Common and Potential Emerging Foodborne Viruses: A Comprehensive Review
Abstract
1. Introduction
2. Common Foodborne Viruses
2.1. Norovirus
2.2. Rotavirus
2.3. Sapovirus
2.4. Astrovirus
2.5. Adenovirus
2.6. Hepatitis A Virus
2.7. Hepatitis E Virus
3. Other Potential Emerging Viruses
3.1. Tick-Borne Encephalitis Virus
3.2. Nipah Virus
3.3. Ebola Virus
3.4. Avian Influenza Virus
3.5. Aichi Virus
3.6. Coronaviruses (SARS-CoV-1, SARS-CoV-2 and MERSCoV)
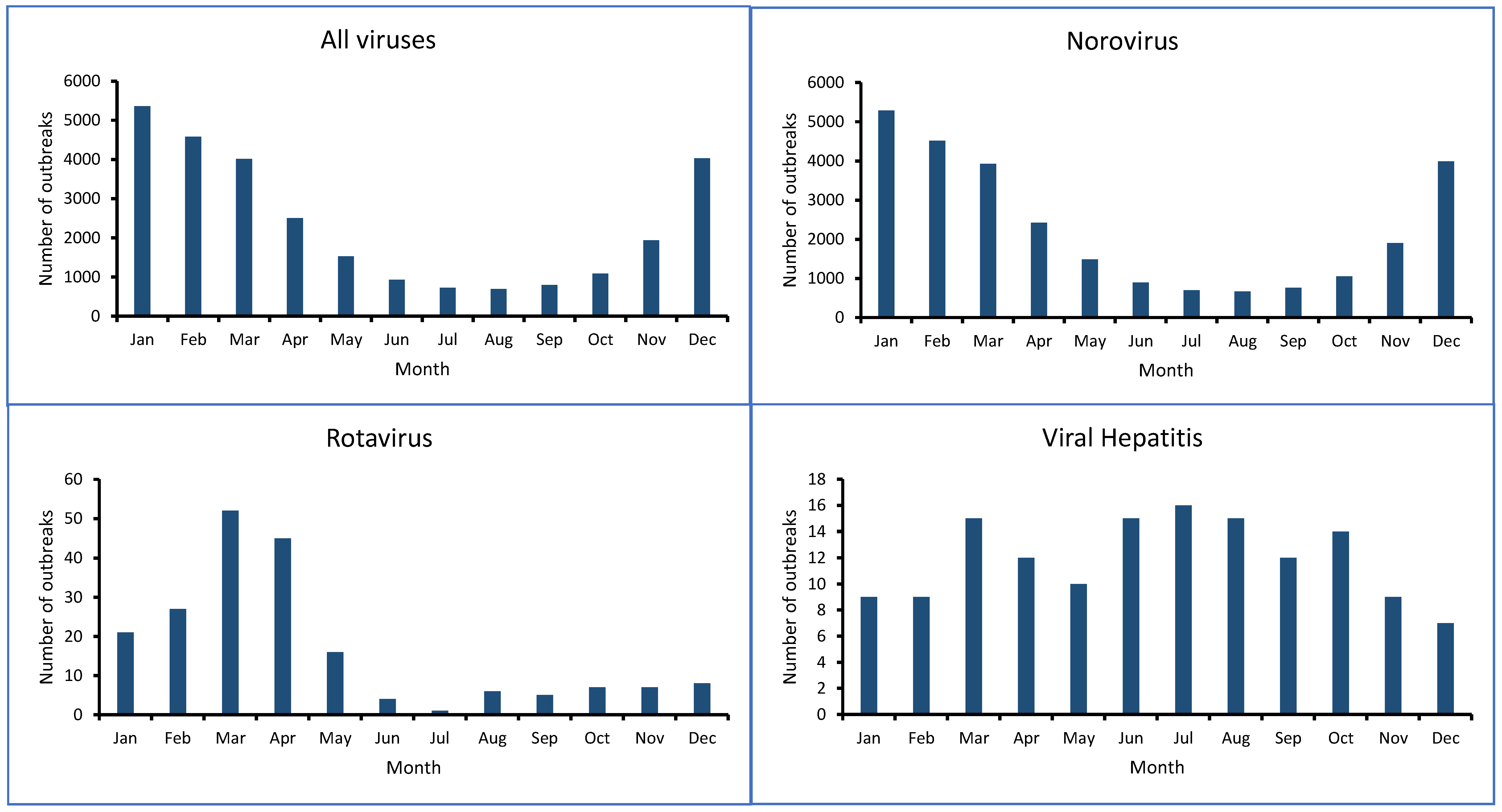
4. Viral Foodborne Outbreaks and Illnesses
5. The Control of Foodborne Viruses in Food Chains
- Heat treatment: cooking or processing food at high temperatures can inactivate most viruses. It has been found that foodborne viruses including hepatitis A virus, norovirus, and hepatitis E virus in foods were efficiently inactivated by heat [286];
- High pressure processing (HPP): the HPP treatment of foods involves treating packaged samples suspended in liquid with pressure which is rapidly released. It was found that HPP is very effective for inactivating food viruses [14];
- UV light: this technology alters the genetic material and the proteins of viruses. UV treatment is an effective method for inactivating viruses on foods or food-processing surfaces. The method is most effective in water and high aw foods [13];
- Cold plasma: cold plasma can be created by the application of an electric field to gases like helium, nitrogen, oxygen, argon, or their mixtures, which are partially or completely ionized to form reactive chemical species. Cold plasma successfully inactivated foodborne viruses including hepatitis A virus and norovirus without affecting the quality attributes of foods. This new option has significant potential value for use in the food industry [15,287];
- Pulsed Electric Field (PEF): PEF is a technique that generates a short time electrical treatment by using a pulse electric field. Although few studies have investigated the inhibitory effect of PEF against foodborne viruses, this technology may have the potential to be applied in a variety of foods [12];
- Sanitizers: sanitizers including chlorine, hydrogen peroxide and ozone showed significant efficiency in the viral decontamination of fresh produce. However, activity depended on the sanitizer type and concentration, food item, type of virus, inoculation level, and method used for decontamination [11];
- Lactic acid bacteria: Fermenting foods with lactic acid bacteria can create an acidic environment and may produce antiviral bacteriocins that could potentially be used as food additives that are hostile to viruses [288].
- Cleaning and disinfecting regular environmental surfaces touched by various individuals. The proper washing of vegetables and fruits should occur before consumption. Only potable water should contact food. Sources of water must be protected from all types of untreated wastewater contamination;
- Increasing the awareness of safety issues regarding foodborne viruses among workers at different stages of responsibility in the supply chain;
- Emphasizing good hand washing with appropriate sanitizers located near the sink. Food preparation equipment and surfaces must be disinfected regularly. Hand washing with soap and maintaining good sanitary hygiene will certainly help in reducing viral contamination [290];
- Reinforcing strict personal hygiene practices for everyone since symptomatic, colonized or asymptomatic individuals can transmit pathogens.
- Displaying clearly visible signs accompanied by frequent verbal and written reminders for food handlers to frequently wash hands after visiting the toilet and before consuming foods.
- Educating food workers and handlers about gastrointestinal illness symptoms;
- Educating the public at large about microbial safety guidelines and hygiene rules;
- Workers who are sick should not be allowed to handle the equipment involved in food processing. Employees must be made aware that at the beginning of gastrointestinal illness symptoms, it is necessity to stop working, and only re-continue work after symptoms ceased after at least 2 days;
- Retailers, distributers, and manufacturers must have an effective system in place for appropriate recalls and enhanced trace-back systems for assumed contaminated water or foods;
- Developing precise interventions for reducing the frequency of viral foodborne illness outbreaks by focusing on shellfish, produce, and food workers;
- Facilitating improvement in viral diagnostics, including the development of efficient, rapid and sensitive viral detection methods;
- Developing specific, effective viral vaccines, and antiviral sanitizers and drugs;
- Developing efficient cell culture systems and robust animal models for the recovery and identification of human foodborne viral agents.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015; World Health Organization: Geneva, Switzerland, 2015; ISBN 978-92-4-156516-5. [Google Scholar]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.-A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne Illness Acquired in the United States—Major Pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef]
- USFDA. What You Need to Know about Foodborne Illnesses. Available online: https://www.fda.gov/food/consumers/what-you-need-know-about-foodborne-illnesses (accessed on 28 December 2022).
- Upfold, N.S.; Luke, G.A.; Knox, C. Occurrence of Human Enteric Viruses in Water Sources and Shellfish: A Focus on Africa. Food Envron. Virol. 2021, 13, 1–31. [Google Scholar] [CrossRef]
- CDC Burden of Foodborne Illness: Findings|Estimates of Foodborne Illness|CDC. 2018. Available online: https://www.cdc.gov/foodborneburden/2011-foodborne-estimates.html (accessed on 10 April 2023).
- Greening, G.E.; Cannon, J.L. Human and Animal Viruses in Food (Including Taxonomy of Enteric Viruses). In Viruses in Foods; Goyal, S.M., Cannon, J.L., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 5–57. ISBN 978-3-319-30721-3. [Google Scholar]
- Soares, V.M.; dos Santos, E.A.R.; Tadielo, L.E.; Cerqueira-Cézar, C.K.; da Cruz Encide Sampaio, A.N.; Eisen, A.K.A.; de Oliveira, K.G.; Padilha, M.B.; de Moraes Guerra, M.E.; Gasparetto, R.; et al. Detection of Adenovirus, Rotavirus, and Hepatitis E Virus in Meat Cuts Marketed in Uruguaiana, Rio Grande Do Sul, Brazil. One Health 2022, 14, 100377. [Google Scholar] [CrossRef]
- Bhilegaonkar, K.N.; Kolhe, R.P. Transfer of Viruses Implicated in Human Disease through Food. In Present Knowledge in Food Safety; Elsevier: Amsterdam, The Netherlands, 2023; pp. 786–811. ISBN 978-0-12-819470-6. [Google Scholar]
- Cliver, D.O. Control of Viral Contamination of Food and Environment. Food Environ. Virol. 2009, 1, 3–9. [Google Scholar] [CrossRef]
- Pexara, A.; Govaris, A. Foodborne Viruses and Innovative Non-Thermal Food-Processing Technologies. Foods 2020, 9, 1520. [Google Scholar] [CrossRef]
- Bosch, A.; Gkogka, E.; Le Guyader, F.S.; Loisy-Hamon, F.; Lee, A.; van Lieshout, L.; Marthi, B.; Myrmel, M.; Sansom, A.; Schultz, A.C.; et al. Foodborne Viruses: Detection, Risk Assessment, and Control Options in Food Processing. Int. J. Food Microbiol. 2018, 285, 110–128. [Google Scholar] [CrossRef]
- Ezzatpanah, H.; Gómez-López, V.M.; Koutchma, T.; Lavafpour, F.; Moerman, F.; Mohammadi, M.; Raheem, D. New Food Safety Challenges of Viral Contamination from a Global Perspective: Conventional, Emerging, and Novel Methods of Viral Control. Compr. Rev. Food Sci. Food Safe 2022, 21, 904–941. [Google Scholar] [CrossRef]
- Gómez-López, V.M.; Jubinville, E.; Rodríguez-López, M.I.; Trudel-Ferland, M.; Bouchard, S.; Jean, J. Inactivation of Foodborne Viruses by UV Light: A Review. Foods 2021, 10, 3141. [Google Scholar] [CrossRef] [PubMed]
- Govaris, A.; Pexara, A. Inactivation of Foodborne Viruses by High-Pressure Processing (HPP). Foods 2021, 10, 215. [Google Scholar] [CrossRef] [PubMed]
- Jenns, K.; Sassi, H.P.; Zhou, R.; Cullen, P.J.; Carter, D.; Mai-Prochnow, A. Inactivation of Foodborne Viruses: Opportunities for Cold Atmospheric Plasma. Trends Food Sci. Technol. 2022, 124, 323–333. [Google Scholar] [CrossRef]
- Shahi, S.; Khorvash, R.; Goli, M.; Ranjbaran, S.M.; Najarian, A.; Mohammadi Nafchi, A. Review of Proposed Different Irradiation Methods to Inactivate Food-processing Viruses and Microorganisms. Food Sci. Nutr. 2021, 9, 5883–5896. [Google Scholar] [CrossRef]
- Seymour, I.J.; Appleton, H. Foodborne Viruses and Fresh Produce. J. Appl. Microbiol. 2001, 91, 759–773. [Google Scholar] [CrossRef]
- CDC Norovirus Virus Classification|CDC. 2021. Available online: https://www.cdc.gov/norovirus/lab/virus-classification.html (accessed on 23 November 2022).
- Parra, G.I. Emergence of Norovirus Strains: A Tale of Two Genes. Virus Evol. 2019, 5, vez048. [Google Scholar] [CrossRef]
- Pogan, R.; Dülfer, J.; Uetrecht, C. Norovirus Assembly and Stability. Curr. Opin. Virol. 2018, 31, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Verhoef, L.; Hewitt, J.; Barclay, L.; Ahmed, S.M.; Lake, R.; Hall, A.J.; Lopman, B.; Kroneman, A.; Vennema, H.; Vinjé, J.; et al. Norovirus Genotype Profiles Associated with Foodborne Transmission, 1999–2012. Emerg. Infect. Dis. 2015, 21, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Cornejo-Sánchez, T.; Soldevila, N.; Coronas, L.; Alsedà, M.; Godoy, P.; Razquín, E.; Sabaté, S.; Guix, S.; Rodríguez Garrido, V.; Bartolomé, R.; et al. Epidemiology of GII.4 and GII.2 Norovirus Outbreaks in Closed and Semi-Closed Institutions in 2017 and 2018. Sci. Rep. 2023, 13, 1659. [Google Scholar] [CrossRef] [PubMed]
- Lysén, M.; Thorhagen, M.; Brytting, M.; Hjertqvist, M.; Andersson, Y.; Hedlund, K.-O. Genetic Diversity among Food-Borne and Waterborne Norovirus Strains Causing Outbreaks in Sweden. J. Clin. Microbiol. 2009, 47, 2411–2418. [Google Scholar] [CrossRef] [PubMed]
- Kambhampati, A.K.; Calderwood, L.; Wikswo, M.E.; Barclay, L.; Mattison, C.P.; Balachandran, N.; Vinjé, J.; Hall, A.J.; Mirza, S.A. Spatiotemporal Trends in Norovirus Outbreaks in the United States, 2009–2019. Clin. Infect. Dis. 2023, 76, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.L.; Bonifacio, J.; Bucardo, F.; Buesa, J.; Bruggink, L.; Chan, M.C.-W.; Fumian, T.M.; Giri, S.; Gonzalez, M.D.; Hewitt, J.; et al. Global Trends in Norovirus Genotype Distribution among Children with Acute Gastroenteritis. Emerg. Infect. Dis. 2021, 27, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, S.; Von Seidlein, L.; Wang, X. The Epidemiology of Norovirus Gastroenteritis in China: Disease Burden and Distribution of Genotypes. Front. Med. 2020, 14, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lucero, Y.; Matson, D.O.; Ashkenazi, S.; George, S.; O’Ryan, M. Norovirus: Facts and Reflections from Past, Present, and Future. Viruses 2021, 13, 2399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ghosh, S.; Li, M.; Altan-Bonnet, N.; Shuai, D. Vesicle-Cloaked Rotavirus Clusters Are Environmentally Persistent and Resistant to Free Chlorine Disinfection. Environ. Sci. Technol. 2022, 56, 8475–8484. [Google Scholar] [CrossRef]
- Teunis, P.F.M.; Le Guyader, F.S.; Liu, P.; Ollivier, J.; Moe, C.L. Noroviruses Are Highly Infectious but There Is Strong Variation in Host Susceptibility and Virus Pathogenicity. Epidemics 2020, 32, 100401. [Google Scholar] [CrossRef] [PubMed]
- Bányai, K.; Estes, M.K.; Martella, V.; Parashar, U.D. Viral Gastroenteritis. Lancet 2018, 392, 175–186. [Google Scholar] [CrossRef]
- Lopman, B.A.; Steele, D.; Kirkwood, C.D.; Parashar, U.D. The Vast and Varied Global Burden of Norovirus: Prospects for Prevention and Control. PLoS Med. 2016, 13, e1001999. [Google Scholar] [CrossRef] [PubMed]
- Velebit, B.; Djordjevic, V.; Milojevic, L.; Babic, M.; Grkovic, N.; Jankovic, V.; Yushina, Y. The Common Foodborne Viruses: A Review. IOP Conf. Ser. Earth Environ. Sci. 2019, 333, 012110. [Google Scholar] [CrossRef]
- Bosch, A.; Pintó, R.M.; Guix, S. Foodborne Viruses. Curr. Opin. Food Sci. 2016, 8, 110–119. [Google Scholar] [CrossRef]
- Bintsis, T. Foodborne Pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef]
- de Graaf, M.; van Beek, J.; Koopmans, M.P.G. Human Norovirus Transmission and Evolution in a Changing World. Nat. Rev. Microbiol. 2016, 14, 421–433. [Google Scholar] [CrossRef]
- Robilotti, E.; Deresinski, S.; Pinsky, B.A. Norovirus. Clin. Microbiol. Rev. 2015, 28, 134–164. [Google Scholar] [CrossRef]
- Todd, K.; Tripp, R. Human Norovirus: Experimental Models of Infection. Viruses 2019, 11, 151. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Weber, S.G. Rotavirus Infection in Adults. Lancet Infect. Dis. 2004, 4, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Bishop, R. Discovery of Rotavirus: Implications for Child Health. J. Gastroenterol. Hepatol. 2009, 24, S81–S85. [Google Scholar] [CrossRef]
- Franco, M.A.; Greenberg, H.B. Rotaviruses, Noroviruses, and Other Gastrointestinal Viruses. In Goldman’s Cecil Medicine; Elsevier: Amsterdam, The Netherlands, 2012; pp. 2144–2147. ISBN 978-1-4377-1604-7. [Google Scholar]
- Bachofen, C. Selected Viruses Detected on and in Our Food. Curr. Clin. Micro. Rpt. 2018, 5, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Tse, H.; Chan, W.-M.; Li, K.S.M.; Lau, S.K.P.; Woo, P.C.Y.; Yuen, K.-Y. Discovery and Genomic Characterization of a Novel Bat Sapovirus with Unusual Genomic Features and Phylogenetic Position. PLoS ONE 2012, 7, e34987. [Google Scholar] [CrossRef] [PubMed]
- Kang, G. Viral Diarrhea. In International Encyclopedia of Public Health; Elsevier: Amsterdam, The Netherlands, 2008; pp. 518–526. ISBN 978-0-12-373960-5. [Google Scholar]
- Payne, S. Family Astroviridae. In Viruses; Elsevier: Amsterdam, The Netherlands, 2017; pp. 125–128. ISBN 978-0-12-803109-4. [Google Scholar]
- Marshall, D.L.; Dickson, J.S.; Nguyen, N.H. Ensuring Food Safety in Insect Based Foods: Mitigating Microbiological and Other Foodborne Hazards. In Insects as Sustainable Food Ingredients; Elsevier: Amsterdam, The Netherlands, 2016; pp. 223–253. ISBN 978-0-12-802856-8. [Google Scholar]
- Rodríguez-Lázaro, D.; Cook, N.; Ruggeri, F.M.; Sellwood, J.; Nasser, A.; Nascimento, M.S.J.; D’Agostino, M.; Santos, R.; Saiz, J.C.; Rzeżutka, A.; et al. Virus Hazards from Food, Water and Other Contaminated Environments. FEMS Microbiol. Rev. 2012, 36, 786–814. [Google Scholar] [CrossRef] [PubMed]
- Di Cola, G.; Fantilli, A.C.; Pisano, M.B.; Ré, V.E. Foodborne Transmission of Hepatitis A and Hepatitis E Viruses: A Literature Review. Int. J. Food Microbiol. 2021, 338, 108986. [Google Scholar] [CrossRef]
- Feinstone, S.M. History of the Discovery of Hepatitis A Virus. Cold Spring Harb. Perspect. Med. 2019, 9, a031740. [Google Scholar] [CrossRef]
- Fiore, A.E. Hepatitis A Transmitted by Food. Clin. Infect. Dis. 2004, 38, 705–715. [Google Scholar] [CrossRef]
- Liu, G.-D. Full-Length Genome of Wild-Type Hepatitis A Virus (DL3) Isolated in China. World J. Gastroenterol. 2003, 9, 499. [Google Scholar] [CrossRef]
- Ahmad, I.; Holla, R.P.; Jameel, S. Molecular Virology of Hepatitis E Virus. Virus Res. 2011, 161, 47–58. [Google Scholar] [CrossRef]
- Dalton, H.R.; Izopet, J. Transmission and Epidemiology of Hepatitis E Virus Genotype 3 and 4 Infections. Cold Spring Harb. Perspect. Med. 2018, 8, a032144. [Google Scholar] [CrossRef]
- Das, A.; Rivera-Serrano, E.E.; Yin, X.; Walker, C.M.; Feng, Z.; Lemon, S.M. Cell Entry and Release of Quasi-Enveloped Human Hepatitis Viruses. Nat. Rev. Microbiol. 2023, 21, 573–589. [Google Scholar] [CrossRef]
- Kamar, N.; Dalton, H.R.; Abravanel, F.; Izopet, J. Hepatitis E Virus Infection. Clin. Microbiol. Rev. 2014, 27, 116–138. [Google Scholar] [CrossRef]
- Kenney, S.P.; Meng, X.-J. Hepatitis E Virus Genome Structure and Replication Strategy. Cold Spring Harb. Perspect. Med. 2019, 9, a031724. [Google Scholar] [CrossRef]
- Kirkwood, C.D.; Dobscha, K.R.; Steele, A.D. Hepatitis E Should Be a Global Public Health Priority: Recommendations for Improving Surveillance and Prevention. Expert Rev. Vaccines 2020, 19, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Serrano, E.E.; González-López, O.; Das, A.; Lemon, S.M. Cellular Entry and Uncoating of Naked and Quasi-Enveloped Human Hepatoviruses. eLife 2019, 8, e43983. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.J.; Wikswo, M.E.; Manikonda, K.; Roberts, V.A.; Yoder, J.S.; Gould, L.H. Acute Gastroenteritis Surveillance through the National Outbreak Reporting System, United States. Emerg. Infect. Dis. 2013, 19, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Sukhrie, F.H.A.; Teunis, P.; Vennema, H.; Copra, C.; Thijs Beersma, M.F.C.; Bogerman, J.; Koopmans, M. Nosocomial Transmission of Norovirus Is Mainly Caused by Symptomatic Cases. Clin. Infect. Dis. 2012, 54, 931–937. [Google Scholar] [CrossRef]
- da Silva Poló, T.; Peiró, J.R.; Mendes, L.C.N.; Ludwig, L.F.; de Oliveira-Filho, E.F.; Bucardo, F.; Huynen, P.; Melin, P.; Thiry, E.; Mauroy, A. Human Norovirus Infection in Latin America. J. Clin. Virol. 2016, 78, 111–119. [Google Scholar] [CrossRef]
- Le Guyader, F.S.; Atmar, R.L.; Le Pendu, J. Transmission of Viruses through Shellfish: When Specific Ligands Come into Play. Curr. Opin. Virol. 2012, 2, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Villabruna, N.; Koopmans, M.P.G.; De Graaf, M. Animals as Reservoir for Human Norovirus. Viruses 2019, 11, 478. [Google Scholar] [CrossRef] [PubMed]
- CDC How Norovirus Spreads. 2023. Available online: https://www.cdc.gov/norovirus/about/transmission.html (accessed on 18 June 2023).
- Alsved, M.; Fraenkel, C.-J.; Bohgard, M.; Widell, A.; Söderlund-Strand, A.; Lanbeck, P.; Holmdahl, T.; Isaxon, C.; Gudmundsson, A.; Medstrand, P.; et al. Sources of Airborne Norovirus in Hospital Outbreaks. Clin. Infect. Dis. 2020, 70, 2023–2028. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Tang, J.; Li, Y. Airborne or Fomite Transmission for Norovirus? A Case Study Revisited. IJERPH 2017, 14, 1571. [Google Scholar] [CrossRef]
- Canales, R.A.; Reynolds, K.A.; Wilson, A.M.; Fankem, S.L.M.; Weir, M.H.; Rose, J.B.; Abd-Elmaksoud, S.; Gerba, C.P. Modeling the Role of Fomites in a Norovirus Outbreak. J. Occup. Environ. Hyg. 2019, 16, 16–26. [Google Scholar] [CrossRef]
- Todd, E.; Grieg, J. Viruses of Foodborne Origin: A Review. Virus Adapt. Treat. 2015, 7, 25–45. [Google Scholar] [CrossRef]
- Raymond, P.; Paul, S.; Perron, A.; Deschênes, L.; Hara, K. Extraction of Human Noroviruses from Leafy Greens and Fresh Herbs Using Magnetic Silica Beads. Food Microbiol. 2021, 99, 103827. [Google Scholar] [CrossRef]
- Food Standards Agency FSA Research Suggests New Higher Estimates for the Role of Food in UK Illness. Available online: https://www.food.gov.uk/news-alerts/news/fsa-research-suggests-new-higher-estimates-for-the-role-of-food-in-uk-illness (accessed on 23 November 2022).
- Roth, A.N.; Karst, S.M. Norovirus Mechanisms of Immune Antagonism. Curr. Opin. Virol. 2016, 16, 24–30. [Google Scholar] [CrossRef]
- Neznanov, N.; Kondratova, A.; Chumakov, K.M.; Angres, B.; Zhumabayeva, B.; Agol, V.I.; Gudkov, A.V. Poliovirus Protein 3A Inhibits Tumor Necrosis Factor (TNF)-Induced Apoptosis by Eliminating the TNF Receptor from the Cell Surface. J. Virol. 2001, 75, 10409–10420. [Google Scholar] [CrossRef]
- Esseili, M.A.; Wang, Q.; Saif, L.J. Binding of Human GII.4 Norovirus Virus-like Particles to Carbohydrates of Romaine Lettuce Leaf Cell Wall Materials. Appl. Environ. Microbiol. 2012, 78, 786–794. [Google Scholar] [CrossRef]
- Gao, X.; Esseili, M.A.; Lu, Z.; Saif, L.J.; Wang, Q. Recognition of Histo-Blood Group Antigen-Like Carbohydrates in Lettuce by Human GII.4 Norovirus. Appl. Environ. Microbiol. 2016, 82, 2966–2974. [Google Scholar] [CrossRef] [PubMed]
- Esseili, M.A.; Gao, X.; Boley, P.; Hou, Y.; Saif, L.J.; Brewer-Jensen, P.; Lindesmith, L.C.; Baric, R.S.; Atmar, R.L.; Wang, Q. Human Norovirus Histo-Blood Group Antigen (HBGA) Binding Sites Mediate the Virus Specific Interactions with Lettuce Carbohydrates. Viruses 2019, 11, 833. [Google Scholar] [CrossRef] [PubMed]
- Esseili, M.A.; Saif, L.J.; Farkas, T.; Wang, Q. Feline Calicivirus, Murine Norovirus, Porcine Sapovirus, and Tulane Virus Survival on Postharvest Lettuce. Appl. Environ. Microbiol. 2015, 81, 5085–5092. [Google Scholar] [CrossRef]
- Esseili, M.A.; Meulia, T.; Saif, L.J.; Wang, Q. Tissue Distribution and Visualization of Internalized Human Norovirus in Leafy Greens. Appl. Environ. Microbiol. 2018, 84, e00292-18. [Google Scholar] [CrossRef]
- Trudel-Ferland, M.; Goetz, C.; Girard, M.; Curt, S.; Mafu, A.A.; Fliss, I.; Jean, J. Physicochemical Parameters Affecting Norovirus Adhesion to Ready-To-Eat Foods. Appl. Environ. Microbiol. 2021, 87, e01396-21. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.-N.; Park, S.Y.; Bae, S.-C.; Oh, M.-H.; Ha, S.-D. Survival of Norovirus Surrogate on Various Food-Contact Surfaces. Food Environ. Virol. 2014, 6, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, R.; Jones, M.K.; Schneider, R.G.; Sreedharan, A.; Schneider, K.R. Preventing Foodborne Illness: Norovirus. FSHN0518, Food Science and Human Nutrition Department, University of Florida, UF/IFAS Extension. 2018. Available online: https://www.nifa.usda.gov/sites/default/files/resource/Preventing-Foodborne-Illness-Norovirus.pdf (accessed on 23 November 2022).
- Ahmed, H.; Maunula, L.; Korhonen, J. Reduction of Norovirus in Foods by Nonthermal Treatments: A Review. J. Food Prot. 2020, 83, 2053–2073. [Google Scholar] [CrossRef]
- LeClair, C.E.; McConnell, K.A. Rotavirus. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Uprety, T.; Wang, D.; Li, F. Recent Advances in Rotavirus Reverse Genetics and Its Utilization in Basic Research and Vaccine Development. Arch. Virol. 2021, 166, 2369–2386. [Google Scholar] [CrossRef]
- Gastañaduy, P.A.; Hall, A.J.; Parashar, U.D. Rotavirus. In Foodborne Infections and Intoxications; Elsevier: Amsterdam, The Netherlands, 2013; pp. 303–311. ISBN 978-0-12-416041-5. [Google Scholar]
- Crawford, S.E.; Ramani, S.; Tate, J.E.; Parashar, U.D.; Svensson, L.; Hagbom, M.; Franco, M.A.; Greenberg, H.B.; O’Ryan, M.; Kang, G.; et al. Rotavirus Infection. Nat. Rev. Dis. Primers 2017, 3, 17083. [Google Scholar] [CrossRef]
- Kuang, X.; Gong, X.; Zhang, X.; Pan, H.; Teng, Z. Genetic Diversity of Group A Rotavirus in Acute Gastroenteritis Outpatients in Shanghai from 2017 to 2018. BMC Infect. Dis. 2020, 20, 596. [Google Scholar] [CrossRef]
- Zhao, S.; Jin, X.; Zang, L.; Liu, Z.; Wen, X.; Ran, X. Global Infection Rate of Rotavirus C during 1980–2022 and Analysis of Critical Factors in the Host Range Restriction of Virus VP4. Viruses 2022, 14, 2826. [Google Scholar] [CrossRef]
- Omatola, C.A.; Olaniran, A.O. Rotaviruses: From Pathogenesis to Disease Control—A Critical Review. Viruses 2022, 14, 875. [Google Scholar] [CrossRef]
- CDC Learn More about Rotavirus Symptoms. 2021. Available online: https://www.cdc.gov/rotavirus/about/symptoms.html (accessed on 23 November 2022).
- Amimo, J.O.; Raev, S.A.; Chepngeno, J.; Mainga, A.O.; Guo, Y.; Saif, L.; Vlasova, A.N. Rotavirus Interactions With Host Intestinal Epithelial Cells. Front. Immunol. 2021, 12, 793841. [Google Scholar] [CrossRef] [PubMed]
- CDC Rotavirus Clinical Information|CDC. 2021. Available online: https://www.cdc.gov/rotavirus/clinical.html (accessed on 23 November 2022).
- Neethirajan, S.; Ahmed, S.R.; Chand, R.; Buozis, J.; Nagy, É. Recent Advances in Biosensor Development for Foodborne Virus Detection. Nanotheranostics 2017, 1, 272–295. [Google Scholar] [CrossRef] [PubMed]
- Osaili, T.M.; Hasan, F.; Al-Nabulsi, A.A.; Olaimat, A.N.; Ayyash, M.; Obaid, R.S.; Holley, R. A Worldwide Review of Illness Outbreaks Involving Mixed Salads/Dressings and Factors Influencing Product Safety and Shelf Life. Food Microbiol. 2023, 112, 104238. [Google Scholar] [CrossRef] [PubMed]
- Davidson, P.C.; Kuhlenschmidt, T.B.; Bhattarai, R.; Kalita, P.K.; Kuhlenschmidt, M.S. Investigation of Rotavirus Survival in Different Soil Fractions and Temperature Conditions. J. Environ. Prot. 2013, 4, 34107. [Google Scholar] [CrossRef]
- Leblanc, D.; Gagné, M.-J.; Poitras, É.; Brassard, J. Persistence of Murine Norovirus, Bovine Rotavirus, and Hepatitis A Virus on Stainless Steel Surfaces, in Spring Water, and on Blueberries. Food Microbiol. 2019, 84, 103257. [Google Scholar] [CrossRef] [PubMed]
- Leong, Y.K.; Xui, O.C.; Chia, O.K. Survival of SA11 Rotavirus in Fresh Fruit Juices of Pineapple, Papaya, and Honeydew Melon. J. Food Prot. 2008, 71, 1035–1037. [Google Scholar] [CrossRef]
- De Oliveira-Tozetto, S.; Santiso-Bellón, C.; Ferrer-Chirivella, J.M.; Navarro-Lleó, N.; Vila-Vicent, S.; Rodríguez-Díaz, J.; Buesa, J. Epidemiological and Genetic Characterization of Sapovirus in Patients with Acute Gastroenteritis in Valencia (Spain). Viruses 2021, 13, 184. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, N.; Song, C.; Oka, T.; Miki, M.; Murakami, K.; Iwasaki, K.; Katayama, K.; Murata, K. Atomic Structure of the Human Sapovirus Capsid Reveals a Unique Capsid Protein Conformation in Caliciviruses. J. Virol. 2022, 96, e00298-22. [Google Scholar] [CrossRef]
- Hansman, G.S.; Saito, H.; Shibata, C.; Ishizuka, S.; Oseto, M.; Oka, T.; Takeda, N. Outbreak of Gastroenteritis Due to Sapovirus. J. Clin. Microbiol. 2007, 45, 1347–1349. [Google Scholar] [CrossRef]
- Magwalivha, M.; Kabue, J.-P.; Traore, A.N.; Potgieter, N. Prevalence of Human Sapovirus in Low and Middle Income Countries. Adv. Virol. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Logue, C.M.; Barbieri, N.L.; Nielsen, D.W. Pathogens of Food Animals. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2017; Volume 82, pp. 277–365. ISBN 978-0-12-812633-2. [Google Scholar]
- Tang, X.; Hu, Y.; Zhong, X.; Xu, H. Molecular Epidemiology of Human Adenovirus, Astrovirus, and Sapovirus Among Outpatient Children with Acute Diarrhea in Chongqing, China, 2017–2019. Front. Pediatr. 2022, 10, 826600. [Google Scholar] [CrossRef] [PubMed]
- Chaaithanya, I.K.; Bhattacharya, D.; Patil, T.; Ghargi, K.V.; Kalal, S.; Roy, S. Etiology of Non-Rotaviral Diarrhea in Hospitalized Children Under Five Years of Age. Indian J. Pediatr. 2020, 87, 571–572. [Google Scholar] [CrossRef] [PubMed]
- Becker-Dreps, S.; González, F.; Bucardo, F. Sapovirus: An Emerging Cause of Childhood Diarrhea. Curr. Opin. Infect. Dis. 2020, 33, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Inns, T.; Wilson, D.; Manley, P.; Harris, J.P.; O’Brien, S.J.; Vivancos, R. What Proportion of Care Home Outbreaks Are Caused by Norovirus? An Analysis of Viral Causes of Gastroenteritis Outbreaks in Care Homes, North East England, 2016–2018. BMC Infect. Dis. 2020, 20, 2. [Google Scholar] [CrossRef]
- Rouhani, S.; Peñataro Yori, P.; Paredes Olortegui, M.; Lima, A.A.; Ahmed, T.; Mduma, E.R.; George, A.; Samie, A.; Svensen, E.; Lima, I.; et al. The Epidemiology of Sapovirus in the Etiology, Risk Factors, and Interactions of Enteric Infection and Malnutrition and the Consequences for Child Health and Development Study: Evidence of Protection Following Natural Infection. Clin. Infect. Dis. 2022, 75, 1334–1341. [Google Scholar] [CrossRef]
- Kobayashi, S.; Fujiwara, N.; Yasui, Y.; Yamashita, T.; Hiramatsu, R.; Minagawa, H. A Foodborne Outbreak of Sapovirus Linked to Catered Box Lunches in Japan. Arch. Virol. 2012, 157, 1995–1997. [Google Scholar] [CrossRef]
- Richards, G.P. Shellfish-Associated Enteric Virus Illness: Virus Localization, Disease Outbreaks and Prevention. In Viruses in Foods; Goyal, S.M., Cannon, J.L., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 185–207. ISBN 978-3-319-30721-3. [Google Scholar]
- El-Heneidy, A.; Ware, R.S.; Lambert, S.B.; Grimwood, K. Sapovirus Infections in an Australian Community-Based Healthy Birth Cohort during the First 2 Years of Life. Clin. Infect. Dis. 2023, 76, 1043–1049. [Google Scholar] [CrossRef]
- Vielot, N.A.; González, F.; Reyes, Y.; Zepeda, O.; Blette, B.; Paniagua, M.; Toval-Ruíz, C.; Diez-Valcarce, M.; Hudgens, M.G.; Gutiérrez, L.; et al. Risk Factors and Clinical Profile of Sapovirus-Associated Acute Gastroenteritis in Early Childhood: A Nicaraguan Birth Cohort Study. Pediatr. Infect. Dis. J. 2021, 40, 220–226. [Google Scholar] [CrossRef]
- Haramoto, E.; Kitajima, M.; Kishida, N.; Katayama, H.; Asami, M.; Akiba, M. Occurrence of Viruses and Protozoa in Drinking Water Sources of Japan and Their Relationship to Indicator Microorganisms. Food. Environ. Virol. 2012, 4, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Becker-Dreps, S.; Bucardo, F.; Vinjé, J. Sapovirus: An Important Cause of Acute Gastroenteritis in Children. Lancet Child Adolesc. Health 2019, 3, 758–759. [Google Scholar] [CrossRef] [PubMed]
- Moresco, V.; Charatzidou, A.; Oliver, D.M.; Weidmann, M.; Matallana-Surget, S.; Quilliam, R.S. Binding, Recovery, and Infectiousness of Enveloped and Non-Enveloped Viruses Associated with Plastic Pollution in Surface Water. Environ. Pollut. 2022, 308, 119594. [Google Scholar] [CrossRef] [PubMed]
- Esseili, M.A.; Chin, A.; Saif, L.; Miller, S.A.; Qu, F.; Lewis Ivey, M.L.; Wang, Q. Postharvest Survival of Porcine Sapovirus, a Human Norovirus Surrogate, on Phytopathogen-Infected Leafy Greens. J. Food Prot. 2015, 78, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- Niendorf, S.; Mas Marques, A.; Bock, C.-T.; Jacobsen, S. Diversity of Human Astroviruses in Germany 2018 and 2019. Virol. J. 2022, 19, 221. [Google Scholar] [CrossRef]
- Haga, K.; Takai-Todaka, R.; Kato, A.; Nakanishi, A.; Katayama, K. Neonatal Fc Receptor Is a Functional Receptor for Human Astrovirus. bioRxiv 2022, 516297. [Google Scholar] [CrossRef]
- Fu, J.; Yu, F.; Li, H.; Shen, L.; Tian, Y.; Jia, L.; Zhang, D.; Yang, P.; Wang, Q.; Gao, Z. Acute Gastroenteritis Outbreaks Caused by Human Astrovirus, 1978–2021: A Systematic Review. Biosaf. Health 2023, 5, 120–125. [Google Scholar] [CrossRef]
- Parrón, I.; Plasencia, E.; Cornejo-Sánchez, T.; Jané, M.; Pérez, C.; Izquierdo, C.; Guix, S.; Domínguez, À.; on behalf of the Working Group for the Study of Acute Gastroenteritis Outbreaks in Catalonia Human. Astrovirus Outbreak in a Daycare Center and Propagation among Household Contacts. Viruses 2021, 13, 1100. [Google Scholar] [CrossRef]
- Razizadeh, M.H.; Pourrostami, K.; Kachooei, A.; Zarei, M.; Asghari, M.; Hamldar, S.; Khatami, A. An Annoying Enteric Virus: A Systematic Review and Meta-analysis of Human Astroviruses and Gastrointestinal Complications in Children. Rev. Med. Virol. 2022, 32, e2389. [Google Scholar] [CrossRef]
- Vu, D.-L.; Sabrià, A.; Aregall, N.; Michl, K.; Sabrià, J.; Rodriguez Garrido, V.; Goterris, L.; Bosch, A.; Pintó, R.M.; Guix, S. A Spanish Case-Control Study in <5 Year-Old Children Reveals the Lack of Association between MLB and VA Astrovirus and Diarrhea. Sci. Rep. 2020, 10, 1760. [Google Scholar] [CrossRef]
- Jacobsen, S.; Höhne, M.; Marques, A.M.; Beslmüller, K.; Bock, C.-T.; Niendorf, S. Co-Circulation of Classic and Novel Astrovirus Strains in Patients with Acute Gastroenteritis in Germany. J. Infect. 2018, 76, 457–464. [Google Scholar] [CrossRef]
- Schultz-Cherry, S. Astroviruses☆. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2014; p. B9780128012383025393. ISBN 978-0-12-801238-3. [Google Scholar]
- Huang, D.; Wang, Z.; Zhang, F.; Wang, T.; Zhang, G.; Sai, L. Molecular and Clinical Epidemiological Features of Human Astrovirus Infections in Children with Acute Gastroenteritis in Shandong Province, China. J. Med. Virol. 2021, 93, 4883–4890. [Google Scholar] [CrossRef]
- Okitsu, S.; Khamrin, P.; Hikita, T.; Shimizu-Onda, Y.; Thongprachum, A.; Hayakawa, S.; Maneekarn, N.; Ushijima, H. Molecular Epidemiology of Classic, MLB, and VA Astroviruses in Children with Acute Gastroenteritis, 2014–2021: Emergence of MLB3 Strain in Japan. Microbiol. Spectr. 2023, 11, e00700-23. [Google Scholar] [CrossRef]
- Moser, L.; Schultz-Cherry, S. Astroviruses. In Encyclopedia of Virology; Elsevier: Amsterdam, The Netherlands, 2008; pp. 204–210. ISBN 978-0-12-374410-4. [Google Scholar]
- Vasickova, P.; Dvorska, L.; Lorencova, A.; Pavlik, I. Viruses as a Cause of Foodborne Diseases: A Review of the Literature. Vet. Med. 2005, 50, 89–104. [Google Scholar] [CrossRef]
- Mattison, C.P.; Vinjé, J.; Parashar, U.D.; Hall, A.J. Rotaviruses, Astroviruses, and Sapoviruses as Foodborne Infections. In Foodborne Infections and Intoxications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 327–344. ISBN 978-0-12-819519-2. [Google Scholar]
- Crenshaw, B.J.; Jones, L.B.; Bell, C.R.; Kumar, S.; Matthews, Q.L. Perspective on Adenoviruses: Epidemiology, Pathogenicity, and Gene Therapy. Biomedicines 2019, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Damon, C.F.; Platts-Mills, J.A. Pediatric Acute Gastroenteritis Associated with Adenovirus 40/41 in Low-Income and Middle-Income Countries. Curr. Opin. Infect. Dis. 2020, 33, 398–403. [Google Scholar] [CrossRef]
- Gaensbauer, J.T.; Lamb, M.; Calvimontes, D.M.; Asturias, E.J.; Kamidani, S.; Contreras-Roldan, I.L.; Dominguez, S.R.; Robinson, C.C.; Zacarias, A.; Berman, S.; et al. Identification of Enteropathogens by Multiplex PCR among Rural and Urban Guatemalan Children with Acute Diarrhea. Am. J. Trop. Med. Hyg. 2019, 101, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Arowolo, K.O.; Ayolabi, C.I.; Lapinski, B.; Santos, J.S.; Raboni, S.M. Epidemiology of Enteric Viruses in Children with Gastroenteritis in Ogun State, Nigeria. J. Med. Virol. 2019, 91, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Pratte-Santos, R.; Miagostovich, M.P.; Fumian, T.M.; Maciel, E.L.; Martins, S.A.; Cassini, S.T.; Keller, R. High Prevalence of Enteric Viruses Associated with Acute Gastroenteritis in Pediatric Patients in a Low-Income Area in Vitória, Southeastern Brazil: PRATTE-SANTOS et al. J. Med. Virol. 2019, 91, 744–750. [Google Scholar] [CrossRef]
- Maunula, L.; Rönnqvist, M.; Åberg, R.; Lunden, J.; Nevas, M. The Presence of Norovirus and Adenovirus on Environmental Surfaces in Relation to the Hygienic Level in Food Service Operations Associated with a Suspected Gastroenteritis Outbreak. Food Environ. Virol. 2017, 9, 334–341. [Google Scholar] [CrossRef]
- Kumthip, K.; Khamrin, P.; Ushijima, H.; Maneekarn, N. Enteric and Non-Enteric Adenoviruses Associated with Acute Gastroenteritis in Pediatric Patients in Thailand, 2011 to 2017. PLoS ONE 2019, 14, e0220263. [Google Scholar] [CrossRef]
- Khanal, S.; Ghimire, P.; Dhamoon, A. The Repertoire of Adenovirus in Human Disease: The Innocuous to the Deadly. Biomedicines 2018, 6, 30. [Google Scholar] [CrossRef]
- Wißmann, J.E.; Kirchhoff, L.; Brüggemann, Y.; Todt, D.; Steinmann, J.; Steinmann, E. Persistence of Pathogens on Inanimate Surfaces: A Narrative Review. Microorganisms 2021, 9, 343. [Google Scholar] [CrossRef] [PubMed]
- Binder, A.M.; Biggs, H.M.; Haynes, A.K.; Chommanard, C.; Lu, X.; Erdman, D.D.; Watson, J.T.; Gerber, S.I. Human Adenovirus Surveillance—United States, 2003–2016. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 1039–1042. [Google Scholar] [CrossRef] [PubMed]
- CDC Symptoms of Adenovirus. 2022. Available online: https://www.cdc.gov/adenovirus/symptoms.html (accessed on 27 May 2023).
- O’Shea, H.; Blacklaws, B.A.; Collins, P.J.; McKillen, J.; Fitzgerald, R. Viruses Associated With Foodborne Infections. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2019; p. B9780128096338902735. ISBN 978-0-12-809633-8. [Google Scholar]
- Kujawski, S.A.; Lu, X.; Schneider, E.; Blythe, D.; Boktor, S.; Farrehi, J.; Haupt, T.; McBride, D.; Stephens, E.; Sakthivel, S.K.; et al. Outbreaks of Adenovirus-Associated Respiratory Illness on 5 College Campuses in the United States, 2018–2019. Clin. Infect. Dis. 2021, 72, 1992–1999. [Google Scholar] [CrossRef] [PubMed]
- Elmahdy, E.M.; Shaheen, M.N.F.; Rizk, N.M.; Saad-Hussein, A. Quantitative Detection of Human Adenovirus and Human Rotavirus Group A in Wastewater and El-Rahawy Drainage Canal Influencing River Nile in the North of Giza, Egypt. Food Environ. Virol. 2020, 12, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, V.; Chen, J.-S.; Hsu, G.-J.; Chen, H.-P.; Chao, H.-C.; Huang, S.-W.; Tsai, I.-S.; Hsu, B.-M. Surveillance of Adenovirus and Norovirus Contaminants in the Water and Shellfish of Major Oyster Breeding Farms and Fishing Ports in Taiwan. Pathogens 2022, 11, 316. [Google Scholar] [CrossRef] [PubMed]
- Chigbu, D.; Labib, B. Pathogenesis and Management of Adenoviral Keratoconjunctivitis. IDR 2018, 11, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Gholipour, S.; Hosseini, M.; Nikaeen, M.; Hadi, M.; Sarmadi, M.; Saderi, H.; Hassanzadeh, A. Quantification of Human Adenovirus in Irrigation Water-Soil-Crop Continuum: Are Consumers of Wastewater-Irrigated Vegetables at Risk? Environ. Sci. Pollut. Res. 2022, 29, 54561–54570. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-Y.; Kim, W.-K.; Cho, S.; Park, K.; Kim, J.; Lee, S.-H.; Lee, J.; Lee, Y.-S.; Kim, J.H.; Byun, K.S.; et al. Genotyping and Molecular Diagnosis of Hepatitis A Virus in Human Clinical Samples Using Multiplex PCR-Based Next-Generation Sequencing. Microorganisms 2022, 10, 100. [Google Scholar] [CrossRef]
- Koff, R.S. Feinstone SM, Kapikian AZ, Purcell RH. Hepatitis A: Detection by Immune Electron Microscopy of a Virus like Antigen Associated with Acute Illness [Science 1973;182:1026–1028]. J. Hepatol. 2002, 37, 2–6. [Google Scholar] [CrossRef]
- Castaneda, D.; Gonzalez, A.J.; Alomari, M.; Tandon, K.; Zervos, X.B. From Hepatitis A to E: A Critical Review of Viral Hepatitis. World J. Gastroenterol. 2021, 27, 1691–1715. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Immunological Basis for Immunization Series: Module 18: Hepatitis A; World Health Organization: Geneva, Switzerland, 2019; ISBN 978-92-4-151632-7. [Google Scholar]
- Cao, G.; Jing, W.; Liu, J.; Liu, M. The Global Trends and Regional Differences in Incidence and Mortality of Hepatitis A from 1990 to 2019 and Implications for Its Prevention. Hepatol. Int. 2021, 15, 1068–1082. [Google Scholar] [CrossRef] [PubMed]
- CDC Hepatitis A|CDC. 2021. Available online: https://www.cdc.gov/vaccines/pubs/pinkbook/hepa.html (accessed on 20 June 2023).
- FDA. Hepatitis A Virus (HAV). 2021. Available online: https://www.fda.gov/food/foodborne-pathogens/hepatitis-virus-hav (accessed on 23 November 2022).
- WHO. Hepatitis A and E. Available online: http://www.emro.who.int/health-topics/hepatitis/introduction.html (accessed on 26 April 2023).
- Patterson, J.; Abdullahi, L.; Hussey, G.D.; Muloiwa, R.; Kagina, B.M. A Systematic Review of the Epidemiology of Hepatitis A in Africa. BMC Infect. Dis. 2019, 19, 651. [Google Scholar] [CrossRef] [PubMed]
- WHO. Hepatitis E. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-e (accessed on 23 November 2022).
- Randazzo, W.; Sánchez, G. Hepatitis A Infections from Food. J. Appl. Microbiol. 2020, 129, 1120–1132. [Google Scholar] [CrossRef]
- Shieh, Y.C.; Cromeans, T.L.; Sobsey, M.D. VIRUSES|Hepatitis Viruses Transmitted by Food, Water, and Environment. In Encyclopedia of Food Microbiology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 738–744. ISBN 978-0-12-384733-1. [Google Scholar]
- CDC. Hepatitis A|Disease Directory|Travelers’ Health|CDC. Available online: https://wwwnc.cdc.gov/travel/diseases/hepatitis-a (accessed on 23 November 2022).
- Migueres, M.; Lhomme, S.; Izopet, J. Hepatitis A: Epidemiology, High-Risk Groups, Prevention and Research on Antiviral Treatment. Viruses 2021, 13, 1900. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Shieh, Y.C. Survival of Hepatitis A Virus on Two-Month Stored Freeze-Dried Berries. J. Food Prot. 2021, 84, 2084–2091. [Google Scholar] [CrossRef] [PubMed]
- Sattar, S.A.; Tetro, J.; Bidawid, S.; Farber, J. Foodborne Spread of Hepatitis A: Recent Studies on Virus Survival, Transfer and Inactivation. Can. J. Infect. Dis. 2000, 11, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Khattab, E.; Shaltout, F.; Sabik, I. Hepatitis A virus related to foods. Benha Vet. Med. J. 2021, 40, 174–179. [Google Scholar] [CrossRef]
- Fierro, N.A.; Realpe, M.; Meraz-Medina, T.; Roman, S.; Panduro, A. Hepatitis E Virus: An Ancient Hidden Enemy in Latin America. World J. Gastroenterol. 2016, 22, 2271–2283. [Google Scholar] [CrossRef]
- Smith, D.; Izopet, J.; Nicot, F.; Simmonds, P.; Jameel, S.; Meng, X.-J.; Norder, H.; Okamoto, H.; Van Der Poel, W.H.M.; Reuter, G.; et al. Update: Proposed Reference Sequences for Subtypes of Hepatitis E Virus (Species Orthohepevirus A). J. Gen. Virol. 2020, 101, 692–698. [Google Scholar] [CrossRef]
- Smith, D.; Simmonds, P.; members of the International Committee on the Taxonomy of Viruses Study Group; Jameel, S.; Emerson, S.U.; Harrison, T.J.; Meng, X.-J.; Okamoto, H.; Van Der Poel, W.H.M.; Purdy, M.A. Consensus Proposals for Classification of the Family Hepeviridae. J. Gen. Virol. 2014, 95, 2223–2232. [Google Scholar] [CrossRef]
- Debing, Y.; Moradpour, D.; Neyts, J.; Gouttenoire, J. Update on Hepatitis E Virology: Implications for Clinical Practice. J. Hepatol. 2016, 65, 200–212. [Google Scholar] [CrossRef]
- Harrison, L.; DiCaprio, E. Hepatitis E Virus: An Emerging Foodborne Pathogen. Front. Sustain. Food Syst. 2018, 2, 14. [Google Scholar] [CrossRef]
- Kamar, N.; Izopet, J.; Dalton, H.R. Chronic Hepatitis e Virus Infection and Treatment. J. Clin. Exp. Hepatol. 2013, 3, 134–140. [Google Scholar] [CrossRef]
- Pischke, S.; Hartl, J.; Pas, S.D.; Lohse, A.W.; Jacobs, B.C.; Van Der Eijk, A.A. Hepatitis E Virus: Infection beyond the Liver? J. Hepatol. 2017, 66, 1082–1095. [Google Scholar] [CrossRef]
- Khuroo, M.; Khuroo, M.; Khuroo, N. Transmission of Hepatitis E Virus in Developing Countries. Viruses 2016, 8, 253. [Google Scholar] [CrossRef]
- Pavio, N.; Doceul, V.; Bagdassarian, E.; Johne, R. Recent Knowledge on Hepatitis E Virus in Suidae Reservoirs and Transmission Routes to Human. Vet. Res. 2017, 48, 78. [Google Scholar] [CrossRef]
- Bi, H.; Yang, R.; Wu, C.; Xia, J. Hepatitis E Virus and Blood Transfusion Safety. Epidemiol. Infect. 2020, 148, e158. [Google Scholar] [CrossRef]
- Yugo, D.; Meng, X.-J. Hepatitis E Virus: Foodborne, Waterborne and Zoonotic Transmission. IJERPH 2013, 10, 4507–4533. [Google Scholar] [CrossRef]
- Williams, T.P.E.; Kasorndorkbua, C.; Halbur, P.G.; Haqshenas, G.; Guenette, D.K.; Toth, T.E.; Meng, X.J. Evidence of Extrahepatic Sites of Replication of the Hepatitis E Virus in a Swine Model. J. Clin. Microbiol. 2001, 39, 3040–3046. [Google Scholar] [CrossRef]
- Meng, X.-J. Zoonotic and Foodborne Transmission of Hepatitis E Virus. Semin. Liver Dis. 2013, 33, 41–49. [Google Scholar] [CrossRef]
- Cook, N.; D’Agostino, M.; Johne, R. Potential Approaches to Assess the Infectivity of Hepatitis E Virus in Pork Products: A Review. Food Environ. Virol. 2017, 9, 243–255. [Google Scholar] [CrossRef]
- Koutsoumanis, K.P.; Lianou, A.; Sofos, J.N. Food Safety: Emerging Pathogens. In Encyclopedia of Agriculture and Food Systems; Elsevier: Amsterdam, The Netherlands, 2014; pp. 250–272. ISBN 978-0-08-093139-5. [Google Scholar]
- Petrović, T.; D’Agostino, M. Viral Contamination of Food. In Antimicrobial Food Packaging; Elsevier: Amsterdam, The Netherlands, 2016; pp. 65–79. ISBN 978-0-12-800723-5. [Google Scholar]
- Hrdy, J.; Vasickova, P. Virus Detection Methods for Different Kinds of Food and Water Samples—The Importance of Molecular Techniques. Food Control 2022, 134, 108764. [Google Scholar] [CrossRef]
- Hamza, I.A.; Jurzik, L.; Überla, K.; Wilhelm, M. Methods to detect infectious human enteric viruses in environmental water samples. Int. J. Hyg. Environ. Health 2011, 214, 424–436. [Google Scholar] [CrossRef]
- USFDA. Q5A(R2) Viral Safety Evaluation of Biotechnology Products Derived from Cell Lines of Human or Animal Origin. 2024. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q5ar2-viral-safety-evaluation-biotechnology-products-derived-cell-lines-human-or-animal-origin (accessed on 16 January 2024).
- Cisak, E.; Wójcik-Fatla, A.; Zając, V.; Sroka, J.; Buczek, A.; Dutkiewicz, J. Prevalence of Tick-Borne Encephalitis Virus (TBEV) in Samples of Raw Milk Taken Randomly from Cows, Goats and Sheep in Eastern Poland. Ann. Agric. Environ. Med. 2010, 17, 283–286. [Google Scholar]
- Paulsen, K.M.; Stuen, S.; das Neves, C.G.; Suhel, F.; Gurung, D.; Soleng, A.; Stiasny, K.; Vikse, R.; Andreassen, Å.K.; Granquist, E.G. Tick-Borne Encephalitis Virus in Cows and Unpasteurized Cow Milk from Norway. Zoonoses Public Health 2019, 66, 216–222. [Google Scholar] [CrossRef]
- Brockmann, S.; Oehme, R.; Buckenmaier, T.; Beer, M.; Jeffery-Smith, A.; Spannenkrebs, M.; Haag-Milz, S.; Wagner-Wiening, C.; Schlegel, C.; Fritz, J.; et al. A Cluster of Two Human Cases of Tick-Borne Encephalitis (TBE) Transmitted by Unpasteurised Goat Milk and Cheese in Germany, May 2016. Eurosurveillance 2018, 23, 17-00336. [Google Scholar] [CrossRef]
- Buczek, A.M.; Buczek, W.; Buczek, A.; Wysokińska-Miszczuk, J. Food-Borne Transmission of Tick-Borne Encephalitis Virus—Spread, Consequences, and Prophylaxis. IJERPH 2022, 19, 1812. [Google Scholar] [CrossRef]
- Kríz, B.; Benes, C.; Daniel, M. Alimentary Transmission of Tick-Borne Encephalitis in the Czech Republic (1997–2008). Epidemiol. Mikrobiol. Imunol. 2009, 58, 98–103. [Google Scholar]
- Taba, P.; Schmutzhard, E.; Forsberg, P.; Lutsar, I.; Ljøstad, U.; Mygland, Å.; Levchenko, I.; Strle, F.; Steiner, I. EAN Consensus Review on Prevention, Diagnosis and Management of Tick-borne Encephalitis. Eur. J. Neurol. 2017, 24, 1214. [Google Scholar] [CrossRef]
- Aditi; Shariff, M. Nipah Virus Infection: A Review. Epidemiol. Infect. 2019, 147, e95. [Google Scholar] [CrossRef]
- Chua, K.B. Nipah Virus Outbreak in Malaysia. J. Clin. Virol. 2003, 26, 265–275. [Google Scholar] [CrossRef]
- Luby, S.; Rahman, M.; Hossain, M.; Blum, L.; Husain, M.; Gurley, E.; Khan, R.; Ahmed, B.-N.; Rahman, S.; Nahar, N.; et al. Foodborne Transmission of Nipah Virus, Bangladesh. Emerg. Infect. Dis. 2006, 12, 1888–1894. [Google Scholar] [CrossRef]
- de Wit, E.; Prescott, J.; Falzarano, D.; Bushmaker, T.; Scott, D.; Feldmann, H.; Munster, V.J. Foodborne Transmission of Nipah Virus in Syrian Hamsters. PLoS Pathog. 2014, 10, e1004001. [Google Scholar] [CrossRef]
- Arunkumar, G.; Chandni, R.; Mourya, D.T.; Singh, S.K.; Sadanandan, R.; Sudan, P.; Bhargava, B.; Nipah Investigators People and Health Study Group; Gangakhedkar, R.R.; Gupta, N.; et al. Outbreak Investigation of Nipah Virus Disease in Kerala, India, 2018. J. Infect. Dis. 2019, 219, 1867–1878. [Google Scholar] [CrossRef]
- Sivanandy, P.; Jun, P.H.; Man, L.W.; Wei, N.S.; Mun, N.F.K.; Yii, C.A.J.; Ying, C.C.X. A Systematic Review of Ebola Virus Disease Outbreaks and an Analysis of the Efficacy and Safety of Newer Drugs Approved for the Treatment of Ebola Virus Disease by the US Food and Drug Administration from 2016 to 2020. J. Infect. Public Health 2022, 15, 285–292. [Google Scholar] [CrossRef]
- CDC. Ebola (Ebola Virus Disease)|CDC. 2021. Available online: https://www.cdc.gov/vhf/ebola/index.html (accessed on 23 November 2022).
- Mann, E.; Streng, S.; Bergeron, J.; Kircher, A. A Review of the Role of Food and the Food System in the Transmission and Spread of Ebolavirus. PLoS Neglected Trop. Dis. 2015, 9, e0004160. [Google Scholar] [CrossRef]
- Onyekuru, N.A.; Ume, C.O.; Ezea, C.P.; Chukwuma Ume, N.N. Effects of Ebola Virus Disease Outbreak on Bush Meat Enterprise and Environmental Health Risk Behavior Among Households in South-East Nigeria. J. Prim. Prev. 2020, 41, 603–618. [Google Scholar] [CrossRef]
- Amonsin, A.; Choatrakol, C.; Lapkuntod, J.; Tantilertcharoen, R.; Thanawongnuwech, R.; Suradhat, S.; Suwannakarn, K.; Theamboonlers, A.; Poovorawan, Y. Influenza Virus (H5N1) in Live Bird Markets and Food Markets, Thailand. Emerg. Infect. Dis. 2008, 14, 1739–1742. [Google Scholar] [CrossRef]
- Shibata, A.; Hiono, T.; Fukuhara, H.; Sumiyoshi, R.; Ohkawara, A.; Matsuno, K.; Okamatsu, M.; Osaka, H.; Sakoda, Y. Isolation and Characterization of Avian Influenza Viruses from Raw Poultry Products Illegally Imported to Japan by International Flight Passengers. Transbound. Emerg. Dis. 2018, 65, 465–475. [Google Scholar] [CrossRef]
- Tumpey, T.M.; Suarez, D.L.; Perkins, L.E.L.; Senne, D.A.; Lee, J.; Lee, Y.-J.; Mo, I.-P.; Sung, H.-W.; Swayne, D.E. Characterization of a Highly Pathogenic H5N1 Avian Influenza A Virus Isolated from Duck Meat. J. Virol. 2002, 76, 6344–6355. [Google Scholar] [CrossRef]
- CDC Technical Report: Highly Pathogenic Avian Influenza A(H5N1) Viruses. 2023. Available online: https://www.cdc.gov/flu/avianflu/spotlights/2022-2023/h5n1-technical-report.htm (accessed on 26 April 2023).
- Ambert-Balay, K.; Lorrot, M.; Bon, F.; Giraudon, H.; Kaplon, J.; Wolfer, M.; Lebon, P.; Gendrel, D.; Pothier, P. Prevalence and Genetic Diversity of Aichi Virus Strains in Stool Samples from Community and Hospitalized Patients. J. Clin. Microbiol. 2008, 46, 1252–1258. [Google Scholar] [CrossRef]
- Yamashita, T.; Sakae, K.; Tsuzuki, H.; Suzuki, Y.; Ishikawa, N.; Takeda, N.; Miyamura, T.; Yamazaki, S. Complete Nucleotide Sequence and Genetic Organization of Aichi Virus, a Distinct Member of the Picornaviridae Associated with Acute Gastroenteritis in Humans. J. Virol. 1998, 72, 8408–8412. [Google Scholar] [CrossRef]
- Yamashita, T.; Sakae, K.; Ishihara, Y.; Isomura, S.; Utagawa, E. Prevalence of Newly Isolated, Cytopathic Small Round Virus (Aichi Strain) in Japan. J. Clin. Microbiol. 1993, 31, 2938–2943. [Google Scholar] [CrossRef]
- Yamashita, T.; Sakae, K.; Kobayashi, S.; Ishihara, Y.; Miyake, T.; Mubina, A.; Isomura, S. Isolation of Cytopathic Small Round Virus (Aichi Virus) from Pakistani Children and Japanese Travelers from Southeast Asia. Microbiol. Immunol. 1995, 39, 433–435. [Google Scholar] [CrossRef]
- Le Guyader, F.S.; Le Saux, J.-C.; Ambert-Balay, K.; Krol, J.; Serais, O.; Parnaudeau, S.; Giraudon, H.; Delmas, G.; Pommepuy, M.; Pothier, P.; et al. Aichi Virus, Norovirus, Astrovirus, Enterovirus, and Rotavirus Involved in Clinical Cases from a French Oyster-Related Gastroenteritis Outbreak. J. Clin. Microbiol. 2008, 46, 4011–4017. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Silva, P.A.; Hauroeder, B.; Diedrich, S.; Cardoso, D.D.P.; Schreier, E. Molecular Characterization of the First Aichi Viruses Isolated in Europe and in South America. Arch. Virol. 2006, 151, 1199–1206. [Google Scholar] [CrossRef]
- Sdiri-Loulizi, K.; Hassine, M.; Aouni, Z.; Gharbi-Khelifi, H.; Sakly, N.; Chouchane, S.; Guédiche, M.N.; Pothier, P.; Aouni, M.; Ambert-Balay, K. First Molecular Detection of Aichi Virus in Sewage and Shellfish Samples in the Monastir Region of Tunisia. Arch. Virol. 2010, 155, 1509–1513. [Google Scholar] [CrossRef]
- Terio, V.; Bottaro, M.; Di Pinto, A.; Fusco, G.; Barresi, T.; Tantillo, G.; Martella, V. Occurrence of Aichi Virus in Retail Shellfish in Italy. Food Microbiol. 2018, 74, 120–124. [Google Scholar] [CrossRef]
- Chathappady House, N.N.; Palissery, S.; Sebastian, H. Corona Viruses: A Review on SARS, MERS and COVID-19. Microbiol Insights 2021, 14, 117863612110024. [Google Scholar] [CrossRef]
- Law, S.; Leung, A.W.; Xu, C. Severe Acute Respiratory Syndrome (SARS) and Coronavirus Disease-2019 (COVID-19): From Causes to Preventions in Hong Kong. Int. J. Infect. Dis. 2020, 94, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Peiris, M.; Poon, L.L.M. Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS) (Coronaviridae). In Encyclopedia of Virology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 814–824. ISBN 978-0-12-814516-6. [Google Scholar]
- WHO. Coronavirus Disease (COVID-19)—World Health Organization. 2022. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 23 November 2022).
- Jia, M.; Taylor, T.M.; Senger, S.M.; Ovissipour, R.; Bertke, A.S. SARS-CoV-2 Remains Infectious on Refrigerated Deli Food, Meats, and Fresh Produce for up to 21 Days. Foods 2022, 11, 286. [Google Scholar] [CrossRef] [PubMed]
- Olaimat, A.N.; Shahbaz, H.M.; Fatima, N.; Munir, S.; Holley, R.A. Food Safety during and after the Era of COVID-19 Pandemic. Front. Microbiol. 2020, 11, 1854. [Google Scholar] [CrossRef]
- Dai, M.; Li, H.; Yan, N.; Huang, J.; Zhao, L.; Xu, S.; Wu, J.; Jiang, S.; Pan, C.; Liao, M. Long-Term Survival of SARS-CoV-2 on Salmon as a Source for International Transmission. J. Infect. Dis. 2021, 223, 537–539. [Google Scholar] [CrossRef]
- Dhakal, J.; Jia, M.; Joyce, J.D.; Moore, G.A.; Ovissipour, R.; Bertke, A.S. Survival of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and Herpes Simplex Virus 1 (HSV-1) on Foods Stored at Refrigerated Temperature. Foods 2021, 10, 1005. [Google Scholar] [CrossRef]
- Esseili, M.A.; Mann, A.; Narwankar, R.; Kassem, I.I.; Diez-Gonzalez, F.; Hogan, R.J. SARS-CoV-2 Remains Infectious for at Least a Month on Artificially-Contaminated Frozen Berries. Food Microbiol. 2022, 107, 104084. [Google Scholar] [CrossRef]
- Feng, X.-L.; Li, B.; Lin, H.-F.; Zheng, H.-Y.; Tian, R.-R.; Luo, R.-H.; Liu, M.-Q.; Jiang, R.-D.; Zheng, Y.-T.; Shi, Z.-L.; et al. Stability of SARS-CoV-2 on the Surfaces of Three Meats in the Setting That Simulates the Cold Chain Transportation. Virol. Sin. 2021, 36, 1069–1072. [Google Scholar] [CrossRef]
- van Doremalen, N.; Bushmaker, T.; Karesh, W.B.; Munster, V.J. Stability of Middle East Respiratory Syndrome Coronavirus in Milk. Emerg. Infect. Dis. 2014, 20, 1263–1264. [Google Scholar] [CrossRef] [PubMed]
- van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Glasbrenner, D.C.; Choi, Y.W.; Middleton, J.K. SARS-CoV-2 Persistence on Common Food Covering Materials: Plastic Wrap, Fruit Wax, and Cardboard Takeout Containers. J. Appl. Microbiol. 2023, 134, lxac071. [Google Scholar] [CrossRef]
- Liu, P.; Yang, M.; Zhao, X.; Guo, Y.; Wang, L.; Zhang, J.; Lei, W.; Han, W.; Jiang, F.; Liu, W.J.; et al. Cold-Chain Transportation in the Frozen Food Industry May Have Caused a Recurrence of COVID-19 Cases in Destination: Successful Isolation of SARS-CoV-2 Virus from the Imported Frozen Cod Package Surface. Biosaf. Health 2020, 2, 199–201. [Google Scholar] [CrossRef]
- Pang, X.; Ren, L.; Wu, S.; Ma, W.; Yang, J.; Di, L.; Li, J.; Xiao, Y.; Kang, L.; Du, S.; et al. Cold-Chain Food Contamination as the Possible Origin of COVID-19 Resurgence in Beijing. Natl. Sci. Rev. 2020, 7, 1861–1864. [Google Scholar] [CrossRef]
- Li, F.; Wang, J.; Liu, Z.; Li, N. Surveillance of SARS-CoV-2 Contamination in Frozen Food-Related Samples—China, July 2020–July 2021. China CDC Wkly 2022, 4, 465–470. [Google Scholar] [CrossRef]
- Alvis-Chirinos, K.; Angulo-Bazán, Y.; Escalante-Maldonado, O.; Fuentes, D.; Palomino-Rodriguez, M.G.; Gonzales-Achuy, E.; Mormontoy, H.; Hinojosa-Mamani, P.; Huamán-Espino, L.; Aparco, J.P. Presence of SARS-CoV-2 on Food Surfaces and Public Space Surfaces in Three Districts of Lima, Peru. Braz. J. Med. Biol. Res. 2022, 55, e12003. [Google Scholar] [CrossRef]
- Guo, M.; Yan, J.; Hu, Y.; Xu, L.; Song, J.; Yuan, K.; Cheng, X.; Ma, S.; Liu, J.; Wu, X.; et al. Transmission of SARS-CoV-2 on Cold-Chain Food: Precautions Can Effectively Reduce the Risk. Food Environ. Virol. 2022, 14, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Sadat, A.; Abdi, R.; Colaruotolo, L.A.; Francavilla, A.; Petker, K.; Nasr, P.; Moraveji, M.; Cruz, G.; Huang, Y.; et al. Detection of SARS-CoV-2 on Surfaces in Food Retailers in Ontario. Curr. Res. Food Sci. 2021, 4, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Dewey-Mattia, D.; Manikonda, K.; Hall, A.J.; Wise, M.E.; Crowe, S.J. Surveillance for Foodborne Disease Outbreaks—United States, 2009–2015. MMWR Surveill. Summ. 2018, 67, 1–11. [Google Scholar] [CrossRef] [PubMed]
- USFDA Foodborne Pathogens. 2020. Available online: https://www.fda.gov/food/outbreaks-foodborne-illness/foodborne-pathogens (accessed on 28 December 2022).
- Koopmans, M.; Duizer, E. Foodborne Viruses: An Emerging Problem. Int. J. Food Microbiol. 2004, 90, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, G.; Bosch, A. Survival of Enteric Viruses in the Environment and Food. In Viruses in Foods; Goyal, S.M., Cannon, J.L., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 367–392. ISBN 978-3-319-30721-3. [Google Scholar]
- Miranda, R.C.; Schaffner, D.W. Virus Risk in the Food Supply Chain. Curr. Opin. Food Sci. 2019, 30, 43–48. [Google Scholar] [CrossRef]
- CDC National Outbreak Reporting System (NORS) Dashboard|CDC. 2022. Available online: https://wwwn.cdc.gov/norsdashboard/ (accessed on 23 November 2022).
- Thomas, M.K.; Murray, R.; Flockhart, L.; Pintar, K.; Pollari, F.; Fazil, A.; Nesbitt, A.; Marshall, B. Estimates of the Burden of Foodborne Illness in Canada for 30 Specified Pathogens and Unspecified Agents, Circa 2006. Foodborne Pathog. Dis. 2013, 10, 639–648. [Google Scholar] [CrossRef]
- Government of Canada Public Health Notices. Available online: https://www.canada.ca/en/public-health/services/public-health-notices.html (accessed on 17 June 2023).
- European Food Safety Authority; European Centre for Disease Prevention and Control, the European Union One Health 2021 Zoonoses Report. EFSA 2022, 20, e07666. [CrossRef]
- Hashemi, M.; Salayani, M.; Afshari, A.; Samadi Kafil, H.; Noori, S.M.A. The Global Burden of Viral Food-Borne Diseases: A Systematic Review. CPB 2023, 24, 1657–1672. [Google Scholar] [CrossRef]
- Adak, G.K.; Meakins, S.M.; Yip, H.; Lopman, B.A.; O’Brien, S.J. Disease Risks from Foods, England and Wales, 1996–2000. Emerg. Infect. Dis. 2005, 11, 365–372. [Google Scholar] [CrossRef]
- Havelaar, A.H.; Haagsma, J.A.; Mangen, M.-J.J.; Kemmeren, J.M.; Verhoef, L.P.B.; Vijgen, S.M.C.; Wilson, M.; Friesema, I.H.M.; Kortbeek, L.M.; van Duynhoven, Y.T.H.P.; et al. Disease Burden of Foodborne Pathogens in the Netherlands, 2009. Int. J. Food Microbiol. 2012, 156, 231–238. [Google Scholar] [CrossRef]
- O’Brien, S.J. Foodborne Diseases: Prevalence of Foodborne Diseases in Europe. In Encyclopedia of Food Safety; Elsevier: Amsterdam, The Netherlands, 2014; pp. 302–311. ISBN 978-0-12-378613-5. [Google Scholar]
- CDC Norovirus Worldwide|CDC. 2021. Available online: https://www.cdc.gov/norovirus/trends-outbreaks/worldwide.html (accessed on 23 November 2022).
- Mattison, C.P.; Dunn, M.; Wikswo, M.E.; Kambhampati, A.; Calderwood, L.; Balachandran, N.; Burnett, E.; Hall, A.J. Non-Norovirus Viral Gastroenteritis Outbreaks Reported to the National Outbreak Reporting System, USA, 2009–2018. Emerg. Infect. Dis. 2021, 27, 560–564. [Google Scholar] [CrossRef]
- Calduch, E.N.; Cattaert, T.; Verstraeten, T. Model Estimates of Hospitalization Discharge Rates for Norovirus Gastroenteritis in Europe, 2004–2015. BMC Infect Dis 2021, 21, 757. [Google Scholar] [CrossRef] [PubMed]
- Todd, E. Foodborne Disease in the Middle East. In Water, Energy & Food Sustainability in the Middle East; Murad, S., Baydoun, E., Daghir, N., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 389–440. ISBN 978-3-319-48919-3. [Google Scholar]
- Kreidieh, K.; Charide, R.; Dbaibo, G.; Melhem, N.M. The Epidemiology of Norovirus in the Middle East and North Africa (MENA) Region: A Systematic Review. Virol. J. 2017, 14, 220. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, S.K.; De Andrade, J.D.S.R.; Miagostovich, M.P.; Fumian, T.M. Virological and Epidemiological Features of Norovirus Infections in Brazil, 2017–2018. Viruses 2021, 13, 1724. [Google Scholar] [CrossRef] [PubMed]
- Barrabeig, I.; Rovira, A.; Buesa, J.; Bartolomé, R.; Pintó, R.; Prellezo, H.; Domínguez, À. Foodborne Norovirus Outbreak: The Role of an Asymptomatic Food Handler. BMC Infect. Dis. 2010, 10, 269. [Google Scholar] [CrossRef] [PubMed]
- CDC Norovirus Laboratory Diagnosis|CDC. 2021. Available online: https://www.cdc.gov/norovirus/lab/diagnosis.html (accessed on 23 November 2022).
- Government of Canada Public Health Notice—Outbreak of Norovirus and Gastrointestinal Illnesses Linked to Raw Oysters. Available online: https://www.canada.ca/en/public-health/services/public-health-notices/2018/outbreak-norovirus-infections-linked-raw-oysters.html (accessed on 23 November 2022).
- CDC Multistate Norovirus Outbreak Linked to Raw Oysters from Texas|CDC. 2022. Available online: https://www.cdc.gov/norovirus/outbreaks/index.html (accessed on 23 November 2022).
- Maritschnik, S.; Kanitz, E.E.; Simons, E.; Höhne, M.; Neumann, H.; Allerberger, F.; Schmid, D.; Lederer, I. A Food Handler-Associated, Foodborne Norovirus GII.4 Sydney 2012-Outbreak Following a Wedding Dinner, Austria, October 2012. Food Environ. Virol. 2013, 5, 220–225. [Google Scholar] [CrossRef]
- Halliday, M.L.; Kang, L.-Y.; Zhou, T.-K.; Hu, M.-D.; Pan, Q.-C.; Fu, T.-Y.; Huang, Y.-S.; Hu, S.-L. An Epidemic of Hepatitis A Attributable to the Ingestion of Raw Clams in Shanghai, China. J. Infect. Dis. 1991, 164, 852–859. [Google Scholar] [CrossRef]
- Wheeler, C.; Vogt, T.M.; Armstrong, G.L.; Vaughan, G.; Weltman, A.; Nainan, O.V.; Dato, V.; Xia, G.; Waller, K.; Amon, J.; et al. An Outbreak of Hepatitis A Associated with Green Onions. N. Engl. J. Med. 2005, 353, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, J.J.; Schemmerer, M.; Oberkofler, H.; Kerschner, H.; Sinha, P.; Koidl, C.; Allerberger, F. Hepatitis A Outbreak in Europe: Imported Frozen Berry Mix Suspected to Be the Source of At Least One Infection in Austria in 2013. Food Environ. Virol 2014, 6, 297–300. [Google Scholar] [CrossRef] [PubMed][Green Version]
- CDC Organic Strawberries Hepatitis A Outbreak|CDC. 2022. Available online: https://www.cdc.gov/hepatitis/outbreaks/2022/hav-contaminated-food/index.htm (accessed on 23 November 2022).
- FDA. Outbreak Investigation of Hepatitis A Virus Infections: Frozen Strawberries (February 2023). 2023. Available online: https://www.fda.gov/food/outbreaks-foodborne-illness/outbreak-investigation-hepatitis-virus-infections-frozen-strawberries-february-2023 (accessed on 23 November 2022).
- Zhang, X.-L.; Li, W.-F.; Yuan, S.; Guo, J.-Y.; Li, Z.-L.; Chi, S.-H.; Huang, W.-J.; Li, X.-W.; Huang, S.-J.; Shao, J.-W. Meta-Transcriptomic Analysis Reveals a New Subtype of Genotype 3 Avian Hepatitis E Virus in Chicken Flocks with High Mortality in Guangdong, China. BMC Vet. Res. 2019, 15, 131. [Google Scholar] [CrossRef] [PubMed]
- Said, B.; Ijaz, S.; Kafatos, G.; Booth, L.; Thomas, H.L.; Walsh, A.; Ramsay, M.; Morgan, D.; on behalf of the Hepatitis E Incident Investigation Team. Hepatitis E Outbreak on Cruise Ship. Emerg. Infect. Dis. 2009, 15, 1738–1744. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Han, Y.; Xin, H.; Liu, W.; Song, Q.; Li, Z.; Gao, S.; Jiang, F.; Cao, J.; Bi, S.; et al. Hepatitis E Outbreak in a Mechanical Factory in Qingdao City, China. Int. J. Infect. Dis. 2019, 86, 191–196. [Google Scholar] [CrossRef] [PubMed]
- CDC Foodborne Outbreak of Group A Rotavirus Gastroenteritis among College Students—District of Columbia, March–April 2000. 2000. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm4950a2.htm (accessed on 23 November 2022).
- NICD. NICD Communiqué 2018. 2018. Available online: https://www.nicd.ac.za/archives/ (accessed on 23 November 2022).
- Calder, L.; Simmons, G.; Thornley, C.; Taylor, P.; Pritchard, K.; Greening, G.; Bishop, J. An Outbreak of Hepatitis A Associated with Consumption of Raw Blueberries. Epidemiol. Infect. 2003, 131, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Donnan, E.J.; Fielding, J.E.; Gregory, J.E.; Lalor, K.; Rowe, S.; Goldsmith, P.; Antoniou, M.; Fullerton, K.E.; Knope, K.; Copland, J.G.; et al. A Multistate Outbreak of Hepatitis A Associated with Semidried Tomatoes in Australia, 2009. Clin. Infect. Dis. 2012, 54, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Gallot, C.; Grout, L.; Roque-Afonso, A.-M.; Couturier, E.; Carrillo-Santisteve, P.; Pouey, J.; Letort, M.-J.; Hoppe, S.; Capdepon, P.; Saint-Martin, S.; et al. Hepatitis A Associated with Semidried Tomatoes, France, 2010. Emerg. Infect. Dis. 2011, 17, 566–567. [Google Scholar] [CrossRef]
- Petrignani, M.; Harms, M.; Verhoef, L.; van Hunen, R.; Swaan, C.; van Steenbergen, J.; Boxman, I.; Peran, I.; Sala, R.; Ober, H.; et al. Update: A Food-Borne Outbreak of Hepatitis A in the Netherlands Related to Semi-Dried Tomatoes in Oil, January-February 2010. Euro Surveill 2010, 15, 19572. [Google Scholar] [CrossRef]
- Swinkels, H.M.; Kuo, M.; Embree, G.; Fraser Health Environmental Health Investigation Team; Andonov, A.; Henry, B.; Buxton, J.A. Hepatitis A Outbreak in British Columbia, Canada: The Roles of Established Surveillance, Consumer Loyalty Cards and Collaboration, February to May 2012. Eurosurveillance 2014, 19, 20792. [Google Scholar] [CrossRef][Green Version]
- Harries, M.; Monazahian, M.; Wenzel, J.; Jilg, W.; Weber, M.; Ehlers, J.; Dreesman, J.; Mertens, E. Foodborne Hepatitis A Outbreak Associated with Bakery Products in Northern Germany, 2012. Eurosurveillance 2014, 19, 20992. [Google Scholar] [CrossRef] [PubMed][Green Version]
- European Food Safety Authority. Tracing of Food Items in Connection to the Multinational Hepatitis A Virus Outbreak in Europe. EFSA 2014, 12, 3821. [Google Scholar] [CrossRef]
- Collier, M.G.; Khudyakov, Y.E.; Selvage, D.; Adams-Cameron, M.; Epson, E.; Cronquist, A.; Jervis, R.H.; Lamba, K.; Kimura, A.C.; Sowadsky, R.; et al. Outbreak of Hepatitis A in the USA Associated with Frozen Pomegranate Arils Imported from Turkey: An Epidemiological Case Study. Lancet Infect. Dis. 2014, 14, 976–981. [Google Scholar] [CrossRef] [PubMed]
- CDC Hepatitis A Infections Linked to Frozen Strawberries|CDC. 2016. Available online: https://www.cdc.gov/hepatitis/outbreaks/2016/hav-strawberries.htm (accessed on 23 November 2022).
- State of Hawaii Hepatitis A Outbreak 2016|Disease Outbreak Control Division. 2016. Available online: https://health.hawaii.gov/docd/hepatitis-a-outbreak-2016/ (accessed on 23 November 2022).
- Franklin, N.; Camphor, H.; Wright, R.; Stafford, R.; Glasgow, K.; Sheppeard, V. Outbreak of Hepatitis A Genotype IB in Australia Associated with Imported Frozen Pomegranate Arils. Epidemiol. Infect. 2019, 147, e74. [Google Scholar] [CrossRef] [PubMed]
- Enkirch, T.; Eriksson, R.; Persson, S.; Schmid, D.; Aberle, S.W.; Löf, E.; Wittesjö, B.; Holmgren, B.; Johnzon, C.; Gustafsson, E.X.; et al. Hepatitis A Outbreak Linked to Imported Frozen Strawberries by Sequencing, Sweden and Austria, June to September 2018. Eurosurveillance 2018, 23, 1800528. [Google Scholar] [CrossRef]
- Yan, B.; Chen, P.; Feng, Y.; Lu, J.; Meng, X.; Xu, Q.; Xu, A.; Zhang, L. A Community-Wide Epidemic of Hepatitis A Virus Genotype IA Associated with Consumption of Shellfish in Yantai, Eastern China, January to March 2020. Hum. Vaccines Immunother. 2022, 18, 2106081. [Google Scholar] [CrossRef]
- Government of Canada Outbreak of Hepatitis A Infections Linked to Frozen Mangoes. Available online: https://www.canada.ca/en/public-health/services/public-health-notices/2021/outbreak-hepatitis-a-infections-frozen-mangoes.html (accessed on 23 November 2022).
- New Zealand Ministry of Health Hepatitis A and Frozen Berries. Available online: https://www.health.govt.nz/our-work/diseases-and-conditions/hepatitis-and-frozen-berries (accessed on 23 November 2022).
- Prato, R.; Lopalco, P.L.; Chironna, M.; Barbuti, G.; Germinario, C.; Quarto, M. Norovirus Gastroenteritis General Outbreak Associated with Raw Shellfish Consumption in South Italy. BMC Infect. Dis. 2004, 4, 37. [Google Scholar] [CrossRef]
- Falkenhorst, G.; Krusell, L.; Lisby, M.; Madsen, S.B.; Böttiger, B.E.; Mølbak, K. Imported Frozen Raspberries Cause a Series of Norovirus Outbreaks in Denmark, 2005. Eurosurveillance 2005, 10, E050922.2. [Google Scholar] [CrossRef]
- Hjertqvist, M.; Johansson, A.; Svensson, N.; Abom, P.E.; Magnusson, C.; Olsson, M.; Hedlund, K.O.; Andersson, Y. Four Outbreaks of Norovirus Gastroenteritis after Consuming Raspberries, Sweden, June-August 2006. Eurosurveillance 2006, 11, E060907.1. [Google Scholar] [CrossRef] [PubMed]
- Maunula, L.; Roivainen, M.; Keränen, M.; Mäkela, S.; Söderberg, K.; Summa, M.; von Bonsdorff, C.H.; Lappalainen, M.; Korhonen, T.; Kuusi, M.; et al. Detection of Human Norovirus from Frozen Raspberries in a Cluster of Gastroenteritis Outbreaks. Eurosurveillance 2009, 14, 19435. [Google Scholar] [CrossRef] [PubMed]
- Ethelberg, S.; Lisby, M.; Böttiger, B.; Schultz, A.C.; Villif, A.; Jensen, T.; Olsen, K.E.; Scheutz, F.; Kjelsø, C.; Muller, L. Outbreaks of Gastroenteritis Linked to Lettuce, Denmark, January 2010. Eurosurveillance 2010, 15, 19484. [Google Scholar] [CrossRef] [PubMed]
- Mäde, D.; Trübner, K.; Neubert, E.; Höhne, M.; Johne, R. Detection and Typing of Norovirus from Frozen Strawberries Involved in a Large-Scale Gastroenteritis Outbreak in Germany. Food Environ. Virol. 2013, 5, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Fisman, D. Seasonality of Viral Infections: Mechanisms and Unknowns. Clin. Microbiol. Infect. 2012, 18, 946–954. [Google Scholar] [CrossRef]
- Sorensen, J.P.R.; Aldous, P.; Bunting, S.Y.; McNally, S.; Townsend, B.R.; Barnett, M.J.; Harding, T.; La Ragione, R.M.; Stuart, M.E.; Tipper, H.J.; et al. Seasonality of Enteric Viruses in Groundwater-Derived Public Water Sources. Water Res. 2021, 207, 117813. [Google Scholar] [CrossRef]
- Stals, A.; Baert, L.; Van Coillie, E.; Uyttendaele, M. Extraction of Food-Borne Viruses from Food Samples: A Review. Int. J. Food Microbiol. 2012, 153, 1–9. [Google Scholar] [CrossRef]
- Cassedy, A.; Parle-McDermott, A.; O’Kennedy, R. Virus Detection: A Review of the Current and Emerging Molecular and Immunological Methods. Front. Mol. Biosci. 2021, 8, 637559. [Google Scholar] [CrossRef]
- Mackay, I.M.; Arden, K.E.; Nitsche, A. Real-Time PCR in Virology. Nucleic Acids Res. 2002, 30, 1292–1305. [Google Scholar] [CrossRef]
- Bozkurt, H.; D’souza, D.H.; Davidson, P.M. Thermal Inactivation of Foodborne Enteric Viruses and Their Viral Surrogates in Foods. J. Food Prot. 2015, 78, 1597–1617. [Google Scholar] [CrossRef]
- Pexara, A. Inactivation of Foodborne Viruses by the Cold Plasma Technology. J. Hell. Vet. Med. Soc. 2022, 73, 3553–3560. [Google Scholar] [CrossRef]
- Mokoena, M.P.; Omatola, C.A.; Olaniran, A.O. Applications of Lactic Acid Bacteria and Their Bacteriocins against Food Spoilage Microorganisms and Foodborne Pathogens. Molecules 2021, 26, 7055. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, M. Foodborne Viruses. FEMS Microbiol. Rev. 2002, 26, 187–205. [Google Scholar] [CrossRef]
- Pal, M.; Ayele, Y. Emerging Role of Foodborne Viruses in Public Health. Biomed. Res. Int. 2020, 5, 1–4. [Google Scholar]
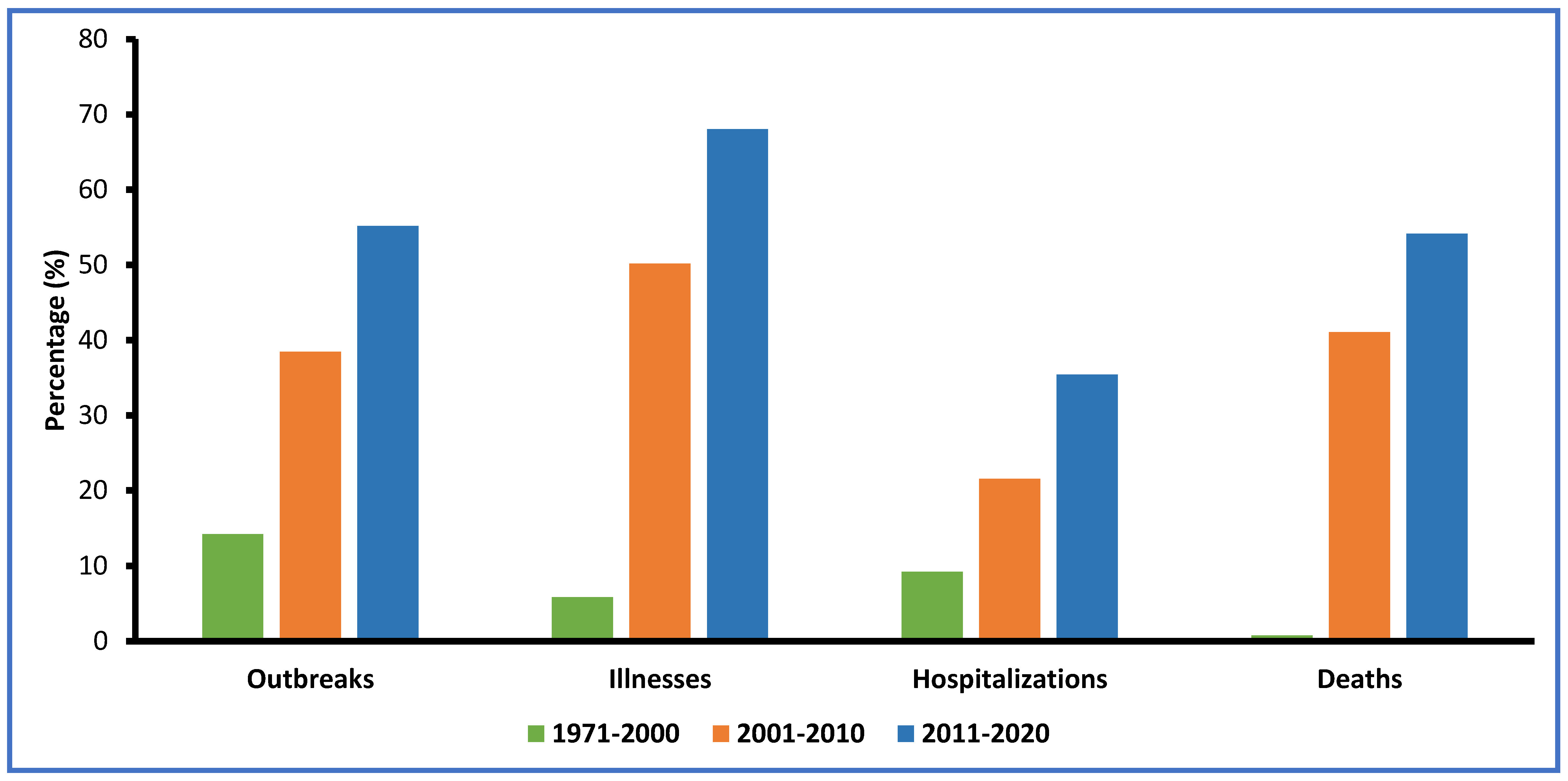
| Discovery Date | Particle/ Genome | Genus/ Family | Structure and Size | Disease Caused | Incubation Period | Duration | Transmission | Symptoms | Prevention and Control | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Norovirus | ||||||||||
| 1968 | Non-enveloped/ssRNA | Norovirus/Caliciviridae | Size of 7.5–7.7 kb length and a diameter of 27 nm | Gastroenteritis | 0.5–3 days | 2–3 days | Person-to-person contact, fecal–oral transmission, foodborne transmission, waterborne transmission | Vomiting, watery diarrhea, abdominal cramps, fever, headache, mucus in stool, myalgia and chills | Proper hand hygiene, washing fruits and vegetables before preparing and eating, preventing infected persons from preparing food for others, cleaning and disinfecting surfaces | [10,18,33,34,35,36,37] |
| Rotavirus | ||||||||||
| 1973 | Non-enveloped/segmented dsRNA | Rotavirus/Reoviridae | Large, icosahedral, and a triple-layered protein coat, up to 76.5 nm in diameter | Gastroenteritis | 2 (1–4) days | 3–8 (up to 22) days | Fecal–oral route | Vomiting, fever, abdominal pain, severe watery diarrhea | Routine vaccination of infants | [10,33,38,39,40] |
| Sapovirus | ||||||||||
| 1977 | Non-enveloped/ssRNA | Sapovirus/Caliciviridae | Small (27–40 nm), genome of about 7.5–8.5 kb in length | Gastroenteritis | 0.5–2 days | 2–6 days | Fecal–oral route | Diarrhea, vomiting, nausea, abdominal cramps, chills, headache, myalgia and malaise | Cooking shellfish adequately, proper hygienic practices and sanitize surfaces with a chlorine solution | [10,41,42] |
| Astrovirus | ||||||||||
| 1975 | Non-enveloped single-stranded RNA | Astrovirus/Astroviridae | Genome approximately 7 kb in size, and 38–40 nm in diameter | Gastroenteritis | 3–5 days | 2–3 days; recurrence possible 7–10 days later | Person-to-person contact fecal–oral route via contaminated water or food, | Nausea, diarrhea, vomiting, malaise, abdominal pain, and fever | Avoidance of shellfish from polluted waters, decontamination of food contact surfaces and good hand hygiene | [10,43,44] |
| Adenovirus | ||||||||||
| 1953 | Non-enveloped double-stranded DNA with an icosahedral capsid | Mastadenovirus/Adenoviridae | Diameter of 70–100 nm, genome 28–45 kb long | Gastroenteritis, conjunctivitis | 2–14 days | 1–2 weeks | Respiratory or environmental routes, waterborne spread, fecal–oral route | Fever, headache, abdominal pain, vomiting, and diarrhea | Good hygiene practices, chlorinate swimming pools | [6,10,45,46] |
| Hepatitis A | ||||||||||
| 1973 | Non-enveloped or quasi-enveloped/ssRNA | Hepatovirus/Picornaviridae | Size of 7.5 kb and a diameter of 27 nm | Hepatitis | 2–4 weeks (15–50 days) | 2 months | Fecal–oral route | Nausea, anorexia, diarrhea, vomiting, malaise, and myalgia. Other symptoms may be present, such as: light-colored stools, dark-colored urine, jaundice | Vaccination, good personal hygiene, avoidance of eating raw shellfish, prevent infected persons from preparing food for others, cooking foods and heating drinks for at least 1 min at 85 °C (185 °F) inactivates hepatitis A virus | [10,33,47,48,49,50] |
| Hepatitis E | ||||||||||
| 1983 | Non-enveloped or quasi-enveloped/ssRNA | Orthohepevirus/Hepeviridae | Diameter of 27–34 nm, size of ∼7.2 kb in length | Hepatitis | 2 weeks to 2 months | 4 weeks (2–18 weeks) | Fecal–oral from water and food | Jaundice, vomiting, diarrhea, and abdominal pain | Good sanitation, vaccination which is only available in China | [10,33,51,52,53,54,55,56,57] |
| Foodborne Virus | Contamination Route | Outbreaks | Illnesses | Hospitalizations | Deaths |
|---|---|---|---|---|---|
| Norovirus | Foodborne | 6662 | 164,740 | 1664 | 17 |
| Waterborne | 130 | 21,341 | 77 | 1 | |
| Person-to-person | 18,996 | 663,775 | 9652 | 887 | |
| Environmental contact | 58 | 2679 | 20 | 1 | |
| Animal contact | 0 | 0 | 0 | 0 | |
| Unknown | 1775 | 46,162 | 753 | 51 | |
| Viral Hepatitis | Foodborne | 109 | 3051 | 479 | 11 |
| Waterborne | 34 | 894 | 17 | 0 | |
| Person-to-person | 0 | 0 | 0 | 0 | |
| Environmental contact | 0 | 0 | 0 | 0 | |
| Animal contact | 0 | 0 | 0 | 0 | |
| Unknown | 0 | 0 | 0 | 0 | |
| Rotavirus | Foodborne | 17 | 449 | 7 | 7 |
| Waterborne | 1 | 1761 | 0 | 0 | |
| Person-to-person | 156 | 3515 | 125 | 11 | |
| Environmental contact | 0 | 0 | 0 | 0 | |
| Animal contact | |||||
| Unknown | 25 | 512 | 10 | 2 | |
| Adenovirus | Foodborne | 2 | 11 | 0 | 0 |
| Waterborne | 4 | 708 | 1 | 0 | |
| Person-to-person | 16 | 350 | 29 | 3 | |
| Environmental contact | 0 | 0 | 0 | 0 | |
| Animal contact | 0 | 0 | 0 | 0 | |
| Unknown | 3 | 36 | 0 | 0 | |
| Astrovirus | Foodborne | 3 | 49 | 1 | 0 |
| Waterborne | 0 | 0 | 0 | 0 | |
| Person-to-person | 25 | 1505 | 7 | 0 | |
| Environmental contact | 0 | 0 | 0 | 0 | |
| Animal contact | 0 | 0 | 0 | 0 | |
| Unknown | 5 | 80 | 0 | 0 | |
| Sapovirus | Foodborne | 20 | 294 | 3 | 0 |
| Waterborne | 0 | 0 | 0 | 0 | |
| Person-to-person | 157 | 6926 | 26 | 2 | |
| Environmental contact | 0 | 0 | 0 | 0 | |
| Animal contact | 0 | 0 | 0 | 0 | |
| Unknown | 22 | 952 | 7 | 1 | |
| Other viruses | Foodborne | 103 | 3049 | 24 | 0 |
| Waterborne | 1 | 36 | 0 | 0 | |
| Person-to-person | 0 | 0 | 0 | 0 | |
| Environmental contact | 0 | 0 | 0 | 0 | |
| Animal contact | 0 | 0 | 0 | 0 | |
| Unknown | 0 | 0 | 0 | 0 | |
| Unknown viruses | Foodborne | 0 | 0 | 0 | 0 |
| Waterborne | 3 | 7 | 0 | 0 | |
| Person-to-person | 0 | 0 | 0 | 0 | |
| Environmental contact | 0 | 0 | 0 | 0 | |
| Animal contact | 0 | 0 | 0 | 0 | |
| Unknown | 0 | 0 | 0 | 0 |
| Virus | Year | Country | Food item | Illnesses | Hospitalizations | Deaths | Reference |
|---|---|---|---|---|---|---|---|
| Hepatitis A virus | 2002 | New Zealand | Raw blueberries | 81 | 18 | 1 | [260] |
| 2003 | USA | Green onions | 601 | 124 | 3 | [251] | |
| 2009 | Australia | Semi-dried tomatoes | 562 | 253 | 1 | [261] | |
| 2010 | France | Semi-dried tomatoes | 59 | 28 | 0 | [262] | |
| 2010 | Netherlands | Semi-dried tomatoes | 13 | 0 | 0 | [263] | |
| 2012 | Canada | Frozen pomegranate arils | 9 | 0 | 0 | [264] | |
| 2012 | Germany | Bakery products | 83 | ND | ND | [265] | |
| 2013 | 10 European countries | Frozen blackberries and redcurrants | 1444 | ND | 0 | [266] | |
| 2013 | USA | Pomegranate seeds | 165 | 71 | 0 | [267] | |
| 2016 | USA | Frozen strawberries | 143 | 56 | 0 | [268] | |
| 2016 | USA | Raw scallops | 292 | 74 | 0 | [269] | |
| 2018 | Australia | Frozen pomegranate arils | 30 | 25 | 1 | [270] | |
| 2018 | Australia, Sweden | Frozen berries | 34 | ND | ND | [271] | |
| 2020 | China | Shellfish | 110 | ND | ND | [272] | |
| 2021 | Canada | Frozen mangoes | 3 | 2 | 0 | [273] | |
| 2022 | New Zealand | Raw blueberries | 32 | 14 | 0 | [274] | |
| 2022 | USA | Fresh organic strawberries | 19 | 13 | 0 | [253] | |
| 2022 | Canada | Fresh organic strawberries | 9 | 0 | [233] | ||
| 2023 | USA | Frozen organic strawberries | 9 | 3 | 0 | [254] | |
| Norovirus | 2002 | Italy | Raw mussels | 103 | ND | ND | [275] |
| 2005 | Denmark | Frozen raspberries | 400 | 23 | 0 | [276] | |
| 2006 | Sweden | Frozen raspberries | 12 | ND | ND | [277] | |
| 2009 | Finland | Frozen raspberries | 46 | ND | ND | [278] | |
| 2010 | Denmark | Lettuce | 264 | ND | ND | [279] | |
| 2012 | Germany | Frozen strawberries | 11,000 | 38 | ND | [280] | |
| 2016 | USA | Unknown | 45 | 0 | 0 | [231] | |
| 2018 | USA | Oysters | 100 | 1 | 0 | [231] | |
| 2018 | USA | Raw oysters | 16 | 2 | 0 | [231] | |
| 2018 | Canada | Raw oysters | 176 | ND | 0 | [247] | |
| 2022 | USA | Raw oysters | 192 | ND | 0 | [248] | |
| 2022 | Canada | Spot prawns | 60 | ND | 0 | [233] | |
| 2022 | Canada | Raw oysters | 339 | ND | 0 | [233] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olaimat, A.N.; Taybeh, A.O.; Al-Nabulsi, A.; Al-Holy, M.; Hatmal, M.M.; Alzyoud, J.; Aolymat, I.; Abughoush, M.H.; Shahbaz, H.; Alzyoud, A.; et al. Common and Potential Emerging Foodborne Viruses: A Comprehensive Review. Life 2024, 14, 190. https://doi.org/10.3390/life14020190
Olaimat AN, Taybeh AO, Al-Nabulsi A, Al-Holy M, Hatmal MM, Alzyoud J, Aolymat I, Abughoush MH, Shahbaz H, Alzyoud A, et al. Common and Potential Emerging Foodborne Viruses: A Comprehensive Review. Life. 2024; 14(2):190. https://doi.org/10.3390/life14020190
Chicago/Turabian StyleOlaimat, Amin N., Asma’ O. Taybeh, Anas Al-Nabulsi, Murad Al-Holy, Ma’mon M. Hatmal, Jihad Alzyoud, Iman Aolymat, Mahmoud H. Abughoush, Hafiz Shahbaz, Anas Alzyoud, and et al. 2024. "Common and Potential Emerging Foodborne Viruses: A Comprehensive Review" Life 14, no. 2: 190. https://doi.org/10.3390/life14020190
APA StyleOlaimat, A. N., Taybeh, A. O., Al-Nabulsi, A., Al-Holy, M., Hatmal, M. M., Alzyoud, J., Aolymat, I., Abughoush, M. H., Shahbaz, H., Alzyoud, A., Osaili, T., Ayyash, M., Coombs, K. M., & Holley, R. (2024). Common and Potential Emerging Foodborne Viruses: A Comprehensive Review. Life, 14(2), 190. https://doi.org/10.3390/life14020190








