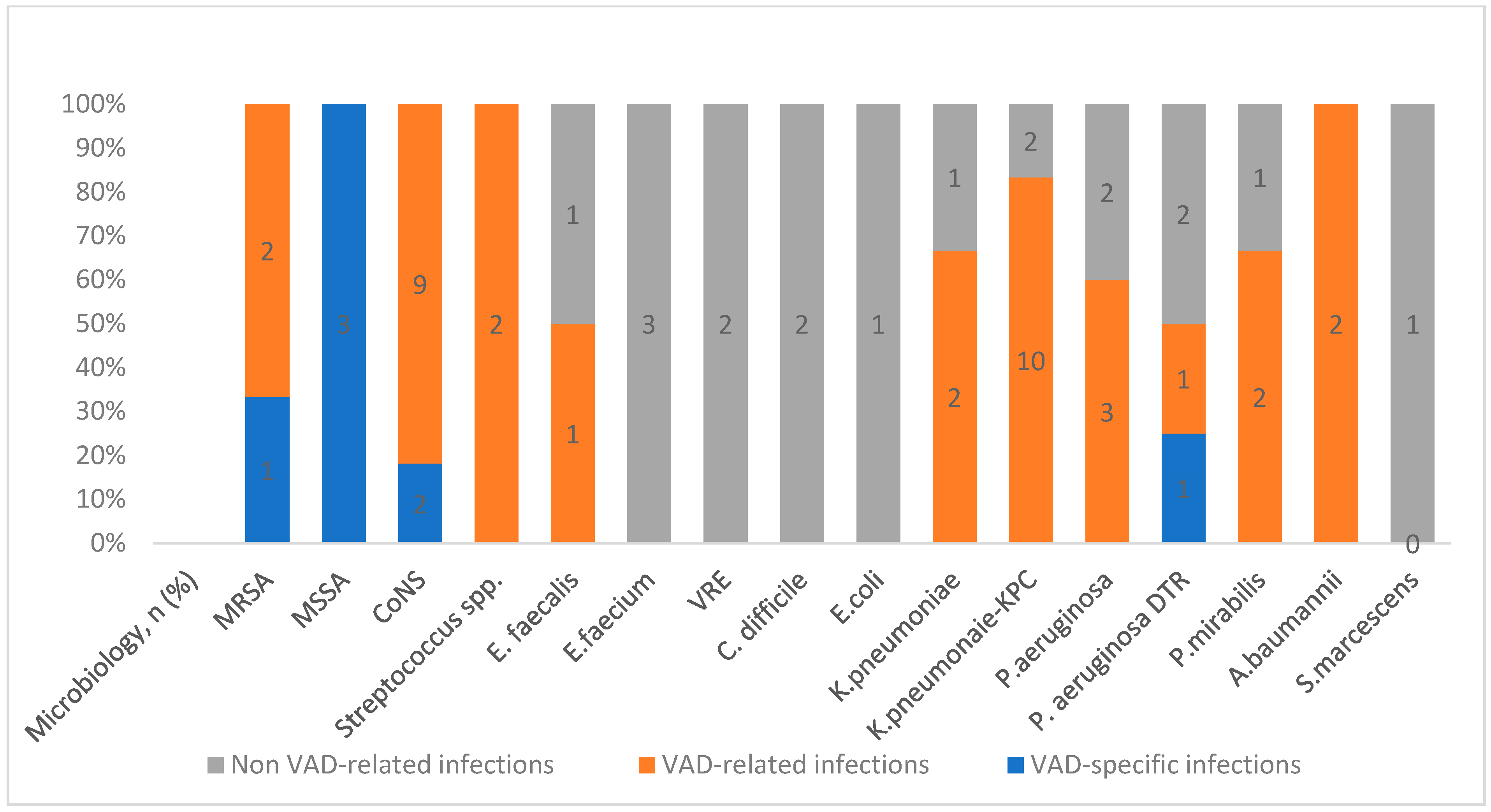
3.3. Epidemiology of Nosocomial Infections
3.4. Risk Factors for MDR Infections
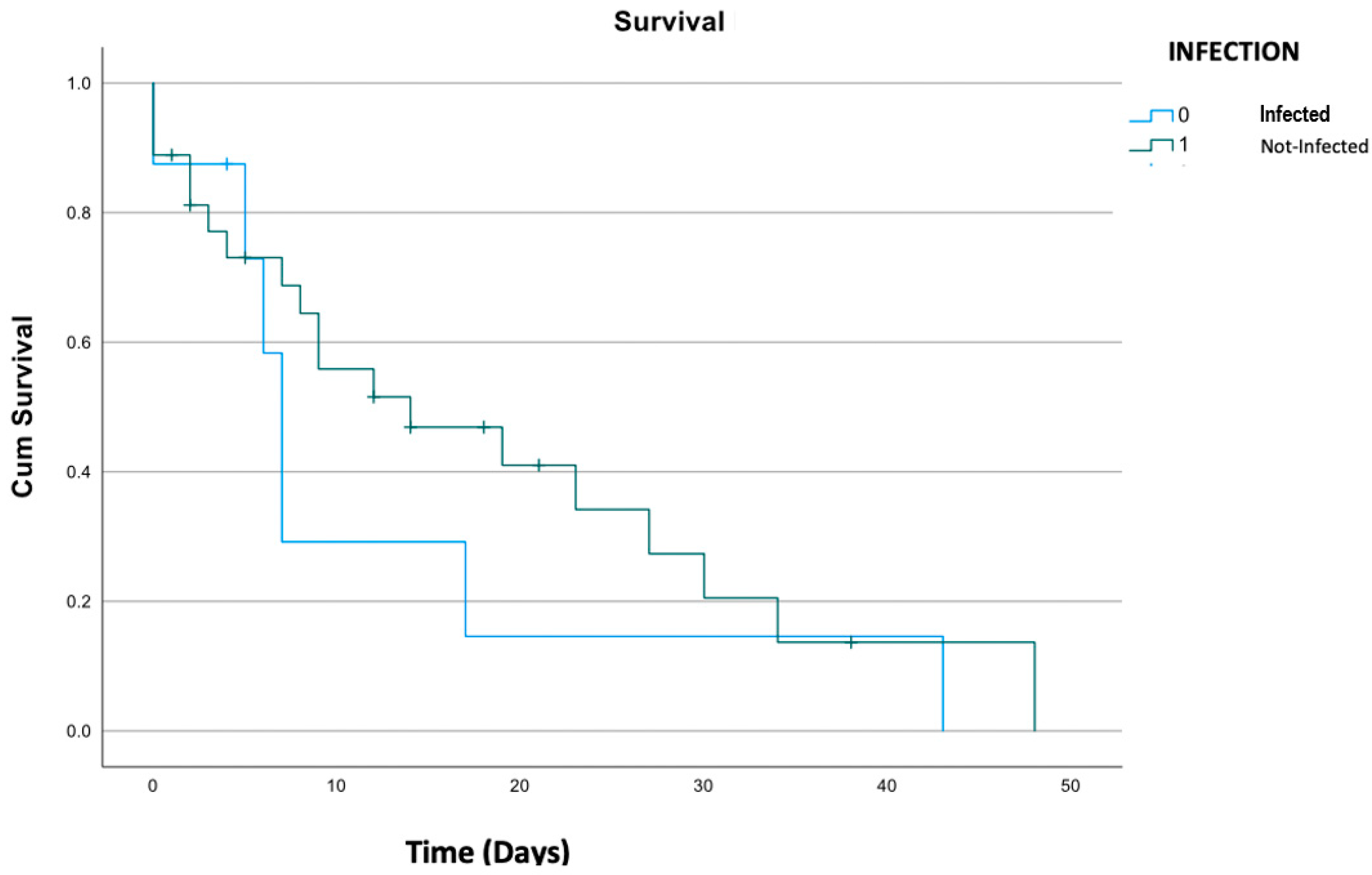
3.5. Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dunlay, S.M.; Roger, V.L. Understanding the Epidemic of Heart Failure: Past, Present, and Future. Curr. Heart Fail. Rep. 2014, 11, 404–415. [Google Scholar] [CrossRef]
- Conrad, N.; Judge, A.; Tran, J.; Mohseni, H.; Hedgecott, D.; Crespillo, A.P.; Allison, M.; Hemingway, H.; Cleland, J.G.; McMurray, J.J.V.; et al. Temporal trends and patterns in heart failure incidence: A population-based study of 4 million individuals. Lancet 2018, 391, 572–580. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failureDeveloped by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Metra, M.; Teerlink, J.R. Heart failure. Lancet 2017, 390, 1981–1995. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Williamitis, C.A.; Slaughter, M.S. Comparison of continuous-flow and pulsatile-flow left ventricular assist devices: Is there an advantage to pulsatility? Ann. Cardiothorac. Surg. 2014, 3, 573. [Google Scholar] [CrossRef] [PubMed]
- de By, T.M.M.H.; Mohacsi, P.; Gahl, B.; Zittermann, A.; Krabatsch, T.; Gustafsson, F.; Leprince, P.; Meyns, B.; Netuka, I.; Caliskan, K.; et al. The Eu-ropean Registry for Patients with Mechanical Circulatory Support (EUROMACS) of the Eu-ropean Association for Cardio-Thoracic Surgery (EACTS): Second report. Eur. J. Cardio-Thorac. Surg. 2018, 53, 309–316. [Google Scholar] [CrossRef]
- Molina, E.J.; Shah, P.; Kiernan, M.S.; Cornwell, W.K.; Copeland, H.; Takeda, K.; Fernandez, F.G.; Badhwar, V.; Habib, R.H.; Jacobs, J.P.; et al. The Society of Thoracic Surgeons Intermacs 2020 Annual Report. Ann. Thorac. Surg. 2021, 111, 778–792. [Google Scholar] [CrossRef]
- Fragasso, T.; Ricci, Z.; Grutter, G.; Albanese, S.; Varano, C.; Amodeo, A.; Cogo, P. Incidence of healthcare-associated infections in a pediatric population with an extracorporeal ventricular as-sist device. Artif. Organs 2011, 35, 1110–1114. [Google Scholar] [CrossRef]
- Garner, J.S.; Jarvis, W.R.; Emori, T.G.; Horan, T.C.; Hughes, J.M. CDC definitions for nosocomial infections, 1988. Am. J. Infect. Control 1988, 16, 128–140. [Google Scholar] [CrossRef]
- Gordon, R.J.; Weinberg, A.D.; Pagani, F.D.; Slaughter, M.S.; Pappas, P.S.; Naka, Y.; Goldstein, D.J.; Dembitsky, W.P.; Giacalone, J.C.; Ferrante, J.; et al. Prospective, multicenter study of ventricular assist device infections. Circulation 2013, 127, 691–702. [Google Scholar] [CrossRef]
- Blanco-Guzman, M.O.; Wang, X.; Vader, J.M.; Olsen, M.A.; Dubberke, E.R. Epidemiology of Left Ventricular Assist Device Infections: Findings From a Large Nonregistry Cohort. Clin. Infect. Dis. 2021, 72, 190–197. [Google Scholar] [CrossRef]
- Mehra, M.R.; Goldstein, D.J.; Uriel, N.; Cleveland, J.C., Jr.; Yuzefpolskaya, M.; Salerno, C.; Walsh, M.N.; Milano, C.A.; Patel, C.B.; Ewald, G.A.; et al. Two-Year Outcomes with a Magnetically Levitated Cardiac Pump in Heart Failure. N. Engl. J. Med. 2018, 378, 1386–1395. [Google Scholar] [CrossRef] [PubMed]
- Nienaber, J.J.; Kusne, S.; Riaz, T.; Walker, R.C.; Baddour, L.M.; Wright, A.J.; Park, S.J.; Vikram, H.R.; Keating, M.R.; Arabia, F.A.; et al. Clinical manifestations and management of left ventricular assist device-associated infections. Clin. Infect. Dis. 2013, 57, 1438–1448. [Google Scholar] [CrossRef]
- Simon, D.; Fischer, S.; Grossman, A.; Downer, C.; Hota, B.; Heroux, A.; Trenholme, G. Left Ventricular Assist Device—Related Infection: Treatment and Outcome. Clin. Infect. Dis. 2005, 40, 1108–1115. [Google Scholar] [CrossRef]
- Dettbarn, E.; Prenga, M.; Stein, J.; Müller, M.; Hoermandinger, C.; Schoenrath, F.; Falk, V.; Potapov, E.; Mulzer, J.; Knierim, J. Driveline infections in left ventricular assist devices-Incidence, epidemiology, and staging proposal. Artif. Organs 2024, 48, 83–90. [Google Scholar] [CrossRef]
- Köhler, A.K.; Körperich, H.; Morshuis, M.; Freytag, C.C.; Gummert, J.; Burchert, W.; Preuss, R.; Körfer, J. Pre-operative risk factors for driveline infection in left ventricular-assist device patients. ESC Heart Fail. 2022, 9, 3995–4002. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multi-drug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Donahey, E.E.; Polly, D.M.; Vega, J.D.; Lyon, M.; Butler, J.; Nguyen, D.; Pekarek, A.; Wittersheim, K.; Kilgo, P.; Paciullo, C.A.; et al. Multidrug-Resistant Organism Infections in Patients with Left Ventricular Assist Devices. Tex. Heart Inst. J. 2015, 42, 522. [Google Scholar] [CrossRef]
- Mekontso-Dessap, A.; Kirsch, M.; Vermes, E.; Brun-Buisson, C.; Loisance, D.; Houël, R. Nosocomial infections occurring during receipt of circulatory support with the paracorporeal ventricular assist system. Clin. Infect. Dis. 2002, 35, 1308–1315. [Google Scholar] [CrossRef]
- Aslam, S.; Xie, R.; Cowger, J.; Kirklin, J.K.; Chu, V.H.; Schueler, S.; de By, T.; Gould, K.; Morrissey, O.; Lund, L.H.; et al. Bloodstream infections in mechanical circulatory support device recipients in the International Society of Heart and Lung Transplantation Mechanically Assisted Circulation Support Registry: Epidemiology, risk factors, and mortality. J. Heart Lung Transplant. 2018, 37, 1013–1020. [Google Scholar] [CrossRef]
- Hannan, M.M.; Xie, R.; Cowger, J.; Schueler, S.; de By, T.; Dipchand, A.I.; Chu, V.H.; Cantor, R.S.; Koval, C.E.; Krabatsch, T.; et al. Epidemiology of infection in mechanical circulatory support: A global analysis from the ISHLT Mechanically Assisted Circulatory Support Registry. J. Heart Lung Transplant. 2019, 38, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.M.; Schmitt, S.K.; Jacobs, M.; Smedira, N.M.; Goormastic, M.; Banbury, M.K.; Yeager, M.; Serkey, J.; Hoercher, K.; McCarthy, P.M. Noso-comial bloodstream infections in patients with implantable left ventricular assist devices. Ann. Thorac. Surg. 2001, 72, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Corcione, S.; De Benedetto, I.; Shbaklo, N.; Ranzani, F.; Pinna, S.M.; Castiglione, A.; Scabini, S.; Bianco, G.; Cavallo, R.; Mirabella, S.; et al. Ten Years of KPC-Kp Bloodstream Infections Experience: Impact of Early Appropriate Empirical Therapy on Mortality. Biomedicines 2022, 10, 3268. [Google Scholar] [CrossRef] [PubMed]
- Giacobbe, D.R.; Corcione, S.; Salsano, A.; Del Puente, F.; Pinna, S.M.; De Rosa, F.G.; Mikulska, M.; Santini, F.; Viscoli, C. Current and emerging pharmacotherapy for the treatment of infections following open-heart surgery. Expert Opin. Pharmacother. 2019, 20, 751–772. [Google Scholar] [CrossRef] [PubMed]
- Maraolo, A.E.; Corcione, S.; Grossi, A.; Signori, A.; Alicino, C.; Hussein, K.; Trecarichi, E.M.; Viale, P.; Timsit, J.-F.; Veeraraghavan, B.; et al. The Impact of Carbapenem Resistance on Mortality in Patients with Klebsiella Pneumoniae Bloodstream In-fection: An Individual Patient Data Meta-Analysis of 1952 Patients. Infect. Dis. Ther. 2021, 10, 541–558. [Google Scholar] [CrossRef]
- Koval, C.E.; Stosor, V.; AST ID Community of Practice. Ventricular assist device-related infections and solid organ transplantation-Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13552. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Dufendach, K.A.; Hong, Y.; Thoma, F.W.; Kilic, A. Infectious complications following contemporary left ventricular assist device implantation. J. Card. Surg. 2022, 37, 2297–2306. [Google Scholar] [CrossRef]
- Gelijns, A.C.; Moskowitz, A.J.; Acker, M.A.; Argenziano, M.; Geller, N.L.; Puskas, J.D.; Perrault, L.P.; Smith, P.K.; Kron, I.L.; Michler, R.E.; et al. Management Practices and Major Infections After Cardiac Surgery. J. Am. Coll. Cardiol. 2014, 64, 372–381. [Google Scholar] [CrossRef]
- Yarboro, L.T.; Bergin, J.D.; Kennedy, J.L.; Ballew, C.C.; Benton, E.M.; Ailawadi, G.; Kern, J.A. Technique for minimizing and treating driveline infections. Ann. Cardiothorac. Surg. 2014, 3, 557–562. [Google Scholar] [CrossRef]
- Seretny, J.; Pidborochynski, T.; Buchholz, H.; Freed, D.H.; MacArthur, R.; Dubyk, N.; Cunliffe, L.; Zelaya, O.; Conway, J. Decreasing driveline infections in patients supported on ventricular assist devices: A care pathway approach. BMJ Open Qual. 2022, 11, e001815. [Google Scholar] [CrossRef]
- Snydman, D.R.; Kusne, S.; Staley, L.; Arabia, F. Prevention and Infection Management in Mechanical Circulatory Support Device Recipients. Clin. Infect. Dis. 2017, 64, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.S.; Siliprandi, E.M.O.; Karsburg, L.L.; Berlesi, F.P.; Carvalho, O.L.D.F.; Rosa, D.S.D.; Santos, R.P.D. Surgical Site Infection Prevention Bundle in Cardiac Surgery. Arq. Bras. Cardiol. 2019, 112, 769–774. [Google Scholar] [CrossRef] [PubMed]
| Baseline Characteristics | Overall | Nosocomial Infection (n = 32) | No Infection (n = 32) | p Value | Multivariate Analysis (95%CI) |
|---|---|---|---|---|---|
| Age (years, IQR) | 61 (56–65) | 62.5 (61.5–65) | 62.5 (61.5–65) | 0.458 | |
| Males (%) | 54 (84%) | 27 (84.4%) | 27 (84.4%) | 0.340 | |
| Hypertension (%) | 26 (40%) | 12 (37.5%) | 14 (43.8%) | 0.728 | |
| Diabetes (%) | 11 (17%) | 4 (12.5%) | 7 (21.9%) | 0.356 | |
| Dyslipidemia (%) | 18 (28%) | 7 (21.9%) | 11 (34.4%) | 0.448 | |
| COPD (%) | 5 (6%) | 3 (9.4%) | 2 (6.3%) | 0.999 | |
| Chronic kidney disease (%) | 18 (28%) | 8 (25.0%) | 10 (31.3%) | 0.999 | |
| INTERMACS Level (%) | |||||
| 1 | 7 (10.9%) | 5 (15.6%) | 2 (6.3%) | ||
| 2 | 13 (20.3%) | 9 (28.1%) | 4 (12.5%) | ||
| 3 | 34 (53.1%) | 14 (43.8%) | 20 (62.5%) | 0.259 | |
| 4 | 8 (12.5%) | 2 (6.3%) | 6 (18.8%) | ||
| 5 | 1(1.6%) | 1 (3.1%) | 0 (0%) | ||
| 6 | 1 (1.6%) | 1 (3.1%) | 0 (0%) | ||
| Indication for LVAD (%) | |||||
| BTT | 22 (34.4%) | 11 (34.0%) | 11 (34.0%) | ||
| BTC | 17 (26.6%) | 11 (34.0%) | 6 (34.4%) | 0.814 | |
| DT | 25 (39.1%) | 10 (31.1%) | 15 (46.9%) | ||
| Type of LVAD support | |||||
| Hearthware | 45 (70%) | 27 (84.4%) | 19 (59.4%) | ||
| Heartmate II | 17 (26.6%) | 12 (37.5%) | 5 (15.6%) | 0.750 | |
| Jarvic 2000 | 2 (3.1%) | 0 (0%) | 2 (6.3%) | ||
| Cardiac disease (%) | |||||
| Dilated CM | 31 (48.4%) | 19 (59.4%) | 12 (37.5%) | 0.909 | |
| Ischemic CM | 32 (50.0%) | 20 (62.5%) | 12 (37.5%) | ||
| Valvular | 1 (1.6%) | 1 (3.1%) | 0 (0%) | ||
| Ventricular support pre- | |||||
| implant (%) | |||||
| IABP | 22 (32.8%) | 13 (40.6%) | 9 (28.1%) | 0.584 | |
| ECMO | 7 (10.9%) | 6 (18.8%) | 1 (3.1%) | ||
| Other cardiovascular surgery during LVAD implant | 5 (7.8%) | 3 (9.4%) | 2 (6.3%) | 0.452 | |
| Weight, (kg, IQR) | 70 (60.0–79.5) | 69.0 (60.0–80.0) | 69.0 (60.0–75.5) | 0.345 | |
| Mean length of surgery (min, IQR) | 242.5 (210.0–294.0) | 257.5 (210.0–338.75) | 235.0 (210.0–282.0) | 0.080 | |
| Mean ICU (days) | 4 (3.0–11.5) | 9.0 (3.0–24.75) | 4.0 (2.0–5.0) | <0.0001 | Sig. 0.022, OR 1.224; 1.049, 1.429 |
| Mean time of mechanical ventilation (h) | 18 (9.0–33.0) | 23.0 (12.25–100) | 11.0 (8.0–21.0) | 0.070 | Sig. 0.622, OR 0.99; 0.973, 1.013 |
| Mean length of hospital stay (days) | 37.5 (28–56) | 50.5 (34.0–61.75) | 31.0 (23.75–45.75) | <0.001 | Sig. 0.119, OR 1.031; 0.992, 1.070 |
| Mean time of ECC (min) | 76 (59.3–105.3) | 79.0 (60.0–109.0) | 66 (55.0–101.5) | 0.272 | Sig. 0.470, OR 0.99; 0.962, 1.018 |
| CVVH (%) | 12 (18.8%) | 10 (31.3%) | 2 (6.3%) | 0.022 | Sig. 0.879, OR 0.88; 0.194, 4.069 |
| In-hospital mortality, n (%) | 4 (6.25%) | 3 (9.4%) | 1 (3.1%) | 0.613 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mornese Pinna, S.; Corcione, S.; Cavallone, E.; Shbaklo, N.; Vita, D.; De Benedetto, I.; Montrucchio, G.; Pasero, D.; Trompeo, A.C.; Costamagna, A.; et al. Epidemiology and Risk Factors for Nosocomial Infections in Left Ventricular Assist Device Recipients. Life 2024, 14, 270. https://doi.org/10.3390/life14020270
Mornese Pinna S, Corcione S, Cavallone E, Shbaklo N, Vita D, De Benedetto I, Montrucchio G, Pasero D, Trompeo AC, Costamagna A, et al. Epidemiology and Risk Factors for Nosocomial Infections in Left Ventricular Assist Device Recipients. Life. 2024; 14(2):270. https://doi.org/10.3390/life14020270
Chicago/Turabian StyleMornese Pinna, Simone, Silvia Corcione, Elena Cavallone, Nour Shbaklo, Davide Vita, Ilaria De Benedetto, Giorgia Montrucchio, Daniela Pasero, Anna Chiara Trompeo, Andrea Costamagna, and et al. 2024. "Epidemiology and Risk Factors for Nosocomial Infections in Left Ventricular Assist Device Recipients" Life 14, no. 2: 270. https://doi.org/10.3390/life14020270
APA StyleMornese Pinna, S., Corcione, S., Cavallone, E., Shbaklo, N., Vita, D., De Benedetto, I., Montrucchio, G., Pasero, D., Trompeo, A. C., Costamagna, A., Brazzi, L., Rinaldi, M., Boffini, M., & De Rosa, F. G. (2024). Epidemiology and Risk Factors for Nosocomial Infections in Left Ventricular Assist Device Recipients. Life, 14(2), 270. https://doi.org/10.3390/life14020270









