Vitamin D Levels as a Marker of Severe SARS-CoV-2 Infection
Abstract
1. Introduction
2. Materials and Methods
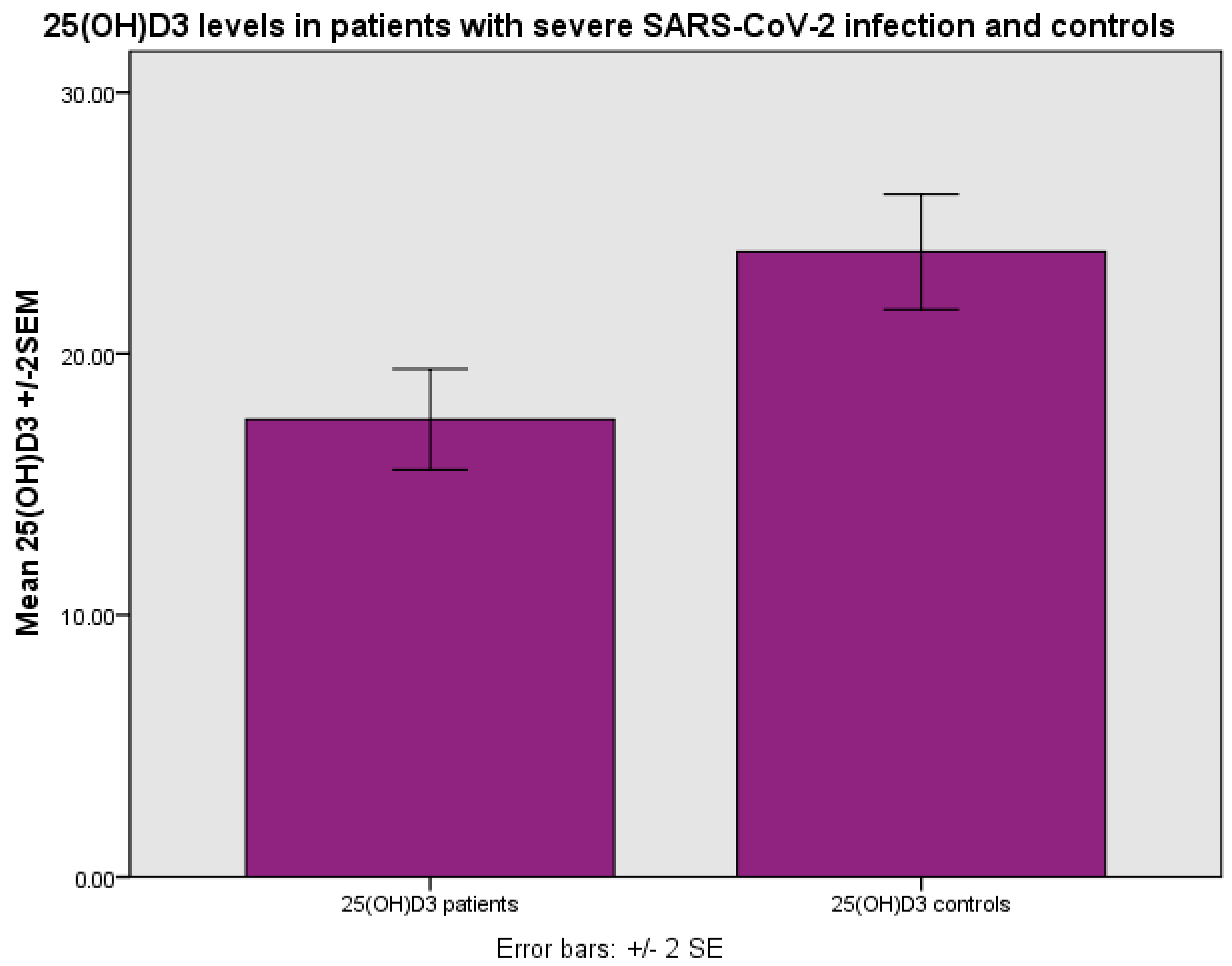
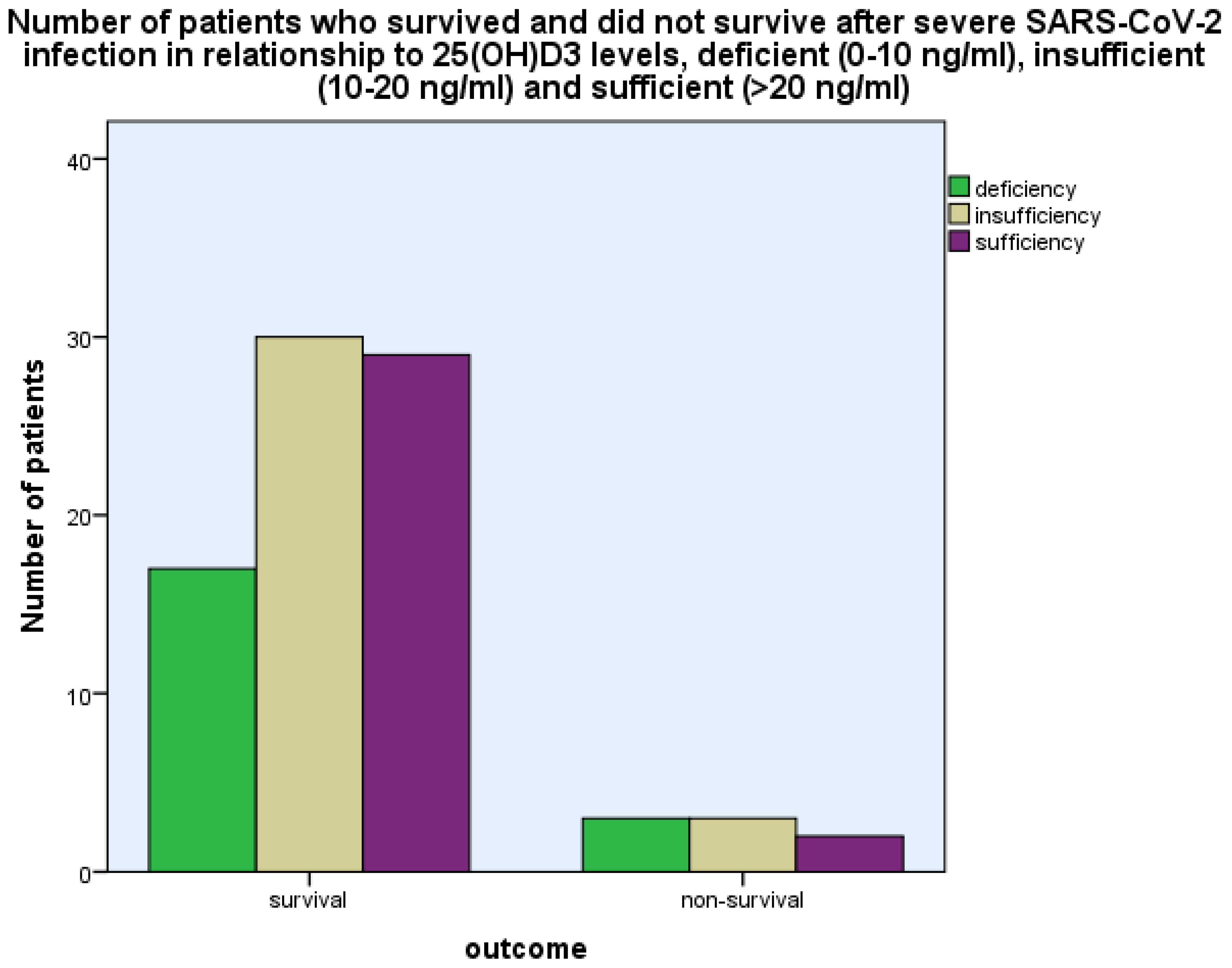
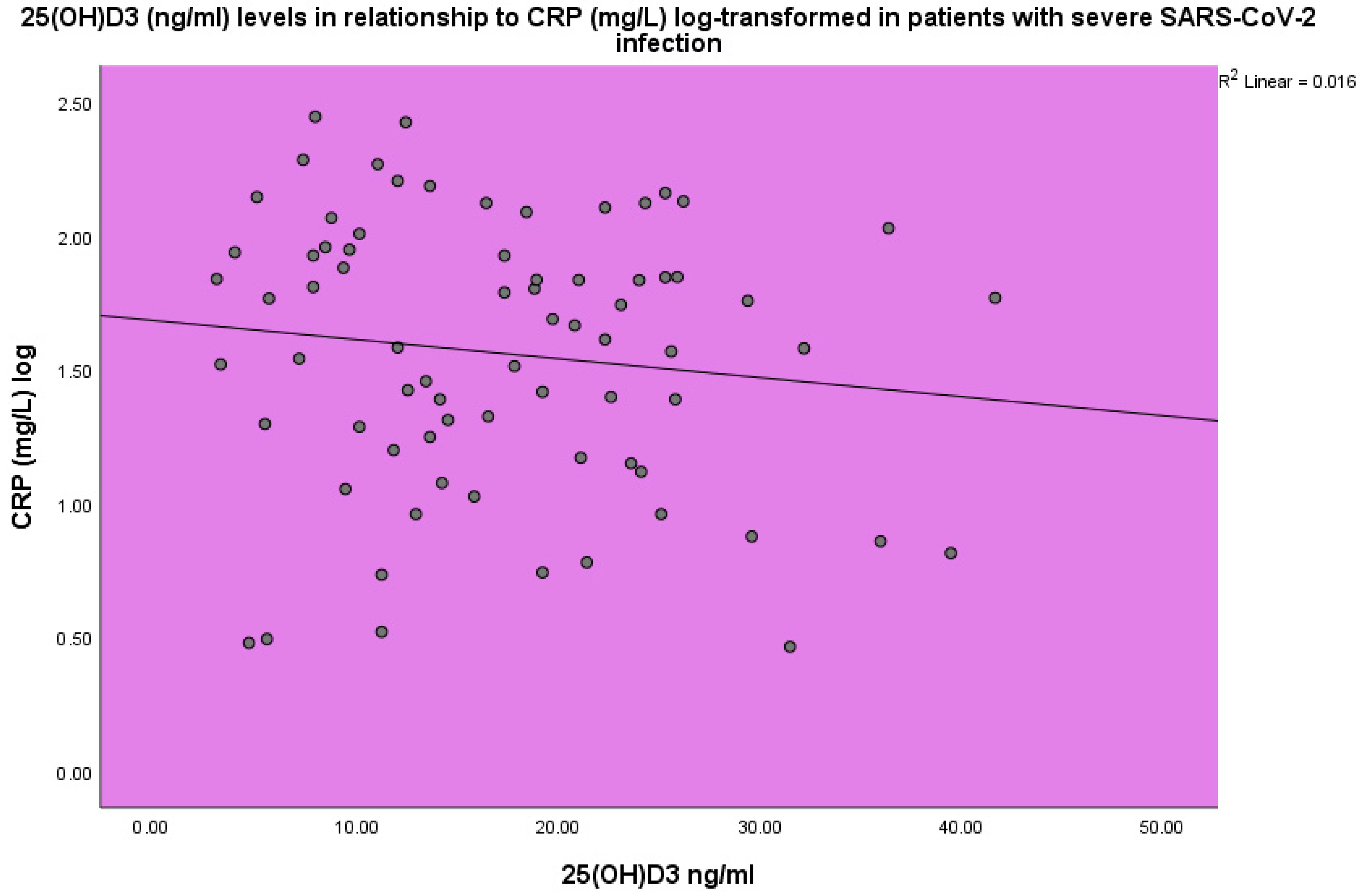
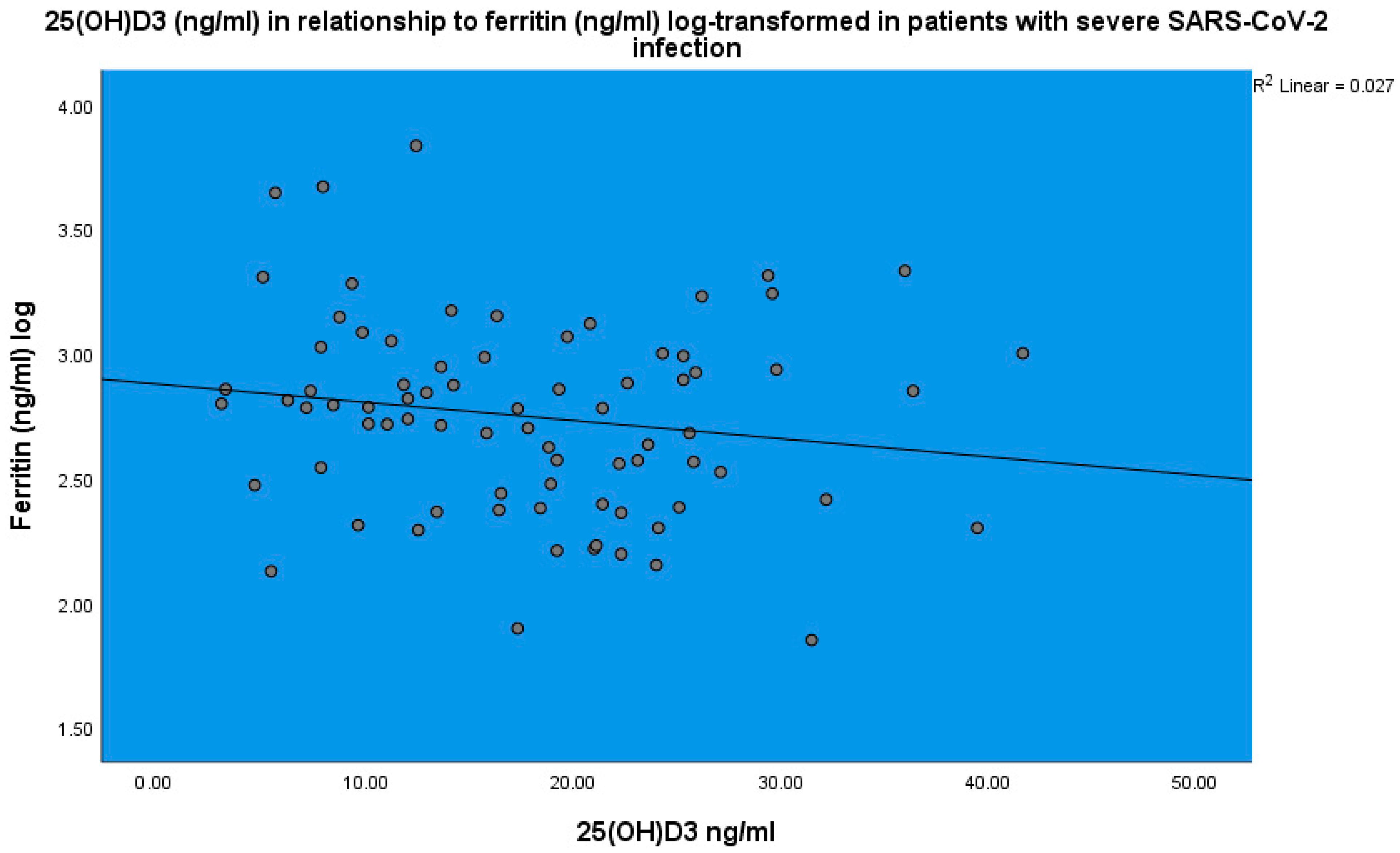
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farrag, M.A.; Amer, H.M.; Bhat, R.; Hamed, M.E.; Aziz, I.M.; Mubarak, A.; Dawoud, T.M.; Almalki, S.G.; Alghofaili, F.; Alnemare, A.K.; et al. SARS-CoV-2: An Overview of Virus Genetics, Transmission, and Immunopathogenesis. Int. J. Environ. Res. Public Health 2021, 18, 6312. [Google Scholar] [CrossRef] [PubMed]
- Shivalkar, S.; Pingali, M.S.; Verma, A.; Singh, A.; Singh, V.; Paital, B.; Das, D.; Varadwaj, P.K.; Samanta, S.K. Outbreak of COVID-19: A Detailed Overview and Its Consequences. Adv. Exp. Med. Biol. 2021, 1353, 23–45. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, J.S.; Bhatti, G.K.; Khullar, N.; Reddy, A.P.; Reddy, P.H. Therapeutic Strategies in the Development of Anti-viral Drugs and Vaccines Against SARS-CoV-2 Infection. Mol. Neurobiol. 2020, 57, 4856–4877. [Google Scholar] [CrossRef] [PubMed]
- Chiodini, I.; Gatti, D.; Soranna, D.; Merlotti, D.; Mingiano, C.; Fassio, A.; Adami, G.; Falchetti, A.; Eller-Vainicher, C.; Rossini, M.; et al. Vitamin D Status and SARS-CoV-2 Infection and COVID-19 Clinical Outcomes. Front. Public Health 2021, 9, 736665. [Google Scholar] [CrossRef] [PubMed]
- Feentved Ødum, S.L.; Kongsbak-Wismann, M. Vitamin D and SARS-CoV-2. Basic. Clin. Pharmacol. Toxicol. 2023, 133, 6–15. [Google Scholar] [CrossRef] [PubMed]
- D’Ecclesiis, O.; Gavioli, C.; Martinoli, C.; Raimondi, S.; Chiocca, S.; Miccolo, C.; Bossi, P.; Cortinovis, D.; Chiaradonna, F.; Palorini, R.; et al. Vitamin D and SARS-CoV2 infection, severity and mortality: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0268396. [Google Scholar] [CrossRef]
- Boulkrane, M.S.; Ilina, V.; Melchakov, R.; Fedotova, J.; Drago, F.; Gozzo, L.; Das, U.N.; Abd El-Aty, A.M.; Baranenko, D. COVID-19 Disease and Vitamin D: A Mini-Review. Front. Pharmacol. 2020, 11, 604579. [Google Scholar] [CrossRef]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Charoenngam, N.; Shirvani, A.; Holick, M.F. Vitamin D for skeletal and non-skeletal health: What we should know. J. Clin. Orthop. Trauma 2019, 10, 1082–1093. [Google Scholar] [CrossRef]
- Shapira, Y.; Agmon-Levin, N.; Shoenfeld, Y. Mycobacterium tuberculosis, autoimmunity, and vitamin D. Clin. Rev. Allergy Immunol. 2010, 38, 169–177. [Google Scholar] [CrossRef]
- Kim, E.W.; Teles, R.M.B.; Haile, S.; Liu, P.T.; Modlin, R.L. Vitamin D status contributes to the antimicrobial activity of macrophages against Mycobacterium leprae. PLoS Negl. Trop. Dis. 2018, 12, e0006608. [Google Scholar] [CrossRef]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef]
- Daneshkhah, A.; Agrawal, V.; Eshein, A.; Subramanian, H.; Roy, H.K.; Backman, V. Evidence for possible association of vitamin D status with cytokine storm and unregulated inflammation in COVID-19 patients. Aging Clin. Exp. Res. 2020, 32, 2141–2158. [Google Scholar] [CrossRef]
- Pinheiro, M.M.; Fabbri, A.; Infante, M. Cytokine storm modulation in COVID-19: A proposed role for vitamin D and DPP-4 inhibitor combination therapy (VIDPP-4i). Immunotherapy 2021, 13, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Sassi, F.; Tamone, C.; D’Amelio, P. Vitamin D: Nutrient, Hormone, and Immunomodulator. Nutrients 2018, 10, 1656. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F. A Network-Based Analysis Reveals the Mechanism Underlying Vitamin D in Suppressing Cytokine Storm and Virus in SARS-CoV-2 Infection. Front. Immunol. 2020, 11, 590459. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.; Casado, G.; Vigón, L.; Rodríguez-Mora, S.; Mateos, E.; Ramos-Martín, F.; López-Wolf, D.; Sanz-Moreno, J.; Ryan-Murua, P.; Taboada-Martínez, M.L.; et al. Changes in the immune response against SARS-CoV-2 in individuals with severe COVID-19 treated with high dose of vitamin D. Biomed. Pharmacother. 2022, 150, 112965. [Google Scholar] [CrossRef]
- Rawat, D.; Roy, A.; Maitra, S.; Shankar, V.; Khanna, P.; Baidya, D.K. Vitamin D supplementation and COVID-19 treatment: A systematic review and meta-analysis. Diabetes Metab. Syndr. 2021, 15, 102189. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.; Kim, H. Mini-Review on the Roles of Vitamin C, Vitamin D, and Selenium in the Immune System against COVID-19. Molecules 2020, 25, 5346. [Google Scholar] [CrossRef] [PubMed]
- Tomazini, B.M.; Maia, I.S.; Cavalcanti, A.B.; Berwanger, O.; Rosa, R.G.; Veiga, V.C.; Avezum, A.; Lopes, R.D.; Bueno, F.R.; Silva, M.; et al. Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients With Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19: The CoDEX Randomized Clinical Trial. JAMA 2020, 324, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Andreakos, E.; Papadaki, M.; Serhan, C.N. Dexamethasone, pro-resolving lipid mediators and resolution of inflammation in COVID-19. Allergy 2021, 76, 626–628. [Google Scholar] [CrossRef]
- Gao, Y.D.; Ding, M.; Dong, X.; Zhang, J.J.; Kursat Azkur, A.; Azkur, D.; Gan, H.; Sun, Y.L.; Fu, W.; Li, W.; et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy 2021, 76, 428–455. [Google Scholar] [CrossRef] [PubMed]
- McPadden, J.; Warner, F.; Young, H.P.; Hurley, N.C.; Pulk, R.A.; Singh, A.; Durant, T.J.S.; Gong, G.; Desai, N.; Haimovich, A.; et al. Clinical characteristics and outcomes for 7,995 patients with SARS-CoV-2 infection. PLoS ONE 2021, 16, e0243291. [Google Scholar] [CrossRef] [PubMed]
- Pran, L.; Baijoo, S.; Slim, H. Viral infection-induced thrombosis, novel coronavirus. J. Vasc. Surg. 2020, 72, 764–765. [Google Scholar] [CrossRef] [PubMed]
- Helms, J.; Tacquard, C.; Severac, F.; Leonard-Lorant, I.; Ohana, M.; Delabranche, X.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Fagot Gandet, F.; et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020, 46, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, N.R.; Phatak, S.; Sharma, V.R.; Agarwal, S.K. COVID-19 and thrombotic microangiopathies. Thromb. Res. 2021, 202, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Bobescu, E.; Marceanu, L.G.; Covaciu, A.; Vladau, L.A. Thrombosis, an important piece in the COVID-19 puzzle: From pathophysiology to therapy. Anatol. J. Cardiol. 2021, 25, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Porta-Etessam, J.; Yus, M.; González García, N.; Valcarcel, A.; Barrado-Cuchillo, J.; Pérez-Somarriba, J. Brain inflammatory thrombogenic vasculopathy related with SARS-CoV-2 infection (Vasculopatía trombogénica inflamatoria del cerebro relacionada con la infección del SARS-CoV-2). Neurologia 2020, 35, 701–703. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Nappi, P.; Gambardella, I.; Avtaar Singh, S.S. Thromboembolic Disease and Cardiac Thrombotic Complication in COVID-19: A Systematic Review. Metabolites 2022, 12, 889. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Elalamy, I.; Kastritis, E.; Sergentanis, T.N.; Politou, M.; Psaltopoulou, T.; Gerotziafas, G.; Dimopoulos, M.A. Hematological findings and complications of COVID-19. Am. J. Hematol. 2020, 95, 834–847. [Google Scholar] [CrossRef]
- Qeadan, F.; Tingey, B.; Gu, L.Y.; Packard, A.H.; Erdei, E.; Saeed, A.I. Prognostic Values of Serum Ferritin and D-Dimer Trajectory in Patients with COVID-19. Viruses 2021, 13, 419. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.X.; He, D.R. Low Vitamin D Levels Are Associated With the Development of Deep Venous Thromboembolic Events in Patients With Ischemic Stroke. Clin. Appl. Thromb. Hemost. 2018, 24, 69s–75s. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, S.; Mishra, A.; Ashraf, M.Z. Emerging Role of Vitamin D and its Associated Molecules in Pathways Related to Pathogenesis of Thrombosis. Biomolecules 2019, 9, 649. [Google Scholar] [CrossRef]
- Arosio, P.; Levi, S. Ferritin, iron homeostasis, and oxidative damage. Free Radic. Biol. Med. 2002, 33, 457–463. [Google Scholar] [CrossRef] [PubMed]
- McCullough, K.; Bolisetty, S. Iron Homeostasis and Ferritin in Sepsis-Associated Kidney Injury. Nephron 2020, 144, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Nairz, M.; Weiss, G. Iron in infection and immunity. Mol. Aspects Med. 2020, 75, 100864. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, J.; Zaritsky, J.J.; Sea, J.L.; Chun, R.F.; Lisse, T.S.; Zavala, K.; Nayak, A.; Wesseling-Perry, K.; Westerman, M.; Hollis, B.W.; et al. Suppression of iron-regulatory hepcidin by vitamin D. J. Am. Soc. Nephrol. 2014, 25, 564–572. [Google Scholar] [CrossRef]
- Cheng, L.; Li, H.; Li, L.; Liu, C.; Yan, S.; Chen, H.; Li, Y. Ferritin in the coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. J. Clin. Lab. Anal. 2020, 34, e23618. [Google Scholar] [CrossRef]
- Mahroum, N.; Alghory, A.; Kiyak, Z.; Alwani, A.; Seida, R.; Alrais, M.; Shoenfeld, Y. Ferritin-from iron, through inflammation and autoimmunity, to COVID-19. J. Autoimmun. 2022, 126, 102778. [Google Scholar] [CrossRef]
- Farrell, C.J.; Martin, S.; McWhinney, B.; Straub, I.; Williams, P.; Herrmann, M. State-of-the-art vitamin D assays: A comparison of automated immunoassays with liquid chromatography-tandem mass spectrometry methods. Clin. Chem. 2012, 58, 531–542. [Google Scholar] [CrossRef]
- Stang, L.J.; Mitchell, L.G. Fibrinogen. Methods Mol. Biol. 2013, 992, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.H.; Lai, W.Y.; Lin, Y.Y.; Luo, Y.H.; Lin, Y.T.; Chen, H.K.; Chen, Y.M.; Lai, Y.C.; Kuo, L.C.; Chen, S.D.; et al. Clinical manifestation and disease progression in COVID-19 infection. J. Chin. Med. Assoc. 2021, 84, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Gui, X.; Gao, S.; Ke, H.; Xiong, Y. Clinical progression and changes of chest CT findings among asymptomatic and pre-symptomatic patients with SARS-CoV-2 infection in Wuhan, China. Expert. Rev. Respir. Med. 2021, 15, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Golob, J.L.; Lugogo, N.; Lauring, A.S.; Lok, A.S. SARS-CoV-2 vaccines: A triumph of science and collaboration. JCI Insight 2021, 6, e149187. [Google Scholar] [CrossRef]
- Patel, R.; Kaki, M.; Potluri, V.S.; Kahar, P.; Khanna, D. A comprehensive review of SARS-CoV-2 vaccines: Pfizer, Moderna & Johnson & Johnson. Hum. Vaccin. Immunother. 2022, 18, 2002083. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Tzou, P.L.; Nouhin, J.; Bonilla, H.; Jagannathan, P.; Shafer, R.W. SARS-CoV-2 Antiviral Therapy. Clin. Microbiol. Rev. 2021, 34, e0010921. [Google Scholar] [CrossRef] [PubMed]
- Imai, M.; Ito, M.; Kiso, M.; Yamayoshi, S.; Uraki, R.; Fukushi, S.; Watanabe, S.; Suzuki, T.; Maeda, K.; Sakai-Tagawa, Y.; et al. Efficacy of Antiviral Agents against Omicron Subvariants BQ.1.1 and XBB. N. Engl. J. Med. 2023, 388, 89–91. [Google Scholar] [CrossRef]
- Takashita, E.; Kinoshita, N.; Yamayoshi, S.; Sakai-Tagawa, Y.; Fujisaki, S.; Ito, M.; Iwatsuki-Horimoto, K.; Halfmann, P.; Watanabe, S.; Maeda, K.; et al. Efficacy of Antiviral Agents against the SARS-CoV-2 Omicron Subvariant BA.2. N. Engl. J. Med. 2022, 386, 1475–1477. [Google Scholar] [CrossRef]
- Mohan, M.; Cherian, J.J.; Sharma, A. Exploring links between vitamin D deficiency and COVID-19. PLoS Pathog. 2020, 16, e1008874. [Google Scholar] [CrossRef]
- Bassatne, A.; Basbous, M.; Chakhtoura, M.; El Zein, O.; Rahme, M.; El-Hajj Fuleihan, G. The link between COVID-19 and VItamin D (VIVID): A systematic review and meta-analysis. Metabolism 2021, 119, 154753. [Google Scholar] [CrossRef]
- Hsieh, M.C.; Hsiao, P.J.; Liao, M.T.; Hou, Y.C.; Chang, Y.C.; Chiang, W.F.; Wu, K.L.; Chan, J.S.; Lu, K.C. The Role of Vitamin D in SARS-CoV-2 Infection and Acute Kidney Injury. Int. J. Mol. Sci. 2022, 23, 7368. [Google Scholar] [CrossRef]
- Azzam, A.Y.; Ghozy, S.; Azab, M.A. Vitamin D and its’ role in Parkinson’s disease patients with SARS-CoV-2 infection. A review article. Interdiscip. Neurosurg. 2022, 27, 101441. [Google Scholar] [CrossRef] [PubMed]
- Radujkovic, A.; Hippchen, T.; Tiwari-Heckler, S.; Dreher, S.; Boxberger, M.; Merle, U. Vitamin D Deficiency and Outcome of COVID-19 Patients. Nutrients 2020, 12, 2757. [Google Scholar] [CrossRef] [PubMed]
- AlSafar, H.; Grant, W.B.; Hijazi, R.; Uddin, M.; Alkaabi, N.; Tay, G.; Mahboub, B.; Al Anouti, F. COVID-19 Disease Severity and Death in Relation to Vitamin D Status among SARS-CoV-2-Positive UAE Residents. Nutrients 2021, 13, 1714. [Google Scholar] [CrossRef] [PubMed]
- Decyk, A.; Kobylińska, M.; Antosik, K.; Kurowska, K. Vitamin D in SARS-CoV-2 infection. Rocz. Panstw. Zakl. Hig. 2022, 73, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, D.; Locatelli, M.; Briguglio, M.; Lombardi, G. Is there a link between vitamin D status, SARS-CoV-2 infection risk and COVID-19 severity? Cell Biochem. Funct. 2021, 39, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Bolívar, V.; García-Fontana, B.; García-Fontana, C.; Muñoz-Torres, M. Vitamin D and COVID-19: Where are we now? Postgrad. Med. 2023, 135, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.Y.; Liu, W.C.; Zheng, J.Q.; Lu, C.L.; Hou, Y.C.; Zheng, C.M.; Song, J.Y.; Lu, K.C.; Chao, Y.C. Immunological Aspects of SARS-CoV-2 Infection and the Putative Beneficial Role of Vitamin-D. Int. J. Mol. Sci. 2021, 22, 5251. [Google Scholar] [CrossRef]
- Dennison, C.L.; de Oliveira, L.B.; Fraga, L.A.O.; Lima, R.E.S.; Ferreira, J.A.; Clennon, J.A.; de Mondesert, L.; Stephens, J.; Magueta, E.B.; Castelo Branco, A.; et al. Mycobacterium leprae-helminth co-infections and vitamin D deficiency as potential risk factors for leprosy: A case-control study in south-eastern Brazil. Int. J. Infect. Dis. 2021, 105, 261–266. [Google Scholar] [CrossRef]
- Papagni, R.; Pellegrino, C.; Di Gennaro, F.; Patti, G.; Ricciardi, A.; Novara, R.; Cotugno, S.; Musso, M.; Guido, G.; Ronga, L.; et al. Impact of Vitamin D in Prophylaxis and Treatment in Tuberculosis Patients. Int. J. Mol. Sci. 2022, 23, 3860. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.C.; Zheng, C.M.; Lu, C.L.; Lin, Y.F.; Shyu, J.F.; Wu, C.C.; Lu, K.C. Vitamin D and immune function in chronic kidney disease. Clin. Chim. Acta 2015, 450, 135–144. [Google Scholar] [CrossRef]
- Channappanavar, R.; Perlman, S. Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017, 39, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, J.; Chen, J.; Luo, Q.; Zhang, Q.; Zhang, H. Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol. Med. Rep. 2017, 16, 7432–7438. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Baylink, D.J.; Chen, C.S.; Reeves, M.E.; Xiao, J.; Lacy, C.; Lau, E.; Cao, H. The importance of vitamin d metabolism as a potential prophylactic, immunoregulatory and neuroprotective treatment for COVID-19. J. Transl. Med. 2020, 18, 322. [Google Scholar] [CrossRef] [PubMed]
- Raisi-Estabragh, Z.; Martineau, A.R.; Curtis, E.M.; Moon, R.J.; Darling, A.; Lanham-New, S.; Ward, K.A.; Cooper, C.; Munroe, P.B.; Petersen, S.E.; et al. Vitamin D and coronavirus disease 2019 (COVID-19): Rapid evidence review. Aging Clin. Exp. Res. 2021, 33, 2031–2041. [Google Scholar] [CrossRef] [PubMed]
- Waldron, J.L.; Ashby, H.L.; Cornes, M.P.; Bechervaise, J.; Razavi, C.; Thomas, O.L.; Chugh, S.; Deshpande, S.; Ford, C.; Gama, R. Vitamin D: A negative acute phase reactant. J. Clin. Pathol. 2013, 66, 620–622. [Google Scholar] [CrossRef]
- Klein, R.G.; Arnaud, S.B.; Gallagher, J.C.; Deluca, H.F.; Riggs, B.L. Intestinal calcium absorption in exogenous hypercortisonism. Role of 25-hydroxyvitamin D and corticosteroid dose. J. Clin. Investig. 1977, 60, 253–259. [Google Scholar] [CrossRef]
- Zhu, A.; Kuznia, S.; Boakye, D.; Schöttker, B.; Brenner, H. Vitamin D-Binding Protein, Bioavailable, and Free 25(OH)D, and Mortality: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 3894. [Google Scholar] [CrossRef]
- Speeckaert, M.M.; Delanghe, J.R. Commentary: Vitamin D Status in Relation to the Clinical Outcome of Hospitalized COVID-19 Patients. Front. Med. 2022, 9, 922820. [Google Scholar] [CrossRef]
- Karcioglu Batur, L.; Hekim, N. The role of DBP gene polymorphisms in the prevalence of new coronavirus disease 2019 infection and mortality rate. J. Med. Virol. 2021, 93, 1409–1413. [Google Scholar] [CrossRef] [PubMed]
- Annweiler, C.; Beaudenon, M.; Gautier, J.; Simon, R.; Dubée, V.; Gonsard, J.; Parot-Schinkel, E. COvid-19 and high-dose VITamin D supplementation TRIAL in high-risk older patients (COVIT-TRIAL): Study protocol for a randomized controlled trial. Trials 2020, 21, 1031. [Google Scholar] [CrossRef] [PubMed]
- Annweiler, C.; Beaudenon, M.; Gautier, J.; Gonsard, J.; Boucher, S.; Chapelet, G.; Darsonval, A.; Fougère, B.; Guérin, O.; Houvet, M.; et al. High-dose versus standard-dose vitamin D supplementation in older adults with COVID-19 (COVIT-TRIAL): A multicenter, open-label, randomized controlled superiority trial. PLoS Med. 2022, 19, e1003999. [Google Scholar] [CrossRef] [PubMed]
- Bilezikian, J.P.; Formenti, A.M.; Adler, R.A.; Binkley, N.; Bouillon, R.; Lazaretti-Castro, M.; Marcocci, C.; Napoli, N.; Rizzoli, R.; Giustina, A. Vitamin D: Dosing, levels, form, and route of administration: Does one approach fit all? Rev. Endocr. Metab. Disord. 2021, 22, 1201–1218. [Google Scholar] [CrossRef] [PubMed]
- Kostoglou-Athanassiou, I.; Pantazi, E.; Kontogiannis, S.; Kousouris, D.; Mavropoulos, I.; Athanassiou, P. Vitamin D in acutely ill patients. J. Int. Med. Res. 2018, 46, 4246–4257. [Google Scholar] [CrossRef]
- Kostoglou-Athanassiou, I.; Athanassiou, P.; Lyraki, A.; Raftakis, I.; Antoniadis, C. Vitamin D and rheumatoid arthritis. Ther. Adv. Endocrinol. Metab. 2012, 3, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, M.J.; Kushner, I.; Epstein, M. The constellation of vitamin D, the acute-phase response, and inflammation. Cleve. Clin. J. Med. 2023, 90, 85–89. [Google Scholar] [CrossRef]
- Perricone, C.; Bartoloni, E.; Bursi, R.; Cafaro, G.; Guidelli, G.M.; Shoenfeld, Y.; Gerli, R. COVID-19 as part of the hyperferritinemic syndromes: The role of iron depletion therapy. Immunol. Res. 2020, 68, 213–224. [Google Scholar] [CrossRef]
- Ruscitti, P.; Berardicurti, O.; Di Benedetto, P.; Cipriani, P.; Iagnocco, A.; Shoenfeld, Y.; Giacomelli, R. Severe COVID-19, Another Piece in the Puzzle of the Hyperferritinemic Syndrome. An Immunomodulatory Perspective to Alleviate the Storm. Front. Immunol. 2020, 11, 1130. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, Y.C.; Kang, Y.L.; Ren, Y.Q.; Miao, H.J.; Wang, F. High-Volume Hemofiltration in Critically Ill Patients With Secondary Hemophagocytic Lymphohistiocytosis/Macrophage Activation Syndrome: A Prospective Study in the PICU. Pediatr. Crit. Care Med. 2016, 17, e437–e443. [Google Scholar] [CrossRef]
- Volfovitch, Y.; Tsur, A.M.; Gurevitch, M.; Novick, D.; Rabinowitz, R.; Mandel, M.; Achiron, A.; Rubinstein, M.; Shoenfeld, Y.; Amital, H. The intercorrelations between blood levels of ferritin, sCD163, and IL-18 in COVID-19 patients and their association to prognosis. Immunol. Res. 2022, 70, 817–828. [Google Scholar] [CrossRef]
- Perkins, M.V.; Joseph, S.B.; Dittmer, D.P.; Mackman, N. Cardiovascular Disease and Thrombosis in HIV Infection. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.M.; Spinler, S.A. COVID-19 and thrombosis: From bench to bedside. Trends Cardiovasc. Med. 2021, 31, 143–160. [Google Scholar] [CrossRef]
- Savla, S.R.; Prabhavalkar, K.S.; Bhatt, L.K. Cytokine storm associated coagulation complications in COVID-19 patients: Pathogenesis and Management. Expert. Rev. Anti Infect. Ther. 2021, 19, 1397–1413. [Google Scholar] [CrossRef] [PubMed]
- Rostami, M.; Mansouritorghabeh, H. D-dimer level in COVID-19 infection: A systematic review. Expert. Rev. Hematol. 2020, 13, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Nugroho, J.; Wardhana, A.; Maghfirah, I.; Mulia, E.P.B.; Rachmi, D.A.; A’Yun, M.Q.; Septianda, I. Relationship of D-dimer with severity and mortality in SARS-CoV-2 patients: A meta-analysis. Int. J. Lab. Hematol. 2021, 43, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Bhansali, A.; Khare, N.; Suri, V.; Yaddanapudi, N.; Sachdeva, N.; Puri, G.D.; Malhotra, P. Short term, high-dose vitamin D supplementation for COVID-19 disease: A randomised, placebo-controlled, study (SHADE study). Postgrad. Med. J. 2022, 98, 87–90. [Google Scholar] [CrossRef]
- Cimmino, G.; Conte, S.; Morello, M.; Pellegrino, G.; Marra, L.; Morello, A.; Nicoletti, G.; De Rosa, G.; Golino, P.; Cirillo, P. Vitamin D Inhibits IL-6 Pro-Atherothrombotic Effects in Human Endothelial Cells: A Potential Mechanism for Protection against COVID-19 Infection? J. Cardiovasc. Dev. Dis. 2022, 9, 27. [Google Scholar] [CrossRef]
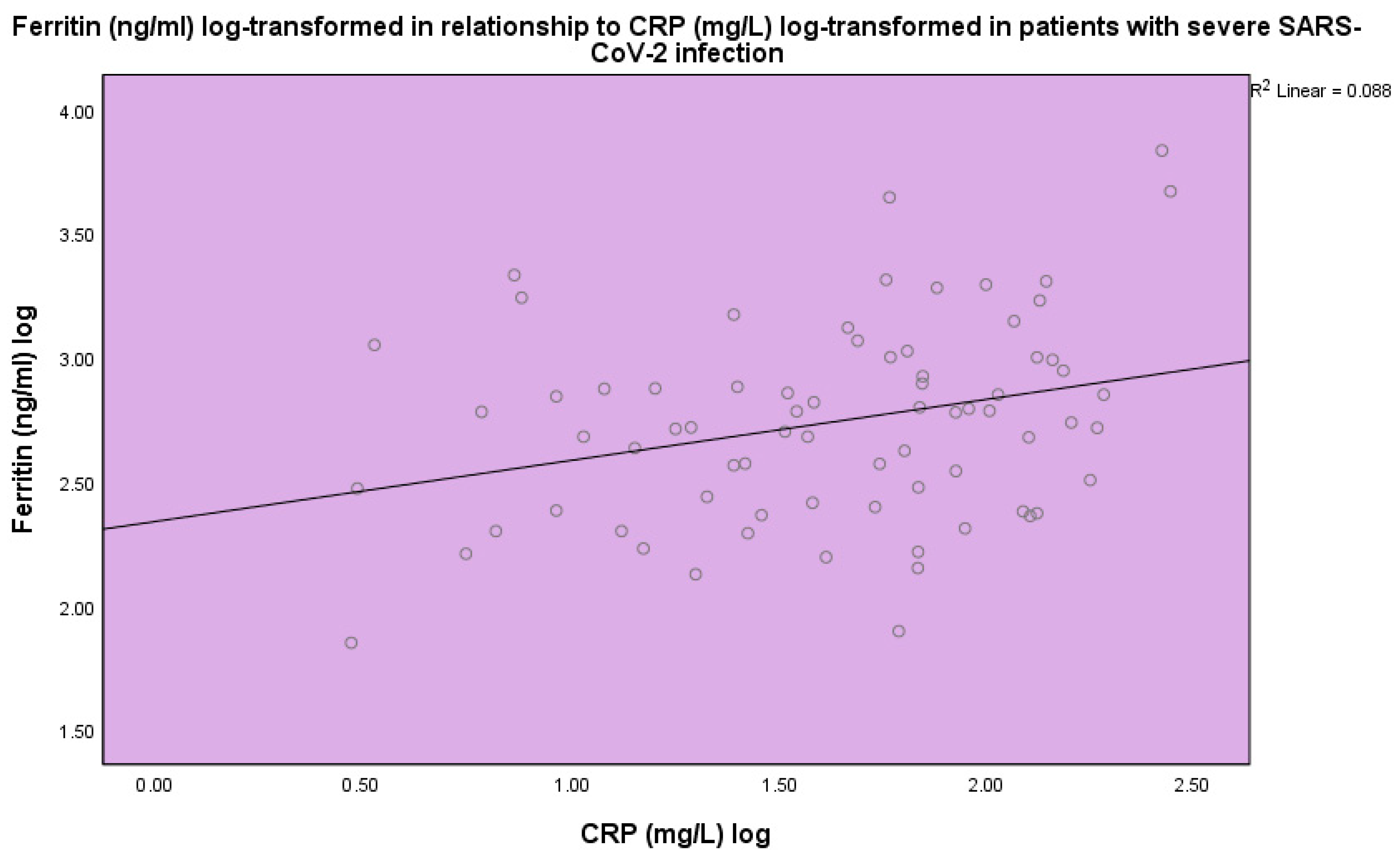

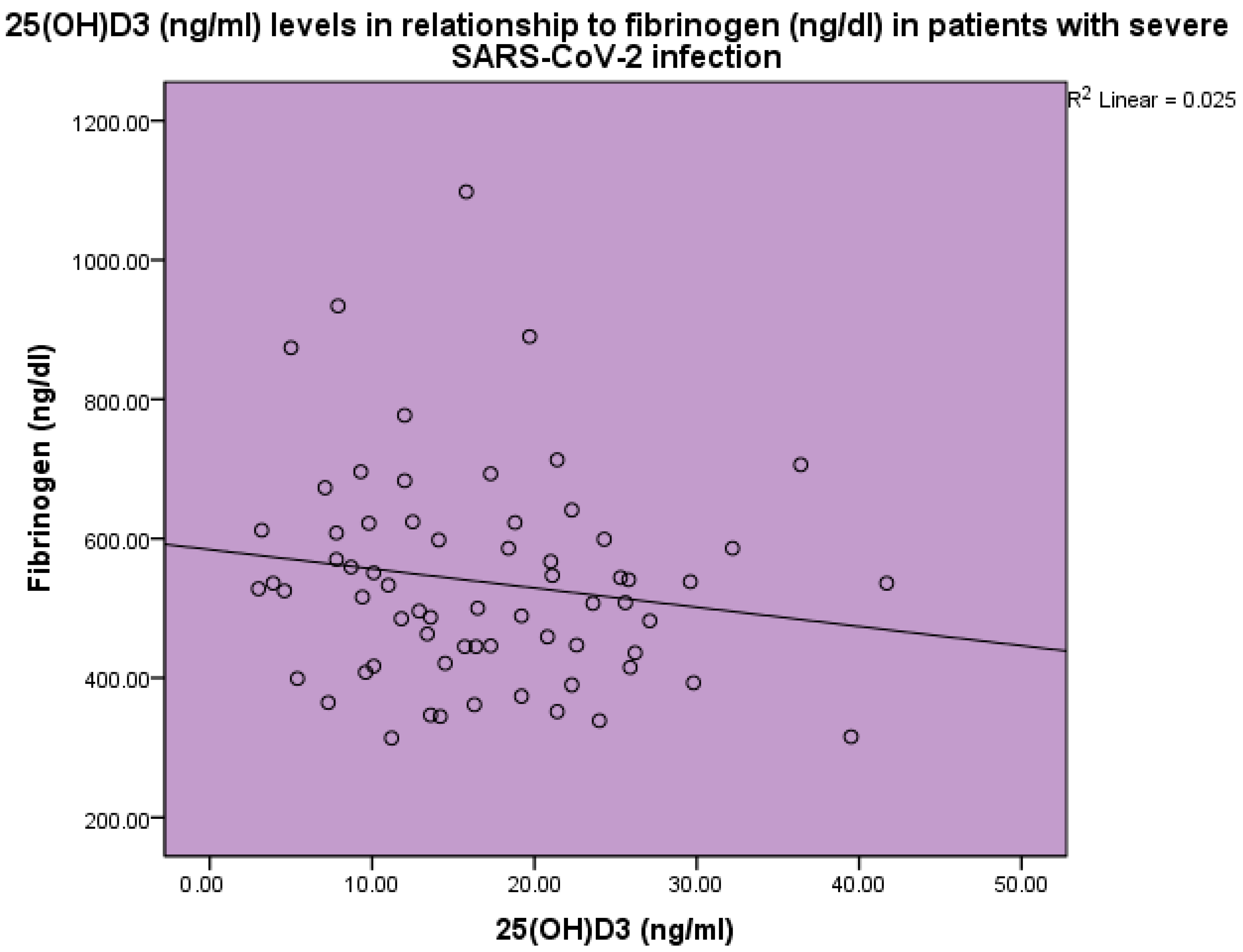
| Normal Range | Minimum | Maximum | Mean | Std. Deviation | |
|---|---|---|---|---|---|
| Age (years) | 21.0 | 97.0 | 67.7 | 16.9 | |
| Sex (male/female) | 47/41 | ||||
| White blood cell count (cells/μL) | 4.6–10.2 | 0.71 | 17,300.0 | 6259.6 | 3373.5 |
| Neutrophils (%) | 37.6 | 89.9 | 70.5 | 13.1 | |
| CRP (mg/L) | <5.0 | 2.9 | 280.0 | 65.1 | 60.6 |
| ESR (mm/1 h) | <15 | 15.0 | 108.0 | 44.6 | 24.4 |
| 25(OH)D3 (ng/mL) | 3.0 | 41.7 | 17.4 | 8.8 | |
| Ferritin (ng/mL) | 20–300 | 71.0 | 6821.0 | 851.9 | 1032.6 |
| d-dimers (μg/L) | 0–550 | 170.0 | 5835.0 | 1690.9 | 1479.5 |
| Fibrinogen (ng/dL) | 180–350 | 314.0 | 1098.0 | 543.1 | 157.2 |
| TSH (μIU/mL) | 0.35–4.94 | 0.36 | 3.36 | 1.17 | 0.78 |
| FT3 (pg/mL) | 1.58–3.91 | 2.2 | 5.1 | 3.7 | 0.66 |
| K (mmol/L) | 3.5–5.2 | 2.2 | 5.1 | 3.7 | 0.6 |
| PT (s) | 10.0 | 14.3 | 11.7 | 1.0 | |
| APTT (s) | 26–39 | 20.9 | 59.3 | 31.1 | 5.1 |
| INR | 0.86 | 1.29 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Athanassiou, L.; Kostoglou-Athanassiou, I.; Nikolakopoulou, S.; Konstantinou, A.; Mascha, O.; Siarkos, E.; Samaras, C.; Athanassiou, P.; Shoenfeld, Y. Vitamin D Levels as a Marker of Severe SARS-CoV-2 Infection. Life 2024, 14, 210. https://doi.org/10.3390/life14020210
Athanassiou L, Kostoglou-Athanassiou I, Nikolakopoulou S, Konstantinou A, Mascha O, Siarkos E, Samaras C, Athanassiou P, Shoenfeld Y. Vitamin D Levels as a Marker of Severe SARS-CoV-2 Infection. Life. 2024; 14(2):210. https://doi.org/10.3390/life14020210
Chicago/Turabian StyleAthanassiou, Lambros, Ifigenia Kostoglou-Athanassiou, Sofia Nikolakopoulou, Alexandra Konstantinou, Olga Mascha, Evangelos Siarkos, Charilaos Samaras, Panagiotis Athanassiou, and Yehuda Shoenfeld. 2024. "Vitamin D Levels as a Marker of Severe SARS-CoV-2 Infection" Life 14, no. 2: 210. https://doi.org/10.3390/life14020210
APA StyleAthanassiou, L., Kostoglou-Athanassiou, I., Nikolakopoulou, S., Konstantinou, A., Mascha, O., Siarkos, E., Samaras, C., Athanassiou, P., & Shoenfeld, Y. (2024). Vitamin D Levels as a Marker of Severe SARS-CoV-2 Infection. Life, 14(2), 210. https://doi.org/10.3390/life14020210








